| Case Report | ||
Open Vet J. 2023; 13(2): 247-252 Open Veterinary Journal, (2023), Vol. 13(2): 247–252 Original Research Echocardiographic diagnosis of aorto-left ventricular tunnel with supravalvular pulmonic stenosis in a Shih-tzu dogDaji Noh1, Hyun-guk Shin2, Sooyoung Choi3, Hojung Choi4, Youngwon Lee4 and Kija Lee1*1College of Veterinary Medicine, Kyungpook National University, Daegu, Korea 224 Africa Animal Medical Center, Daejeon, Korea 3College of Veterinary Medicine, Kangwon National University, Chuncheon, Korea 4College of Veterinary Medicine, Chungnam National University, Daejeon, Korea *Corresponding Author: Kija Lee. College of Veterinary Medicine, Kyungpook National University, Daegu, Korea. Email: leekj [at] knu.ac.kr Submitted: 17/10/2022 Accepted: 14/01/2023 Published: 24/02/2023 © 2023 Open Veterinary Journal
AbstractBackground: The aorto-left ventricular tunnel (ALVT) is a congenital extracardiac channel that connects the ascending aorta to the left ventricle. Case Description: A 2-year-old Shih-tzu dog presented with mild exercise intolerance. Echocardiography revealed an abnormal slit-like tunnel structure connecting the ascending aorta to the left ventricle, with diastolic blood flow from the aorta to the left ventricle. Echogenic membranous stenosis was observed in the main pulmonary artery. Based on these findings, the dog was diagnosed with ALVT and type I supravalvular pulmonic stenosis. Conclusion: This is the first case report of ALVT in veterinary medicine that describes diagnostic imaging findings. ALVT should be considered in dogs with an aortic regurgitation murmur and can be detected by echocardiography. Keywords: Aortoventricular tunnel, Congenital heart disease, Dog, Echocardiography, Pulmonic stenosis. IntroductionAn aorto-ventricular tunnel is a congenital abnormal extracardiac channel that connects the ascending aorta to the left or right ventricle, leading to progressive ventricular dilation. To our knowledge, there has been no report of aorto-left ventricular tunnel (ALVT) in dogs and it is extremely rare in humans, accounting for approximately 0.001% of all congenital cardiac diseases (Kathare et al., 2015). In this case report, we present the first case of ALVT in veterinary medicine that describes diagnostic imaging findings. Case DetailsA 2–year-old intact female Shih-tzu dog weighing 3.6 kg presented with a history of mild exercise intolerance. The owner was aware that the patient had an abnormal cardiac murmur since she was 3 months old. Auscultation revealed a decrescendo pandiastolic murmur at the left sternal edge. No abnormalities were detected in the complete blood count, serum biochemical analysis, or electrolyte examination. Thoracic radiography revealed mild left ventriculomegaly and bulging of the ascending aorta. The cardiac size is within normal range with the value of vertebral heart score of 10.0 and vertebral left atrial score of 2.2 (Fig. 1). Transthoracic echocardiography (Affiniti 50; Philips Medical Systems, WA, USA) was performed with a sector-array transducer (4–12 MHz). An abnormal slit-like tunnel structure, 2.2 mm in internal diameter, connecting the ascending aorta to the left ventricle was detected above the sinutubular junction. The opening of this structure was located in the base of the ventriculoaortic junction between the anterior mitral leaflet and non-coronary cusp. The morphology and motion of both the mitral and arterial valves were normal, with no evidence of aortic stenosis, mitral regurgitation, or valvular vegetation (Supplementary video I). During the diastolic phase, a regurgitant jet was detected along the aortic cusps with a 2.8 m/second peak velocity and 32 mmHg pressure gradient and along the anterior mitral leaflet with a 2.3 m/second peak velocity and 22 mmHg pressure gradient from the tunnel opening to the left ventricle (Fig. 2, Supplementary Video I). There was mild left ventricular eccentric hypertrophy with an internal diameter in diastole and systole of 23.7 mm (reference range: 16.70–18.93 mm) and 12.2 mm (reference range: 9.04–10.98 mm), respectively. The normalized value of the end diastolic left ventricular interior dimension was 1.63 (reference range: <1.7). The interventricular septum thickness in diastole and systole was 4.9 mm (reference range: 5.70–7.53 mm) and 6.1 mm (reference range: 8.60–10.64 mm), respectively. The thickness of the left posterior wall during diastole and systole was 4.6 mm (reference range: 4.53–6.03 mm) and 7.0 mm (reference range: 7.97–9.55 mm), respectively. Left ventricular systolic function was normal based on fractional shortening of 48.5% and an ejection fraction of 61.6% obtained by Simpson’s method of discs.
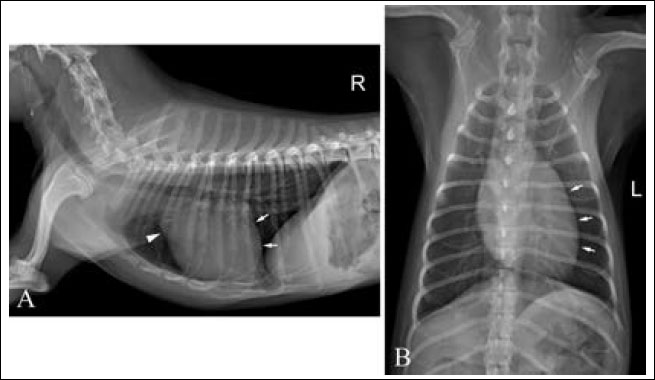
Fig. 1. Right lateral (A) and ventrodorsal (B) thoracic radiographs. mild left-sided enlargement of the cardiac silhouette (white arrows) and mild bulging of ascending aorta (arrowhead) are seen. The cardiac size is within normal range with the value of vertebral heart score of 10.0 and vertebral left atrial score of 2.2.
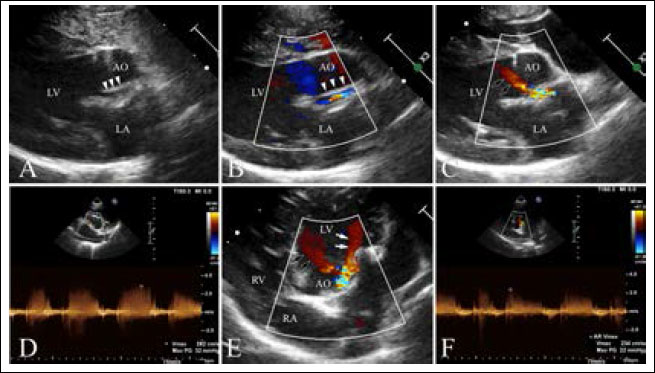
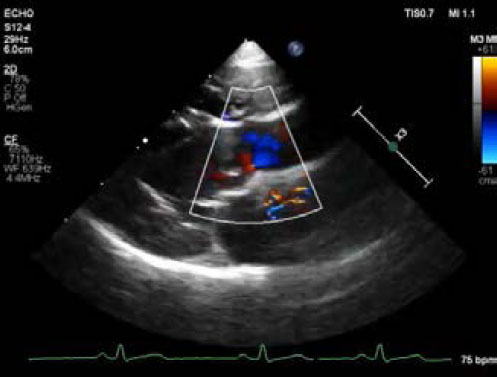
Fig. 2. Echocardiography of right parasternal five chamber view (A–C), oblique right parasternal short axis view of the heart base (D), and oblique left apical long axis view (E,F). (A, B) an abnormal slit-like tunnel structure (arrowheads) next to the aorta is shown. (A,C) The opening of this tunnel structure is located in the base of the ventriculoaortic junction between the non-coronary cusps and anterior mitral leaflet. (D–F) during the diastolic phase, a regurgitant jet is detected along the aortic cusps (black arrows; 2.8 m/second peak velocity, 32 mmHg pressure gradient) and along the anterior mitral leaflet (white arrows; 2.3 m/second peak velocity, 22 mmHg pressure gradient) from the tunnel opening to the left ventricle. In the main pulmonary artery, there was abnormal discrete tissue approximately 7.0 mm away from the pulmonary valve, consistent with a supravalvular stenotic lesion. Color flow and continuous-wave Doppler showed turbulent blood flow originating at the level of the stenotic lesion with a 3.8 m/second peak velocity (57 mmHg pressure gradient). No marked post-stenotic dilation of the main pulmonary artery was observed (Fig. 3, Supplementary Video II). The right atrium and ventricle were normal in size and appearance. Based on these echocardiographic findings, the patient was diagnosed with ALVT with supravalvular pulmonic stenosis. Additional diagnostic investigations, including angiography and cardiac computed tomography, were recommended for surgical planning. However, the owner refused further investigation or medical or surgical treatment. Three months after diagnosis, we attempted but failed to contact the owner by telephone for follow-up. DiscussionThe etiology of ALVT remains unclear; however, it is considered a developmental abnormality. According to the development theory of McKay (2007), the cushions forming the aortic and pulmonary sinuses with their valve leaflet are normally separated by an extracardiac tissue plane due to regression of the surrounding muscle. The coronary arteries are also initially encased by the cuff of the myocardium and grow through it to join the aortic sinuses. Failure to develop this tissue plane may result in a tunnel above the aortic sinuses. This theory could also explain why the proximal coronary artery and valve leaflet are frequently involved in ALVT (McKay, 2007). The type of ALVT may be relevant for treatment and prognosis in veterinary practice. Hovaguimian et al. (1988) classified ALVT into four anatomic types: (1) type I, a simple tunnel with a slit-like opening at the aortic end without valve distortion; (2) type II, a large extracardiac aneurysm of the tunnel with an oval-shaped opening at the aortic end with or without valve distortion; (3) type III, an intracardiac aneurysm of the septal portion of the tunnel with or without right ventricular outflow tract obstruction; and (4) type IV, a combined form of types II and III. According to this previous study, patients with type I ALVT were asymptomatic, in contrast to patients with other types of ALVT (Hovaguimian et al., 1988). In addition, no type II patients died after medical management, indicating the low severity of the pathology in type I compared to the other types (Hovaguimian et al., 1988). In the present case, an abnormal slit-like tunnel structure with normal aortic and mitral valve leaflet was identified on echocardiography, which was consistent with type I ALVT. The patient in this study showed only mild exercise intolerance. In humans, patients with ALVT have symptoms of heart failure due to non-valvular aortic regurgitation and diastolic steal (Kathare et al., 2015). The age at presentation, clinical severity, and progression of heart failure are related to the cross-sectional area of tunnel and the severity of aortic regurgitation, with outcomes ranging from fetal death to asymptomatic in adult life, although most patients show heart failure during infancy or early childhood, leading to death in the first year (Hovaguimian et al., 1988; Kouchoukos et al., 2003; Kathare et al., 2015). In particular, the presence of aortic valvular stenosis, severe dysplasia, or atresia constitutes a lethal combination of malformations (McKay et al., 2002). In this dog, the shape of the ALVT was a simple, slit-like tunnel with mild aortic regurgitation and normal aortic valvular morphology that is presumed to be related to mild clinical signs up to 2 years of age.
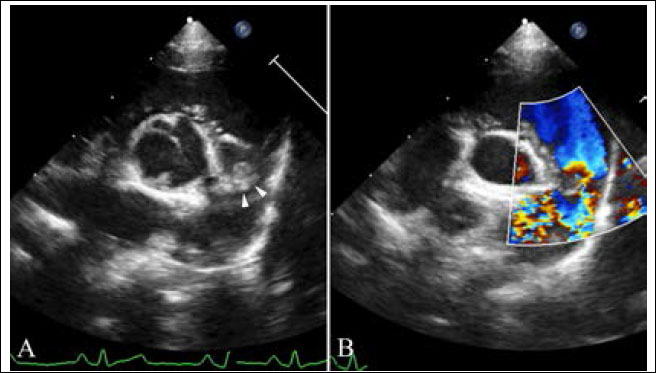
Fig. 3. Echocardiography of right parasternal short axis view showing the right ventricular outflow tract. (A) The supravalvular lesion (arrowheads) is shown distal to the pulmonary valve. (B) color flow Doppler ultrasound shows turbulent blood flow originating at the level of the supravalvular stenotic lesion. Echocardiography is the single most important modality of choice for diagnosing ALVT (McKay et al., 2002; McKay, 2007; Kathare et al., 2015). Two-dimensional echocardiography of the parasternal long-axis view can be used to image the tunnel structure next to the aorta and its openings. In the left apical view, left ventricular dilation and hypertrophy, variable impairment of fractional shortening, and a conserved cardiac structure can be observed (McKay et al., 2002). Color flow Doppler imaging shows blood flow from the aorta to the left ventricle in diastole and the opposite direction in systole, passing through the abnormal channel (McKay et al., 2002; McKay, 2007). Cardiac catheterization with angiography is additionally performed only in cases with inadequate information about the anatomy of the coronary artery or to exclude uncommon associated cardiac anomalies (McKay et al., 2002; Kouchoukos et al., 2003; Sadeghpour et al., 2006; McKay, 2007). In our case, there was an apparent tunnel structure next to the aorta and its opening to the left ventricle, with diastolic blood flow from the aorta to the left ventricle. The reason for the absence of apparent left ventricular hypertrophy and impaired systolic function was thought to be mild ALVT, as shown by mild clinical signs. Although ALVT was not confirmed by surgery or necropsy, in this case, the echocardiographic findings were matched to those in humans and sufficient to diagnose ALVT. In our case, ALVT coexisted with supravalvular pulmonary stenosis. In humans, the reported cardiac anomalies associated with ALVT are proximal coronary anomaly, aortic valvular dysplasia, bicuspid valve with aortic stenosis, pulmonary valve stenosis, and subvalvular pulmonary obstruction due to the tunnel, but not supravalvular pulmonic stenosis (Kathare et al., 2015). Similar to ALVT, supravalvular pulmonic stenosis is also rare and there have been few case reports in dogs (MacGregor et al., 2006; Luciani et al., 2011; Treseder and Jung, 2017). Supravalvular pulmonic stenosis can be classified into four types. Type I is a single constriction of the main, right, or left pulmonary arteries of various lengths. Type II has stenosis at the main pulmonary artery bifurcation, involving the distal portion of the main pulmonary artery. Type III includes multiple stenoses of the pulmonary artery segments at their ostia, and type IV includes multiple stenoses involving peripheral segments (MacGregor et al., 2006). According to these classifications and peak systolic pressure gradients, our case was consistent with mild-to-moderate type I supravalvular pulmonic stenosis. Due to the rarity and limited information on ALVT and supravalvular pulmonic stenosis, it was difficult to infer their etiological association. However, similar to other congenital heart diseases, it is important to confirm other cardiac anomalies when diagnosing ALVT. Surgery or transcatheter tunnel closure is the treatment option, and medical management is only for the control of heart failure while awaiting surgery in ALVT cases. Because of progressive congestive heart failure and aortic regurgitation due to progressive damage to the aortic valve in most patients, early surgical intervention is required, even in asymptomatic cases (Hovaguimian et al., 1988; McKay et al., 2002; Kouchoukos et al., 2003; McKay, 2007; Kathare et al., 2015). Transcatheter tunnel closure is performed only when the tunnel is located away from the coronary ostia with an intact aortic valvular structure without significant aortic regurgitation; however, in some cases, surgical intervention is preferred in humans (McKay et al., 2002; McKay, 2007; Kathare et al., 2015). Although treatment was not performed in our case, a better prognosis would be expected if surgical intervention or transcatheter tunnel closure for ALVT was performed because of an intact aortic valvular structure with mild aortic regurgitation. We presented a case of ALVT with type I supravalvular pulmonic stenosis in a dog. The limitations of the present case were that surgical intervention was not performed, and long-term follow-up was lost. However, this case is still valuable for reporting the first case of ALVT and describing the echocardiographic findings in veterinary medicine. ALVT should be considered in dogs with a pandiastolic murmur, and echocardiography may play a key role in its diagnosis. Conflict of interestThe authors declare that there is no conflict of interest. Author contributionsThis work was carried out in collaboration between all authors. D.N. examined the patient, established the diagnosis, and wrote the first draft. H.S. referred the patient and searched the literature. S.C., H.C., and Y.L. searched the literature and helped in final diagnosis. K.L. helped in the final diagnosis and reviewed and editing the manuscript. All authors read and approved the manuscript. ReferencesHovaguimian, H., Cobanoglu, A. and Starr, A. 1988. Aortico-left ventricular tunnel: a clinical review and new surgical classification. Ann. Thorac. Surg. 45, 106–112. Kathare, P., Subramanyam, R.G., Dash, K.D, Muthuswamy, K.S., Raghu, K. and Koneti, N.R. 2015. Diagnosis and management of aorto-left ventricular tunnel. Ann. Pediatr. Cardiol. 8, 103–107. Kouchoukos, N.T., Blackstone, E.H., Hanley, F.L. and Kirkilin, J.K. 2003. Congenital sinus of Valsalva aneurysm and aortico-left ventricular tunnel. In Kirkilin/Barratt-Boyes cardiac surgery, Eds., Kouchoukos, N.T, Blackstone, E.H., Hanley, F.L. and Kirkilin, J.K. Ann Arbor, MI: Elsevier, pp: 1326–1341. Luciani, A., Sconza, S. and Guglielmini, C. 2011. Supravalvular PA stenosis (PAS) of probable congenital origin. J. Am. Vet. Med. Assoc. 239, 1415–1416. MacGregor, J.M., Winter, M.D., Keating, J., Keating, J., Tidwell, A.S. and Brown, D.J. 2006. Peripheral pulmonary artery stenosis in a four-month-old West Highland White Terrier. Vet. Radiol. Ultrasound. 47, 345–350. McKay, R. 2007. Aorto-ventricular tunnel. Orphanet. J. Rare. Dis. 2, 41. McKay, R., Anderson, R.H. and Cook, A.C. 2002. The aorto-ventricular tunnels. Cardiol. Young. 12, 563–580. Sadeghpour, A., Peighambari, M., Dalirrooyfard, M., Esmailzadeh, M., Maleki, M., Noohi, F., Ojaghi, M. and Samiei, N. 2006. Aorta-to-left ventricle tunnel associated with noncompaction left ventricle. J. Am. Soc. Echocardiogr. 19, 1073. Treseder, J.R. and Jung, S.W. 2017. Balloon dilation of congenital supravalvular pulmonic stenosis in a dog. J. Vet. Sci. 18, 111–114. Supplementary Material
Supplementary Video II. Transthoracic echocardiography of right parasternal short axis view of the heart base. Note abnormal supravalvular lesion distal to the pulmonary valve, consistent with a supravalvular stenosis. There is no marked post-stenotic dilation of the main pulmonary artery. Elongated anterior pulmonary leaflet is seen.
Supplementary Video I. Transthoracic echocardiography of right parasternal five chamber view showing aorto-left ventricular tunnel. Note an abnormal slit-like tunnel structure next to the aorta with regurgitant jet flow from the tunnel opening to the left ventricle during the diastolic phase. The morphology and motion of the mitral and arterial valves are normal. | ||
| How to Cite this Article |
| Pubmed Style Noh D, Shin H, Choi S, Choi H, Lee Y, Lee K. Echocardiographic diagnosis of aorto-left ventricular tunnel with supravalvular pulmonic stenosis in a Shih-tzu dog. Open Vet J. 2023; 13(2): 247-252. doi:10.5455/OVJ.2023.v13.i2.14 Web Style Noh D, Shin H, Choi S, Choi H, Lee Y, Lee K. Echocardiographic diagnosis of aorto-left ventricular tunnel with supravalvular pulmonic stenosis in a Shih-tzu dog. https://www.openveterinaryjournal.com/?mno=121488 [Access: April 25, 2024]. doi:10.5455/OVJ.2023.v13.i2.14 AMA (American Medical Association) Style Noh D, Shin H, Choi S, Choi H, Lee Y, Lee K. Echocardiographic diagnosis of aorto-left ventricular tunnel with supravalvular pulmonic stenosis in a Shih-tzu dog. Open Vet J. 2023; 13(2): 247-252. doi:10.5455/OVJ.2023.v13.i2.14 Vancouver/ICMJE Style Noh D, Shin H, Choi S, Choi H, Lee Y, Lee K. Echocardiographic diagnosis of aorto-left ventricular tunnel with supravalvular pulmonic stenosis in a Shih-tzu dog. Open Vet J. (2023), [cited April 25, 2024]; 13(2): 247-252. doi:10.5455/OVJ.2023.v13.i2.14 Harvard Style Noh, D., Shin, . H., Choi, . S., Choi, . H., Lee, . Y. & Lee, . K. (2023) Echocardiographic diagnosis of aorto-left ventricular tunnel with supravalvular pulmonic stenosis in a Shih-tzu dog. Open Vet J, 13 (2), 247-252. doi:10.5455/OVJ.2023.v13.i2.14 Turabian Style Noh, Daji, Hyunguk Shin, Sooyoung Choi, Hojung Choi, Youngwon Lee, and Kija Lee. 2023. Echocardiographic diagnosis of aorto-left ventricular tunnel with supravalvular pulmonic stenosis in a Shih-tzu dog. Open Veterinary Journal, 13 (2), 247-252. doi:10.5455/OVJ.2023.v13.i2.14 Chicago Style Noh, Daji, Hyunguk Shin, Sooyoung Choi, Hojung Choi, Youngwon Lee, and Kija Lee. "Echocardiographic diagnosis of aorto-left ventricular tunnel with supravalvular pulmonic stenosis in a Shih-tzu dog." Open Veterinary Journal 13 (2023), 247-252. doi:10.5455/OVJ.2023.v13.i2.14 MLA (The Modern Language Association) Style Noh, Daji, Hyunguk Shin, Sooyoung Choi, Hojung Choi, Youngwon Lee, and Kija Lee. "Echocardiographic diagnosis of aorto-left ventricular tunnel with supravalvular pulmonic stenosis in a Shih-tzu dog." Open Veterinary Journal 13.2 (2023), 247-252. Print. doi:10.5455/OVJ.2023.v13.i2.14 APA (American Psychological Association) Style Noh, D., Shin, . H., Choi, . S., Choi, . H., Lee, . Y. & Lee, . K. (2023) Echocardiographic diagnosis of aorto-left ventricular tunnel with supravalvular pulmonic stenosis in a Shih-tzu dog. Open Veterinary Journal, 13 (2), 247-252. doi:10.5455/OVJ.2023.v13.i2.14 |