| Original Article | ||
Open Vet J. 2022; 12(1): 23-32 Open Veterinary Journal, (2022), Vol. 12(1): 23–32 Original Research Exposure to low-dose bisphenol A induces spleen damage in a murine model: Potentially through oxidative stress?Taher Shaibi1*, Hanan N. Balug1, Mohamed E. Ben-Othman3, Ahmeda E. Benjama4, Mohamed Elhensheri5, Bashir A. Lwaleed6 and Mohamed A. Al-Griw41Department of Zoology, Faculty of Science, University of Tripoli, Tripoli, Libya 2Department of Chemistry, Faculty of Science, University of Tripoli, Tripoli, Libya 3Department of Clinical Pathology, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya 4Department of Histology and Genetics, Faculty of Medicine, University of Tripoli, Tripoli, Libya 5Department of Research of Oral and Maxillofacial Surgery, University Medical Centre Rostock, Rostock, Germany 6School of Health Sciences, University of Southampton, Southampton, UK *Corresponding Author: Taher Shaibi. Department of Zoology, Faculty of Science, University of Tripoli, Tripoli, Libya. Email: t.shaibi [at] uot.edu.ly Submitted: 10/09/2021 Accepted: 29/11/2021 Published: 07/01/2022 © 2022 Open Veterinary Journal
AbstractBackground: During early life, exposure to environmental toxicants, including endocrine disruptor bisphenol A (BPA), can be detrimental to the immune system. To our knowledge, a few researches have looked at the effects of developing BPA exposures on the spleen. Aim: The murine model was developed to investigate the underlying molecular mechanisms and mode of BPA actions on the spleen subsequent to prolonged early-life exposure to BPA. Methods: Immature (3-week-old) male and female Swiss Albino mice were intraperitoneally injected with 50 μg/kg BPA in corn oil or corn oil alone for 6 weeks. Mouse spleens were harvested and examined histologically at 10 weeks old (adulthood). Results: We observed neurobehavioral impairments and a significant increase in peripheral monocyte and lymphocyte counts in mice (males and females). Moreover, several spleen abnormalities in both male and female mice were observed in adulthood. BPA-treated mice’s histopathological results revealed toxicity in the form of significantly active germinal centers of the white pulp and a few apoptotic cells. There was also a notable invasion of the red pulp by eosinophils and lymphocytes that were significantly higher than normal. Agarose gel electrophoresis provided further evidence of internucleosomal DNA fragmentation and apoptosis in the splenic tissues of BPA-treated mice compared to controls. In addition, there were increased levels of the lipid peroxidation malondialdehyde end-product, a marker of oxidative lipid damage, in the spleens of BPA-treated mice compared to controls. Conclusion: Our study provides evidence that oxidative stress injury induced by early-life exposures to BPA could contribute to a range of splenic tissue damages during adulthood. Keywords: Apoptosis, Bisphenol A, Murine model, Oxidative stress, Spleen damage. IntroductionEnvironmental exposures to toxicants continue to be a major worldwide public health issue. A growing body of research has cautioned against the extensive use of environmental toxicants due to their longevity in the environment and their accumulation in the living tissues of organisms (Camarca et al., 2016; Maamar et al., 2016; Al-Griw et al., 2017; Gassman, 2017). It is estimated that exposures cause approximately 24% of human abnormalities/diseases to toxicants, with the risk of abnormalities/diseases being passed on to future generations without direct exposure (Hou et al., 2012). Previous works revealed that environmental exposure to toxicants during intrauterine life, postnatal-life, early-life, and/or germ cell plays a significant role in determining the mature phenotype and vulnerability to abnormalities/diseases in later life (Skinner et al., 2013). Evaluating the chemical toxicity, such as cadmium, mercury, dioxin, trichloroethane, and bisphenol A (BPA), shows that some of these substances immediately affect the ecosystem. On the contrary, the others can result in subtle alterations delayed in the expressions (Al-Griw et al., 2016). The environmental toxicant BPA, known as an endocrine-disrupting chemical, has been studied concerning cancers, metabolic disturbances, and reproductive and developmental toxicity (Dong et al., 2013; Camarca et al., 2016). People of all ages are inadvertently exposed to BPA regularly due to its widespread use in the manufacturing of plastic containers for food and beverage (Gassman, 2017; Gear and Belcher, 2017). Several studies demonstrated that BPA attributed exposures to cytotoxic, genotoxic, and carcinogenic consequences; nevertheless, their findings are still hotly disputed. There is no clear consensus on BPA’s safety or function in human illnesses. It has been shown that developmental exposure to BPA may compromise the immune responses later on in life (Camarca et al., 2016; Gassman, 2017). Specifically, in vitro BPA exposure increased the proliferation of murine splenocytes (Dong et al., 2013). Experiments carried out to investigate the impact of BPA exposures on murine spleens showed that BPA has a dose- and sex-specific effect on the cellular and micro-anatomical structures of the spleen, showing minor alterations in immunomodulatory and hematopoietic functions, irrespective of gender (Gear and Belcher, 2017). One of the contributing factors may be the lack of understanding of molecular mechanisms and mode of actions that designate the various pleiotropic effects seen during BPA exposure in both humans and wildlife (Acconcia et al., 2015). Increasing evidence links BPA exposure to oxidative stress (Asimakopoulos et al., 2016; Maamar et al., 2016). Furthermore, pathological studies involved oxidative stress as a cause of cell death (Li et al., 2010). Oxidative stress may affect cell integrity but only when antioxidant mechanisms are compromised and therefore become unable to deal with the generation of free radicals (Wang et al., 2013). A variety of reactive oxygen species (ROS)-mediated modifications of proteins was documented in many diseases (Wang et al., 2013). ROS have been linked to the development of autoimmune disorders by disrupting immune function, causing autoantigen generation by oxidative modification, and inducing the creation of autoantibodies (Li et al., 2010). According to mounting evidence, ROS-modified proteins can trigger an autoimmune response and contribute to disease development (Ben Mansour et al., 2010). Indeed, autoimmune illness patients have greater amounts of malondialdehyde (MDA)/4-hydroxynonenal (HNE)-modified proteins and protein carbonyls, indicating that these oxidatively changed proteins have a role in autoimmune disorders (Ben Mansour et al., 2010). ROS formations, such as the superoxide anion (O2-) and hydroxyl (HO-) radicals, can lead to alterations in the enzyme activity, decreased DNA repair, impaired utilization of oxygen, glutathione depletion, and lipid depletion peroxidation. Some of these alterations induced by oxidative stress are recognized to be characteristic features of cell death (Khan et al., 2001). To the best of our knowledge, a few studies investigated the impacts of developmental BPA exposures on the splenic tissue architecture in later life. Since the spleen includes vascular and lymphoid components and is a major site for activating primary immune responses (Suttie, 2006; Dong et al., 2013), there is increased interest in ascertaining molecular mechanisms and mode of actions underlying spleen disorders induced by BPA. The impacts of early-life exposure to low-dose BPA on the peripheral blood components and spleens of male and female mice at adulthood were examined in a novel murine animal model focusing on oxidative stress. Materials and MethodsStudy design and animal husbandryA total of 36 immature (3-week-old) male and female Swiss Albino mice weighing 14 ± 2 g were used in this study. The animals were bred in the animal house of the Zoology Department, Faculty of Sciences, University of Tripoli (Tripoli, Libya); they were housed under normal lighting conditions (12-hour cycle) and temperature (26°C ± 2°C). The animals were fed with a standard diet and water ad libitum. Mice were selected as the preferred animals for these experiments because of the resemblance of the mouse and human spleen in terms of anatomy and hemodynamics with a slight splenic microstructure. They were randomly divided into 3 groups, including 12 mice in each: sham control, vehicle control, and BPA (Sigma-Aldrich Chemical; St. Louis, MO) 50 μg/kg group, BPA was dissolved in corn oil. The sham control group did not receive treatment, while the vehicle control group was given corn oil. The dose of BPA (50 μg/kg) was chosen based on previous studies (Sadowski et al., 2014). The animals were dosed for 6 weeks intraperitoneally (ip); subsequently, they were euthanized under anesthesia using sodium pentobarbital (Sigma-Aldrich Chemical; St. Louis, MO) at the age of 10 weeks. Clinical assessmentThe clinical assessment included animal survival, body weight, and locomotor activity. During the exposure period, mice were observed twice daily for any abnormal clinical signs or behavior that may result from toxicity. To exclude out non-BPA-related deaths, night fatalities were recorded. Motor behavior was measured throughout the study course as previously described (Al-Griw et al., 2015). Peripheral blood and tissue harvestBlood was withdrawn from the inferior vena cava. Sera from mice were obtained following blood clotting and centrifugation, and samples were stored in a small aliquot at −20°C until batch-wise analysis. The animals were then sacrificed, and the spleen of each mouse was then rapidly excised and weighed. Tissue samples were quickly frozen at −70°C for subsequent measurements of oxidative stress biomarker. Other portions of the splenic tissues were immediately used for the investigations detailed below. Histopathology processingThe spleens were first preserved in 10% formalin immediately. Histological slides were prepared and identified as previously described (Al-Griw et al., 2016; Maamar et al., 2016). Peripheral blood leukocyte countWhite blood cells (WBCs) were counted as previously described (Maamar et al., 2016) on a blood film from control and BPA-treated mice. Differential WBC counts were carried out under the high power of the microscope (Leica), field by field. Lipid oxidative stress measurementLipid peroxidation levels in spleens were determined spectrophotometrically as a concentration of final lipid peroxidation products, which upon reacting with thiobarbituric acid (TBARS) form a color complex (TBARS-reactive substances) (Zhang et al., 2004). Splenic tissues were homogenized with a tissue homogenizer (IKA, RW 20. n, Germany) in ice-cold 10% (w/v) phosphate-buffered saline solution. After centrifugation, a 500 ml aliquot of spleen homogenate samples was mixed with 2 ml TCA–TBA–HCl reagent (TBARS 0.37%, 0.24 N HCl, and 15% TCA), heated for 15 minutes at 100°C, and then cooled. The supernatant was removed after centrifugation at 3,000 rpm for 10 minutes, and the absorbance was measured at 532 nm. The calibration curve was obtained using different concentrations of 1, 1, 3, 3-tetra methoxy propane as standard to determine the TBA–MDA adducts concentration in the tissue samples. DNA isolation and electrophoresisDNA was isolated using a QIAamp DNA Minikit (Qiagen, Germany). Briefly, up to 25 mg of the tissue samples were ground into small pieces and homogenized in a DNA lysis buffer. Proteinase K (2 mg/ml) was then incubated in the same buffer overnight at 56°C. Samples were treated with RNase A (20 mg/ml), purified on the spin column, and eluted with Tris/EDTA buffer. The extracted DNA was measured by UV spectrophotometry (BioPhotometer, Eppendorf, UK), with an absorbance of A260/A280 nm ratios at pH 8.0. To determine the integrity of the extracted DNA, 3 μg of each DNA extract were fractionated by electrophoresis on 1.5% agarose gel. The gel was stained with Gel Red™ (Sigma-Aldrich, Germany), and the DNA bands were visualized under a UV light source. MicroscopyThe spleen structure under light microscopy (Leica, Germany) was studied and imaged using a low-power objective. Two independent investigators investigated all histological assessments blindly. StatisticsStatistical analyses were carried out using Statistical Package for the Social Sciences (SPSS) version 20 (IBM SPSS version 20.0 Inc., Chicago, IL). Data are expressed as means ± SEM. Normality was assessed using the Kolmogorov–Smirnov test. Two-way analysis of variance (ANOVA), followed by a post-hoc test for multiple comparisons (Dunnett’s), was used to determine the statistical significance between the treatment groups and vehicle control within male and female mice. p-values <0.05 were considered statistically significant. Ethical approvalThis study was carried out in compliance with the international guidelines set out in the Declaration of Helsinki (Anonymous, 2014). All efforts were made to meet the ethical standards of experimentation, such as minimizing pain during animal handling and experiments and reducing the number of animals used. Ethical approval was obtained from Bioethics Committee of the Biotechnology Research Center with approval number BEC-BTRC 10-2019. The refinement process was carried out by trained personnel utilizing recognized methodologies. In addition, every effort was made to provide the greatest possible housing, enrichment, and analgesic conditions for the animals. ResultsEffects of BPA on animal survivalThroughout the study, no mortality was recorded among the studied groups, except one death in the control group, 3 weeks from the start of the experiments. Effects of BPA on motor activityExposure to low-dose BPA was significantly (p < 0.05) associated with abnormalities in the neurobehavioral performance of male and female mice at adulthood compared to controls. BPA-treated mice displayed turning motor activity 2 hours after treatment, while the vehicle did not affect motor behavior (Fig. 1). Effects of BPA on body and spleen weightsAll animals in this study had their body weights tracked from about 3 weeks of life to the end of the trial, with final body and spleen weights recorded for examination at necropsy (~10 weeks of age). ANOVA revealed significant changes in body weights between controls and the BPA group (p < 0.05) for body weights (Fig. 2A). However, there was no sex-specific effect on the animal body weights in all mice groups. For spleen weights (Fig. 2B), there was a significant (p < 0.05) increase in the spleen weights of BPA-exposed mice when compared to controls. There were no significant differences between the spleen weight of males and females in the two groups of mice. Effects of BPA on peripheral blood cellular componentsThe quantitative analysis showed a significant (p < 0.05) increase in the total WBC counts (leukocytosis) in the adult male and female mice exposed to BPA compared to controls (Fig. 3A and B). Specifically, we observed a significant increase in the lymphocyte and monocyte counts in the adult males and females exposed to BPA compared to the control group (data not shown). Conversely, neutrophils, eosinophils, and basophils counts were within the normal range (data not shown). There were no differences in the total WBC counts between males and females in all mice groups. Histopathological investigation of the splenic tissuesHistopathological assessment of BPA-treated splenic tissues was blindly carried out, considering splenic tissue architecture changes and cell population changes. There were no obvious histopathological alterations in the spleens of male and female control mice (Fig. 4A). Mainly, the control spleens exhibited normal morphologic white and red pulp structures except for some apoptotic lymphocytes in the germinal centers and mildly activated megakaryocytes represented in increased counts of primitive cells. The red pulp was infiltrated by mature lymphocytes and a few eosinophils, including mild apoptotic changes (Fig. 4A, panels i–iii`). The splenic tissues from BPA-treated mice showed highly activated germinal centers of the white pulp with minimal apoptotic features and a prominent megakaryocytosis and lymphohistiocytic infiltrate of the red pulp, and comparatively increased counts of eosinophils and mature lymphocytes were detected (Fig. 4A, panels iii and iii`).
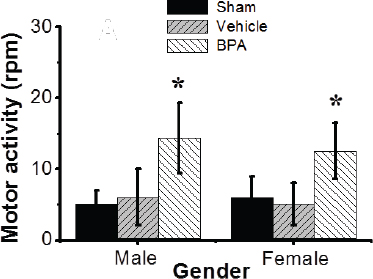
Fig. 1. Effects of BPA exposure on neurobehavioral performance. Motor behavior in males and females exposed to control or BPA conditions. Data are shown as mean ± SEM (n=12 per group). *Significant differences from same sex control (p ≤ 0.05) by Dunnett’s multiple comparison test.
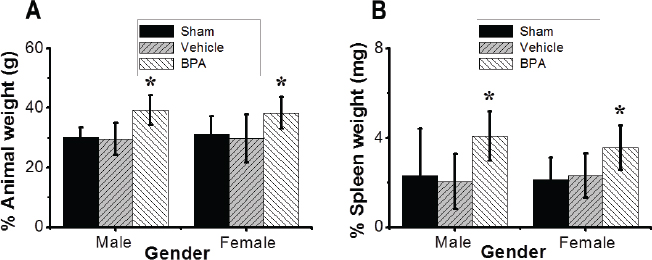
Fig. 2. Effects of BPA exposure on body and spleen weights. (A) Animal body weight of males and females exposed to control or BPA conditions. (B) Spleen weight of adult males and females exposed to control or BPA conditions. Data are shown as mean ± SEM (n=12 per group). *Significantly different from same sex control (p ≤ 0.05) by Dunnett’s multiple comparison test. Additionally, a histiocytosis was represented by a focal replacement of the red pulp and parts of the white pulp, by increased macrophage counts. Multifocal erythrocytes extravasation and multiple hemosiderosis were detected in the red pulp (Fig. 4A, panels ii and iii). There were also apoptotic changes in the germinal centers of the white pulp. In addition, the walls of the central arterioles were ruptured and showed increasingly narrow lumens. Furthermore, quantitative analysis showed a statistically significant increase in spleen abnormalities in BPA-exposed mice compared to the control group (Fig. 4B). There were no differences in the microanatomical structure between males and females in all mice groups.
Fig. 3. Effects of BPA exposure on the peripheral blood cellular components. (A) Total WBC counts in the peripheral blood film of different mice groups. (B) The count of total WBC counts in different groups. Data are shown as mean ± SEM (n=12 per group). *Significantly different from same sex control (p ≤ 0.05) by Dunnett’s multiple comparison test.
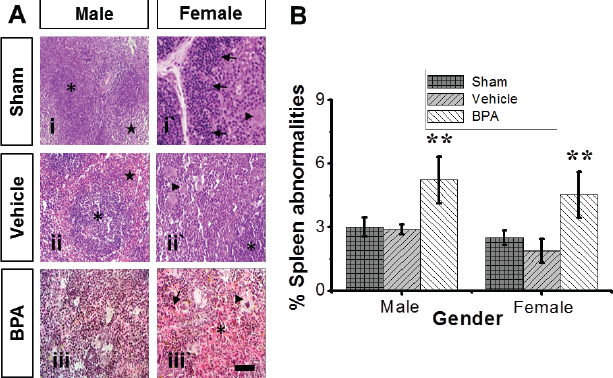
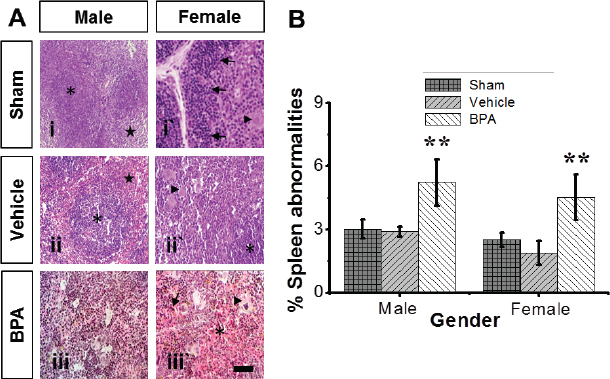
Fig. 4. Representative photomicrographs of the adult male and female microanatomical structures and histopathological findings of spleens in control and BPA-treated mice. (A) H&E-stained sections show lymphohistiocytic infiltrate of red pulp with multifocal erythrocytes extravasation (asterisk), multifocal hemosiderosis (arrow), and prominent extramedullary hematopoiesis of the spleen (arrowhead) in male and female BPA-treated mice (panels iii and iii`) compared to male and female control mice (panels i and ii`), revealing prominent lymph follicles (asterisk) and red pulp (stern) with easily discernable periarteriolar lymphocyte sheaths (arrow) and extramedullary hematopoiesis of the spleen (arrowhead). Scale bars indicate 100 μm, 100×. (B) Percentages of mice with spleen abnormalities. Data are shown as mean ± SEM (n=12 per group). *Significantly different from same sex control (p ≤ 0.05) by Dunnett’s multiple comparison test.
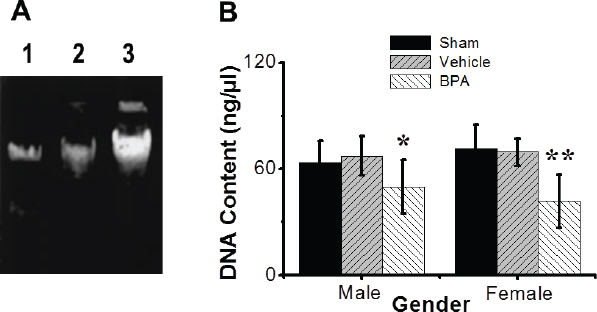
Fig. 5. Effects of BPA exposures on DNA biomarkers (quality and integrity) of splenic tissues. (A) Agarose gel electrophoresis of DNA isolated from splenic tissues of the male and female control and BPA-treated groups. Lane 1, sham control group; lane 2, vehicle control group; and lane 3, BPA-treated group. Almost no DNA degradation was detected in controls. (B) Quantification of DNA concentration. DNA content (ng/μl) was examined with mean ± SEM presented (n=12 per group). *Significantly different from same sex control (p ≤ 0.05) by Dunnett’s multiple comparison test.
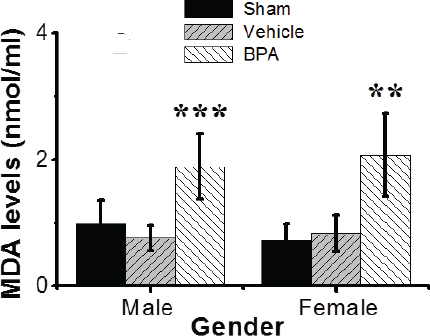
Fig. 6. Effects of BPA exposure on oxidative stress biomarker (MDA) levels (nmol/ml) in the splenic tissues of control and BPA-treated groups. MDA levels (nmol/ml) were examined with the mean ± SEM presented (n=12 per group). Data are shown mean ± SEM (n=12 per group). *Significantly different from same sex control (p ≤ 0.05) by Dunnett’s multiple comparison test. Analysis of DNA fragmentationNuclear DNA from mouse groups was analyzed by agarose gel electrophoresis (Fig. 5A). DNA from the sham and vehicle control-operated spleens was largely intact and exhibited no/little internucleosomal DNA fragmentation (Fig. 5A, lanes 1 and 2). However, DNA from the BPA-exposed group exhibited a characteristic nucleosome ladder, which might have resulted from DNA endonucleolytic digestion (Fig. 5A, lane 3). A smear pattern resulting from random DNA degradation suggests that necrosis might have occurred concurrently with apoptosis. There was no difference in the DNA integrity between adult males and females in all mouse groups. Quantitative analysis showed that BPA-exposed mice exhibited a significant decrease in the DNA concentration, i.e., 1.4-fold, compared to control mice. Still, the vehicle alone had no significant effect when administrated under control conditions (Fig. 5B). There was no difference in the DNA concentrations between adult males and females in all mice groups investigated. Effects of BPA on oxidative stress indices in the spleensHere we found that early-life exposures to low-dose BPA resulted in a significant (p < 0.01) increase in the levels of spleen MDA, a marker of oxidative lipid damage, compared to the control group (Fig. 6). DiscussionA growing body of evidence indicates that exposure to environmental chemicals can cause serious detrimental effects on different body systems (Camarca et al., 2016; Maamar et al., 2016; Al-Griw et al., 2017; Gassman, 2017). The endocrine-disrupting chemical BPA has specifically received increased attention due to its worldwide distribution with large exposure (Camarca et al., 2016). Human exposure is thought to be virtually ubiquitous (Gassman, 2017). Numerous studies have suggested links between BPA exposures and metabolic disorders, such as obesity and diabetes, and cardiovascular diseases (Inadera, 2015; Gassman, 2017). Due to the lack of knowledge of mechanisms and modes of action that might explain the varied and pleiotropic effects reported after BPA, this study aims to use an in vivo model to elucidate the possible molecular mechanism of splenic tissue injury induced by the environmentally relevant level of BPA exposures using a novel murine animal model. Our study demonstrates that early-life exposures to a low dose of the environmental toxicant BPA resulted in abnormalities in the spleens of male and female mice at adulthood based on the nontoxic a pharmacological dose 0.1% of the oral LD50 for BPA (Vom-Saal and Hughes, 2005; Sadowski et al., 2014). These findings are based on careful evolutions using different approaches, including spleen histology, oxidative stress biomarker MDA, and nuclear alterations, related to splenic tissue damage molecular mechanisms in all mice groups studied. For example, BPA induced alterations in peripheral blood cell counts. Lipid oxidative stress biomarker and apoptosis were significantly increased in the BPA-treated mice compared to controls. The in vivo models used to study the toxicity of chemicals are of great importance because animal systems are extremely complicated, and the interaction of chemical compounds with biological components could lead to unique bio-distributions and some disturbances in immune responses and metabolism. As a result, when assessing the immunomodulatory actions of endocrine disruptors in a specific organ or cell type, choosing an appropriately sensitive animal model to evaluate the toxicity of environmental chemicals with adverse health risks at birth or later in life has proven to be an essential factor for generating informative data. Immunotoxicity screening studies must be able to distinguish sex-specific immunomodulatory effects of endocrine disruptors due to the known sexually dimorphic nature of immunological responses and concerns related to individual susceptibility. As a result, based on known differences in responses and sensitivities, all endpoints studied here were addressed individually for male and female mice. Exposure to BPA was found in both male and female mice, with sex-specific effects of BPA exposures observed in both males and females (Suttie, 2006; Gear and Belcher, 2017). Exposure to environmental toxicants can significantly affect human health even at low environmental doses, which may not be apparent at higher doses used in traditional toxicological studies (Camarca et al., 2016). The US Environmental Protection Agency and the European Food Safety Authority have established a tolerable daily intake, or reference dose, of 50 mg/kg/day for BPA as a “safe dose.” However, several researchers suggested that BPA at such a dose causes various adverse effects (Vom-Saal and Hughes, 2005; Sadowski et al., 2014). Fetuses and/or newborns are more sensitive than adults, and exposure to chemicals during critical stages of development may cause irreversible long-lasting consequences (Newbold, 2004; Koike et al., 2018). Despite this body of evidence, the health risks associated with chronic and low dose exposures to BPA remain controversial (Gassman, 2017). In the present study, we chose an in vivo murine model to investigate the long-term impacts of early-life exposures to low-dose BPA (50 μg/kg/day, ip, for 6 weeks) on the spleens of male and female mice in adulthood. Our study shows that BPA exposure associates negatively with increased body and spleen weights of adult male and female mice. Prenatal and early postnatal BPA exposures have been linked to increased body weight in rats in several studies (Magliano and Lyons, 2013; Picard and Turnbull, 2013). On the contrary, it was reported that perinatal exposure to BPA at 2.4 μg/kg/day was associated with increased body weight in adulthood. Still, postnatal exposure (from weaning to adulthood) was not (Akingbemi et al., 2004). The BPA toxic effects on the spleen are dependent on the doses and route of administration (Yildiz and Barlas, 2013). For example, BPA induced functional and structural changes in the liver, kidney, and spleen after rats aged 4–5 weeks were exposed to 125 mg BPA/kg/day via oral route for 13 weeks (Yildiz and Barlas, 2013). There was no effect of BPA on the liver and kidney when mice were exposed to 5 mg BPA/kg/day in dietary, while the toxic effects were found when exposed to 50 or 600 mg BPA/kg/day (Tyl et al., 2008; Dong et al., 2013). Accumulating evidence indicates that neurobehavioral disorders arise from environmental toxicants (Kulig, 1987; Al-Griw et al., 2015). BPA has been shown to negatively impact neurological development and neurobehavioral disorders (Sathyanarayana et al., 2011; Inadera, 2015). Here we found that early-life BPA exposure is significantly associated with neurobehavioral abnormalities in male and female mice in adulthood. It is noted that BPA increases motor activity in male and female mice at the dose used in these experiments and causes turning behavior. Still, vehicles do not alter locomotion in male and female control groups, suggesting the BPA has neurotoxicity. It has been reported that exposure to a dose between 4 and 40,000 μg/kg/day BPA from conception until 12–14 weeks of age-induced microstructural changes in mouse spleens (Gear and Belcher, 2017). These findings also show that BPA has dose- and sex-dependent influences on the cellular and microanatomical structures of the spleen, revealing modest changes in immunomodulatory and hematological activities. In agreement with other reports, this study showed that BPA exposures induce splenomegaly in adult male and female mice, possibly due to inflammatory processes. In addition, our data showed serious histological and microstructural changes in the spleens of male and female mice exposed to BPA. There were also changes in the splenic tissue architecture; however, alterations were noticed in the control group. Such changes included lymphocyte depletion in the white pulp associated with many aggregates of apoptotic cells. Furthermore, the core arterioles’ walls broke, revealing progressively smaller lumens. There were also significant alterations in the red pulp, such as increased macrophages, neutrophils, and pyknotic cell nests. The detection of cell pyknosis in the spleen may be related to increased splenocyte susceptibility to apoptosis, which may be an important mechanism of autoimmune diseases and immune senescence (Hsu and Mountz, 2003). Similar spleen changes were previously reported (Maamar et al., 2016), and all of these changes may be attributed to a loss of infiltration efficiency. The BPA-treated group had a significant increase in the number of apoptotic splenocytes, while controls had only rare apoptotic cells. A limitation of this study is that the cell death mechanism(s) triggered by BPA was not investigated. A splenocyte cell death was viewed to occur by either apoptosis or necrosis following spleen injury (Li et al., 2010). Exposures to environmental toxicant BPA can disrupt the immune system even at lower doses (Rogers et al., 2013; Schug et al., 2013; Camarca et al., 2016; Koike et al., 2018; Özaydın et al., 2018). It is important to note that there are other mechanisms through which BPA might affect the immune cells independent mitochondrial function. For example, how does BPA induce oxidative stress in the spleen and give rise to mitochondrial integrity changes? BPA induced mitochondrial dysfunction in the liver concurrent with increases in hepatic oxidative stress (Moon, 2012). Mitochondria are very susceptible to oxidative stress due to their double membrane-enclosed structures (Picard and Turnbull, 2013). Thus, mitochondrial dysfunction can induce abnormal cell metabolism or even apoptosis (Dong et al., 2013). An in vitro study showed that BPA could induce apoptosis via damage to the mitochondria in rat cells (Lin, 2013). Several studies have indicated that the possible mechanism of BPA in immune regulation is mainly through activity at estrogen receptors on immune cells (Miao, 2008). Recently, the association between BPA exposures and oxidative stress has become a focus of study for an additional potential mechanism of toxicity. Studies have shown that BPA exposures were associated with an increased risk of asthma; both oxidative stress and modulated inflammation are thought to be involved in the underlying mechanisms that relate to BPA exposures (Chepelev, 2013; Selgrade et al., 2013; Gassman, 2017). Induction of ROS by BPA may contribute significantly to its toxicity (Gassman, 2017). It has been reported that ROS-modified proteins, such as carbonyls and lipid peroxidation-derived aldehydes, including MDA and HNE-protein adducts, may elicit an autoimmune response and contribute to disease pathogenesis (Ben Mansour et al., 2010; Wang et al., 2013). Patients with autoimmune diseases had greater amounts of MDA/HNE-modified proteins and protein carbonyls (Ben Mansour et al., 2010). Investigations exploring the production of oxidative stress by BPA, like other parts of BPA research, have produced inconsistent results concerning pro-oxidant/antioxidant activity (Babu et al., 2013; Gassman, 2017). To offer new evidence for the role of oxidative stress in BPA-induced spleen abnormalities, we investigated the oxidative stress marker MDA in the splenic tissues compared with controls. BPA exposures showed a significant increase in the splenic tissue MDA level, where BPA could generate free radicals and induce autoimmune disorders (Wang et al., 2013). The results demonstrated that oxidative stress is a potential mechanism mediating spleen abnormalities. The findings and conclusions of our study indicated that BPA does not have sex-specific impacts in the spleen consistent with alterations in hematopoietic and immunomodulatory functions in both sexes. Taken together, it is plausible to assume that the oxidative stresses induced by early-life exposures to low-dose BPA may contribute to spleen abnormalities in male and female mice in adulthood. ConclusionThis study found that early-life exposure to a low dose of BPA promotes spleen damage in adult male and female mice. In addition, it induced changes in peripheral blood components and increased oxidative stress damage to splenic tissues. This injury may be useful as early-stage biomarkers of toxicant exposure and adult-onset abnormality/disease. Although not designed for risk assessment, these findings have implications for people exposed to various toxicants. The degree of environmentally induced phenotypic variations implicated in various disease etiologies remains unknown. Thus, a robust and proper understanding of the molecular basis of disease processes, including the role of environmental epigenetics, could provide insights into new diagnostic and/or therapeutic options for certain disease conditions. To explore further the molecular mechanisms and mode of actions of splenic tissue damage induced by BPA exposure, further studies focusing on the potential pathways mediating splenocyte death and the effect of such damage on the quantity and the characteristics of circulating blood cells would be required. AcknowledgmentThe authors would like to express their deepest appreciation to Sana Algahmasi for her expert technical assistance. Conflict of interestThe authors have no conflict of interest to declare. FundingThis work was funded by the University of Tripoli. Authors’ contributionsMAA and HNB conception, design, and organization of the study; MAA, HNB, and ROA contributed to the conduct of the study; ROA and HNB acquisition of data; MAA, HNB, and ROA analysis and interpretation of data; MAA, TS, ROA, MEB, AEB, ME, and BAL drafting of the manuscript and critiquing the output for important intellectual content. All authors discussed the results and commented on the manuscript. ReferencesAcconcia, F., Pallottini, V. and Marino, M. 2015. Molecular mechanisms of action of BPA. Dose Response 13, 1559325815610582. Akingbemi, B.T., Sottas, C.M., Koulova, A.I., Klinefelter, G.R. and Hardy, M.P. 2004. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endocrinology 145, 592–603. Al-Griw, A.M., Treesh, S.A., Alghazeer, R.O. and Regeai, S.O. 2017. Environmentally toxicant exposures induced intragenerational transmission of liver abnormalities in mice. Open Vet. J. 7, 244–253. Al-Griw, M.A., Al-Ghazeer, R.O., Al-Azreg, S.A. and Bennour, E.M. 2016. Cellular and molecular etiology of hepatocyte injury in a murine model of environmentally induced liver abnormality. Open Vet. J. 6, 150–157. Al-Griw, M.A., Elnfati, A.H., Salama, N.M., Maamar, M.S., Treesh, S.A. and Shaibi, T. 2015. Mode of cell death in mouse brain following early exposure to low-dose trichloroethane: apoptosis or necrosis. Am. J. Biol. Life Sci. 3, 232–240. Anonymous. 2014. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J. Am. Coll. Dent. 81, 14–18. Asimakopoulos, A.G., Xue, J., De Carvalho, B.P., Lyer, A., Abualnaja, K.O., Yaghmoor, S.S., Kumosani, T.A. and Kannan, K. 2016. Urinary biomarkers of exposure to 57 xenobiotics and its association with oxidative stress in a population in Jeddah, Saudi Arabia. Environ. Res. 50, 573–581. Babu, S., Uppu, S., Claville, M.O. and Uppu, R.M. 2013. Prooxidant actions of bisphenol A (BPA) phenoxyl radicals: implications to BPA-related oxidative stress and toxicity. Toxicol. Mech. Methods 23, 273–280. Ben Mansour, R., Lassoued, S., Elgaied, A., Haddouk, S., Marzouk, S., Bahloul, Z., Masmoudi, H., Attia, H., Aïfa, M.S. and Fakhfakh, F. 2010. Enhanced reactivity to malondialdehydemodified proteins by systemic lupus erythematosus autoantibodies. Scand. J. Rheumatol. 39, 247–253. Camarca, A., Gianfrani, C., CimminoI, A.F., Bruzzese, D. and Scerbo, R. 2016. Human peripheral blood mononuclear cell function and dendritic cell differentiation are affected by bisphenol-aexposure. PLoS One 11, e0161122. Chepelev, N.L. 2013. Bisphenol A activates the Nrf1/2-anti-oxidant response element pathway in HEK 293 cells. Chem. Res. Toxicol. 26, 498–506. Dong, Y., Zhai, L., Zhang, L., Jia., L. and Wang, X. 2013. Bisphenol A impairs mitochondrial function in spleens of mice via oxidative stress. Mol. Cell. Toxicol. 9, 401–406. Gassman, N.R. 2017. Induction of oxidative stress by bisphenol A and its pleiotropic effects. Environ. Mol. Mutagen. 58, 60–71. Gear, R.B. and Belcher, S.M. 2017. Impacts of bisphenol A and ethinyl estradiol on male and female cd-1 mouse spleen. Sci. Rep. 7, 856. Hou, L., Zhang, X., Wang, D. and Baccarelli, A. 2012. Environmental chemical exposures and human epigenetics. Int. J. Epidemiol. 41, 79–105. Hsu, H.C. and Mountz, J.D. 2003. Origin of late-onset autoimmune disease. Immunol. Allergy Clin. N. Am. 23, 65–82. Inadera, H. 2015. Neurological effects of bisphenol A and its analogues. Int. J. Med. Sci. 12, 926–936. Khan, M.F., Wu, X. and Ansari, G. 2001. Anti-malondialdehyde antibodies in MRL+/+ mice treated with trichloroethene and dichloroacetyl chloride: possible role of lipid peroxidation in autoimmunity. Toxicol. Appl. Pharmacol. 170, 88–92. Koike, E., Yanagisawa, R., Win-Shwe, T.T. and Takano, H. 2018. Exposure to low-dose bisphenol A during the juvenile period of development disrupts the immune system and aggravates allergic airway inflammation in mice. Int. J. Immunopathol. Pharmacol. 32, 1–14. Kulig, B.M. 1987. The effects of chronic trichloroethylene exposure on neurobehavioral functioning in the rat. Neurotoxicol. Teratol. 9, 171–178. Li, J.L., Li, S., Li, S., Tang, Z.X., Xu, S.W. and Wang, X.L. 2010. Oxidative stress-mediated cytotoxicity of cadmium in chicken splenic lymphocytes. Pol. J. Environ. Stud. 19, 947–956. Lin, Y. 2013. Exposure to bisphenol A induces dysfunction of insulin secretion and apoptosis through the damage of mitochondria in rat insulinoma(INS-1) cells. Cell Death Dis. 4, e460. Maamar, M.S., Al-Griw, M.A., Al-Ghazeer, R.O., Al-Azreg, S.A., Salama, N.M. and Bennour, E.M. 2016. Oxidative stress mediated cytotoxicity of trichloroethane in a model of murine splenic injury. Am. J. Biosci. 4, 1–8. Magliano, D.J. and Lyons, J.G. 2013. Bisphenol A and diabetes, insulin resistance, cardiovascular disease and obesity: controversy in a(plastic) cup? J. Clin. Endocrinol. Metab. 98, 502–504. Miao, S. 2008. Influence of bisphenol A on devel-oping rat estrogen receptors and some cytokines in rats: a two-generational study. J. Toxicol. Environ. Health 71, 1000–1008. Moon, M.K. 2012. Bisphenol A impairs mitochondrial function in the liver at doses below the no observed adverse effect level. J. Korean Med. Sci. 27, 644–652. Newbold, R.R. 2004. Lessons learned from perinatal exposure to diethylstilbestrol. Toxicol. Appl. Pharmacol. 199, 142–150. Özaydın, T., Öznurlu, Y., Sur, E., İ., Ç. and D., U. 2018. The effects of bisphenol A on some plasma cytokine levels and distribution of CD8+ and CD4+ T lymphocytes in spleen, ileal Peyer’s patch and bronchus associated lymphoid tissue in rats. Acta Histochem. 120, 728–733. Picard, M. and Turnbull, D.M. 2013. Linking the metabolic state and mitochondrial DNA in chronic disease, health, and aging. Diabetes 62, 672–678. Rogers, J.A., Metz, L. and Yong, V.W. 2013. Review: endocrine disrupting chemicals and immune responses: a focus on bisphenol-A and its potential mechanisms. Mol. Immunol. 53, 421–430. Sadowski, R.N., Wise, L.M., Park, P.Y., Schantz, S.L. and Jurask, J.M. 2014. Early Exposure to bisphenol a alters neuron and glia number in the rat prefrontal cortex of adult males, but not females. Neuroscience 279, 122–131. Sathyanarayana, S., Braun, J.M., Yolton, K., Liddy, S. and Lanphear, B.P. 2011. Case report: high prenatal bisphenol a exposure and infant neonatal neurobehavior. Environ. Health Perspect. 119, 1170–1175. Schug, T.T., Heindel, J.J., Camacho, L., Delclos, K.B., Howard, P., Johnson, A.F. and Bucher, J.R. 2013. A new approach to synergize academic and guideline-compliant research: the CLARITY-BPA research program. Reprod. Toxicol. 40, 35–40. Selgrade, M.K., Blain, R.B., Fedak, K.M. and Cawley, M.A. 2013. Potential risk of asthma associated with in utero exposure to xenobiotics. Birth Defects Res. C Embryo Today 99, 1–13. Skinner, M.K., Manikkam, M., Tracey, R., Guerrero-Bosagna, C., Haque, M. and Nilsson, E.E. 2013. Ancestral dichlorodiphenyltrichloroethane (DDT) exposure promotes epigenetic transgenerational inheritance of obesity. BMC Med. 11, 228–243. Suttie, A.W. 2006. Histopathology of the spleen. Toxicol. Pathol. 34, 466–503. Tyl, R.W., Myers, C.B., Marr, M.C., Sloan, C.S., Castillo, N.P., Veselica, M.M., Seely, J.C., Dimond, S.S., Van Miller, J.P., Shiotsuka, R.N., Beyer, D., Hentges, S.G. and Waechter, J.M., Jr. 2008. Two-generation reproductive toxicity study of dietary bisphenol A in CD-1 (Swiss) mice. Toxicol. Sci 104, 362–384. Vom-Saal, F.S. and Hughes, C. 2005. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ. Health Perspect. 113, 926–933. Wang, G., Wang, J., Ma, H., Ansari, G.A.S. and Khan, M.F. 2013. N-Acetylcysteine protects against trichloroethene-mediated autoimmunity by attenuating oxidative stress. Toxicol. Appl. Pharmacol. 273, 189–195. Yildiz, N. and Barlas, N. 2013. Hepatic and renal functions in growing male rats after bisphenol A and octylphenol exposure. Hum. Exp. Toxicol. 32, 675–786. Zhang, Y.T., Zheng, Q.S., Pan, J. and Zheng, R.L. 2004. Oxidative damage of biomolecules in mouse liver induced by morphine and protected by antioxidants. Basic Clin. Pharmacol. Toxicol. 95, 53–58. | ||
| How to Cite this Article |
| Pubmed Style Shaibi T, HNB, Alghazeer RO, MEB, Benjama AE, Elhensheri M, Lwaleed BA, Al-Griw MA. Exposure to low-dose bisphenol A induces spleen damage in a murine model: Potentially through oxidative stress?. Open Vet J. 2022; 12(1): 23-32. doi:10.5455/OVJ.2022.v12.i1.4 Web Style Shaibi T, HNB, Alghazeer RO, MEB, Benjama AE, Elhensheri M, Lwaleed BA, Al-Griw MA. Exposure to low-dose bisphenol A induces spleen damage in a murine model: Potentially through oxidative stress?. https://www.openveterinaryjournal.com/?mno=122437 [Access: April 20, 2024]. doi:10.5455/OVJ.2022.v12.i1.4 AMA (American Medical Association) Style Shaibi T, HNB, Alghazeer RO, MEB, Benjama AE, Elhensheri M, Lwaleed BA, Al-Griw MA. Exposure to low-dose bisphenol A induces spleen damage in a murine model: Potentially through oxidative stress?. Open Vet J. 2022; 12(1): 23-32. doi:10.5455/OVJ.2022.v12.i1.4 Vancouver/ICMJE Style Shaibi T, HNB, Alghazeer RO, MEB, Benjama AE, Elhensheri M, Lwaleed BA, Al-Griw MA. Exposure to low-dose bisphenol A induces spleen damage in a murine model: Potentially through oxidative stress?. Open Vet J. (2022), [cited April 20, 2024]; 12(1): 23-32. doi:10.5455/OVJ.2022.v12.i1.4 Harvard Style Shaibi, T., , . H. N. B., Alghazeer, . R. O., , . M. E. B., Benjama, . A. E., Elhensheri, . M., Lwaleed, . B. A. & Al-Griw, . M. A. (2022) Exposure to low-dose bisphenol A induces spleen damage in a murine model: Potentially through oxidative stress?. Open Vet J, 12 (1), 23-32. doi:10.5455/OVJ.2022.v12.i1.4 Turabian Style Shaibi, Taher, Hanan N Balog, Rabia O Alghazeer, Mohamed E Ben-Othman, Ahmeda E Benjama, Mohamed Elhensheri, Bashir A Lwaleed, and Mohamed A Al-Griw. 2022. Exposure to low-dose bisphenol A induces spleen damage in a murine model: Potentially through oxidative stress?. Open Veterinary Journal, 12 (1), 23-32. doi:10.5455/OVJ.2022.v12.i1.4 Chicago Style Shaibi, Taher, Hanan N Balog, Rabia O Alghazeer, Mohamed E Ben-Othman, Ahmeda E Benjama, Mohamed Elhensheri, Bashir A Lwaleed, and Mohamed A Al-Griw. "Exposure to low-dose bisphenol A induces spleen damage in a murine model: Potentially through oxidative stress?." Open Veterinary Journal 12 (2022), 23-32. doi:10.5455/OVJ.2022.v12.i1.4 MLA (The Modern Language Association) Style Shaibi, Taher, Hanan N Balog, Rabia O Alghazeer, Mohamed E Ben-Othman, Ahmeda E Benjama, Mohamed Elhensheri, Bashir A Lwaleed, and Mohamed A Al-Griw. "Exposure to low-dose bisphenol A induces spleen damage in a murine model: Potentially through oxidative stress?." Open Veterinary Journal 12.1 (2022), 23-32. Print. doi:10.5455/OVJ.2022.v12.i1.4 APA (American Psychological Association) Style Shaibi, T., , . H. N. B., Alghazeer, . R. O., , . M. E. B., Benjama, . A. E., Elhensheri, . M., Lwaleed, . B. A. & Al-Griw, . M. A. (2022) Exposure to low-dose bisphenol A induces spleen damage in a murine model: Potentially through oxidative stress?. Open Veterinary Journal, 12 (1), 23-32. doi:10.5455/OVJ.2022.v12.i1.4 |