| Original Article | ||
Open Vet J. 2022; 12(2): 290-302 Open Veterinary Journal, (2022), Vol. 12(2): 290–302 Original Research Epidemiological investigations on microbial infection and crystals causing feline lower urinary tract disease in tomcats in Ismailia, EgyptAhmed E. Mahmoud1, Mamdouh M. El-Maghraby1, Reham M. Eltarabili2 and Essam S. Soliman3*1Internal Medicine Division, Department of Animal Medicine, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt 2Department of Bacteriology, Immunology, and Mycology, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt 3Animal, Poultry, and Environmental Hygiene Division, Department of Animal Hygiene, Zoonosis, and Animal Behavior, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt *Corresponding Author: Essam S. Soliman. Department of Animal Hygiene, Zoonosis & Animal Behavior, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt. Email: soliman.essam [at] vet.suez.edu.eg Submitted: 30/12/2021 Accepted: 01/04/2022 Published: 21/04/2022 © 2022 Open Veterinary Journal
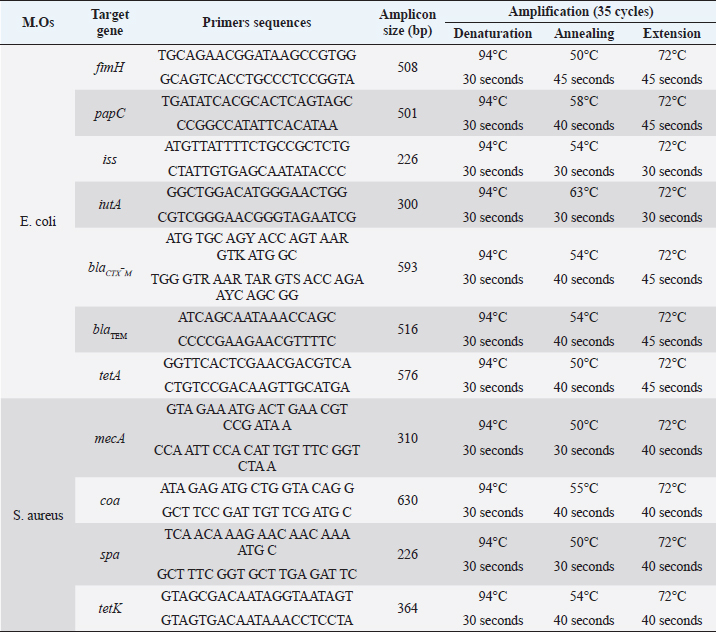
AbstractBackground: Feline lower urinary tract disease (FLUTD) is a common disorder associated with the dysfunction of the urinary bladder or urethra in tomcats. Aim: A prospective study was carried out on the point prevalence and odds ratio (OR) of the FLUTD in Shirazi and Baladi tomcats at Ismailia Governorate, Egypt, recording the prominent clinical manifestation and identifying the antibiogram, virulence, and antimicrobial resistance genes of the causative microorganisms. Methods: A total number of 420 tomcats admitted to the veterinary clinics of Ismailia during the period June 2020 to May 2021 were examined for FLUTD. A total of 1,260 urine samples were collected and analyzed. Results: Hematuria, dysuria, and pollakiuria were the most evident signs recorded in a total of 120 tomcats diagnosed with FLUTD. The diagnosed cases of FLUTD were associated with causes like crystals (35.83%), pyogenic microorganisms (19.16%), and mixed cases (45.00%). The prevalence revealed highly significant (p < 0.01) increases in the cases caused by Escherichia coli, E. coli mixed cases, and calcium oxalate at >4 years; Staphylococcus aureus at ≤ 2 years; amorphous urate and phosphate at 2–4 and >4 years in Shirazi and ≤2 years in Baladi; triple phosphate at ≤2 years in Shirazi and >4 years in Baladi; and S. aureus mixed cases at ≤2 years. The OR of FLUTD revealed higher odds of associations with E. coli, E. coli mixed cases, S. aureus, amorphous urate, and triple phosphate, as well as lower odds with S. aureus, calcium oxalate, amorphous phosphate, and S. aureus mixed cases. Isolated E. coli revealed higher resistance to amoxicillin (AMX, 83.4%), ceftriaxone (83.4%), ceftazidime (CAZ, 75.0%), and cefoxitin (FOX, 50.0%), and S. aureus to oxacillin (100%), FOX (100%), AMX (85.8%), CAZ (76.2%), and FOX (50.0%). S. aureus-detected virulence genes were mecA, coa, spa, and tetK, and E. coli were fimH, iss, iutA, papC, blaTEM, blaCTX-M, and tetA. About 100% of E. coli and 76.1% of S. aureus isolates exhibited multidrug resistance. Conclusion: FLUTD in tomcats is associated with higher odds in E. coli, E. coli mixed cases, and triple phosphate at older ages (>4 years) with high antimicrobial resistance in the microbial isolates contributing to the disease. Keywords: Antibiotic resistance, Crystals, Feline lower urinary tract disease, Prevalence, Virulence. IntroductionFeline lower urinary tract disease (FLUTD) is not a specific disease but rather is a term used to describe the dysfunction of the urinary bladder or urethra of cats and has been commonly reported with a prevalence of up to 8% (Adam and Dean, 2009; Lund et al., 2012). FLUTD has been revealed in some cases of urethral obstruction associated with oliguria and anuria caused by bacterial cystitis, neoplasia, urolithiasis, anatomical abnormalities, and urethral plugs (Saevik et al., 2011; Dorsch et al., 2014; Forrester and Towell, 2015). The prevalence of urinary tract infections (UTI) is associated with the cat’s age and to some extent breed as the prevalence increase with older age (Eggertsdottir et al., 2011; Martinez-Ruzafa et al., 2012). The prevalence has been defined as the proportion of the population affected with a particular disease at a particular time and is usually expressed as a percentage (Bruce et al., 2017). Prevalence (P) can also be expressed as the multiplication of the incidence by the duration of the study (Rothman, 2012). Point prevalence (PP) is a more specific proportion for the number of cases developed at a particular time to the number of the given population at this time (Viera, 2008; Brink, 2021). Odds ratio (OR) is known by the association of the exposure to a certain predisposing cause to an outcome (Carneiro, 2011; Motulsky, 2018). OR usually measures the strength of the association to the presence and absence of the cause (Noordzij et al., 2017). OR values are expressed as OR > 1 when the exposure is associated with higher odds of outcome and OR < 1 when the exposure is associated with lower odds of outcome (Szklo and Nieto, 2019). Escherichia coli (E. coli) is the principal etiologic agent of UTI causing up to 90% (Loh and Sivalingam, 2007), and is recognized as uropathogenic E. coli (UPEC) (Schwartz et al., 2013). UPEC have various virulence factors, such as fimbriae for bacterial adhesion and invasion, iron-acquisition systems for surviving in the iron-deficient environment, flagella, and toxins for bacterial dissemination. Virulence genes are found on transmissible genes (plasmid) and/or the chromosome (Farshad et al., 2012). Extraintestinal disease E. coli (ExPEC) has numerous virulence factors that cannot be established by traditional diagnostics and is a prominent cause of UTIs. Urinary E. coli isolated from cats have shared similarities with ExPEC isolated from humans (Johnson et al., 2001). ExPEC isolates have unique virulence factors which enable them to invade host surfaces, damage host tissues, and prevent or reverse host defenses (Johnson and Stell, 2000). Staphylococci are nonmotile, nonspore-forming, and opportunistic cocci widely found on mammalian skin, producing various illnesses ranging from mild, soft tissue infections to life-threatening conditions, such as bacteremia and endocarditis (Chang et al., 2003). Staphylococcus aureus and S. schleifer are the most prevalent pathogenic species (Yamashita et al., 2005). Staphylococcus aureus is a common cause of UTIs in the general population (Siegman-Igra, 2005). Cats can play a part in S. aureus transmission and recurring human methicillin-resistant S. aureus (MRSA) infections (Litster et al., 2007). The current study aims to conduct a prospective epidemiological study on the PP and OR associated with the FLUTD, as well as the associated antibiogram, virulence, and the antimicrobial resistance genes of E. coli and S. aureus in Ismailia Governorate, Egypt, regarding breed and age during the period from June 2020 to May 2021. Material and MethodsStudy area and designThe study was carried out in small animal clinics in Ismailia Governorate which is located in the northeastern part of Egypt with coordinates of 30°36ʹ15.37″N and 32°16ʹ20.1″E. A prospective epidemiological study was designed to investigate the PP and OR of predisposing risk factors associated with FLUTD of tomcats in Ismailia Governorate, Egypt, considering tomcat’s breed and age during the period from June 2020 to May 2021. SamplingA total number of 420 tomcats were admitted to the clinics during the study period. Out of them, 120 tomcats (28.57%) were suffering from urine retention. All the cats were recorded for the breed, age, and some other environmental influences, such as feeding habits, housing system, housing pattern, vaccination history, deworming actions, and disease and medication history. The cats were all fed on commercial dry feed and housed individually with the owners in their houses. The clinical examinations had been carried out according to the procedures described by Cote et al. (2010). A total of 1,260 urine samples (three urine samples per case) were collected from all admitted cats via cystocentesis to run a complete urine analysis and detect the predisposing causes for the development of FLUTD (pyogenic organisms, crystals, or both). Urine analysisThe urine samples (1,260 samples) were examined in a clinicopathological laboratory in Ismailia Governorate, Egypt. The recorded major causes, like crystals and pyogenic causes, were surveyed for calculating the frequencies. The result reports were categorized in a Microsoft Excel sheet (Microsoft Excel 365) for statistical analysis. Epidemiological measuresThe epidemiological measures included PP and OR and they were calculated as recommended by Thrusfield and Christley’s (2018) modifications. PP rates of the diseased-specific tomcats admitted to clinics were calculated as follows: PP rate=(α / µ) × 100 where α is the number of cats admitted with the FLUTD during the study period and µ is the number of susceptible cats admitted considering breed and age during the same period. OR investigated the risk of contracting FLUTD in the exposed cats’ population concerning breed and age as follows: OR=[(a / b) / (c / d)] where (a / b) is the odds of exposure among the cases (in presence of dependent b) and (c / d) is the odds of exposure among the controls (in absence of dependent b). Isolation of E. coli and S. aureusEscherichia coli and S. aureus strains were categorized into strains related to pyogenic infection and strains related to mixed infection. Standard bacteriological and biochemical investigations were carried out to isolate and identify E. coli and S. aureus strains. Escherichia coli strains were streaked onto MacConkey and eosine methylene blue agar medium (Oxoid) and incubated at 37°C for 24 hours. Blood agar was used to detect the hemolytic activity related to the pathogenicity of E. coli. The purified colonies were placed into a nutrient agar slant, incubated at 37°C for 24 hours, and maintained for further identification at −80°C in 10% glycerol (v/v). Isolated colonies have been purified and biochemically tested to validate their identity as E. coli according to Quinn et al. (2002). Staphylococcus aureus was streaked onto blood agar, nutrient agar, and mannitol salt agar plates (Oxoid, Hampshire, UK). The streaked plates were then incubated at 37°C for 24–48 hours. Staphylococcus aureus circular, convex, and golden yellow colonies were collected and maintained for further examination at −80°C in 10% glycerol (v/v). Isolated colonies were purified and biochemical tests were conducted to confirm their identity as S. aureus according to Quinn et al. (2002). Antibiotic susceptibilityTwenty-four isolates of E. coli and 21 isolates of S. aureus (one sample-one strain) were analyzed for their antibiotic susceptibility using the Kirby–Bauer disk diffusion method, and zone diameters were interpreted according to the Clinical and Laboratory Standards Institute (CLSI). The antibiotics used in the presented study were as follows: amoxicillin (AMX, 30 μg), AMX and clavulanic acid (AMC, 10 μg), oxacillin (OXA, 25 μg), ceftazidime (CAZ, 30 μg), ceftriaxone (CTR, 30 μg), cefoxitin (FOX, 30 μg), meropenem (MEM, 10 μg), Nitrofurntin (NIT, 25 μg), gentamicin (GEN, 10 μg), norfloxacin (NOR, 10 μg), amikacin (AK, 30 μg), doxycycline (DOX, 10 μg), trimethoprim-sulfamethoxazole (SXT, 25 μg), and aztreonam (ATM, 30 μg) (Oxoid, Basingstoke, UK). The diameter of the inhibition zone was estimated as described by CLSI 27. The phenotypic resistance patterns are classified into PDR, extensive drug-resistant (XDR), and multidrug resistance (MDR). Bacterial extraction and PCR amplificationEscherichia coli and S. aureus genomic DNAs were extracted following QIAamp DNA Mini Kit instructions from the manufacturer (Qiagen, Germany, GmbH). Specific primers were employed to amplify fimH, papC, iutA, and iss operons by PCR. Primers’ details and predicted sizes of the amplified products and specific annealing temperatures are shown in Table 1. Amplification of bacterial DNA was carried out in a total volume of 50 μl containing 5 μl of 10x PCR reaction buffer, 1 μl 200 μM (of each dNTP) of dNTP mix (10 mM), 10 μl of bacterial DNA, 0.4 μl 2 U of Taq DNA polymerase (5 U/μl), 30 pmol of each used primer (0.1–0.6 μM), and then sterile ddH2O was added (up to 50 μl). Table 1. Primers and amplicon sizes of the amplified products and specific annealing temperatures.
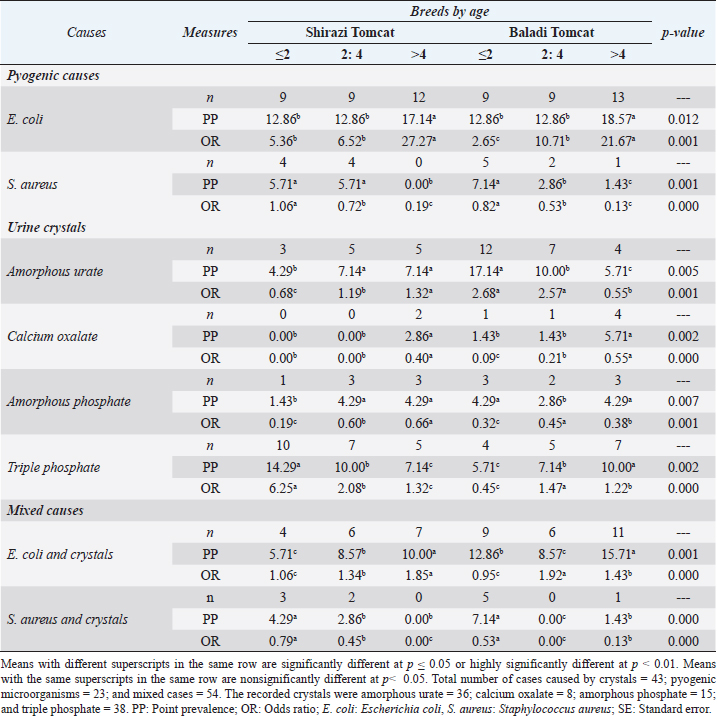
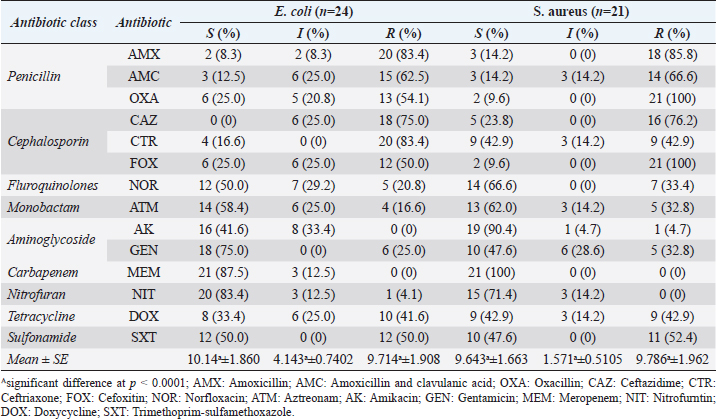
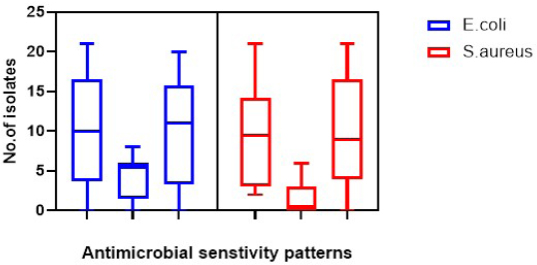
The amplification was carried out in thermal protocols. Initial denaturation at 94°C for 10 min was required, followed by 35 cycles at a specific temperature (Table 1), and extension at 72°C for 1 min. A 10-μl aliquot of PCR was subjected to a 2% agarose gel electrophoresis, then stained with an ethidium bromide solution. Amplified DNA fragments of specific sizes were detected by UV-induced fluorescence. Statistical analysisStatistical analysis was carried out using the Statistical Package for the Social Sciences (SPSS) version 20.0 software (SPSS, 2016). The data were analyzed using multifactorial analysis of variance (two-tailed analysis of variance) to determine the influence of breed and age on the urine analysis pictures in the examined tomcats. The statistical model empathized as follows: Yijk=µ + αi + βj + (αβ)ij + Ɛijk where Yijk is the measurement of dependent variables, µ is the overall mean, αi is the fixed effect of the breed, βj is the fixed effect of age, (αβ)ij is the interactions of the breeds by tomcats’ age, and Ɛijk is the random error. Ethical approvalThe materials, protocol, and study design were approved by the Scientific Research Ethics Committee on Animal and Poultry Researches at the Faculty of Veterinary Medicine, Suez Canal University, Egypt, with approval number (2021050). The examined tomcats were subjected to the examination humanly and carefully, taking all the necessary precautions to minimize the pain during sample collection. Some of the owners witnessed the examinations and sampling for more clarity and obvious consent for sampling of the animal for research purposes. ResultsClinical examinationsThe clinical examinations of the tomcats admitted to the clinics revealed a prevalence of 28.57% for FLUTD-infected animals (a total of 120 diseased animals out of the 420 examined animals). The prominent recorded clinical signs in the diseased animals were hematuria, dysuria, stranguria, pollakiuria, periuria, vocalization when trying to urinate, urinating in unusual places, excessive licking of the genital area, aggression associated with a large, and distended bladder on abdominal palpation. All of the previous symptoms were concurrent with a history of anuria or oliguria and in some cases passage of a few drops of blood-stained urine for more than 2 days was noted. Urine analysisThe FLUTD in the infected 120 tomcats out of the examined 420 animals was related to some predisposing factors that were revealed in the urine analysis, such as crystals (n=43, PPTotal=35.83%), pyogenic microorganisms (n=23, PPTotal=19.16%), and mixed cases (n=54, PPTotal=45%). The total recorded crystals were amorphous urate (n=36, PPTotal=30.00%, PPSpecific=83.72%), calcium oxalate (n=8, PPTotal=6.66%, PPSpecific=18.60%), amorphous phosphate (n=15, PPTotal=12.50%, PPSpecific=34.88%), and triple phosphate (n=38, PPTotal=31.66%, PPSpecific=88.37%). The isolated pyogenic microorganisms were E. coli (n=61, PPTotal=50.83%) and S. aureus (n=16, PPTotal=13.33%). Epidemiological investigationsThe prevalence reveals in Table 2 highly significant (p < 0.01) increases of E. coli, E. coli mixed cases, and calcium oxalate crystals in tomcats older than 4 years in both breeds with no significant differences between the breeds. Highly significant (p < 0.01) increases of prevalence were recorded in S. aureus in ≤2 and 2–4 years Shirazi with no significant differences between the two ages and in ≤2 years Baladi tomcats. Amorphous urate and phosphate reveal highly significant (p < 0.01) increases in 2–4 and >4 years in Shirazi with no significant differences between the two ages and in ≤2 years in Baladi tomcats. Triple phosphate crystals revealed highly significant (p < 0.01) increases in ≤2 years in Shirazi and >4 years in Baladi tomcats. Staphylococcus aureus mixed cases revealed highly significant (p < 0.01) increases in ≤2 years in both breeds with no significant differences between the breeds. The OR of FLUTD reveals in Table 2 higher odds of associations with E. coli, mixed cases of E. coli, S. aureus (≤2 years in Shirazi), amorphous urate crystals (2–4 and >4 years in Shirazi and ≤2 and 2–4 years Baladi), and triple phosphate crystals (≤2, 2–4, and >4 years in Shirazi and 2–4, and >4 years in Baladi), while OR reveals lower odds of associations with S. aureus (2–4 and >4 years in Shirazi, and <2, 2–4, and >4 years in Baladi), amorphous urate (<2 years in Shirazi and >4 years in Baladi), calcium oxalate, amorphous phosphate, triple phosphate (<2 years in Baladi), mixed cases of S. aureus. Antimicrobial susceptibility of the recovered isolatesThe antimicrobial susceptibility testing revealed (Table 3) a high resistance pattern of the E. coli isolates to AMX (83.4%), CTR (83.4%), CAZ (75.0%), and FOX (50.0%). The examined strains were highly susceptible to MEM (87.5%), nitrofurantoin (83.4%), and gentamycin (75.0%) as described in Table 4 and Figure 1. Statistically, the resistance of retrieved isolates to various tested antimicrobial agents was significantly different (p < 0.05). Table 2. Prevalence and odds ratio of FLUTD’s contributing causes (pyogenic, crystals, and mixed cases) in Shirazi and Baladi tomcats.
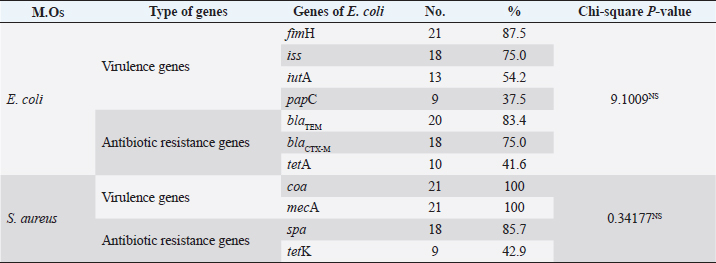
The antimicrobial susceptibility testing revealed that the S. aureus-recovered isolates exhibited a high resistance pattern to penicillins: OXA (100%), cephalosporins: FOX (100%), AMX (85.8%), CAZ (76.2%), and FOX (50.0%). In addition, the examined strains were highly susceptible to MEM (100%), AK (90.4%), nitrofurantoin (71.4%), and NOR (66.6%) as described in Table 3 and Figure 1. Statistically, the resistance of retrieved isolates to various tested antimicrobial agents was significantly different (p < 0.05). MAR index values, in this study, showed multiple-resistant patterns, indicating recovered isolates originated from high-risk contamination. The distribution of the virulence genes and antibiotic-resistance genes among the recovered strainsVirulence genes and antibiotic resistance genes (Table 4) were detected successfully in 21 S. aureus isolates. The most frequent S. aureus virulence genes were mecA (100%), coa (100%), spa (85.7%), and tetK (42.9%) (Fig. 2). The distribution of virulence genes and antibiotic-resistant genes among the examined strains was statistically not significantly different. Table 3. Antimicrobial susceptibility of the recovered isolates.
Table 4. The distribution of the virulence genes and antibiotic resistance genes among the recovered strains of E. coli and S. aureus.
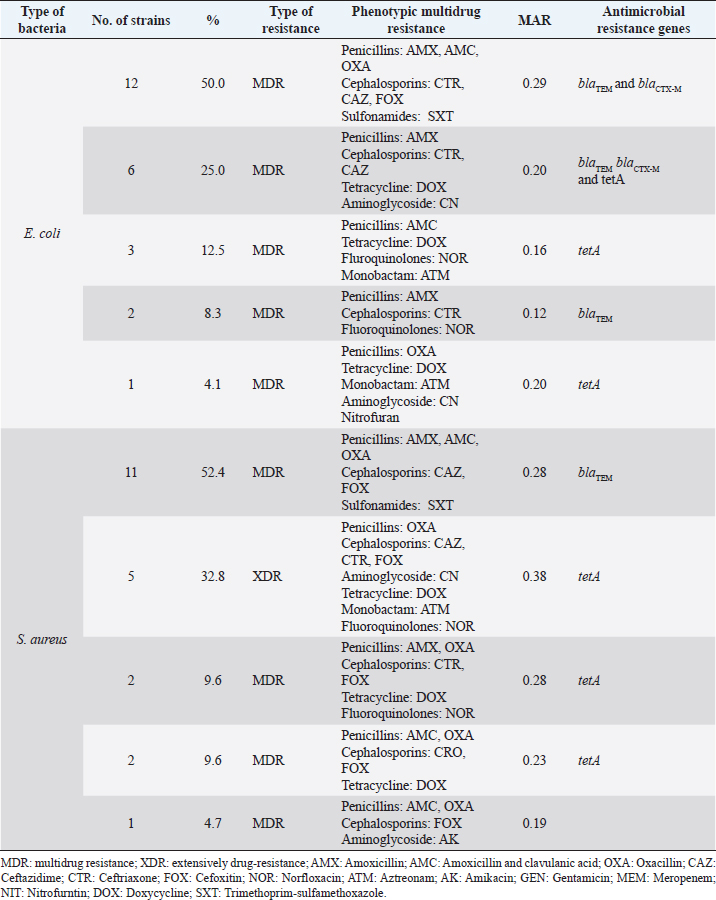
Virulence genes and antibiotic resistance genes (Table 4) were detected successfully in 24 E. coli isolates. The most frequent E. coli virulence genes were fimH (100%), iss (75%), iutA (54.2%), papC (37.5%), blaTEM (83.4%), blaCTX-M (75%), and tetA (41.6%) (Figs. 3 and 4). The distribution of virulence-determinant genes and antibiotic-resistant genes among the examined strains is statistically not significantly different. A combination between phenotypic multidrug resistance and AMR genesAbout 100% of E. coli isolates were multidrug-resistant (MDR, Table 5) to various antimicrobial agents and 32.8% of S. aureus were XDR to seven antimicrobial agents, and 76.1% of S. aureus were multidrug resistance (MDR) to various antimicrobial agents. DiscussionFLUTD can be considered a term to describe the affections of the urinary bladder and/or urethra of tomcats rather than to be considered a disease. The clinical manifestations are similar to other diseases and affections of the lower urinary tract, which is why further investigations should be carried out to differentiate as reported by Lund et al. (2016). The most common and reported clinical manifestations of the FLUTD in tomcats are dysuria (painful urination), pollakiuria (increased urination frequencies), hematuria (blood in urine), periuria (urination outside the litter), self-over-grooming around the perineum, and behavioral vices, like aggression, irritation, and stranguria (blockage of the urethra), following Kim et al. (2018). The current study reveals that FLUTD prevailed as 28.57% (a total of 120 diseased animals out of the 420 examined animals). These infected cases were correlated to the main causes that were detected through the urine analysis as follow: crystals (n=43, PPTotal=35.83%), pyogenic microorganisms (n=23, PPTotal=19.16%), and mixed cases (n=54, PPTotal=45.00%). The most predominant recorded crystals were amorphous urate (n=36, PPTotal=30.00%, PPSpecific=83.72%), calcium oxalate (n=8, PPTotal=6.66%, PPSpecific=18.60%), amorphous phosphate (n=15, PPTotal=12.50%, PPSpecific=34.88%), and triple phosphate (n=38, PPTotal=31.66%, PPSpecific=88.37%). The isolated pyogenic microorganisms were E. coli (n=61, PPTotal=50.83%) and S. aureus (n=16, PPTotal=13.33%).
Fig. 1. Antibiotics’ sensitivity patterns in the isolated E. coli and S. aureus.
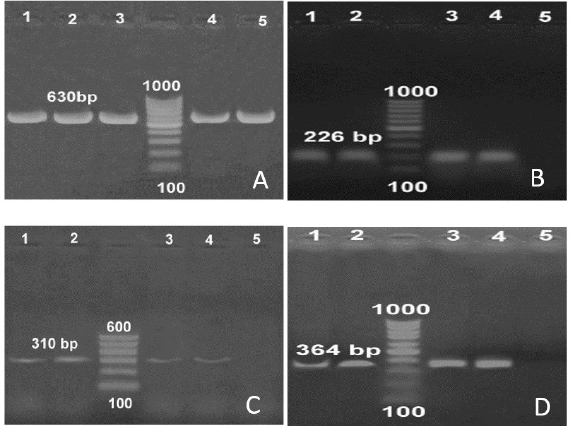
Fig. 2. (A) PCR amplification of the specific gene of S. aureus (coa) at 630 bp. (B) PCR amplification of the specific gene of S. aureus (spa) at 226 bp. (C) PCR amplification of the specific gene of S. aureus (mecA) at 310 bp. (D) PCR amplification of the specific gene of S. aureus (tetK) at 364 bp. On the other hand, FLUTD was reported by Lew-Kojrys et al. (2017) with a prevalence up to 51.9% higher than the recorded prevalence in the current study, and they attributed the high prevalence to idiopathic cystitis in Polish feline populations. They also recorded that FLUTD is associated with numerous factors such as animal factors including breed, sex, age, and neutering conditions, as well as environmental factors including housing and living standards, and diet formulation. Kovarikova et al. (2020) reported a higher prevalence compared to the current study of up to 51.9% of 214 examined males and female cats for FLUTD. They attributed the disease to a urethral obstruction in 23 cases and 100 cases were nonobstructive. Lund and Eggertsdottir (2019) reported studied the occurrence of FLUTD in Norwegian cats during the period 2003–2009. They attributed these cases to nonobstructive feline idiopathic cystitis and urolithiasis. They suggested the application of multimodal environmental modifications and enrichment of cats. Kaul et al. (2020) conducted a questionnaire survey on the incidence of FLUTD in 101 cats from 2010 to 2013. They recorded nearly close numbers to the current study and associated them with pathogenic causes such as 52 cats diagnosed with idiopathic cystitis, 21 with urolithiasis, and 13 with bacterial urinary tract infection.
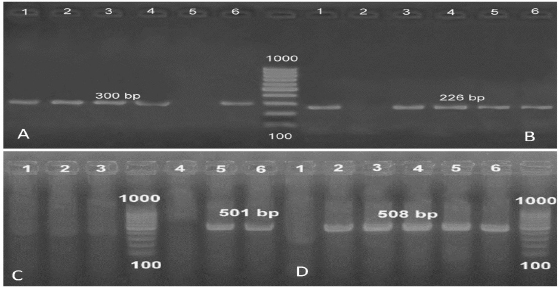
Fig. 3. (A) PCR amplification of the specific gene of E. coli (iutA) at 300 bp. (B) PCR amplification of the specific gene of E. coli (iss) at 226 bp. (C) PCR amplification of the specific gene of E. coli (papC) at 501 bp. (D) PCR amplification of the specific gene of E. coli (fimH) at 508 bp.
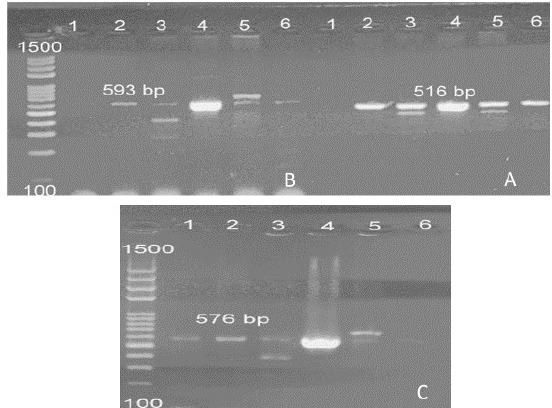
Fig. 4. (A) PCR amplification of the specific gene of E. coli (blaCTX-M) at 593 bp. (B) PCR amplification of the specific gene of E. coli (blaTEM) at 516 bp. (C) PCR amplification of the specific gene of E. coli (tetA) at 576 bp. Table 5. The frequency of the phenotypic multidrug resistance and the antibiotic resistance genes among the retrieved strains of E. coli and S. aureus.
The most often isolated bacterium in dogs and cats with UTI was E. coli. In general, Enterobacteriaceae were responsible for more than half of UTIs in dogs and cats; Simultaneously, Staphylococcus spp. were the second most often detected bacteria; however, the Staphylococcus species found varied greatly across dogs and cats (Hall et al., 2013). Enterobacteriaceae along with Staphylococcus spp. causes approximately 79% of UTIs in dogs and cats (Marques et al., 2018). UTI are the most commonly diagnosed diseases of cats that are inappropriately treated with empirical antibiotics without microbiological testing (De Briyne et al., 2014). The cats are assumed to be potential reservoirs for antimicrobial resistance (AMR) transfer to humans due to the large-scale use of antimicrobial agents and close interaction with humans being extensively treated by broad-spectrum antimicrobial agents (Guardabassi et al., 2004; Lloyd, 2007). The risk of antimicrobial treatment failure could be increased in both animals and humans (Algammal et al., 2020a; Li et al., 2021). The current findings regarding the bacteriological identification of characteristic colonies on eosine methylene blue agar and the biochemical reaction are similar to those of Edwads and Ewing (1972). The in vitro antimicrobial resistance pattern of the recovered E. coli strains exhibited a high resistance pattern to AMX (83.4%), CTR (83.4%), CAZ (75.0%), and FOX (50.0%). In addition, the examined strains were highly susceptible to MEM (87.5%) was consistent with those detected by Prescott et al. (2000) and Patel et al. (1999). In the present study, the PCR proved that the tested strains harbored the virulence-determinant genes (fimH). Adhesion gene was the most common and present in 87.5% UPEC isolates, which is in agreement with studies carried out in Romania (86%) (Vargová et al., 2017) and Mongolia (89.9%) (Munkhdelger, 2017) on UTI. The present study shows the presence of virulence genes characteristic of ExPEC strains in bacterial isolates from nondiarrheic cats, as well as cats with UTI (iss, iutA, and papC), with an incidence of 75.0, 54.2, and 37.5%, respectively. Gregova and Kmet (2020) revealed that the most commonly discovered virulence genes in E. coli strains isolated from animals were iutA, iss, and papC. The presence of these genes has also been confirmed in ExPEC strains derived from UPEC. Pets are natural reservoirs for various pathogens, including ExPEC strains, that are capable of infecting humans, which is a reason for concern (Johnson et al., 2003). The large increase in the discovery of multidrug-resistant and methicillin-resistant S. aureus (MDR MRSA) over time has been a finding because it poses serious therapeutic constraints, which is in agreement with Jay-Russell et al. (2014) who isolated MDR MRSA in cats. The rapid emergence and spread of resistant Staphylococci in cats is a worrying development. Resistant Staphylococci in cats are similar to human Staphylococci that are highly resistant to penicillin, ampicillin, and tetracycline. Notably, the mecA gene was mostly found in Staphylococcus spp. isolated from cats. Nonetheless, MRSA identification in cats with UTIs is a public health hazard because companion animals will be responsible for spreading these bacteria to the household and public surroundings. Tetracyclines were extensively employed for therapy and prevention of bacterial infections in humans, animals, and plants (Malik et al., 2005). The genes tetK and tetL are most often plasmid-borne (Schwarz and Noble, 1999). Staphylococcal virulence factors are generally divided into four categories: exoenzymes, exotoxins, adhesins, and others (Kuroda et al., 2001; Zhang et al., 2003). The coa gene was 100% abundant in the MRSA strains tested. These findings are comparable to those of Akineden et al. (2001). This gene showed no polymorphisms of any size (Algammal et al., 2021). However, in other investigations, amplification of the coagulase gene led to various amplicons, demonstrating the variability of coagulase gene size. The spa gene showed notable gene polymorphisms with various amplicons (140, 270, and 290 bp). The X region of the spa gene is usually replaced variable (up to 24 times) in various strains (Algammal et al., 2020b). ConclusionFLUTD is a common disease affecting tomcats and was detected with high prevalence in older ages >4 and 2–4 years. The OR epidemiological investigations reveal higher odds of associations with E. coli, mixed cases of E. coli and S. aureus, amorphous urate crystals, and triple phosphate crystals, while OR reveals lower odds of associations with S. aureus, amorphous urate, calcium oxalate, amorphous phosphate, triple phosphate, and mixed cases of S. aureus. The antimicrobial susceptibility reveals a high resistance of E. coli and S. aureus to a large number of the tested antibiotic disks. The most frequently detected S. aureus virulence genes were mecA, coa, spa, and tetK, and E. coli were fimH, iss, iutA, papC, blaTEM, blaCTX-M, and tetA. About 100% of E. coli and 76.1% of S. aureus isolates exhibited multidrug resistance and 32.8% of S. aureus were extensively drug-resistant to 7 antimicrobial agents. Authors’ contributionsAEM collected the raw data and urine samples from tomcats admitted to the clinics, carried out the clinical examination, conducted the urine analysis, and took part in writing the manuscript. MME participated in the clinical examination, urine analysis, and took part in writing the manuscript. RME conducted the bacteriological examination, antibiotic sensitivity testing, virulence examination, and took part in writing the manuscript. ESS designed the study design, conducted the epidemiological analysis, took part in writing the manuscript, and reviewed the final edit of the manuscript. AcknowledgmentThe authors sincerely thank the Ismailia Veterinary Clinic, Ismailia Governorate, for providing the opportunity to examine and collect samples from a large number of tomcats during the study. FundingThe authors received no financial support for the research, authorship, and/or publication of this article. Conflict of interestsThe authors declare that they have no financial or personal conflicts which may have inappropriately influenced them in writing this manuscript. ReferencesAdams, V.J. and Dean, R.S. 2009. Morbidity of UK cats: results of a health survey. Proceedings of the 52nd British Small Animal Veterinary Association (BSAVA) Congress; 2009. Akineden, O., Annemüller, C., Hassan, A.A., Lämmer, C., Wolter, W. and Zschöck, M. 2001. Toxin Genes and Other Characteristics of Staphylococcus aureus isolates from Milk of Cows with Mastitis. Clin. Diagn. Lab. Immunol. 8, 959–964. Algammal, A.M., Enany, M.E., El-Tarabili, R.M., Ghobashy, M.O.I. and Helmy Y.A. 2020a. Prevalence, antimicrobial resistance profiles, virulence and enterotoxin-determinant genes of MRSA isolated from subclinical bovine mastitis samples in Egypt. Pathogens 9(5), 1–11. Algammal, A.M., Hashem, H.R., Alfifi, K.J., Hetta, H.F., Sheraba, N.S., Ramadan, H. and El-Tarabili, R.M. 2021. atpD gene sequencing, multidrug resistance traits, virulence-determinants, and antimicrobial resistance genes of emerging XDR and MDR-Proteus mirabilis. Sci Rep. 11(1), 1–15. Algammal, A.M., Hetta, H.F., Batiha, G.E., Hozzein, W.N., El Kazzaz, W.M., Hashem, H.R., Tawfik, A.M. and El-Tarabili, R.M. 2020b. Virulence-determinants and antibiotic-resistance genes of MDR-E. coli isolated from secondary infections following FMD-outbreak in cattle. Sci Rep. 10(1), 1–13. Brinks, R. 2021. Illness-death model in chronic disease epidemiology: characteristics of a related, differential equation and an inverse problem. Article ID 5091096. doi:10.1155/2018/5091096. Bruce, N., Pope, D. and Stanistreet, D. 2017. Quantitative methods for health research: a practical interactive guide to epidemiology and statistics, 2nd ed. Hoboken, NJ: John Wiley & Sons, Ltd. ISBN: 978-1-118-66526-8. doi: 10.1002/9781118665374 Carneiro, I. 2011. Introduction to epidemiology. Ed., Natasha H. 2nd ed.). Maidenhead, Berkshire, New York: Open University Press. ISBN: 978-0-335-24462-1. Chang, F.Y., Peacock, J.E. Jr, Musher, D.M., Triplett, P., MacDonald, B.B., Mylotte, J.M., O’Donnell, A., Wagener, M.M. and Yu, V.L. 2003. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore) 82(5), 333–339. Côté, E., Edwards, N.J., Ettinger, S.J., Fuentes, V.L., MacDonald, K.A., Scansen, B.A., Sisson, D.D. and Abbott, J.A. 2015. Management of incidentally detected heart murmurs in dogs and cats. J. Vet. Cardiol. 17, 245–261. De Briyne, N., Atkinson, J., Borriello, S.P. and Pokludová, L. 2014. Antibiotics used most commonly to treat animals in Europe. Vet. Rec. 175, 325. Dorsch, R., Remer, C., Sauter-Louis, C. and Hartmann, K. 2014. Feline lower urinary tract disease in German cat population: a retrospective analysis of demographic data, causes and clinical signs. Tierarztl. Prax. Ausg. K. Kleintiere. Heimtiere. 42(4), 231–239. Edwards, P.R. and Ewing, W.H. 1972. Identification of Enterobacteriaceae. Minneapolis, MN: Burgess Publishing Co., , pp: 362. Eggertsdottir, A.V., Saevik, B.K., Halvorsen, I. and Sorum, H. 2011. Occurrence of occult bacteriuria in healthy cats. J. Feline Med. Surg. 13, 800–803. Farshad, S., Ranjbar, R., Japoni, A., Hosseini, M., Anvarinejad, M. and Mohammadzadegan, R. 2012. Microbial susceptibility, virulence factors, and plasmid profiles of uropathogenic Escherichia coli strains isolated from children in Jahrom, Iran. Arch. Iran Med. 15(5), 312–316. Forrester, S.D. and Towell, T.L. 2015. Feline idiopathic cystitis. Vet. Clin. North Am. Small Anim. Pract. 45, 783–806. Gregova, G. and Kmet, V. 2020. Antibiotic resistance and virulence of Escherichia coli strains isolated from animal rendering plant. Sci. Rep. 10(1), 17108. Guardabassi, L., Schwarz, S. and Lloyd, D.H. 2004. Pet animals as reservoirs of antimicrobial-resistant bacteria. J. Antimicrob. Chemother. 66, 700–709. Hall, J.L., Holmes, M.A. and Baine, S.J. 2013. Prevalence and antimicrobial resistance of canine urinary tract pathogens. Vet Rec. 173, 549. Jay-Russell, M.T., Hake, A.F., Bengson, Y., Thiptara, A. and Nguyen, T. 2014. Prevalence and characterization of Escherichia coli and Salmonella strains isolated from stray dog and coyote feces in a major leafy greens production region at the United States-Mexico border. PLoS One 9(11), e113433. doi: 10.1371/journal.pone.0113433. Johnson, J.R. and Stell, A.L. 2000. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 181, 261–272. Johnson, J.R., Delavari, P., Stell, A.L., Whittam, T.S., Carlino, U. and Russo, T.A. 2001. Molecular comparison of extraintestinal Escherichia coli isolates of the same electrophoretic lineages from humans and domestic animals. J. Infect. Dis. 183, 154–159. Johnson, J.R., Kaster, N., Kuskowski, M.A. and Ling, G.V. 2003. Identification of urovirulence traits in Escherichia coli by comparison of urinary and rectal E. coli isolates from dogs with urinary tract infection. J. Clin. Microbiol. 41(1), 337–345. Kaul, E., Hartmann, K., Reese, S. and Dorsch, R. 2020. Recurrence rate and long-term course of cats with feline lower urinary tract disease. J. Feline Med. Surg. 22(6), 544–556. Kim, Y., Kim, H., Pfeiffer, D., Brodbelt, D. 2018. Epidemiological study of feline idiopathic cystitis in Seoul, South Korea. J. Feline Med. Surg. 20(10), 913–921. Kovarikova, S., Simerdova, V., Bilek, M., Honzak, D., Palus, V. and Marsalek, P. 2020. Clinicopathological characteristics of cats with signs of feline lower urinary tract disease in Czech Republic. Vet. Med. 65(3), 123–133. Kuroda, M., Ohta, T., Uchiyama, I., Baba, T., Yuzawa, H., Kobayashi, I., Cui, L., Oguchi, A., Aoki, K., Nagai, Y., Lian, J., Ito, T., Kanamori, M., Matsumaru, H., Maruyama, A., Murakami, H., Hosoyama, A., Mizutani-Ui, Y., Takahashi, N.K., Sawano, T., Inoue, R., Kaito, C., Sekimizu, K., Hirakawa, H., Kuhara, S., Goto, S., Yabuzaki, J., Kanehisa, M., Yamashita, A., Oshima, K. and Furuya, K. 2001. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet 357, 1225–1240. Lew-Kojrys, S., Mikulska-Skupien, E., Snarska, A., Krystkiewicz, W. and Pomianowski, A. 2017. Evaluation of clinical signs and causes of lower urinary tract disease in polish cats. Vet. Med. Czech. 62(7), 386–393. Li, Y., Fernández, R., Durán, I., Molina-López, R.A. and Darwich, L. 2021. Antimicrobial resistance in bacteria isolated from cats and dogs from the Iberian Peninsula. Front. Microbiol. 11, 621597. Litster, A., Moss, S.M., Honnery, M. Rees, B. and Trott, D.J. 2007. Prevalence of bacterial species in cats with clinical signs of lower urinary tract disease: recognition of Staphylococcus felis as a possible feline urinary tract pathogen. Vet. Microbiol. 121, 182–188. Lloyd, D.H. 2007. Reservoirs of antimicrobial resistance in pet animals. Clin. Infect. Dis. 45(Suppl. 2), S148–S152. Loh, K.Y. and Sivalingam, N. 2007. Urinary tract infections in pregnancy. Malaysian Fam. Physician 2, 54–57. Lund, H.S. and Eggertsdottir, A.V. 2019. Recurrent episodes of feline lower urinary tract disease with different causes: possible clinical implications. J. Feline Med. Surg. 21(6), 590–594. Lund, H.S., Rimstad, E. and Eggertsdottir, A.V. 2012. Prevalence of viral infections in Norwegian cats with and without feline urinary tract disease. J. Feline Med. Surg. 14(12), 895–899. Lund, H.S., Sævik, B.K., Finstad, Ø.W., Grøntvedt, E.T., Vatne, T. and Eggertsdottir, A.V. 2016. Risk factors for idiopathic cystitis in Norwegian cats: a matched case-control study. J. Feline Med. Surg. 18(6), 483–491. Malik, S., Peng, H. and Barton, M.D. 2005. Antibiotic resistance in Staphylococci associated with cats and dogs. J. Appl. Microbiol. 99, 1283–1293. Marques, C., Belas, A., Franco, A., Aboim, C., Gama, L.T. and Pomba, C. 2018. Increase in antimicrobial resistance and emergence of major international high-risk clonal lineages in dogs and cats with urinary tract infection: 16 year retrospective study. J. Antimicrob. Chemother. 73(2), 377–384. Martinez-Ruzafa, I., Kruger, J.M., Miller, R., Swenson, C.L., Bolin, C.A. and Kaneene, J.B. 2012. Clinical features and risk factors for development of urinary tract infections in cats. J. Feline Med. Surg. 14, 729–740. Motulsky, H. 2018. Intuitive biostatistics: a nonmathematical guide to statistical thinking, 4th ed.. New York: Oxford University Press, pp: 266. ISBN: 978-0-19-064356-0.. Munkhdelger, Y., Gunregjav, N., Dorjpurev, A., Juniichiro, N. and Sarantuya, J. 2017. Detection of virulence genes, phylogenetic group and antibiotic resistance of uropathogenic Escherichia coli in Mongolia. J. Infect. Dev. Ctries. 11(1), 51–57. Noordzij, M., van, Diepen, M., Caskey, F.C. and Jager, K.J. 2017. Relative risk versus absolute risk: one cannot be interpreted without the other. Nephrology, Dialysis, Transpl. 32(Suppl. 2), ii13–ii18. Patel, A., Lloyd, H.D. and Lamport, I.A. 1999. Antimicrobial resistance of feline Staphylococci in south-east England. Vet. Dermatol. 10, 257–261. Prescott, J.F., Baggot, J.D. and Walker, D.R. 2000. Antimicrobial therapy in veterinary medicine, 3rd ed. Ames, IA: Iowa State University Press, pp: 1–771. Quinn, P.J., Markey, B.K., Carter, M.E., Donnelly, W. J. and Leonard, F.C. 2002. Bacterial causes of bovine mastitis. In Veterinary microbiology and microbial disease, 2nd ed. Hoboken, NJ: Wiley-Blackwell, ISBN: 978-1-405-15823-7, pp: 465–475. Rothman, K.J. 2012. Epidemiology: an introduction. Oxford,UK: Oxford University Press, pp: 53. ISBN: 978-0-19-975455-7. Saevik, B.K., Trangerud, C., Ottesen, N., Sorum, H. and Eggertsdottir, A.V. 2011. Causes of lower urinary tract disease in Norwegian cats. J. Feline Med. Surg. 13(6), 410–417. Schwartz, D.J., Kalas, V., Pinkner, J.S., Chen, S.L., Spaulding, C.N., Dodson, K.W. and Hultgren, S.J. 2013. Positively selected FimH residues enhance virulence during urinary tract infection by altering FimH conformation. Proc. Natl. Acad. Sci. U. S. A. 110(39), 15530–15537. Schwarz, S. and Noble, C.W. 1999. Aspects of bacterial resistance to antimicrobials used in veterinary dermatological practice. Vet. Dermatol. 10, 163–176. Siegman-Igra, Y., Reich, P., Orni-Wasserlauf, R., Schwartz, D. and Giladi, M. 2005. The role of vancomycin in the persistence or recurrence of Staphylococcus aureus bacteraemia. Scand. J. Infect. Dis. 37(8), 572–578. SPSS. 2016. Statistical Packages of Social Sciences. Version 21 for windows. Chicago, IL: SPSS. Inc.. Szklo, M. and Nieto, F.J. 2019. Epidemiology: beyond the basics, 4th ed. Burlington, MA: Jones & Bartlett Learning, pp: 488. ISBN: 9781284116595. Thrusfield M. and Christley R. 2018. Veterinary epidemiology, 4th ed. London, UK: Blackwell Science Ltd., pp: 214–256. Vargová, R., Kmetóvá, M., Čurová, K. and Siegfried, L. 2017. Virulence genes in Escherichia coli strains isolated from urine of eldery patients. Biologia 72, 259–266. Viera, A.J. 2008. Odds ratios and risk ratios: what’s the difference and why does it matter? South. Med. J. 101(7), 730–734. Yamashita, K., Shimizu, A., Kawano, J., Uchida, E., Haruna, A. and Igimi, S. 2005. Isolation and characterization of Staphylococci from external auditory meatus of dogs with or without otitis externa with special reference to Staphylococcus schleiferi subsp. coagulans isolates. J. Vet. Med. Sci. 67, 263–268. Zhang, Y.Q., Ren, S.X., Li, H.L., Wang, Y.X., Fu, G., Yang, J., Qin, Z.Q., Miao, Y.G., Wang, W.Y. and Chen, R.S. 2003. Genome-based analysis of virulence genes in a nonbiofilm- forming Staphylococcus epidermidis strain (ATCC 12228). Mol. Microbiol. 49, 1577–1593. | ||
| How to Cite this Article |
| Pubmed Style Mahmoud AE, El-Maghraby MM, Eltarabili RM, Soliman ES, . Epidemiological Investigations on Microbial Infection and Crystals Causing Feline Lower Urinary Tract Disease in Tom-Cats of Ismailia, Egypt. Open Vet J. 2022; 12(2): 290-302. doi:10.5455/OVJ.2022.v12.i2.18 Web Style Mahmoud AE, El-Maghraby MM, Eltarabili RM, Soliman ES, . Epidemiological Investigations on Microbial Infection and Crystals Causing Feline Lower Urinary Tract Disease in Tom-Cats of Ismailia, Egypt. https://www.openveterinaryjournal.com/?mno=139961 [Access: April 20, 2024]. doi:10.5455/OVJ.2022.v12.i2.18 AMA (American Medical Association) Style Mahmoud AE, El-Maghraby MM, Eltarabili RM, Soliman ES, . Epidemiological Investigations on Microbial Infection and Crystals Causing Feline Lower Urinary Tract Disease in Tom-Cats of Ismailia, Egypt. Open Vet J. 2022; 12(2): 290-302. doi:10.5455/OVJ.2022.v12.i2.18 Vancouver/ICMJE Style Mahmoud AE, El-Maghraby MM, Eltarabili RM, Soliman ES, . Epidemiological Investigations on Microbial Infection and Crystals Causing Feline Lower Urinary Tract Disease in Tom-Cats of Ismailia, Egypt. Open Vet J. (2022), [cited April 20, 2024]; 12(2): 290-302. doi:10.5455/OVJ.2022.v12.i2.18 Harvard Style Mahmoud, A. E., El-Maghraby, M. M., Eltarabili, R. M., Soliman, E. S. & (2022) Epidemiological Investigations on Microbial Infection and Crystals Causing Feline Lower Urinary Tract Disease in Tom-Cats of Ismailia, Egypt. Open Vet J, 12 (2), 290-302. doi:10.5455/OVJ.2022.v12.i2.18 Turabian Style Mahmoud, Ahmed E, Mamdouh M. El-Maghraby, Reham M. Eltarabili, Essam S. Soliman, and . 2022. Epidemiological Investigations on Microbial Infection and Crystals Causing Feline Lower Urinary Tract Disease in Tom-Cats of Ismailia, Egypt. Open Veterinary Journal, 12 (2), 290-302. doi:10.5455/OVJ.2022.v12.i2.18 Chicago Style Mahmoud, Ahmed E, Mamdouh M. El-Maghraby, Reham M. Eltarabili, Essam S. Soliman, and . "Epidemiological Investigations on Microbial Infection and Crystals Causing Feline Lower Urinary Tract Disease in Tom-Cats of Ismailia, Egypt." Open Veterinary Journal 12 (2022), 290-302. doi:10.5455/OVJ.2022.v12.i2.18 MLA (The Modern Language Association) Style Mahmoud, Ahmed E, Mamdouh M. El-Maghraby, Reham M. Eltarabili, Essam S. Soliman, and . "Epidemiological Investigations on Microbial Infection and Crystals Causing Feline Lower Urinary Tract Disease in Tom-Cats of Ismailia, Egypt." Open Veterinary Journal 12.2 (2022), 290-302. Print. doi:10.5455/OVJ.2022.v12.i2.18 APA (American Psychological Association) Style Mahmoud, A. E., El-Maghraby, M. M., Eltarabili, R. M., Soliman, E. S. & (2022) Epidemiological Investigations on Microbial Infection and Crystals Causing Feline Lower Urinary Tract Disease in Tom-Cats of Ismailia, Egypt. Open Veterinary Journal, 12 (2), 290-302. doi:10.5455/OVJ.2022.v12.i2.18 |