| Case Report | ||
Open Vet J. 2022; 12(5): 612-617 Open Veterinary Journal, (2022), Vol. 12(5): 612–617 Case Report Eosinophilic pulmonary granulomatosis resembling a pulmonary carcinoma in a dog in Hong KongAngel Almendros1,2*, Sin Yi Lai2 and Antonio Giuliano1,21Veterinary Clinical Sciences, City University of Hong Kong, Kowloon, Hong Kong 2Veterinary Medical Centre, City University of Hong Kong, Kowloon, Hong Kong *Corresponding Author: Angel Almendros. Veterinary Clinical Sciences, City University of Hong Kong, Kowloon, Hong Kong. Email: aalmendr [at] cityu.edu.hk Submitted: 16/04/2022 Accepted: 08/08/2022 Published: 05/09/2022 © 2022 Open Veterinary Journal
AbstractBackground: Canine Eosinophilic Pulmonary Granulomatosis (EPG) is a severe form of eosinophilic pulmonary disease that carries a guarded prognosis, responds poorly to therapy and recurs frequently. Most studies have reported a caudal lobar pulmonary distribution and a poorer prognosis in idiopathic cases. Case Description: A 7-year-old dog was presented for persistent cough, hyporexia, and weight loss. Eosinophilia and basophilia were transiently present, and an antigen test for heartworm disease was negative. Radiographic studies, followed by a computed tomography (CT) scan revealed nodular lesions and a large mass in the left cranial lobar region suggestive of neoplasia. Cytological and histopathological evaluation was consistent with EPG. The dog responded positively to corticosteroids and has since remained free of disease. Conclusion: EPG in dogs can resemble primary pulmonary neoplasia with secondary intra-pulmonary metastasis. Contrary to previous reports, idiopathic EPG can present with a cranial pulmonary distribution and respond positively to therapy. Keywords: Corticosteroids, Eosinophilic pneumonia, Eosinophilic pulmonary granulomatosis, Heartworm disease, Pulmonary masses. IntroductionEosinophilic pulmonary granulomatosis (EPG) has been reported as a form of eosinophilic lung disease (ELD) that presents with nodular pulmonary infiltration and/or masses containing eosinophils, macrophages, plasma cells, and neutrophils (Reinero, 2019). It is not well defined whether EPG is a disease entity in itself, or a type of eosinophilic pneumonia or bronchopneumonia (EBP) (Reinero, 2019). EPG is described as a more severe form of ELD with worse prognosis, frequent recurrences, and high mortality (Neer et al., 1986; Calvert, 1992; Reinero, 2019; Abbott and Allen, 2020). There are only a few reported cases of EPG in the literature (Abbott and Allen, 2020). The reports include only small numbers of dogs or case reports with the largest cohort being 10 dogs and overall, only three studies reported five or more dogs (Calvert, 1988; Fina et al., 2014; Johnson et al., 2019). Most dogs affected young and there is no clear evidence of breed or sex predilection (Fina et al., 2014; Abbott and Allen, 2020). Heartworm has been historically associated with EPG and is suggested these cases have a better prognosis than idiopathic EPG cases (Neer et al., 1986; Katajavuori et al., 2013). Common lesions include parenchymal and bronchiolar nodules and masses affecting most frequently the caudal lung lobes with associated bronchiectasis (Fina et al., 2014). We report a case of idiopathic EPG in a dog, that had no evidence of heartworm or lungworm disease, with a cranial lobar distribution of lesions, and presented for suspected pulmonary neoplasia, that resolved completely following prednisolone treatment. Case DetailsA 7-year-old entire male Bichon Frise was presented for an episode of progressive worsening cough and anorexia. The owner reported that the cough had been present for 4 weeks and was initially occasional, but the cough increased in frequency and the appetite decreased a couple of days previous to the visit. The patient had no previous history of respiratory disease. The dog was up to date with vaccination but was not on any prophylactic treatment for ectoparasites or endoparasites. Heartworm preventative treatment had been last given 18 months prior. On clinical examination, the dog was bright, alert, and responsive, and had no respiratory or cardiovascular abnormalities. His weight was 4.3 kg with an ideal body condition score of 4/9 but had lost 20% of his body weight since the previous visit 18 months prior. Other than mild otitis externa, the rest of the clinical examination was unremarkable. Table 1. Hematology results showing eosinophilia and basophilia in a dog with EPG.
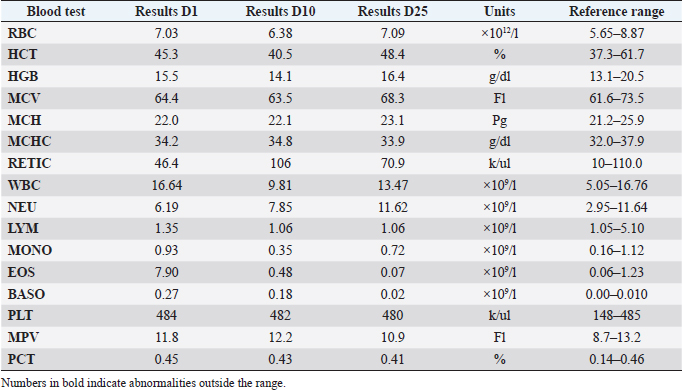
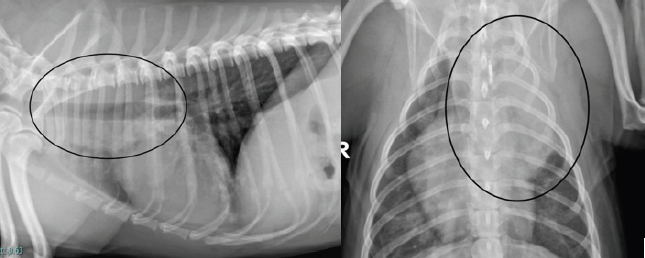
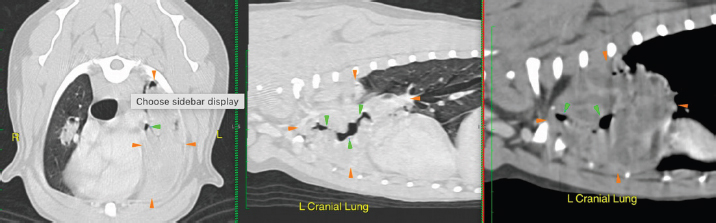
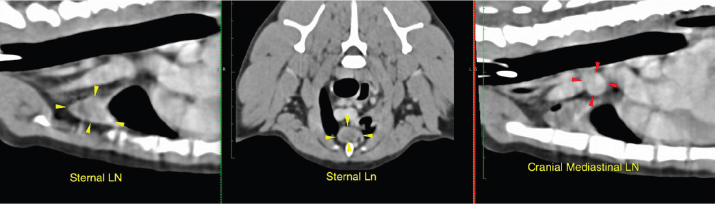
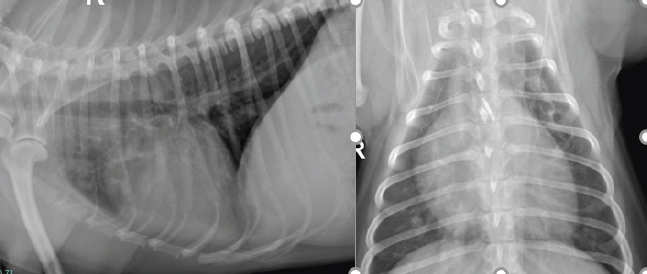
Serum biochemistry was unremarkable. Hematology revealed marked eosinophilia (7.9 × 109/l; reference range 0.06–1.23/l), moderate basophilia (0.27 × 109/l; reference range 0.00–0.1 × 109/l) and moderate elevation of platelets (PLT) (561 K/µl; reference range 148–484 K/µl) (Table 1). Thoracic radiography revealed a large soft tissue opacity in the left cranial lung lobe and mild generalized bronchiolar pattern (Fig. 1). There were no signs of pleural effusion or enlargement or tortuosity of pulmonary vessels. A mild interstitial pattern was noted in the caudal left lung lobe. An antigen heartworm test (Snap 4DX IDDEXX Laboratories) was negative (Table 1), and fecal analysis was negative for parasites too. The patient was treated with antibiotics (amoxicillin/clavulanic acid, 16 mg/kg PO q 12 hours; Clavulox, Pfizer) and beta agonist bronchodilators (terbutaline, 1.2 mg/kg PO q 12 hours; Bricanyl, Astra Zeneca) for 10 days. Additionally, intestinal parasite treatment (febantel 17.4 mg/kg, pyrantel embonate 16.7 mg/kg, and praziquantel 5.8 mg/kg PO once; Drontal, Bayer) and an endo and ectoparasite combination therapy (alfoxolaner 4.3 mg/kg and milbemycin oxime 0.9 mg/kg PO once) were administered against fleas, ticks, heartworms, roundworms, tapeworms, whipworms, and hookworms. Poor clinical response after 10 days of treatment was reported by the owner. The cough frequency was similar but the appetite was slightly better. Hematology and thoracic radiographs were repeated. Eosinophilia had resolved but there was no significant radiographic difference in the size and appearance of the lung masses. Based on the poor treatment response and the imaging findings, pulmonary neoplasia was suspected. The case was referred internally to the oncology department of the teaching hospital. A computed tomography (CT) scan of the chest and abdomen was performed under general anesthesia. A fine needle aspiration and a tru-cut biopsy of the lung mass were also performed under the same general anesthesia following the CT scan. Plain and contrast CT studies of the thorax and abdomen were evaluated by a board-certified radiologist. A large, lobulated mass lesion on the left cranial lung lobe was identified that measured 7.6 × 2.1 × 5.8 (L × W × H) cm and extended to the level of the left main stem bronchus (Fig. 2). The mass surrounded, compressed and distorted all of the bronchi within the cranial subsegment of the left cranial lung and exhibited heterogeneous contrast enhancement with a few non-enhancing fluid pockets. At the right cranial and middle lung lobe, there was irregular nodular thickening which infiltrated into the lumen of the lobar and secondary bronchi, distorting the contour of the airway and causing near complete occlusion and distortion of the airway (Fig. 2). Similar irregular soft tissue thickening was identified in the lobar bronchus of the right middle lung lobe, extending through the distal branches and causing complete occlusion and distortion of the airway (Fig. 2). The sternal lymph node was moderately thickened, measuring approximately 1.1 cm in thickness (Fig. 3). The distinction between the sternal lymph node and the left cranial pulmonary mass was difficult, and it is possible that there was a direct invasion of the mass through the mediastinum pleura. A cranial mediastinal lymph node was moderately thickened and rounded, measuring approximately 1.0 cm (Fig. 3). Remaining lung lobes were well-inflated with no additional soft tissue lesions. CT scan of the abdomen was unremarkable.
Fig. 1. Left lateral and dorsoventral thoracic radiograph reveals a mass in the left cranial dorsal lung field.
Fig. 2. CT scan of the thorax showing an extensive lesion suspected to be a mass affecting the left cranial and middle pulmonary lobes causing distorted narrowed airways. Orange arrowheads demarcate the mass and green arrowheads demarcate the airways.
Fig. 3. CT scan of the thorax showing enlarged sternal and cranial mediastinal lymph nodes. The cytology of the main lung mass revealed marked neutrophilic and eosinophilic inflammation and suggested no overt evidence of neoplasia. The lesion appeared to be primarily inflammatory with a significant eosinophilic component. Histopathologic results of the sample confirmed marked, multifocal, chronic, neutrophilic, and eosinophilic granulomatosis with a fibrous capsule (Figs. 4 and 5). Histochemical stains including, Gram for bacteria, Ziehl-Neelsen for acid-fast organisms, Periodic Acid–Schiff, and Grocott Methenamine Silver for detection of fungal organisms, were applied to the tissue sampled and all failed to reveal any infectious agents. A negative bacterial culture supported the results observed in histochemistry. The result was consistent with pulmonary pyogranuloma compatible with EPG. Corticosteroid treatment was started with a daily dose of prednisolone (Macrolone, Mavlab) at 2 mg/kg PO q 24 hours for 2 weeks.
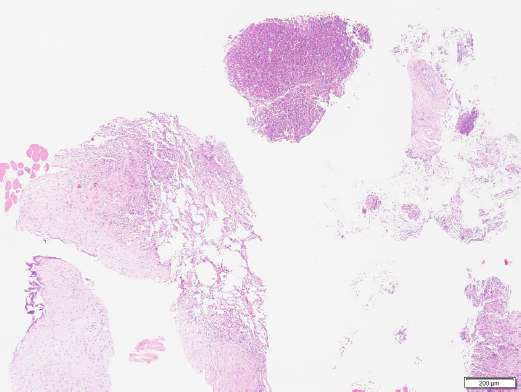
Fig. 4. Histopathology of the pulmonary mass via a Trucut biopsy. Overview revealing fibrosis and an eosinophilic multifocal inflammatory component (40× magnification). Image courtesy of Dr. May TSE from CityU VDL, Hong Kong.
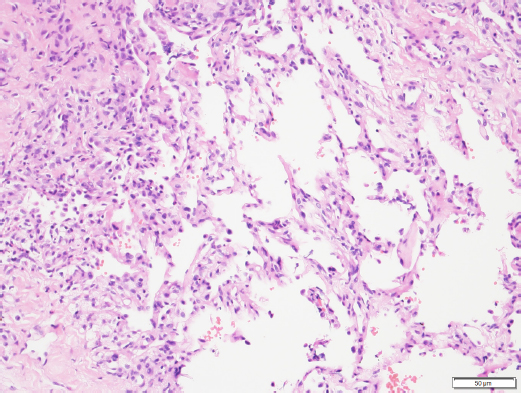
Fig. 5. Histopathology of the pulmonary mass. Fibrosis, eosinophilic multifocal inflammation and alveolar septa (200× oil magnification). Image courtesy of Dr. May TSE from CityU VDL, Hong Kong. At the 2-week revisit, the owner reported the coughing had resolved. Radiographs of the chest were repeated and revealed marked improvement with the larger lung opacity on the left cranial lung field becoming significantly smaller. The dose of prednisolone was reduced to 1 mg/kg PO q 24 hours for 2 more weeks. Continued clinical improvement was reported by the owner and was documented in the thoracic radiographs at the 4-week revisit. Complete resolution of clinical signs and radiographical lesions was observed at week 10 (Fig. 6). The dose of prednisolone was tapered off over the following 8 weeks and then stopped. The dog is currently doing well with no signs of recurrence over 1 year after the initial presentation. DiscussionThis case report describes the diagnosis and treatment of a scarcely documented pulmonary eosinophilic disease in a young dog without evidence of heartworm or lung worm infection. The dog responded positively to corticosteroids treatment achieving complete remission and cure. Only a small number of cases of EPG have been reported in the veterinary literature. Although the association with dirofilariasis has been suggested (Confer et al., 1983; Neer et al., 1986), about 70% of cases have no evidence of infection. Of the cases suspected to be associated with dirofilariasis, however, neither microfilaremia nor adult worms might be present (Confer et al., 1983; Calvert, 1992). In this case, we suggest there was no association with dirofilariasis. There were no previous known infections or compatible clinical findings supportive of the disease. The presentation was not chronic and the radiography and CT did not reveal radiological signs of previous or present heartworm disease (Confer et al., 1983). Additionally, the dog had been treated with moxidectin 18 months prior, and an antigen test was negative. Association with a previous asymptomatic infection, however, cannot be excluded. Diagnostic testing like a bronchoalveolar lavage (BAL) for the presence of other pulmonary parasites, potentially involved in the etiopathogenesis of EPG, was not performed. However, fecal analysis was negative for parasites, and on cytology and histopathology, no larvae or parasites were seen. Angiostrongylus vasorum has not been reported in Hong Kong and this would make it an unlikely differential diagnosis. In veterinary medicine, EPG is described as a more aggressive form of chronic eosinophilic pneumonia (CEP) that presents with granulomatosis (Reinero, 2019). The present case was diagnosed as EPG, however, it is questionable whether this case should be considered a subtype of CEP due to the relatively young age of the patient, the lack of chronicity of signs, and the lack of a more negative prognosis described in advanced stages of non-parasitic CEP, often accompanied by severe bronchiectasis (Fina et al., 2014). Eosinophilia and basophilia, as well as oral lesions, are reported in association with eosinophilic disease and bronchopneumopathy, but the latter was not observed in this case (German et al., 2002). Guarded prognosis with recurrence of granulomatosis has been traditionally reported in most cases of EPG Clercx et al., 2000; Fina et al., 2014). In the present case, there was a rapid and successful response to treatment differently from the usually more aggressive and poorly responsive form reported in previous studies (Neer et al., 1986; Katajavuori et al., 2013; Fina et al., 2014). Recent studies have shown a better prognosis during the management of EPG as was the case in this report (Johnson et al., 2019; Agudelo et al., 2022). A caudal pulmonary lobe distribution of lesions has been described in approximately 75% of the cases reported in the literature (Abbott and Allen, 2020). That included a recent study that assessed CT characteristics in five dogs with EPG where only caudal lobes were affected (Fina et al., 2014). The cranial lobar location of the mass in this case was different from what was observed in the previous studies. There is no known association between cranial or caudal distribution of lesions in EPG cases.
Fig. 6. Left lateral and dorsoventral thoracic radiographs 10 weeks following corticosteroid treatment. The large size of the mass and the radiological appearance of the lesions at the time of initial diagnosis in the present case were suggestive of neoplasia. Previous studies have suggested EPG as a differential diagnosis for pulmonary neoplasia (Fina et al., 2014). Radiographic and clinical features were similar to other unrelated pulmonary granulomatous diseases such as lymphomatoid granulomatosis where coughing, presence of masses, lymphadenopathy, and eosinophilia and basophilia have been also described (Berry et al., 1990). This highlights the importance of histopathology to reach a definitive diagnosis. This case might have been misdiagnosed as neoplasia based on imaging assessment alone and that could have led to euthanasia. Bronchoscopy and BAL might have been useful to better assess the bronchial involvement and rule out lung worms. Bronchointerstitial pattern on radiography, cough, and eosinophilia are reported in association with EBP, and this could have been a differential diagnosis, however, no nodules or masses are reported in this subtype of ELD (Clercx et al., 2000). Like in other reported eosinophilic pulmonary conditions eosinophilic infiltration resolved after treatment (Clercx et al., 2000), however, it is unknown at this stage whether recurrence of eosinophilia might be observed in future visits. The use of corticosteroids is the treatment of choice for eosinophilic pulmonary disease, and this was started once a diagnosis was reached. Corticosteroids reduce inflammation through inhibition of phospholipase A2, stopping the release of inflammatory mediators and arachidonic acid and the migration of inflammatory cells and granulocytes into the airways, decreasing the potential formation of granulomas as they did in the present case. More aggressive immunosuppressive therapy, including the use of cyclophosphamide, has been reported for dogs with large pulmonary nodules, but that was not necessary in this case as the dog is still well and free of disease over a year after diagnosis. AcknowledgmentsThe pathologists Daniela Hernandez Muguiro and May Tse for their contribution to the interpretation of the cytology and the histopathology as well as for providing the image samples. The radiologist Steven Tsai for the CT interpretation. Conflict of interestThe authors declare no conflict of interest. Authors’ contributionsAA wrote the manuscript with equal contributions from SYL and AG. SYL and AG managed the case before and after referral respectively. All authors reviewed the final version of the manuscript and agreed to its submission. ReferencesAbbott, D.E.E. and Allen, A.L. 2020. Canine eosinophilic pulmonary granulomatosis: case report and literature review. J. Vet. Diagn. Invest. 32, 329–335. Agudelo, C.F., Stehlik, L., Filipejova, Z., Koskova, B., Sterbova, M. and Crha, M. 2022. Pulmonary eosinophilic granulomatosis in a dog. Vet. Med. (Praha). 67, 150–155. Berry, C.R., Moore, P.F., Thomas, W.P., Sisson, D. and Koblik, P.D. 1990. Pulmonary lymphomatoid granulomatosis in seven dogs (1976–1987). J. Vet. Intern. Med. 4, 157–166. Calvert, C. 1988. Pulmonary and disseminated eosinophilic granulomatosis in dogs. J. Am. Anim. Hosp. Assoc. 24, 311–320. Calvert, C. 1992. Eosinophilic pulmonary granulomatosis. In Current veterinary therapy XI. Eds., Kirk, R. and Bonagura J. Philadelphia, PA: Saunders, pp: 813–816. Clercx, C., Peeters, D., Snaps, F., Hansen, P., McEntee, K., Detilleux, J., Henroteaux, M. and Day, M.J. 2000. Eosinophilic bronchopneumopathy in dogs. J. Vet. Intern. Med. 14, 282–291. Confer, A.W., Qualls Jr, C.W., MacWilliams, P.S. and Root, C. 1983. Four cases of pulmonary nodular eosinophilic granulomatosis in dogs. Cornell. Vet. 73, 41–51. Fina, C., Vignoli, M., Terragni, R., Rossi, F., Wisner, E. and Saunders, J.H. 2014. Computed tomographic characteristics of eosinophilic pulmonary granulomatosis in five dogs. Vet. Radiol. Ultrasound. 55, 16–22. German, A.J., Holden, D.J., Hall, E.J. and Day, M.J. 2002. Eosinophilic diseases in two Cavalier King Charles spaniels. J. Small. Anim. Pract. 43, 533–538. Johnson, L.R., Johnson, E.G., Hulsebosch, S.E., Dear, J.D. and Vernau, W. 2019. Eosinophilic bronchitis, eosinophilic granuloma, and eosinophilic bronchopneumopathy in 75 dogs (2006–2016). J. Vet. Intern. Med. 33, 2217–2226. Katajavuori, P., Melamies, M. and Rajamäki, M.M. 2013. Eosinophilic pulmonary granulomatosis in a young dog with prolonged remission after treatment. J. Small. Anim. Pract. 54, 40–43. Neer, T., Waldron, D. and Miller, R. 1986. Eosinophilic pulmonary granulomatosis in two dogs and literature review. J. Am. Anim. Hosp. Assoc. 22, 543–599. Reinero, C. 2019. Interstitial lung diseases in dogs and cats part II: known cause and other discrete forms. Vet. J. 243, 55–64. | ||
| How to Cite this Article |
| Pubmed Style AA, Lai SY, AG, . Eosinophilic pulmonary granulomatosis resembling a pulmonary carcinoma in a dog in Hong Kong. Open Vet J. 2022; 12(5): 612-617. doi:10.5455/OVJ.2022.v12.i5.3 Web Style AA, Lai SY, AG, . Eosinophilic pulmonary granulomatosis resembling a pulmonary carcinoma in a dog in Hong Kong. https://www.openveterinaryjournal.com/?mno=17065 [Access: April 24, 2024]. doi:10.5455/OVJ.2022.v12.i5.3 AMA (American Medical Association) Style AA, Lai SY, AG, . Eosinophilic pulmonary granulomatosis resembling a pulmonary carcinoma in a dog in Hong Kong. Open Vet J. 2022; 12(5): 612-617. doi:10.5455/OVJ.2022.v12.i5.3 Vancouver/ICMJE Style AA, Lai SY, AG, . Eosinophilic pulmonary granulomatosis resembling a pulmonary carcinoma in a dog in Hong Kong. Open Vet J. (2022), [cited April 24, 2024]; 12(5): 612-617. doi:10.5455/OVJ.2022.v12.i5.3 Harvard Style , A. A., Lai, S. Y., , A. G. & (2022) Eosinophilic pulmonary granulomatosis resembling a pulmonary carcinoma in a dog in Hong Kong. Open Vet J, 12 (5), 612-617. doi:10.5455/OVJ.2022.v12.i5.3 Turabian Style , Angel Almendros, Sin Yi Lai, Antonio Giuliano, and . 2022. Eosinophilic pulmonary granulomatosis resembling a pulmonary carcinoma in a dog in Hong Kong. Open Veterinary Journal, 12 (5), 612-617. doi:10.5455/OVJ.2022.v12.i5.3 Chicago Style , Angel Almendros, Sin Yi Lai, Antonio Giuliano, and . "Eosinophilic pulmonary granulomatosis resembling a pulmonary carcinoma in a dog in Hong Kong." Open Veterinary Journal 12 (2022), 612-617. doi:10.5455/OVJ.2022.v12.i5.3 MLA (The Modern Language Association) Style , Angel Almendros, Sin Yi Lai, Antonio Giuliano, and . "Eosinophilic pulmonary granulomatosis resembling a pulmonary carcinoma in a dog in Hong Kong." Open Veterinary Journal 12.5 (2022), 612-617. Print. doi:10.5455/OVJ.2022.v12.i5.3 APA (American Psychological Association) Style , A. A., Lai, S. Y., , A. G. & (2022) Eosinophilic pulmonary granulomatosis resembling a pulmonary carcinoma in a dog in Hong Kong. Open Veterinary Journal, 12 (5), 612-617. doi:10.5455/OVJ.2022.v12.i5.3 |