| Case Report | ||
Open Vet J. 2022; 12(6): 868-876 Open Veterinary Journal, (2022), Vol. 12(6): 868–876 Case Report The use of flow cytometry for diagnosis and immunophenotyping in chronic lymphocytic leukemia in a dog: Clinical case reportRosina Sánchez-Solé1*, Graciela Pedreira1, José Manuel Venzal2, Carlos Eduardo Fonseca-Alves3 and Paula Pessina Serdio11Laboratorio de Análisis Clínicos, Facultad de Veterinaria, Universidad de la República, Montevideo, Uruguay 2Laboratorio de Vectores y Enfermedades Transmitidas, Centro Universitario Litoral Norte, Universidad de la República, Salto, Uruguay 3Departamento de Clínica Veterinária, Faculdade de Medicina Veterinária e Zootecnia de Botucatu, Universidade Estadual Paulista Júlio de Mesquita Filho, São Paulo, Brazil Submitted: 26/05/2022 Accepted: 21/10/2022 Published: 19/11/2022 *Corresponding Author: Rosina Sánchez-Solé. Laboratorio de Análisis Clínicos, Facultad de Veterinaria, Universidad de la República, Montevideo, Uruguay. Email: rosinasanchsole [at] gmail.com © 2022 Open Veterinary Journal
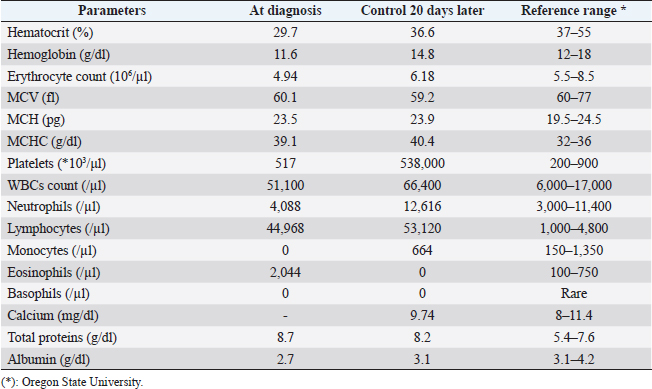
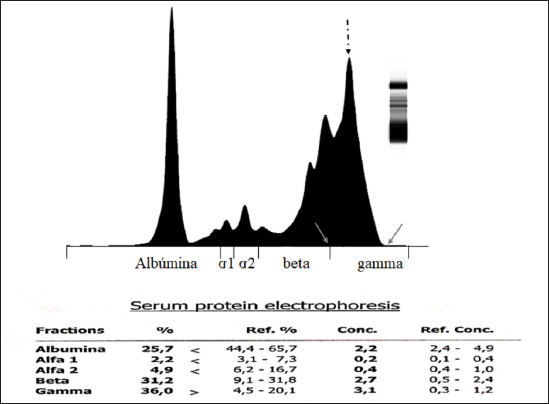
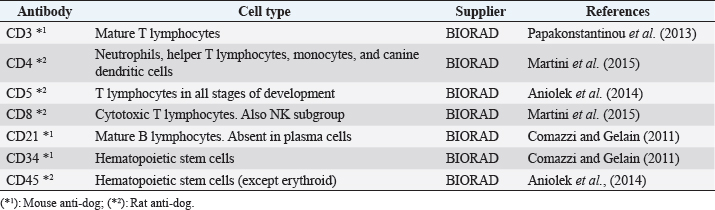
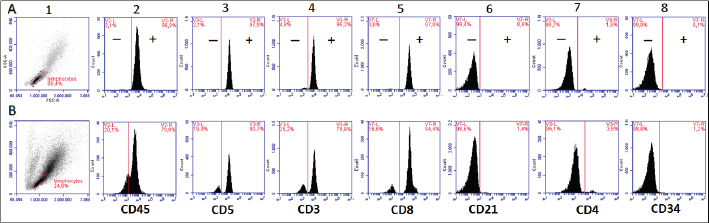
AbstractBackground: Chronic lymphocytic leukemia (CLL) is the most common type of leukemia in dogs. It is characterized by the proliferation of neoplastic lymphocytes in the bone marrow, which are morphologically normal (mature), but non-functional. CLL in canines commonly originates in cytotoxic T lymphocytes (TCD8+), and although there is controversy regarding the prognostic value of the immunophenotype, this cell lineage may be associated with a good prognosis. Case Description: A 10-year-old, entire female, mixed-breed dog was brought to the University Hospital of the Veterinary Faculty (UdelaR) for consultation because a routine pre-surgical check-up revealed lymphocytic leukocytosis, normocytic anemia, and hyperglobulinemia due to an oligoclonal gammopathy. The ultrasound revealed splenomegaly. PCR performed on blood was negative for Ehrlichia canis. Blood and bone marrow flow cytometry was performed to complement the diagnosis and carry out the immunophenotype, which showed CLL of CD8+ T-cell lineage. The clinical suspicion of CLL was confirmed by a myelogram. Chemotherapy treatment based on alkylating agents and glucocorticoids was established. So far, the patient has an overall survival of 13 months with a good response to treatment. Conclusion: The combination of the immunophenotyping test, the myelogram, and the hematological and biochemical profile confirmed the presence of T-CLL in our patient. Flow cytometry, increasingly used in veterinary medicine, allowed us to confirm the diagnosis of CLL originating in cytotoxic T lymphocytes in our patient, through the presence of positive staining of primary antibodies specific for the canine species CD45, CD3, CD5, and CD8 and the absence of staining for CD4, CD21, and CD34. Keywords: Canine, Cytotoxic T lymphocytes, Chronic leukemia, Immunophenotype, Lymphocytosis. IntroductionChronic lymphocytic leukemia (CLL) is a malignant tumor characterized by an expansion of small and mature-appearing lymphocytes in the blood and bone marrow, characterized by being morphologically normal but immunodeficient (Workman and Vernau, 2003). Although the leukemias incidence in dogs is unknown so far, it is known that CLL represents the most common type in both canines and humans, and is the second most diagnosed hematopoietic malignancies in both species, after lymphoma (Nabhan and Rosen, 2014; Rout and Avery, 2017; Avery, 2020). The cause of this disease is unknown, but genetic factors linked to its development are being studied (Rout et al., 2018; Avery, 2020). CLL normally occurs in adult dogs, between 10 and 12 years old (Helfand and Modiano, 2000; Comazzi et al., 2011), and it does not have a sexual predisposition (Workman and Vernau, 2003; Adam et al., 2009). Likewise, small-breed dogs are at higher risk, specifically for CLL originating from B lymphocytes (Bromberek et al., 2016). Although the course of CLL is generally slow and insidious, a subset of dogs has been described that develop a more aggressive disease (described as atypical CLL) with shorter survival (Comazzi et al., 2011). However, most dogs are asymptomatic, and the diagnosis in many cases is incidental (hematological evaluation in patients with other pathologies, or routine checkups in geriatric patients). If any, clinical symptoms are mild and nonspecific (Vail et al., 2019). Lethargy, apathy, hypoxia, weight loss, and vomiting are described as frequent symptoms. Physical examination may show mucosa pallor, lymphadenomegaly, splenomegaly, and to a lesser extent hepatomegaly (Leifer and Matus, 1986; Bromberek et al., 2016). The clinical manifestations are due to the progressive accumulation of neoplastic lymphocytes in the bone marrow, lymph nodes, and other tissues, as well as to the immunological alterations that accompany this process (Workman and Vernau, 2003). The diagnosis of this disease begins with the finding of persistent absolute lymphocytosis in serial blood tests, accompanied or not by cytopenia of the other cell lines, mainly mild anemia and thrombocytopenia (McDonough and Moore, 2000; Dobson et al., 2006; Tasca et al., 2009). Lymphocyte counts range from 6,000 to 100,000 small lymphocytes/µl (Leifer and Matus, 1986), with counts even exceeding one million lymphocytes/µl of blood (Vernau and Moore, 1999). Although alterations in the hematological and biochemical profile are nonspecific, anemia, hypercalcemia, and gammopathies occur in a subset of patients with CLL. Anemia, in addition to being frequently found, has been associated with an unfavorable prognosis in T-cell CLL, but not in B-cell CLL (Comazzi et al., 2011; Rout et al., 2021). CLL is one of the causes of malignant hypercalcemia, whose systemic repercussions must be controlled to prevent the clinical picture from worsening (Bergman, 2012). Another paraneoplastic syndrome is gammopathies that are present in a subset of patients with CLL (Leifer and Matus, 1986; Moore and Avery, 2019). In veterinary medicine, there are currently technological alternatives such as flow cytometry that have quickly become indispensable tools for medical research. In recent years, the uses of this technique have increased, with multiple applications in fields such as immunology, hematology, oncology, pathology, and cell and molecular biology (Tarrant, 2005). However, the most important applications are in the area of onco-hematology, as it occurs in humans (Weiss, 2002). In this sense, this technique is a common element of diagnostic and prognostic investigations in hematology-oncology, allowing blood and/or bone marrow samples to determine the immunophenotyping of leukemias and lymphomas; perform differential bone marrow counts, as well as diagnose anemias and immunodeficiencies in dogs and cats (Reggeti and Brienzle, 2011; Aniolek et al., 2014). Flow cytometry allows a rapid quantitative and qualitative evaluation of the cell population, evaluating the size of the cells, the simultaneous expression of different antigens in the same population, as well as the quantitative evaluation of each marker individually (Gelain et al., 2008; Comazzi and Gelain, 2011; Martini et al., 2015). The identification of surface antigens on lymphocytes (clusters of differentiation, CD), allows their typing and determining the degree of cell maturation (Aniolek et al., 2014; Aresu et al., 2015; Martini et al., 2015). Canine CLL may originate from both B lymphocytes (B-CLL) and T cells (T-CLL), (Rout and Avery, 2017). In canines, unlike what occurs in humans, CLL originated in T lymphocytes, particularly cytotoxic T ymphocytes (CD8), are more frequent. These are characterized by the expression of the surface markers CD45, CD3, CD5, and CD8 (Adam et al., 2009; Comazzi et al., 2011). In general, these neoplastic cytotoxic T lymphocytes are peculiarly larger than normal lymphocytes, they present a small nucleus and abundant cytoplasm (Vernau and Moore, 1999; Rout and Avery, 2017). The definitive diagnosis of CLL is obtained in most cases, by puncturing the bone marrow and performing the corresponding myelogram. It is characterized by a hyperplasia of the lymphoid series with normal morphology, with a predominance of mature-looking lymphocytes (>30%), small in size, and with condensed chromatin (Vernau and Moore, 1999). The presence of azurophilic granules in the cytoplasm of lymphocytes allows us to identify the most frequent subtype in the dog, which is CLL of large granular lymphocytes (Tasca et al., 2009; Comazzi et al., 2011; Rout and Avery, 2017).Chemotherapy is useful in patients with clinical symptoms, with a count greater than 60,000 lymphocytes/µl, severe cytopenia, hepatomegaly, splenomegaly, and/or lymphadenopathy (Comazzi et al., 2011). The prognosis of CLL is good, with survival that can reach 3 years in patients that suffer from it (Leifer and Matus, 1986; Vernau and Moore, 1999; Nelson and Couto, 2010). As far as we know, there are few studies that link how the immunophenotype determines the prognosis, and the little medical literature on the subject is controversial. In this regard, Williams et al. (2008) reported a similar prognosis in both immunophenotypes (T-CLL vs. B-CLL), however, other authors state that survival is higher in canines with T-CLL (Comazzi et al., 2011). On the other hand, it has been reported that exacerbation (blast crisis) and Richter’s syndrome (development of lymphoma in patients with CLL) may occur as complications of this disease, regardless of the immunophenotype (Kantarjian and O’Brien, 2012; Comazzi et al., 2015a). The aim of this study is to show how immunophenotyping through flow cytometry allows a better characterization of the disease, as well as to classify leukemia present in this clinical case. This study also seeks to show the usefulness of this diagnostic tool to differentiate a reactive lymphocytosis from a neoplastic lymphocytosis, this being the main challenge to reaching a definitive diagnosis of CLL. The following is a clinical case of a canine with CLL and its evolution after chemotherapy treatment, treated at the University Hospital of the Veterinary School, UdelaR. Case DetailsA 10-year-old female mixed-breed canine was brought to the University Hospital of the Veterinary School (UdelaR) for consultation. The patient was referred from a private clinic after the routine pre-surgical check-up for ovariectomy that revealed a marked lymphocytosis (15,660 lymphocytes/µl), sustained over time (14,760 lymphocytes/µl, according to studies performed in the 6 to 5 months prior to her referral). Having previously ruled out other possible causes, the clinical veterinarian suggested neutering since the patient had recurrent skin problems that could be related to estrogen production. The clinical examination did not reveal any abnormality in the dog, with the exception of dermatological problems (bilateral alopecia on the flank, and back of the thighs, along with hyperpigmentation of the area). Nothing unusual was observed in the coloration of the mucous membranes, body temperature, and heart or respiratory rate. The palpable lymph nodes did not show changes in size or consistency. The complete blood count revealed alterations in both the red cells (normocytic anemia) and the leukogram (leukocytosis, marked lymphocytosis, eosinophilia, and monocytopenia), which are summarized in Table 1. Calcium levels and renal function values were within the reference ranges for the species. Alterations were neither observed in the concentration of liver enzymes, cholesterol, and bilirubin, however, hyperproteinemia, hyperglobulinemia, and hypoalbuminemia were found. Protein fractions determined by the capillary protein electrophoresis diagram revealed an increase in the beta-gamma fraction and an oligoclonal gammopathy (high and narrow “monoclonal-like” peak superimposed on a broad “polyclonal-like” base) (Fig. 1). PCR was performed on a blood subsample using the commercial kit GeneJET Genomic DNA Purification Kit (Thermo Scientific, Lithuania) following manufacturer’s instructions, to extract DNA. A molecular screening was carried out using primers EHR16SD (5′-GGT-ACC-YAC-AGA-AGA-AGT-CC-3′)/EHR16SR (5′-TAG-CAC-TCA-TCG-TTT-ACA-GC-3′) with PCR conditions described by Parola et al. (2000). This PCR is able to detect a 16S rRNA gene fragment (345 bp) of the Anaplasmataceae family including genera Anaplasma, Ehrlichia, Neorickettsia and Wolbachia, being negative for all of them. Distilled water and an Anaplasma marginale DNA positive sample were included as negative and positive controls in all runs. Five microliters of PCR products were analyzed by electrophoresis into 1.5% agarose gels, stained with GoodViewTM Nucleic Acid. Stain (SBS Genetech Co., Beijing, China), and examined under UV transillumination. The ultrasound study showed multiple spherical anechoic structures in both ovaries, with smooth edges and posterior acoustic reinforcement suggestive of follicles and/or cysts. Mild splenomegaly was observed, with smooth edges, slightly heterogeneous parenchyma, and preserved echogenicity. No alterations were reflected in the other visualized organs. A discrete interstitial lung pattern was observed on the radiographs. None of the imaging studies showed lymphadenopathy. For immunophenotyping, by flow cytometry (Accuri C6, BD BIOSCIENCES, CA) we used samples of satisfactory quality, both in the number of cells (500,000 to 1,000,000 total cells) and in viability (greater than 80%). The latter was determined through negative staining to propidium iodide (Invitrogen, Thermo Fisher Scientific, Waltham, MA). For immunophenotyping, we used primary antibodies specific for the canine species (BIO-RAD, USA) directed against cell differentiation antigens (B or T lymphocytes) (Table 2). The incubation of all antibodies was carried out following the manufacturer’s recommendations, in a dark environment for 45 minutes, at room temperature. Next, the red blood cells were lysed, using 1 ml of lysis buffer (Quicklysis, Visur, Salamanca, Spain), for 10 minutes in the dark. Once the lysis was complete, the nucleated cells were ready to be placed in the flow cytometer. A minimum of 5,000 lymphocytes was acquired and a tube with the same type of sample (blood or marrow puncture) was included as a negative control. The latter underwent the same treatment, but no antibodies were added. Immunophenotyping was consistent with lymphoid leukemia originating from cytotoxic T lymphocytes, characterized by positive marking for CD45, CD3, CD5, and CD8 antigens. The samples were negative for CD4 (T helpers), CD21 (specific for B lymphocytes), and CD34 (Hematopoietic stem cells) (Fig. 2). Table 1. Hematological and serum biochemistry analysis at the time of diagnosis and at the control 20 days later.
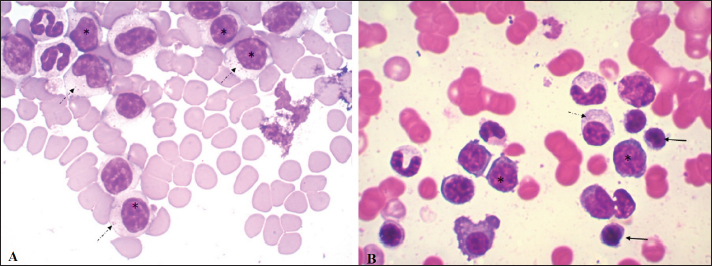
Fig. 1. Capillary protein electrophoresis diagram. The different protein fractions are observed (albumins; alpha-1 globulins, alpha-2 globulins; beta globulins, gamma globulins). A restricted oligoclonal gammopathy is distinguished, characterized by the presence of a broad-based “polyclonal type” beta-gamma peak (solid arrows), superimposed with a “monoclonal type” peak (dashed arrow). The myelogram confirmed the presumptive diagnosis of lymphoid leukemia, showing a cellular bone marrow with 49% small to medium-sized lymphocytes with fine azurophilic granules (Fig. 3). The erythroid, myeloid, and megakaryocytic series did not present qualitative alterations, showing different stages of maturation. Plasma cells were within normal limits. The myeloid-to-erythroid ratio was slightly decreased. Due to the dermatological conditions present in the dog, the presence of parasites (mites) was ruled out through skin scraping in the affected areas. The analysis of the results as a whole allowed us to establish the definitive diagnosis of CLL originating in cytotoxic T lymphocytes. Based on this diagnosis, a treatment based on alkylating agents (Leukeran; chlorambucil, 3 mg/m2/PO/48 hours) and glucocorticoids (prednisone, 2mg/kg/day/PO, gradually reducing the dose until its suspension when lymphocytosis is normalized) was established. Twenty days after starting the treatment, a clinical and analytical control was carried out, finding a clear clinical improvement despite the fact that lymphocytosis persisted in the blood count. It is worth noting that the lymphocyte count had increased, also presenting a mild neutrophilia, however, the erythrocyte values improved and the anemia and eosinophilia initially diagnosed disappeared. The other parameters studied and the abdominal ultrasound did not show significant changes. Table 2. WBCs markers used to detect canine leukemia by flow cytometry.
Fig. 2. Graphic representation of the flow cytometry performed (A, blood; B, bone marrow). Morphologic scatter plot (forward scatter, FSC; side scatter, SSC), (A.1 and B.1). Histograms show that the lymphocyte population studied in both cases was positive for CD45 (A.2, B.2). Diagnosis of CD8+ T CLL; positive for CD5 (A.3, B.3), CD3 (A.4, B.4), CD8 (A.5, B.5) and negative for CD21 (A.6, B.6), CD4 (A.7, B.7) and CD34 (A.8, B.8) antigens.
Fig. 3. Blood smear (A) and bone marrow cytology (B) stained with May-Grunwald Giemsa. In both, the cell population is mainly composed of medium-sized lymphocytes with abundant cytoplasm (*) and fine azurophilic granules (dashed arrow). Some normal lymphocytes are seen (solid arrow). The ultrasound findings, together with the skin scraping without evidence of pathogens (acari, bacteria nor fungi), allowed us to presume that the dermatological problems could result from the ovarian alterations observed. Due to the diagnosis of CLL, the patient’s ovariectomy was postponed until the end of chemotherapy and her subsequent progress. So far, the patient has had 13 months of survival with a good response to treatment. DiscussionCLL is caused by the abnormal proliferation of small lymphocytes that undergo complete and orderly maturation in the bone marrow. The diagnosis of CLL in the reported case was an incidental finding in a routine pre-surgical check-up. This disease is characterized by the presence of lymphocytosis, a hematological alteration that also occurs in immune-mediated diseases, infectious diseases (antigenic stimulation), hypoadrenocorticism, thymoma, and lymphoproliferative disorders, thus requiring a correct differential diagnosis (Avery and Avery, 2007). Most of the causes mentioned, unlike CLL, present lower or transient lymphocytosis and hardly reach 15–20,000 lymphocytes/µl, along with clinical manifestations associated with the primary disease (Rondeau et al., 2005; Rosypal et al., 2005; Dobson et al., 2006; Lathan and Thompson, 2018). Among the infectious causes, chronic canine Ehrlichiosis is an exception, since in some cases we can observe counts that exceed 30,000 lymphocytes/µl (McDonough and Moore, 2000; Heeb et al., 2003; Avery and Avery, 2007). This disease was ruled out in the present case due to a lack of clinical criteria, PCR-negative test, and because in our country, although the vector exists, it has not been infected so far and there are no confirmed diagnoses of this disease. It has been reported that lymphocyte counts greater than 20,000/µl are almost pathognomonic of CLL (Nelson and Couto, 2010) and that those above 30,000 CD8 lymphocytes/µl have lower survival (Williams et al., 2008). However, there are other neoplastic diseases that also present peripheral lymphocytosis as a primary feature, such as acute lymphoblastic leukemia (ALL) and T-zone lymphoma (Avery and Avery, 2007). The differentiation of primary leukemia or lymphoma can be tricky and, in some cases, the patient can present a transformation of lymphoma/leukemia which leaves the patient with the worst prognosis (Comazzi et al., 2015b). In lymphoma, solid lymphoid organs will be involved and leukemia is classically considered a blood cancer. In our clinical approach, we did not find any evidence of a solid lymphoid organ that could bring lymphoma as a diagnosis. In our case, ALL was ruled out due to the absence of lymphoblasts in the patient’s smear and negative CD34 marking on flow cytometry (Wilkerson et al., 2005; Gelain et al., 2008). Flow cytometry is of great importance in differentiating CLL from the lymphocytosis caused in T-zone lymphoma, since the circulating lymphocytes in CLL are CD45+, whereas those in T-zone lymphoma are CD45− (Seelig et al., 2014; Martini et al., 2015, 2016). It is important to highlight that patient’s clinical response was in accordance with our approach. For a T-zone lymphoma or other T-cell non-Hodgkin lymphoma subtype, a different treatment should be applied and the clinical response will be different. The relevance of flow cytometry lies in the fact that it allows ruling out other lymphoproliferative diseases, and the phenotypic classification of CLL according to the cell lineage that originates the tumor. This also confirms the diagnosis, since the fact that most of the cells correspond to a single cell subtype (TCD8+ in this case) is highly suggestive of neoplasm (Nelson and Couto, 2010). CD8+ T CLL is the subtype most frequently observed in canines and was the phenotype found in our case (Tasca et al., 2009; Comazzi et al., 2011). In the veterinary literature, it is common to find this type of cancer classified as T-cell CLL. In dogs, immunophenotype was previously associated with patients’ overall survival, being B-cell lymphocyte leukemia more aggressive than T-cell lymphocytic leukemia (Comazzi et al., 2011). However, due to the low number of patients in each study and the low availability of flow cytometry for immunophenotype in several countries, no difference in overall survival between CD4+ and CD8+ lymphocytic chronic leukemia has been shown (Gonzalez-Rodriguez et al., 2010; Comazzi et al., 2011). The lymphoid hyperplasia found in the myelogram performed on our patient reaffirmed the diagnosis of CLL (Dobson et al., 2006). The anemia observed in the dog is consistent with Tasca et al. (2009), who consider this hematological alteration as a frequent complication in canine oncology patients. In addition to being one of the disorders most frequently associated with CLL, anemia is considered a negative prognostic factor (Comazzi et al., 2011). To our knowledge, the mild eosinophilia observed in our case has not been reported in patients with CLL. However, Ozaki et al. (2006) described eosinophilia as a paraneoplastic condition in close relation to IL-5 synthesis by neoplastic T lymphocytes in patients with intestinal lymphoma. Similarly, the presence of monocytopenia in our case could be associated with lower production of this cell line associated with excessive production of lymphocytes, or be due to an immune-mediated mechanism (Dipaolo et al., 2005). However, we believe this is likely clinically insignificant as if 1 monocyte had been observed on the differential, the monocyte count would fall within the reference interval. Other hematological alterations (thrombocytopenia, neutropenia) associated with CLL have been reported but were not found in the present case (Tasca et al., 2009; Bromberek et al., 2016). The presence of gammopathy in our patient may be paradoxical because CLL in this case occurs in T lymphocytes. However, this phenomenon has been described in several B and T-lymphocyte disorders in humans, including T-cell CLL (Mims, 2018). In a recent study by our work group, gammopathies were observed in T-zone lymphoma (CD45−). Higher levels of gamma globulins were observed in these dogs than in the B lymphoma (LB) and CD45+ T lymphoma groups and higher levels of beta globulins than in the LB group (Sánchez et al., 2021). Additionally, high percentages of monoclonal gammopathy have been reported in canines with CLL, but to our knowledge, the available studies have not determined the lineage (B vs. T), assuming that monoclonal gammopathy is usually related to B-cell neoplasms (Leifer and Matus, 1986; Giraudel et al., 2002; Dobson et al., 2006). The presence of an oligoclonal gammopathy, also known as restricted polyclonal, seen in our patient, is frequently seen in dogs with immune-mediated, infectious, or inflammatory conditions (Tothova et al., 2016). However, this type of electrophoretic tracing has also been associated with lymphoproliferative neoplasms (Moore and Avery, 2019). In general, these neoplastic lymphocytes are seen in the blood, however, the extent of the disease in other organs or tissues should be evaluated. In the present case, mild splenomegaly and slightly heterogeneous parenchyma, possibly associated with infiltration, were found. CLL usually responds well to treatment, with chlorambucil and prednisone the most commonly used drugs in veterinary medicine to treat it. At the moment of this case report publication, the patient is tolerating well the treatment with overall survival of 13 months. The patient keeps doing monthly follow up. ConclusionThis case highlights that combining the immunophenotyping test, the myelogram, and the hematological and biochemical profile is important in the complex diagnostic process of CLL. Flow cytometry, increasingly used in veterinary medicine, is a rapid and minimally invasive diagnostic method that allows not only to confirm of the diagnosis of CLL but also to differentiate it from other reactive processes that cause lymphocytosis. The presence of positive staining of primary antibodies specific for the canine species CD45, CD3, CD5, and CD8 and the absence of staining for CD4, CD21, and CD34, confirmed the diagnosis of CLL originating in cytotoxic T lymphocytes in our patient. AcknowledgmentsWe thank Dr. Alicia Decuadro for referring the clinical case to the University Hospital of the Veterinary School, and for performing the treatment as well as the follow-up of the patient. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionRosina Sánchez conducted the clinical case and wrote the article. Graciela Pedreira and José Venzal performed the laboratory tests. Carlos Fonseca corrected the final version of the manuscript. Paula Pessina assisted in writing and editing the article. ReferencesAdam, F., Villiers, E., Watson, S., Coyne, K. and Blackwood, L. 2009. Clinical pathological and epidemiological assessment of morphologically and immunologically confirmed canine leukaemia. Vet. Comp. Oncol. 7(3), 181–195. Aniolek, O., Gajewski, Z. and Ginzinski, S. 2014. Application of flow cytometry in diagnosing lymphomas in dogs and cats. Centr. Eur. J. Immunol. 39(3), 327–330. Aresu, L., Martini, V., Rossi, F., Vignoli, M., Sampaolo, M., Aricom A., Laganga, P., Pierini, A., Frayssinet, P., Mantovani, R. and Marconato, L. 2015. Canine indolent and aggressive lymphoma: clinical spectrum with histologic correlation. Vet. Comp. Oncol. 13(4), 348–362. Avery, A.C. 2020. The genetic and molecular basis for canine models of human leukemia and lymphoma. Front. Oncol. 10, 23. Avery, C. and Avery, P. 2007. Determining the significance of ersistent lymphocytosis. Vet. Clin. Small. Anim. 37(2), 267–282. Bergman, J.P. 2012. Paraneoplastic hypercalcemia. Top. Companion. Anim. Med. 27(4), 156–158. Bromberek, J.L., Rout, E.D., Agnew, M.R., Yoshimoto, J., Morley, P.S. and Avery, A.C. 2016. Breed distribution and clinical characteristics of B cell chronic lymphocytic leukemia in dogs. J. Vet. Intern. Med. 30(1), 215–222. Comazzi, S. and Gelain, M.E. 2011. Use of flow cytometric immunophenotyping to refine the cytological diagnosis of canine lymphoma. Vet. J. 188(2), 149–155. Comazzi, S., Gelain, M.E., Martini, V., Riondato, F., Miniscalco, B., Marconato, L., Stefanello, D. and Mortarino, M. 2011. Immunophenotype predicts survival time in dogs with chronic lymphocytic leukemia. J. Vet. Intern. Med. 25(1), 100–106. Comazzi, S., Martini, V., Riondato, F., Poggi, A., Stefanello, D., Marconato, L., Albonico, F. and Gelain, M.E. 2015a. Chronic lymphocytic leukemia transformation into high-grade lymphoma: a description of Richter’s syndrome in eight dogs. Vet. Comp. Oncol. 15(2), 366–373. Comazzi, S., Aresu, L. and Marconato, L. 2015b. Transformation of canine lymphoma/leukemia to more aggressive diseases: anecdotes or reality? Front. Vet. Sci. 2, 42. DiPaolo, R.J., Glass, D.D., Bijwaard, K.E. and Shevach, E.M. 2005. CD4+ CD25+ T cells prevent the development of organ specific autoimmune disease by inhibiting the differentiation of autoreactive effector T cells. J. Immunol. 175(11), 7135–7142. Dobson, J., Villiers, E. and Morris, J. 2006. Diagnosis and management of leukaemia in dogs and cats. In. Pract. 28(1), 22–31. Gelain, M.E., Mazzilli, M., Riondato, F., Marconato, L. and Comazzi, S. 2008. Aberrant phenotypes and quantitative antigen expression in different subtypes of canine lymphoma by flow cytometry. Vet. Immunol. Immunopathol. 121(3–4), 179–188. Giraudel, J.M., Pagès, J.P. and Guelfi, J.F. 2002. Monoclonal gammopathies in the dog: a retrospective study of 18 cases (1986–1999) and literature review. J. Am. Anim. Hosp. Assoc. 38(2), 135–147. Gonzalez-Rodriguez, A.P., Contesti, J., Huergo-Zapico, L., Lopez-Soto, A., Fernández-Guizán, A., Acebes-Huerta, A., Gonzalez-Huerta, A.J., Gonzalez, E., Fernandez-Alvarez, C. and Gonzalez, S. 2010. Prognostic significance of CD8 and CD4 T cells in chronic lymphocytic leukemia. Leuk. Lymphoma. 51(10), 1829–1836. Heeb, H.L., Wilkerson, M.J., Chun, R. and Ganta, R.R. 2003. Large granular lymphocytosis, lymphocyte subset inversion, thrombocytopenia, dysproteinemia, and positive Ehrlichia serology in a dog. J. Am. Anim. Hosp. Assoc. 39(4), 379–384. Helfand, S.C. and Modiano, J.F. 2000. Chronic lymphocytic leukemia. In Schalm’s veterinary hematology. Eds., Feldman, B.F., Zinkl, J.G. and Jain, N.C. Philadelphia, PA: Lippincott Williams and Wilkins, pp: 638–641. Kantarjian, H. and O’Brien, S. 2012. The chronic leukemias. In: Cecil textbook of medicine. Eds., Goldman, L. and Schafer, A. I. Philadelphia, PA: Saunders Elsevier, pp: 1209–1218. Lathan, P. and Thompson, A. 2018. Management of hypoadrenocorticism (Addison’s disease) in dogs. Vet. Med (Auckl). 9, 1–10. Leifer, C.E. and Matus, R.E. 1986. Chronic lymphocytic leukemia in the dog: 22 cases (1974-1984). J. Am. Vet. Med. Assoc. 189(2), 214–217. Martini, V., Poggi, A., Riondato, F., Gelain, M. E., Aresu, L. and Comazzi, S. 2015. Flow-cytometric detection of phenotypic aberrancies in canine small clear cell lymphoma. Vet. Comp. Oncol. 13(3), 281–287. Martini, V., Marconato, L., Poggi, A., Riondato, F., Aresu, L., Cozzi, M. and Comazzi, S. 2016. Canine small clear cell/T-zone lymphoma: clinical presentation and outcome in a retrospective case series. Vet. Comp. Oncol. 14(1), 117–126. McDonough, S.P. and Moore, P.F. 2000. Clinical, hematologic, and immunophenotypic characterization of canine large granular lymphocytosis. Vet. Pathol. 37(6), 637–646. Mims, M.P. 2018. Lymphocytosis, lymphocytopenia, hypergammaglobulinemia, and hypogammaglobulinemia. In Hematology, basic principles and practice. Eds., Hoffman, R., Benz, E.J., Silbrstein, L.E., Heslop, H.E., Weitz, J.I., Anastasi, J., Salama, M.E. and Abutalib, S.A. Philadelphia, PA: Elsevier, pp: 682–690. Moore, A.R. and Avery, P.R. 2019. Protein characterization using electrophoresis and immunofixation; a case-based review of dogs and cats. Vet. Clin. Pathol. 48(1), 29–44. Nabhan, C. and Rosen, S. 2014. Chronic lymphocytic leukemia a clinical review. JAMA. 312(21), 2265–2276. Nelson, R.W. and Couto, C.G. 2010. Leucemias. In Medicina Interna de pequeños animals. Eds., Nelson, R.W. and Couto, C.G. Barcelona, Spain: Elsevier, pp: 1187–1194. Ozaki, K., Yamagami, T., Nomura, K. and Narama, I. 2006. T-Cell lymphoma with eosinophilic infiltration involving the intestinal tract in 11 dogs. Vet. Pathol. 43(3), 339–344. Papakonstantinou, S., Berzina, I., Lawlor, A., O’Neill, E.J. and O’Brien, P.J. 2013. Rapid, effective and user-friendly immunophenotyping of canine lymphoma using a personal flow cytometer. Ir. Vet. J. 66(1), 6. Parola, P., Roux, V., Camicas, J.L., Baradji, I., Brouqui, P., and Raoult, D. 2000. Detection of Ehrlichiae in African ticks by polymerase chain reaction. Trans. R. Soc. Trop. Med. Hyg. 94(6), 707–708. Reggeti, F. and Bienzle, D. 2011. Flow cytometry in veterinary oncology. Vet. Pathol. 48(1), 223–235. Rondeau, M.P., Walton, R.M., Bissett, S., Drobatz, K.J. and Washabau, R.J. 2005. Suppurative, nonseptic polyarthropathy in dogs. J. Vet. Intern. Med. 19(5), 654–662. Rosypal, A.C., Troy, G.C., Duncan, R.B., Zajac AM. and Lindsay, D.S. 2005. Utility of diagnostic tests used in diagnosis of infection in dogs experimentally inoculated with a North American isolate of Leishmania infantum infantum. J. Vet. Intern. Med. 19(6), 802–809. Rout, E.D. and Avery, P.R. 2017. Lymphoid neoplasia: correlations between morphology and flow cytometry. Vet. Clin. North. Am. Small. Anim. Pract. 47(1), 53–70. Rout, E.D., Burnett, R.C., Labadie, J.D., Yoshimoto, J.A. and Avery, A.C. 2018. Preferential use of unmutated immunoglobulin heavy variable region genes in boxer dogs with chronic lymphocytic leukemia. PLoS One 13(1), e0191205. Rout, E.D., Labadie, J.D., Yoshimoto, J.A., Avery, P.R., Curran, K.M. and Avery, A.C. 2021. Clinical outcome and prognostic factors in dogs with B-cell chronic lymphocytic leukemia: a retrospective study. J. Vet. Intern. Med. 35(4), 1918–1928. Sánchez-Solé, R., Mosquillo, F., Jark, P., Breijo, M. and Pessina, P. 2021. Hematological and biochemical profiles of canine CD45- T lymphomas are different from other immunophenotypes. Open. Vet. J. 11(4), 734–746. Seelig, D.M., Avery, P., Webb, T., Yoshimoto, J., Bromberek, J., Ehrhart, E.J. and Avery, A.C. 2014. Canine T-zone lymphoma: unique immunophenotypic features, outcome, and population characteristics. J. Vet. Intern. Med. 28(3), 878–886. Tarrant, J.M. 2005. The role of flow cytometry in companion animal diagnostic medicine. Vet. J. 170(3), 278–288. Tasca, S., Carli, E., Caldin, M., Menegazzo, L., Furlanello, T. and Gallego, L.S. 2009. Hematologic abnormalities and flow cytometric immunophenotyping results in dogs with hematopoietic neoplasia: 210 cases (2002-2006). Vet. Clin. Pathol. 38(1), 2–12. Tothova, C., Nagy, O. and Kovac, G. 2016. Serum proteins and their diagnostic utility in veterinary medicine: a review. Vet. Med. (Praha). 61, 475–496. Vail, D.M., Thamm, D.H. and Liptak, J.M. 2019. Hematopoietic tumors. In Small animal clinical oncology. Eds., Withrow and MacEwen’s. St.Louis, MO: Elsevier, pp: 688–772. Vernau, W. and Moore, P.F. 1999. An immunophenotypic study of canine leukemias and preliminary assessment of clonality by polymerase chain reaction. Vet. Immunol. Immunopathol. 69(2–4), 145–164. Weiss, D.J. 2002. Application of flow cytometric techniques to veterinary clinical hematology. Vet. Clin. Pathol. 31(2), 72–82. Wilkerson, M.J., Dolce, K., Koopman, T., Shuman, W., Chun, R., Garrett, L., Barber, L. and Avery, A. 2005. Lineage differentiation of canine lymphoma/leukaemia and aberrant expression of CD molecules. Vet. Immunol. Immunopathol. 106(3–4), 179–196. Williams, M.J., Avery, A.C., Lana, S.E., Hillers, K.R., Bachand, A.M. and Avery, P.R. 2008. Canine lymphoproliferative disease characterized by lymphocytosis: immunophenotypic markers of prognosis. J. Vet. Intern. Med. 22(3), 596–601. Workman, H.C. and Vernau, W. 2003. Chronic lymphocytic leukemia in dogs and cats: the veterinary perspective. Vet. Clin. North. Am. Small. Anim. Pract. 33(6), 1379–1399. | ||
| How to Cite this Article |
| Pubmed Style RSS, GP, JMV, Fonseca-Alves CE, Serdio PP, . The use of flow cytometry for diagnosis and immunophenotyping in a chronic lymphocytic leukemia in a dog: clinical case report. Open Vet J. 2022; 12(6): 868-876. doi:10.5455/OVJ.2022.v12.i6.13 Web Style RSS, GP, JMV, Fonseca-Alves CE, Serdio PP, . The use of flow cytometry for diagnosis and immunophenotyping in a chronic lymphocytic leukemia in a dog: clinical case report. https://www.openveterinaryjournal.com/?mno=43916 [Access: August 31, 2024]. doi:10.5455/OVJ.2022.v12.i6.13 AMA (American Medical Association) Style RSS, GP, JMV, Fonseca-Alves CE, Serdio PP, . The use of flow cytometry for diagnosis and immunophenotyping in a chronic lymphocytic leukemia in a dog: clinical case report. Open Vet J. 2022; 12(6): 868-876. doi:10.5455/OVJ.2022.v12.i6.13 Vancouver/ICMJE Style RSS, GP, JMV, Fonseca-Alves CE, Serdio PP, . The use of flow cytometry for diagnosis and immunophenotyping in a chronic lymphocytic leukemia in a dog: clinical case report. Open Vet J. (2022), [cited August 31, 2024]; 12(6): 868-876. doi:10.5455/OVJ.2022.v12.i6.13 Harvard Style , R. S. S., , G. P., , J. M. V., Fonseca-Alves, C. E., Serdio, P. P. & (2022) The use of flow cytometry for diagnosis and immunophenotyping in a chronic lymphocytic leukemia in a dog: clinical case report. Open Vet J, 12 (6), 868-876. doi:10.5455/OVJ.2022.v12.i6.13 Turabian Style , Rosina Sánchez Solé, Graciela Pedreira, José Manuel Venzal, Carlos Eduardo Fonseca-Alves, Paula Pessina Serdio, and . 2022. The use of flow cytometry for diagnosis and immunophenotyping in a chronic lymphocytic leukemia in a dog: clinical case report. Open Veterinary Journal, 12 (6), 868-876. doi:10.5455/OVJ.2022.v12.i6.13 Chicago Style , Rosina Sánchez Solé, Graciela Pedreira, José Manuel Venzal, Carlos Eduardo Fonseca-Alves, Paula Pessina Serdio, and . "The use of flow cytometry for diagnosis and immunophenotyping in a chronic lymphocytic leukemia in a dog: clinical case report." Open Veterinary Journal 12 (2022), 868-876. doi:10.5455/OVJ.2022.v12.i6.13 MLA (The Modern Language Association) Style , Rosina Sánchez Solé, Graciela Pedreira, José Manuel Venzal, Carlos Eduardo Fonseca-Alves, Paula Pessina Serdio, and . "The use of flow cytometry for diagnosis and immunophenotyping in a chronic lymphocytic leukemia in a dog: clinical case report." Open Veterinary Journal 12.6 (2022), 868-876. Print. doi:10.5455/OVJ.2022.v12.i6.13 APA (American Psychological Association) Style , R. S. S., , G. P., , J. M. V., Fonseca-Alves, C. E., Serdio, P. P. & (2022) The use of flow cytometry for diagnosis and immunophenotyping in a chronic lymphocytic leukemia in a dog: clinical case report. Open Veterinary Journal, 12 (6), 868-876. doi:10.5455/OVJ.2022.v12.i6.13 |