| Original Article | ||
Open Vet J. 2021; 11(2): 319-329 Open Veterinary Journal, (2021), Vol. 11(2): 319–329 Original Research Adrenal cortex stimulation with hCG in spayed female dogs with Cushing’s syndrome: Is the LH-dependent variant possible?Ignacio M. Espiñeira1,2, Patricia N. Vidal3,4, María C. Ghersevich5, Elber A. Soler Arias3, Fernanda Bosetti1,3, María F. Cabrera Blatter1,3, Diego D. Miceli3,6 and Víctor A. Castillo1,3*1Facultad de Ciencias Veterinarias, Cátedra de Clínica Médica de Pequeños Animales, Universidad de Buenos Aires, Buenos Aires, Argentina 2Becario Estímulo UBACyT, Rep. Argentina 3Hospital Escuela de Medicina Veterinaria-U. Endocrinología, Rep. Argentina 4Becaria Proyecto Estratégicos UBACyT, Rep. Argentina 5Facultad de Ciencias Agropecuarias, U. Católica de Córdoba-Argentina, Rep. Argentina 6IByME-CONICET, Rep. Argentina *Corresponding Author: Víctor A. Castillo. Facultad de Ciencias Veterinarias, Universidad de Buenos Aires, Cátedra de Clínica Médica de Pequeños Animales, Buenos Aires, Argentina. Email: vcastill [at] fvet.uba.ar Submitted: 25/01/2021 Accepted: 23/06/2021 Published: 29/06/2021 © 2021 Open Veterinary Journal
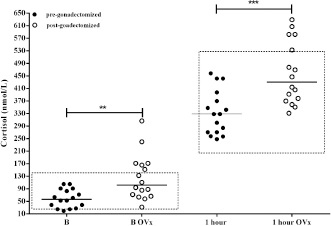
AbstractBackground: The expression and overexpression of luteinizing hormone (LH) receptors in the canine adrenal gland cortex have been reported. Therefore, it was hypothesized that a LH-dependent form of Cushing’s syndrome (CS) could exist in dogs. Aim: To assess whether the adrenal gland post-ovariectomy (OVx) exhibits a greater response to adrenocorticotrophin (ACTH) stimulation; to evaluate whether the adrenal gland responds to human chorionic gonadotropin (hCG) stimulation by increasing the release of cortisol; and to consider whether hCG stimulus testing would be useful as a diagnosis for possible cases of LH-dependent CS. Methods: Cortisol concentrations were measured from healthy female dogs (n=16) at baseline and following ACTH stimulation before and 2 months after gonadectomy (OVx). Cortisol concentrations were also measured for female dogs with CS (n=14) following administration of hCG (5000 IU). A post-hCG cortisol concentration greater than 140 nmol/l was used to define dogs with LH-dependent Cushing’s syndrome. Results: In normal female dogs, both pre- and post-stimulation cortisol concentrations increased following OVx (p=0.002 and p=0.0003, respectively). In female dogs with CS, cortisol concentrations increased following stimulation with hCG in 57% (8/14; p=0.002). Age at the time of OVx was associated (p=0.015) with the cortisol response to hCG [8 (5–9) years vs. 3.5 (2–6) years, p=0.0013). Conclusion: Based on these results, an LH-dependent form of CS occurs in spayed female dogs, and that it is more likely to occur when female dogs are spayed later in life. Keywords: Aberrant receptors, Cushing’s syndrome, Gonadectomy, hCG, Luteinizing hormone. IntroductionCushing’s syndrome (CS) or hyperadrenocorticism is one of the most frequent endocrinopathies in dogs whose prevalence and presentation coincide with the ones included in different authors’ reports (Gallelli et al., 2010; O’Neill et al., 2016; Carotenuto et al., 2019; Martins et al., 2019). In the present, and considering all different etiologies of the syndrome, it can be classified as adrenocorticotrophin (ACTH)-dependent Cushing’s syndrome and ACTH-independent or adrenal Cushing’s syndrome (Kooistra and Galac, 2012; Duan et al., 2015). Within this last group, neoplastic causes (nodes, adenomas, and adrenal cortex carcinomas), bilateral nodular hyperplasia, and expression of receptors that are not normally found or expressed in the adrenal cortex, i.e., aberrant receptors, are considered (Vuorenoja et al., 2007; Bermichtein et al., 2008). First references to the expression and functionality of the LH receptor (LHR) and, therefore, to the action of the luteinizing hormone (LH) over the adrenal cortex were found in postmenopausal women, in which it was observed that no longer after having experienced menopause, they developed the disease (Pabon et al., 1996) with lack of existence of pituitary or suprarenal neoplasia or ACTH-producing neuroendocrine tumor. Lacroix et al. (2001), as well as other authors, argue that chronic stimulation of aberrant receptors causes bilateral adrenal hyperplasia to develop, afterward, nodes (uni- or bilateral), adenomas, and adrenal cortex carcinomas as last stage (Rilianawati et al., 1998; Mazzuco et al., 2006, 2007). In one in vitro study, Feelders et al. (2003) describe the changes taking place due to the LH chronic stimulation over its receptor and in the signaling of the hyperplastic adrenal cortex cells. In veterinary medicine, the first feasible case by an aberrant receptor in dogs was reported by Galac et al. (2008) due to adrenal cortex stimulation by gastric inhibitory peptide or glucose-dependent insulinotropic peptide. Later, Galac et al. (2010) described the presence of different aberrant receptors in the adrenal cortex in healthy dogs and dogs with CS, among them, the LHR ectopic overexpression. Moreover, as the responsible cause of endocrinopathy and adrenal tumor development, LH’s action has been confirmed in ferrets (Schoemaker et al., 2002, 2008). Neutering by ovariectomy (OVx) in female dogs is increasingly common in early ages on account of birth control and prophylactic reasons (Root Kustritz, 2002). Nonetheless, there is great inequality among countries and regions within the same country regarding the age of OVx, depending on idiosyncrasy (Kubinyi et al., 2009). In general, the age of OVx in South America tends to be higher than 2 or 3 years. Regarding CS and the proportion of spayed females, a range has been reported that covers from 61%, inferred from the study by Bennaim et al. (2018), similar to the 64.9% in Martins et al.’s (2019) study, to 86.1% pertaining to O’Neill et al.’s (2016) study. In retrospect, when analyzing medical records of dogs suffering from CS, it was striking that owners reported, in some cases, that they started noticing weight increase with changes in the shape of the abdomen and increasing liquid intake shortly after OVx.Since after OVx, the negative feedback of the gonadal axis is lost, and waiting for an increase in LH, the action of this hormone may be one of the causes of CS in female OVx dogs, which suggests LH-dependent CS. The objective was to evaluate, initially, whether cortisol concentrations are affected after OVx and, according to post-OVx assessment, carry out a second test. Such a test consisted of administering human chorionic gonadotropin hormone (hCG), given its similar LH effect in healthy dogs and dogs with CS (both OVx cases) to determine, firstly, whether it produces any effect over cortisol secretion and, secondly, whether it would be useful for the identification and diagnosis of possible LH-dependent CS. Materials and MethodsStudy 1Study population Sixteen normal weight, 3/5-body condition (WSAVA, 2013), medium-size (weight: 13.4 ± 3.5 kg; age: 6.7 ± 1.6 year) female dogs were referred for spaying from the Responsible Pet Ownership Program (12 mixed-breed females and 4 beagles) after owners gave their consent to participate in the study. Laboratory and clinical examination and cardiorespiratory assessment were carried out a week before surgery to evaluate the animals’ good health conditions. By vaginal smear, it was confirmed that all of them were in anestrus. Two days before spaying, a synthetic ACTH stimulation test (tetracosactrin) was carried out (Synacthen 0.25 mg/ml ®, Novartis Lab.), administering the complete ampoule (0.25 mg) intravenously (Frank et al., 2000). Basal and 1-hour poststimulation cortisol evaluation times were considered. The same test was carried out 60 days after OVx (to avoid immediate postoperative stress and in the subsequent controls), alongside the same pre-surgical routine. Study 2Study population CS-OVx group: OVx dogs with presumptive CS diagnosis were selected based upon clinical signs (weight increase, more prominent abdomen, polyuria and polydipsia, and dermatological symptoms) and blood biochemistry alterations (hepatic enzymes increase and dyslipidemia) that were referred for final diagnosis and treatment. Age at consultation, age in which OVx was carried out, and time from OVx to CS final diagnosis were considered. An immediate abdominal ultrasound was carried out on female selected dogs, exploring adrenal glands, before carrying out diagnostic tests, to include only those which would be apt for the purpose of the study (apart from the hepatomegaly characteristic of CS, there was not supposed to be any other organic alteration). The inclusion criterion was that, by ultrasound, the adrenal gland (Liste et al., 1997) did not present tumor of irregular aspect and bigger than 2 cm, which may raise suspicions of an adrenal carcinoma. Only OVx dogs with increased bilateral gland or presence of node in one or both glands, of normal aspect and smaller than 2 cm, were admitted. The reason for not including animal with suspected adrenal carcinoma was that they might not express or overexpress receptors, both MC2R (for ACTH) and aberrant ones (LHR and GIPR), depending on the cell undifferentiating level (Weiss et al., 1989; Galac et al., 2010). After obtaining owners’ consent, 14 spayed females were selected for the study (10 medium-size mixed-breed, 1 Basenji, 1 Fox Terrier, 1 Beagle, and 1 Poodle). The median age was 9.5 years (range 7–14) and weight 11.9 ± 3.3 kg. The median age in which OVx was carried out was 7 (2–9) years, and the time between OVx and diagnosis was 3.5 (1–12) years. In order to confirm CS diagnosis, synthetic ACTH stimulus was carried out as previously described. Subsequently, suppression by dexamethasone was indicated by means of the cortisol/creatinine ratio in urine (Dexs/CCR) before and after administering 0.1 mg/kg of dexamethasone orally, every 8 hours (Galac et al., 1997; Kooistra and Galac, 2012), considering a normal basal CCR value (pre-dexamethasone) up to 10 × 10–6 and that inhibition occurs (pituitary origin indicator) when there is a decrease in the post-dexamethasone value of over 50% compared to the basal value. It was possible to carry out a magnetic resonance imaging (MRI) of the seller region on 9 out of the 14 selected females (owners of the remaining females did not give their consent on different grounds). hCG testhCG dose preliminary analysis For the preliminary study, 5000 IU of hCG (Gonacor 5000, Ferring Lab.) were administered intramuscularly. First, doses indicated in reproduction were tested in three healthy OVx control female dogs (Kutzler, 2007); later, 2500 IU and, lastly, 5000 IU. Evaluated times were basal time (pre-hCG), 30 minutes, 1 hour, and 2 hours after administration. It was only with 5000 IU that a slight increase was noted after 1 hour that reached its peak and did not differ with the measuring 2 hours, so it was decided to use this last dose in the development of the study and take into account the basal and 1 hour times (data not shown). Control group (CG) Six female dogs from the kennels of the institution (vivarium) were included. Such group comprised two mixed-breeds and four beagles (weight 12.3 ± 2 kg and age 7 ± 2 years), which had been OVx 60 days before the study due to management reasons of the vivarium. Once their health conditions were checked, the ACTH stimulation test was first carried out, and plasma ACTH was measured. Later (7 days), the 5000 IU-hCG stimulation was carried out in a single-dose intramuscular administration (Gonacor 5000, Ferring Lab.), evaluating basal cortisol (pre-hCG) and 1 hour after administration. CS-OVx group 5000 IU of hCG stimulation was carried out on the 14 female dogs included in the study, as previously described. Stimulation was carried out the week after having carried out Dexs/CCR to ensure no inhibition of the adrenal axis due to exogenous corticosteroid. In accordance with the resulting hCG response (with or without considerable cortisol increase), dogs would be separated into two groups: CS-OVxA, with no significant increase, and CS-OVxB, significantly increasing. Evaluated hormonesCortisol was evaluated in plasma and urine (with the corresponding urinary creatinine) and the plasmatic ACTH (pACTH). Samples were obtained by venepuncture of the antebrachium cephalic vein. For cortisol, they were collected in plastic tubes with ethylenediaminetetraacetic acid anticoagulant. For pACTH, a separate refrigerated tube containing aprotinin was used; the sample was immediately centrifuged and frozen at –80 C until its processing. Only basal time was evaluated. Cortisol was measured by the chemiluminescence method (Immulite 1000 ®, Siemens Healthineers, Munro, Argentina) with a 5.5% interassay and 8% intraassay coefficients of variation and a 5.5 nmol/l sensitivity. Cortisol in urine was also evaluated by chemiluminescence, and creatinine was measured by laboratory routine automated method (MetrolabAutoanalyzer Merck, Darmstadt, Germany). Interassay and intraassay coefficients of variation were 8% and 5%, respectively; sensitivity was 11 nmol/l for urinary cortisol and 1.77 μmol/l for urinary creatinine. pACTH was also measured by chemiluminescence (Immulite 1000 ACTH: adrenocorticotropic hormone reagent 100 count 1/Bx, Siemens Healthineers, Munro, Argentina), being the interassay and intraassay coefficients of variation 8% and 7.6%, respectively, and sensitivity was 1.1 pmol/l. In our laboratory, reference intervals for such a method are 2.2–14.3 pmol/l. Statistical analysisThe results of study 1 were compared by non-parametric paired sample test (Wilcoxon U test) between pre- and post-OVx cortisol values. In study 2, basal plasmatic cortisol and hCG poststimulation data were compared by the Wilcoxon U test (intragroup) and Mann–Whitney test (between groups). Primarily, it was evaluated whether there existed a substantial post-hCG cortisol increase in both groups and if there were differences between the CG and the 14 female dogs with CS-OVx. Afterward, the results obtained from the CS-OVxA and CS-OVxB groups were compared. Moreover, delta variation ∆M-m between basal time (minimum value, m) and the highest post-hCG value obtained (maximum, M) was determined, as well as the maximum stimulation percentage datum (%M) of each group. Finally, a contingency table (Fisher’s exact test) was used to determine whether there is a statistical association between the age of OVx, the time elapsed between OVx, and diagnosis of CS and the response obtained to the hCG stimulus. Data are expressed as median and percentiles 10 and 90 (P10 and P90), being the significance level p < 0.05 (GraphPad 6 software, San Diego, CA). Ethical approvalThe Committee for the Use and Care of Laboratory Animals (CICUAL) of the Faculty of Veterinary Sciences of the University of Buenos Aires approved all procedures (Protocol CICUAL #2013/17 and UBACyT 20720170100006BA), and written consent was obtained from all proprietors for carrying out all evaluations and their subsequent publication. ResultsStudy 1None of the female dogs included in the study presented alterations, neither in the pre-surgical exams (all of them were in the anestrus phase) nor 60 days after OVx. After OVx, cortisol concentrations increased significantly (Fig. 1), in both basal value (p=0.002) and 1-hour poststimulation ( p=0.0003). Basal cortisol was over the maximum cut-off limit in 6/16 (37.5 %). Poststimulation cortisol values exceeded 551.8 nmol/l in 4/16 (25%), similar to the hyperresponse that defines CS diagnosis (Gilor and Graves, 2011; Behrend et al., 2013; Bennaim et al., 2019).
Fig. 1. Stimulation with ACTH before and after OVx. Note that after OVx, cortisol concentrations are higher. **p=0.002 between baseline cortisol values; ***p=0.0003 between 1-hour cortisol values. Median is indicated. Each circle corresponds to a studied dog. Delimited area corresponds to normal range of cortisol, pre- and post-ACTH stimulus. Study 2Imaging, ACTH stimulation, Dexs/CCR, and pACTH in female dogs of CG and with CS Results of adrenal ultrasound and MRI (in cases in which it was carried out) and responses to diagnostic tests are shown in Table 1. Ultrasound tests indicated bilateral enlargement of adrenal glands. In two dogs (cases #6 year 9), one node in the left adrenal gland was also found, being, in each case, <1.5 cm. Via MRI (nine dogs), the presence of a hypophysis tumor could only be confirmed in four dogs; one of them reported the previously informed node as well (case #6).In CG, the ACTH stimulation test presented a normal response, basal cortisol being 122.6 (66.42–163.5) nmol/l, and 1 hour 336.7 (252.4–389) nmol/l. The GC pACTH concentration was 6.5 (4.8–10.9) pmol/l. Table 1. Dogs with CS.
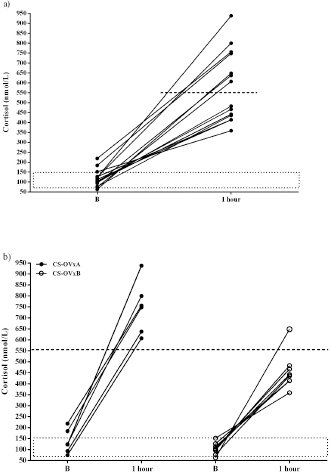
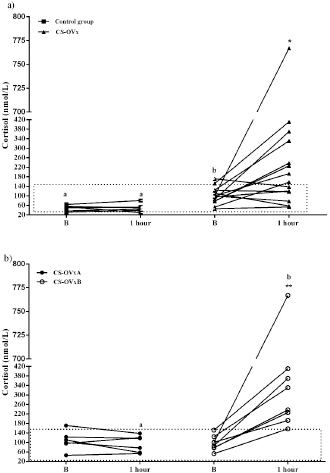
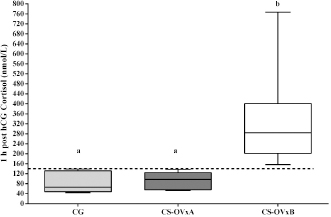
In dogs with CS, the ACTH stimulation study was hyperresponsive in 7/14 dogs (Table 1); cortisol value did not exceed 551.8 nmol/l after 1 hour in the rest of them (Fig. 2). Basal cortisol was 109 (62.9–218.8) nmol/l, and 1 hour after ACTH was 544.9 (358.7–938.1) nmol/l in the CS-OVx. Dexs/CCR test (Table 1) suppressed more than 50% (60.5 [56–83]%) in 4/14 and it was less than 50 % (21 [4–29]%) in 10/14 dogs. pACTH concentration was 13.2 (2.7–17.8) pmol/l. hCG stimulation testThere were no meaningful differences between pre-and 1-hour post-hCG cortisol concentrations in the CG (Fig. 3). Basal cortisol and 1 hour post-hCG were lower compared to the CS-OVx group (p=0.002 and p=0.0004, respectively). Cortisol increased notably (p=0.02) in dogs with CS-OVx after administering hCG (Fig. 3). This indicated that hCG stimulation with 5000 IU acts differently in dogs with CS compared to control dogs. After observing the individual behavior of each dog after hCG, it was noted that in 6/14 there was no significant increase in cortisol, while there was in the remaining eight dogs (seven of these had no hyperresponse to the ACTH test and none had Dexs/CCR inhibition) Those in which there was no substantial cortisol increase 1-hour post-hCG (basal: 105 [45.5–171.1] nmol/l; 1 hour: 97.4 [53.2–138] nmol/l) were identified and grouped as CS-OVxA (n=6). The remaining ones were grouped as CS-OVxB (n=8), as they presented significant differences (p=0.008) between pre- and 1-hour post-hCG cortisol (92.3 [52.4–151.7] nmol/l and 284.2 [157–767] nmol/l, respectively) (Fig. 3).Differences ( p=0.0007) between CS-OVxA and CS-OVxB in cortisol concentration were evidenced only 1-hour post-hCG (Fig. 3). There were no differences between the respective cortisol basal concentrations. Therefore, it was analyzed whether there would be differences between 1-hour post-hCG of CG versus CS-OVxA, not having found significant differences.
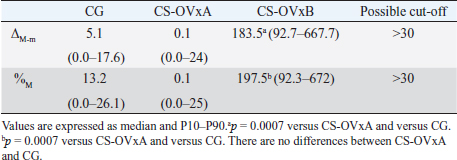
Fig. 2. Stimulation with ACTH. (a) The response in all female dogs with CS-OVx (14); (b) separate responses of resulting CS-OVxA and CS-OVxB groups. It can be observed that not all stimulations (7/14) exceed the cut-off value of 551.8 nmol/l after 1 hour. The delimited area corresponds to basal cortisol reference values. Considering the P90 of the CS-OVxA and CGs (138 and 138.9 nmol/l, respectively) and the P10 of the CS-OVxB group (157 nmol/l), there are no overlap cases observed. A post-hCG cut-off value of 140 nmol/l could be considered (Fig. 4). ∆M-m and %M of the hCG test were determined, being importantly higher ( p=0.0007) in CS-OVxB with regard to CS-OVxA and the CG. There were no differences between CG and CS-OVxA (Table 2). In the same way, as previously described, for ∆M-m and %M (P90), the cut-off values could be set at values >30 for both estimators, as no bitch from the other groups had a ∆M-m or %M that exceeded that value. The P10 for ∆M-m and %M of the CS-OVxB group was 92.7 and 92.3, respectively
Fig. 3. Cortisol concentration with 5000 IU of hCG stimulation test. (a) CG dogs and CS-OVx dogs (group of 14 dogs); (b) resulting CS-OVxA and CS-OVxB groups. (a) No considerable changes were detected in the CG after 1 hour; cortisol level increased compared to its baseline value in CS-OVx dogs (*p=0.02). The basal cortisol concentration in this group was higher than in the GC (a–bp=0.002). (b) **p=0.008; 1-hour cortisol versus baseline cortisol of CS-OVxB; a–bp=0.0007; 1-hour cortisol of CS-OVxA versus 1-hour cortisol of CS-OVxB. There are no differences between basal cortisol concentrations of both groups. The delimited area corresponds to basal cortisol reference values.
Fig. 4. Comparison of cortisol at post-hCG time between the CGs, CS-OVxA, and CS-OVxB. It is expressed as median and 10th and 90th percentiles. The dotted line indicates the value of 140 nmol/l, with no overlap between the maximum values of CS-OVxA and the minimum values of CS-OVxB. (a–a) There are no significant differences; (a versus b) p=0.0007. pACTH concentrations were significantly higher (p=0.003) in CS-OVxA (12.8 [6.2–17.8] pmol/l) than in CS-OVxB (5.6 [2.7–6.6] pmol/l). As shown in Table 1, dogs that reacted notably to hCG stimulus did not react to ACTH stimulus (except for one with both responsive tests, case #9). In none of them, there was dexamethasone suppression.Comparing cortisol concentrations when carrying out the ACTH stimulation test between CG versus CS-OVxa and CS-OVxB, no significant differences were observed between the basal cortisols of CG [122.6 (66.42–163.5) nmol/l] versus CS-OVxA (123.6 [74.5–218.8] nmol/l) and CS-OVxB (103.5 [62.9–151.7] nmol/l). At 1-hour post-ACTH, differences were found between the cortisol of CS-OVxA [751.8 (607–938.1) nmol/l] versus CG [336.7 (252.4–389) nmol/l] (p <0.001) and versus CS-OVxB (438.7 [358.7–648.4 nmol/l) (p < 0.01), with no differences between CG versus CS-OVxB.
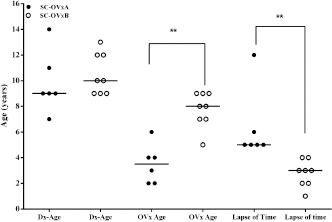
Fig. 5. Age of CS diagnosis, age of OVx, and time between OVx and CS diagnosis between CS-OVxA and CS-OVxB. There are differences only between age of OVx and time lapse and between OVx and CS diagnosis. Line indicates median age. **p=0.0013 (CS-OVxA vs. CS-OVxB) and **p=0.0007 (time lapse). Dx-Age: age at diagnosis; OVx-age: age of ovariectomy. On the other hand, pACTH did not show differences between CG [6.5 (4.8–10.2) pmol/l], CS-OVxB [5.6 (2.7–6.6) pmol/l], and CS-OVxA [12.8 (6.2–17.8) pmol/l] (p < 0.01). Age of diagnosis, age of OVx, and time between OVx and diagnosisIn CS-OVxA, age of diagnosis was 9 (7–14) years; age of OVx, 3.5 (2–6) years; time between OVx and CS diagnosis was 5.5 (5–12) years. In CS-OVxB age of diagnosis was 10 (7–14) years; age of OVx, 8 (5–9) years; time between OVx and CS diagnosis was 3 (1–4) years.Age of diagnosis was the same in both groups (Fig. 5); there were differences in the age of OVx ( p=0.0013) and time between OVx and CS diagnosis ( p=0.0007). Association between hCG-stimulation response and age of OVxIt was verified that there was a considerable statistical association (p=0.009, OR: 0.03; 95%CI=0.001–0.74) between the age of hCG stimulation and age of OVx (it was considered more than 7 year or less than that, as it was the median age of the 14 female dogs). Table 2. ∆-M-m and %M values from hCG stimulation tests carried out on CG and dogs grouped in CS-OVxA and CS-OVxB.
DiscussionThis study noted that basal cortisol level increased after OVx in healthy dogs (study 1) and presented greater ACTH stimulus-response. In study 2, control female dogs did not show cortisol increase after hCG stimulus. On the contrary, it was confirmed that when stimulating CS-OVx dogs with hCG, there was an increase in the hormone in some female dogs, while in others no increase was observed. Such response variation was related to the age in which OVx was carried out and the etiology of CS. Pessina et al. (2009) reported that there exist different responses to ACTH stimulus and low dose dexamethasone suppression, in both males and females. In such a study, it was observed that females have a greater response to exogenous ACTH than males. It was also observed that cortisol and ACTH concentrations vary during the estrous cycle (Gallelli et al., 2015), increasing during prestrus and estrus. Later, Gallelli et al. (2016) conveyed that both corticotropic cell and adrenal cortex cells of female dogs express estrogen receptors alpha, suggesting regulation of sex steroids of adrenal axis, as communicated in previously published studies (Handa and Weiser, 2014). In postmenopausal women, ferrets and mice, LHR overexpression in the adrenal cortex of individuals with CS was described as aberrant (Lacroix et al., 2001; Schoemaker et al., 2002, 2008). Therefore, LH increase occurring in postmenopausal women or by neutering in ferrets and mice is considered responsible for aberrant LHR activation. This results in hypercortisolism, changes in the adrenal morphology, and according to what was hypothesized by different authors, it could progress toward adrenal cortex carcinoma (Lacroix et al., 2001; Schoemaker et al., 2002, 2008). In human, LHRs implication in CS is only significant in a patient with adrenal (ACTH-independent) Cushing’s syndrome. Galac et al. (2010) describe for the first time the LHR overexpression in the adrenal cortex of healthy dogs, dogs with adenoma, and adrenal cortex carcinoma, being located in zona glomerulosa and zona fasciculata. This would explain cortisol increase after OVx (it was confirmed that the ovary had been completely removed) in the first study. However, within reference ranges, it is still higher than the level before OVx. In women, cortisol increase is also described during climacteric and postmenopause, and that replacement with estradiol mitigates adverse effects of such hormone (Woods et al., 2006; Cagnacci et al., 2011; Ycaza Herrera et al., 2017) and inhibits steroidogenesis in ovariectomized rats (Jopeck et al., 2018). Postmenopausal or post-OVx estradiol decrease could be related to adrenal axis deregulation. On the other hand, gonadal axis inhibition is lost, resulting in an LH increase observed after spaying in postpubertal females (Löfstedt and Van Leeuwen, 2002; Buijtels et al., 2006). Therefore, cortisol increase in this group of dogs may be attributed to the combined effect of estradiol decrease and LH increase. After stimulation with ACTH, the greatest increase in cortisol observed, even above its cut-off values in some bitches, could be that a synergistic action between LH and ACTH occurs in cells of the fasciculata zone. Surgical stress cannot be considered a reason for this due to the time elapsed; even the >551.8 nmol/l response in one dog is striking. While this could result from consultation stress, a greater sensitivity was still evidenced in the cells of zona fasciculata when exposed to ACTH stimulus. Unfortunately, apart from arranged monitoring, follow-up examination could be carried out, neither in this dog nor in the rest of the group. The result of the 5000 IU-hCG study in dogs of the CG recently spayed produced no stimulation after hCG. This is in contrast with the CS group, in which it was confirmed that eight dogs showed considerable stimulation. Moreover, age of OVx and time elapsed until carrying out the study would play a major role, as healthy dogs from the vivarium were 2 years younger on average than dogs with CS, and post-OVx time in which the test was carried out was 60 days for the first group, whereas for the CS-OVx group, it was of at least 3.5 years. As later discussed, this is important, as there is a longer action time of LH over the adrenal gland. Considering the CS-OVx population, it can be clearly observed that there is a difference in hCG responses related to the age of OVx and time elapsed between OVx and diagnosis of the disease. Regarding neutering and CS, reports informed that most female dogs with CS were spayed (O’Neill et al., 2016; Bennaim et al., 2018; Martins et al., 2019), but none of such reports indicates the spaying age and its relationship through time (early or late) with the presence of the symptoms of the disease. The age in which dogs were spayed seems to be important, and it may, somehow, resemble what Schoemaker et al. (2000) described in ferrets. Spayed age (8 years, between 5 and 9) in dogs that reacted to hCG stimulus and that are potentially considered LH-dependent CS was 5 years later than those that did not react significantly to hCG stimulation (younger spaying: 3.5 year, between 2 and 6). It is significant that the time-lapse between OVx and CS diagnosed in dogs that reacted to hCG stimulus was 3 year (between 1 and 3), while in the other group, it was 5.5 year (between 5 and 12). This could be related to a longer time of action of both estrogens and LH (with their variations depending on the estrous cycle) over the adrenal cortex, being able to cause changes and the overexpression of LHR in rats as postulated by Apaja et al. (2005). By being spayed at an earlier time, and given the possible estradiol and LH effect over its receptor in the adrenal cortex (Gallelli et al., 2016), the action of this hormone would not be present, and LH by itself would not be enough to produce LHR overexpression and functionality. This hypothesis requires further investigation. Late neutering could be compared to menopause in women and later CS development by LH (Pabon et al., 1996; Lacroix et al., 1999, 2001). The question would be: why would there be such a difference among OVx dogs? In other words, why is it possible for LHR to be overexpressed and functional, resulting in CS in some cases but not in others? A possible explanation could be that in the first place, as stated before, it must be considered a longer time estradiol action and its steroidogenesis regulatory effect over the adrenal cortex in the group in which a later OVx was carried out (Jopeck et al., 2018). The second aspect is the LH role. It has been described that maturity and LHR expression in rats is a process physiologically controlled by gonadotrophins (Apaja et al., 2005). Moreover, Kero et al. (2000) describe that LH increase induces the expression of its receptor and steroidogenesis in the adrenal cortex, depending on the LHR expression level, according to what Jopeck et al. (2018) observed in rats. Meij et al. (1997) report substantially higher LH concentrations in neutered dogs with CS than in unneutered dogs, as it would be reasonable to expect high LH concentration in the female dogs included in this study. Thus, the post-OVx LH increase (carried out in a later stage) may cause the LHR expression in the adrenal cortex of the female dog (with a longer exposure time in years to increases in estrogen during the estrous cycle), resembling that described in postmenopausal women, ferrets, and mice (Lacroix et al., 2001; Apaja et al., 2005; Christopoulos et al., 2005; Schoemaker et al., 2008). It would then be possible that in younger castrated bitches the origin of Cushing’s syndrome is mainly pituitary-dependent, while if they are castrated at an older age, the probability of being of adrenal origin increases, particularly if it is an adrenal cortex tumor and if the RLH has or does not have implications in its development, something that should be investigated in the dog. In reference to the reaction to CS diagnostic tests, pACTH concentrations and hCG test, it was significant that dogs that reacted to hCG were those whose ACTH stimulation reflected <551.8 nmol/l (and without significant differences with the CG) were not suppressed by dexamethasone and presented lower pACTH concentrations so that the cause for this condition should be considered ACTH non-dependent in this group of dogs. In these cases, LH is likely to be considered the cause of hypercortisolism instead of native ACTH. The resulting hypercortisolism causes corticotropic cell inhibition (lower pACTH), which causes dexamethasone not to act as an inhibitor of the adrenal axis, as the glucocorticoid receptor is already blocked decreased (Bosje et al., 2002; Kooistra and Galac, 2012). Should the diagnostic sensitivities described for ACTH stimulation test and dexamethasone suppression (Kooistra et al., 1997; Behrend and Kemppainen, 2001; Behrend et al., 2013; Bennaim et al., 2018, 2019) be considered, many of the false-negative cases for the ACTH stimulation, or there was no cortisol suppression by dexamethasone due to inhibition of the adrenal axis, could be LH-dependent CS. According to the test and its results, they are considered possible adrenal carcinoma, pituitary macroadenoma, erratic behavior, stress, and even the concurrent presence of pituitary and adrenal adenoma (van Bokhorst et al., 2018). It is feasible that in OVx female dogs, hypercortisolism is the result of the native ACTH action and that LH may play a combined stimulatory role. The behavior observed in dogs of experience 1 and case # 9, which presented a hyperresponsive in both stimulation tests, could indicate what was previously mentioned. hCG stimulation proved to be useful to identify females with possible LH-dependent CS, given the observed cortisol increase. Indication and interpretation of such stimulation should be carried out only in dogs with ACTH non-dependent CS, together with the results of the ACTH stimulation, dexamethasone suppression (regardless of the method), and the pACTH concentrations. If the response to ACTH stimulus is <551.8 nmol/l and, there is no dexamethasone suppression alongside a decreased pACTH concentration, the possibility of being LH-dependent is viable. In turn, the prospect of an adrenal tumor suspected of carcinoma or a macroadenoma in the pituitary must have been dismissed by imaging. This is important since it opens the therapeutic possibility with GnRH agonists or antagonists as in ferrets or as suggested in humans (Lacroix et al., 1999; Wagner et al., 2001, 2005). At the time of reporting our findings, two bitches had been treated for 6 months with a GnRH agonist, obtaining encouraging results (disappearance of clinical signs, normalization of CRC and ACTH, and hCG and ACTH stimulations). Another two dogs are with the same treatment, and we await their conclusion to analyze the results and communicate them. Diagnostic sensitivity of the test to discriminate possible LH-dependent CS from those does not make this a useful test to diagnose this specific group. The study requires the evaluation of more cases and deeper investigation regarding their molecular aspects. A considerable number of OVx dogs were dismissed, as only dogs whose owners agreed to be part of the study were included. This would explain that the number of females with and without response to hCG is almost the same. It is not possible to state the real incidence of LH-dependent CS until the further performance of this test on new cases on a routine basis.On the other hand, for ethical reasons, it was not possible to collect adrenal glands or nodes samples to analyze LHR expression and functionality, being able to compare, in this way, the hCG-responding ones to the hCG non-responding ones and, consequently, proving or discarding the previously stated hypothesis. Therefore, it could only be inferred that it would be overexpressed and functional in a group of OVx dogs, based on the response when stimulated with hCG and what was reported by Galac et al. (2010). Including females with possible adrenal carcinoma and males in the study is also pending. ConclusionIn conclusion, we observed an increase in cortisol post OVx. Spaying age and time elapsed between OVx and the presence of CS clinical signs and its diagnosis are important data to be considered, together with the results of ACTH stimulation, dexamethasone suppression and the pACTH concentration. Normally, patient with pituitary-dependent CS didn’t have a response to hCG given that their CS is due to excessive stimulation of their adrenal by their excessive endogenous ACTH Diagnostic imaging (pituitary and adrenal glands) will help in the diagnostic orientation toward the possibility of LH-dependent CS. Finally, hCG stimulation would be useful for the distinction of possible LH-dependent CS cases. Therefore, it would be the last step after dismissing other causes, as previously discussed. Values to be considered are 1-hour post-hCG cortisol concentration > 140 nmol/l, ∆M-m and %M >30. AcknowledgmentThis work was supported by the University of Buenos Aires-UBACyT (code: 20720170100006BA,$ 2018–2021), Argentina. Authors’ contributionsIgnacio M Espiñeira: performance of the hCG test and follow-up of the bitches, both healthy and with CS; case selection and data collection; image analysis; manuscript writing process; and critical analysis. Patricia Vidal: performance of the hCG test in bitches with CS; post-hCG therapeutic evaluation; case selection and data collection; hormone measurement; and critical reading of the manuscript. María C. Ghersevich: performance of the ACTH test in healthy dogs before and after castration; selection of cases and data collection of healthy dogs studied in part 1; critical reading of the manuscript. Elber A. Soler Arias: performance in the endocrinology clinic; selection of cases; hormone measurement in the laboratory and image analysis; and writing process and critical analysis. Fernanda Bosetti: recruitment of cases during endocrinology clinical care destined for research and critical reading of the manuscript. María F. Cabrera Blatter: recruitment of cases during endocrinology clinical care destined for research and critical reading of the manuscript. Diego D. Miceli: hormone measurement in the laboratory and writing process and critical analysis. Víctor A. Castillo: director of the research project and director of the scholarship holders and thesis students; designer of the plan of work and research; analysis of the results obtained; statistical processing and consultant in the statistics departments of the Faculty of Medicine and Veterinary Sciences of the UBA; and critical discussion and writing process. Conflicts of interestThe authors declares that there is no conflicts of interest. ReferencesApaja, P.M., Aatsinki, J.T., Rajaniemi, H.J. and Petäjä-Repo, U.E. 2005. Expression of the mature luteinizing hormone receptor in rodent urogenital and adrenal tissues is developmentally regulated at a posttranslational level. Endocrinol. 146, 3224–3232. Behrend, E.N. and Kemppainen, R.J. 2001. Diagnosis of canine hyperadrenocorticism. Vet. Clin. North Am. Small Anim. Pract. 31, 985–1003. Behrend, E.N., Kooistra, H.S., Nelson, R., Reusch, C.E. and Scott-Moncrieff, J.C. 2013. Diagnosis of spontaneous canine hyperadrenocorticism: 2012. ACVIM consensus statement (small animal). J. Vet. Intern. Med. 27, 1292–1304. Bennaim, M, Shiel, R.E. and Mooney, C.T. 2019. Diagnosis of spontaneous hyperadrenocorticism in dogs. Part 2: adrenal function testing and differentiating tests. Vet. J. 252, 1–11. Bennaim, M., Shiel, R.E., Forde, C. and Mooney, C.T. 2018. Evaluation of individual low dose dexamethasone suppression test patterns in naturally occurring hyperadrenocorticism in dogs. J. Vet. Inter. Med. 32, 967–977. Bermichtein, S., Alevizaki, M. and Huhtaniemi, L. 2008. Is the adrenal cortex a target for gonadotropins? Trends Endocrinol. Metab.19, 231–238. Bosje, J.T., Rijnberk, A., Mol, J.A., Voorhou, G. and Kooistra, H.S. 2002. Plasma concentrations of ACTH precursors correlate with pituitary size and resistance to dexamethasone in dogs with pituitary-dependent hyperadrenocorticism. Domest. Animal. Endocrinol. 22, 201–210. Buijtels, J.J., Beijerink, N.J., Kooistra, H.S., Dieleman, S.J. and Okkens, A.C. 2006. Effects of gonadotrophin releasing hormone administration on the pituitary-ovarian axis in anoestrous vs ovariectomized bitches. Reprod. Domest. Anim. 41, 555–561. Cagnacci, A., Cannoletta, M., Caretto, S., Zanin, R., Xholli, A. and Volpe, A. 2011. Increased cortisol level: a possible link between climacteric symptoms and cardiovascular risk factors. Menopause 18, 273–278. Carotenuto, G., Malerba, E., Dolfini, C, Brugnoli, F., Giannuzzi, P., Semprini, G., Tosolini, P. and Fracassi, F. 2019. Cushing’s syndrome: an epidemiological study based on a canine population of 21,281 dogs. Open Vet. J. 9, 27–32. Christopoulos, S., Bordeau, I. and Lacroix, A. 2005. Clinical and subclinical ACTH-independent macronodular hyperplasia and aberrant hormone receptors. Horm. Res. 64, 119–131. Duan, K., Gomez Hernandez, K. and Mete, O. 2015. Clinicopathological correlates of adrenal Cushing’s syndrome. J. Clin. Pathol. 68, 175–86. Feelders, R.A., Lamberts, S.T.W., Hofland, L.J., van Koetsveld, P.T., Verhoef-Post, M., Themmen, A.P.N., de Jong, F.H., Bonjer, H.J., Clark, A.J., van der Lely, A.J., Wouter, W. and de Herder, W.W. 2003. Luteinizing Hormone (LH)-responsive Cushing’s syndrome: the demonstration of LH receptor messenger ribonucleic acid in hyperplastic adrenal cells, which respond to chorionic gonadotropin and serotonin agonists in vitro. J. Clin. Endocrinol. Metab. 88, 230–237. Frank, L.A., Denovo, R.C., Kraje, A.C. and Oliver, J.W. 2000. Cortisol concentrations following stimulation of healthy and adrenopathic dogs with two doses of tetracosactrin. J. Small Anim. Pract. 41, 308–311. Galac, S., Kars, V.J., Klarenbeek, S., Teerds, K.J., Mol, J.A. and Kooistra, H.S. 2010. Expression of receptors for luteinizing hormone, gastric-inhibitory polypeptide, and vasopressin in normal adrenal glands and cortisol-secreting adrenocortical tumors in dogs. Domest. Animal. Endocrinol. 39, 63–75. Galac, S., Kars, V.J., Voorhout, G., Mol, J.A. and Kooistra, H.S. 2008. ACTH independent hyperadrenocorticism due to food-dependent hypercortisolemia in a dog: a case report. Vet. J. 177, 141–143. Galac, S., Kooistra, H.S., Teske, E. and Rijnberk, A. 1997. Urinary corticoid/creatinine ratios in the differentiation between pituitary-dependent hyperadrenocorticism and hyperadrenocorticism due to adrenocortical tumour in the dog. Vet. Quart. 19, 17–20. Gallelli, M.F., Cabrera Blatter, M.F. and Castillo, V.A. 2010. A comparative study by age and gender of the pituitary adenoma and ACTH and a-MSH secretion in dogs with pituitary-dependent hyperadrenocorticism. Res. Vet. Sci. 88, 33–40. Gallelli, M.F., Lombardo, D., Quiroga, A., Caggiano, N., Soler, E., Vissio, P., Meikle, A. and Castillo, V.A. 2016. Immunohistochemical analysis of the hypothalamic-pituitary-adrenal axis in dogs: sex-linked and seasonal variation. Res. Vet. Sci. 104, 10–16. Gallelli, M.F., Monachesi, N., Miceli, D.D., Cabrera Blatter, M.F., Gómez, N.V., Meikle, A. and Castillo, V.A. 2015. Plasma ACTH, α-MSH and cortisol variations in the dog during the oestrous cycle in different photoperiods. Vet. Med.-Czech. 60, 567–577. Gilor, C.H. and Graves, T.K. 2011. Interpretation of laboratory tests for canine Cushing’s Syndrome. Top. Companion Anim. M. 26, 98–108. Handa, R.J. and Weiser, M.J. 2014. Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Front. Neuroendocrinol. 35, 197–220. Jopeck, K., Tyczewska, M., Ramanjaneya, M., Szyszka, M., Celichowski., Milecka, P., Malendowicz, L.K. and Rucinski, M. 2018. Effect of ACTH and hCG on the expression of gonadotropin-inducible ovarian transcription factor 1 (Giot1) gene in the rat adrenal gland. Int. J. Mol. Sci. 19, 2–21. Kero, J., Poutanen, M., Zhang, F.P., Rahman, N., McNicol, A.M., Nilson, J.H., Keri, R.A. and Huhta niemi, I.T. 2000. Elevated luteinizing hormone induces expression of its receptor and promotes steroidogenesis in the adrenal cortex. J. Clin. Invest. 105, 633–641. Kooistra, H.S. and Galac, S. 2012. Recent advances in the diagnosis of Cushing’s syndrome in dogs. Top. Companion Anim. Med. 27, 21–24. Kooistra, H.S., Okkens, A.C., Bevers, M.M., Popp-Snijders, C., van Haaften, B., Dieleman, S.J. and Schoemaker, J. 1999. Concurrent pulsatile secretion of luteinizing hormone and folliclestimulating hormone during different phases of the estrous cycle and anestrus in beagle bitches. Biol. Reprod. 60, 65–71. Kooistra, H.S., Voorhout, G., Mol, J.A. and Rijnberk, A. 1997. Correlation between impairment of glucocorticoid feedback and the size of the pituitary gland in dogs with pituitary-dependent hyperadrenocorticism. J. Endocrinol. 152, 387–394. Kubinyi, E., Turcsan, B. and Miklosi, A. 2009. Dog and owner demographic characteristics and dog personality trait associations. Behav. Processes. 81, 392–401. Kutzler, M.A. 2007. Estrus induction and synchronization in canids and felids. Theriogenology 68, 354–374. Lacroix, A., Diaye, N., Tremblay, J. and Hamet, P. 2001 Ectopic and abnormal hormone receptors in adrenal Cushing’s syndrome. Endocr. Rev. 22, 75–110. Lacroix, A., Hamet, P. and Boutin, J.M. 1999. Leuprolide acetate therapy in luteinizing hormone-dependent Cushing’s syndrome. New. Engl. J. Med. 341, 1577–1581. Liste, F., Cuevas, M., Gascon, M., de Jalon Garcia, J. and Cuevas, I. 1997. Ultrasonographic diagnosis of an adrenocortical carcinoma in a dog. Vet. Rec.140, 339–341. Löfstedt, R.M. and van Leeuwen, J. 2002. Evaluation of a commercially available luteinizing hormone test for its ability to distinguish between ovariectomized and sexually intact bitches. J. Am. Vet. Med. Assoc. 220, 1331–1335. Martins, F.S.M., Carvalho, G.L.C., Jesus, L., Gomes Pöpl, A. and González, F.H.D. 2019. Epidemiological, clinical, and laboratory aspects in a case series of canine hyperadrenocorticism: 115 cases (2010-2014). Pesqui. Vet. Brasil/Braz. J. Vet. Res. 39, 900–908. Mazzuco, T.L., Chabre, O. and Feige J-J. 2006. Thomas M. Aberrant expression of human luteinizing hormone receptor by adrenocortical cells is sufficient to provoke both hyperplasia and Cushing’s syndrome features. J. Clin. Endocrinol. Metab. 91, 196–203. Mazzuco, T.L., Chabre, O., Feige, J-J. and Thomas, M. 2007. Aberrant GPCR expression is sufficient genetic event to trigger adrenocortical tumorigenesis. Mol. Cell Endocrinol. 265–266, 23–28. Meij, B.P., Mol, J.A., Bevers, M.M. and Rijnberk, A. 1997. Alterations in anterior pituitary function of dogs with pituitary -dependent hyperadrenocorticism. J. Endocrinol. 154, 505–512. O’Neill, D.G., Scudder, C., Faire, J.M., Church, D.B., McGreevy, P.D., Thomson, P.C. and Brodbelt, D.C. 2016. Epidemiology of hyperadrenocorticism among 210,824 dogs attending primary-care veterinary practices in the UK from 2009 to 2014. J. Small. Anim. Pract. 57, 365–373. Pabon, J.E., Li ,X., Lei, Z.M., Sanfilippo, J.S., Yussman, M.A. and Rao, C.V. 1996. Novel presence of luteinizing hormone/chorionic gonadotropin receptors in human adrenal glands. J. Clin. Endocrinol. Metab.81, 2397–2400. Pessina, P., Fernandez-Foren, A., Cueto, E., Delucchi, L., Castillo, V. and Meikle, A. 2009. Cortisol secretion after adrenocorticotrophin (ACTH) and dexamethasone tests in healthy female and male dogs. Acta Vet. Scand. 51, 33–37. Rilianawati, Paukku, T., Kero, J., Zhang, F-P., Rahman, N., Kananen, K. and Huhtaniemi, I. 1998. Direct luteinizing hormone action triggers adrenocortical tumorigenesis in castrated mice transgenic for the murine inhibin α-subunit promoter/simian virus 40 T-antigen fusion gene. Mol. Cell Endocrinol. 12, 801–809. Root Kustritz, M.V. 2002. Early spay-neuter: Clinical considerations. Clin. Tech. Small Anim. Pract. 17, 124–128. Schoemaker, N.J., Kuijten, A.M. and Galac, S. 2008. Luteinizing hormone dependent Cushing’s syndrome in a pet ferret (Mustela putoriusfuro). Domest. Anim. Endocrinol. 34, 278 –283. Schoemaker, N.J., Schuurmans, M., Moorman, H. and Lumeij, J.T. 2000. Correlation between age at neutering and age at onset of hyperadrenocorticism in ferrets. J. Am. Vet. Med. Assoc. 216,195–197. Schoemaker, N.J., Teerds, K.J., Mol, J.A., Lumeij, J.T., Thijssen, J.H.H. and Rijnberk, A. 2002. The role of luteinizing hormone in the pathogenesis of hyperadrenocorticism in neutered ferrets. Mol. Cell Endocrinol. 197,117–125. van Bokhorst, K.L., Kooistra, H.S., Boroffka, S.A.E.B. and Galac, S. 2018. Concurrent pituitary and adrenocortical lesions on computed tomography imaging in dogs with spontaneous hypercortisolism. J. Vet. Intern. Med. 33, 72–78. Vuorenoja, S., Rivero-Müller, A. and Kiiveri, S. 2007. Adrenocortical tumorigenesis, luteinizing hormone receptor and transcription factors GATA-4 and GATA-6. Mol. Cell Endocrinol. 269, 38–45. Wagner, R.A., Bailey, E.M., Schneider, J.F. and Oliver, J.W. 2001. Leuprolide acetate treatment of adrenocortical disease in ferrets. J. Am. Vet. Med. Assoc. 218, 1272–1274. Wagner, R.A., Piché, C.A., Jöchle, W. and Oliver, J.W. 2005. Clinical and endocrine responses to treatment with deslorelin acetate implants in ferrets with adrenocortical disease. Am. J. Vet. Res. 66, 910–914. Weiss, L.M., Medeiross, L.J. and Vickery, A.L. Jr. 1989. Pathologic features of prognostic significance in adrenocortical carcinoma. Am. J. Surg. Pathol.13, 202–206. Woods, N.F., Carr, M.C., Tao, E.Y., Taylor, H.J. and Mitchell, E.S. 2006. Increased urinary cortisol levels during the menopause transition. Menopause 13, 212–221. WSAVA, Global Nutrition Committee. Body Condition Score. 2013. Ycaza Herrera, A., Hodis, H.N., Mack, W.J. and Mather, M. 2017. Estradiol therapy after menopause mitigates effects of stress on cortisol and working memory. J. Clin. Endocrinol. Metab. 102, 4457–4466. | ||
| How to Cite this Article |
| Pubmed Style Espieira IM, Vidal PN, Ghersevich MC, Arias ES, Bosetti F, Blatter MFC, Miceli DD, Castillo VA, . Adrenal cortex stimulation with hCG in spayed female dogs with Cushings syndrome: Is the LH-dependent variant possible?. Open Vet J. 2021; 11(2): 319-329. doi:10.5455/OVJ.2021.v11.i2.17 Web Style Espieira IM, Vidal PN, Ghersevich MC, Arias ES, Bosetti F, Blatter MFC, Miceli DD, Castillo VA, . Adrenal cortex stimulation with hCG in spayed female dogs with Cushings syndrome: Is the LH-dependent variant possible?. https://www.openveterinaryjournal.com/?mno=44689 [Access: April 19, 2024]. doi:10.5455/OVJ.2021.v11.i2.17 AMA (American Medical Association) Style Espieira IM, Vidal PN, Ghersevich MC, Arias ES, Bosetti F, Blatter MFC, Miceli DD, Castillo VA, . Adrenal cortex stimulation with hCG in spayed female dogs with Cushings syndrome: Is the LH-dependent variant possible?. Open Vet J. 2021; 11(2): 319-329. doi:10.5455/OVJ.2021.v11.i2.17 Vancouver/ICMJE Style Espieira IM, Vidal PN, Ghersevich MC, Arias ES, Bosetti F, Blatter MFC, Miceli DD, Castillo VA, . Adrenal cortex stimulation with hCG in spayed female dogs with Cushings syndrome: Is the LH-dependent variant possible?. Open Vet J. (2021), [cited April 19, 2024]; 11(2): 319-329. doi:10.5455/OVJ.2021.v11.i2.17 Harvard Style Espieira, I. M., Vidal, P. N., Ghersevich, M. C., Arias, E. S., Bosetti, F., Blatter, M. F. C., Miceli, D. D., Castillo, V. A. & (2021) Adrenal cortex stimulation with hCG in spayed female dogs with Cushings syndrome: Is the LH-dependent variant possible?. Open Vet J, 11 (2), 319-329. doi:10.5455/OVJ.2021.v11.i2.17 Turabian Style Espieira, Ignacio Manuel, Patricia Noemi Vidal, Mara Carolina Ghersevich, Elber Soler Arias, Fernanda Bosetti, Mara Fernanda Cabrera Blatter, Diego Daniel Miceli, Victor Alejandro Castillo, and . 2021. Adrenal cortex stimulation with hCG in spayed female dogs with Cushings syndrome: Is the LH-dependent variant possible?. Open Veterinary Journal, 11 (2), 319-329. doi:10.5455/OVJ.2021.v11.i2.17 Chicago Style Espieira, Ignacio Manuel, Patricia Noemi Vidal, Mara Carolina Ghersevich, Elber Soler Arias, Fernanda Bosetti, Mara Fernanda Cabrera Blatter, Diego Daniel Miceli, Victor Alejandro Castillo, and . "Adrenal cortex stimulation with hCG in spayed female dogs with Cushings syndrome: Is the LH-dependent variant possible?." Open Veterinary Journal 11 (2021), 319-329. doi:10.5455/OVJ.2021.v11.i2.17 MLA (The Modern Language Association) Style Espieira, Ignacio Manuel, Patricia Noemi Vidal, Mara Carolina Ghersevich, Elber Soler Arias, Fernanda Bosetti, Mara Fernanda Cabrera Blatter, Diego Daniel Miceli, Victor Alejandro Castillo, and . "Adrenal cortex stimulation with hCG in spayed female dogs with Cushings syndrome: Is the LH-dependent variant possible?." Open Veterinary Journal 11.2 (2021), 319-329. Print. doi:10.5455/OVJ.2021.v11.i2.17 APA (American Psychological Association) Style Espieira, I. M., Vidal, P. N., Ghersevich, M. C., Arias, E. S., Bosetti, F., Blatter, M. F. C., Miceli, D. D., Castillo, V. A. & (2021) Adrenal cortex stimulation with hCG in spayed female dogs with Cushings syndrome: Is the LH-dependent variant possible?. Open Veterinary Journal, 11 (2), 319-329. doi:10.5455/OVJ.2021.v11.i2.17 |