| Case Report | ||
Open Vet J. 2022; 12(4): 495-501 Open Veterinary Journal, (2022), Vol. 12(4): 495–501 Case Report Left-dominant arrhythmogenic cardiomyopathy in a Fila Brasileiro dogGuillermo Belerenian1, Pablo Alejandro Donati2,3, Cristian Daniel Rodríguez2, Víctor Castillo1, Juan Manuel Guevara2 and Roberto Walter Israel Olivares4*1Instituto de zoonosis Luis Pasteur, Buenos Aires, Argentina 2UCICOOP, Buenos Aires, Argentina 3Universidad de Buenos Aires, Facultad de Ciencias Veterinarias, Cátedra de Anestesiología y Algiología, Buenos Aires, Argentina 4Servicio de Patología Diagnóstica LAPAVET-ESFA, Cátedra de Patología e Histología, Escuela de Medicina y Cirugía Veterinaria San Francisco de Asís, Universidad Veritas, San José, Costa Rica *Corresponding Author: Roberto Walter Israel Olivares. Servicio de Patología Diagnóstica LAPAVET-ESFA, Cátedra de Patología e Histología, Escuela de Medicina y Cirugía Veterinaria San Francisco de Asís, Universidad Veritas, San José, Costa Rica. Email: rolivares [at] veterinariaveritas.ac.cr Submitted: 03/04/2022 Accepted: 04/07/2022 Published: 01/08/2022 © 2022 Open Veterinary Journal
AbstractBackground: In human medicine, arrhythmogenic left ventricular cardiomyopathy was described as a primary disease of the heart characterized by fibroadipose replacement of the myocardium.. Case Description: We report the case of a dog, with history of syncope and irregular cardiac rhythm. Electrocardiogram, echocardiography, and a 24-hour Holter monitoring showed, respectively, the presence of premature ventricular complexes with right bundle branch block morphology, an increase of the left ventricle end-diastolic diameter with preserved fractional shortening and ejection fraction, and a sinus arrhythmia as baseline rhythm with supraventricular tachycardia episodes and ventricular complexes with left bundle branch block morphology. After the death of the canine, a postmortem examination showed cardiomegaly. Fibroadipose replacement of the septum and both ventricles, with left ventricle myocardial fibrosis, suggestive of previous necrosis, was observed. Conclusion: These findings are suggestive of left-dominant arrhythmogenic cardiomyopathy which, to the best of our knowledge, has not been described in veterinary medicine. Keywords: Left-dominant, Arrhythmogenic cardiomyopathy, Canine. IntroductionIn human medicine, arrhythmogenic right ventricular cardiomyopathy was described as a primary disease of the heart muscle characterized by the adipose or fibroadipose replacement of the right ventricular myocardium (Orgeron and Crosson, 2017). At first, lesions usually involve the classic “Triangle of Dysplasia”, composed of the subtricuspid (or juxtatricuspid) region, the outflow tract, and apex of the right ventricle (Corrado et al., 2017). As the disease progresses, a global right ventricle involvement usually occurs, and in advanced cases, biventricular involvement may be observed (Xu et al., 2010). These structural changes cause electrical instability and induce ventricular arrhythmias by re-entry (Basso et al., 2004). People affected by this disease usually have arrhythmias and sudden death or, less commonly, may develop a systolic failure of the right ventricle and right congestive cardiac failure (Gandjbakhch et al., 2018). Sudden death usually occurs in young people during physical exercise (Mu et al., 2017). In the 1980s, a similar disease was described for boxer dogs, in which there was also adipose or fibro-adipose replacement of some regions of the right ventricle, which was named “Boxer cardiomyopathy” (Harpster, 1991; Basso et al., 2004; Hariu and Carpenter, 2010). Later, similar diseases were described in other breeds, such as Siberian Husky, Labrador Retriever, Bull mastiff, dachshund, and bulldog (Nakao et al., 2011; Palermo et al., 2011). In human medicine, a variant characterized by the exclusive involvement of the left ventricle was previously described (Sen-Chowdhry et al., 2008; Calkins and Tandri, 2015). In this presentation, adipose tissue replacement occurs in the free wall of the left ventricle in the midventricular (or subepicardial) region, affecting the ventricle circumferentially (Sen-Chowdhry et al., 2008). The septum can also be affected, although this presentation is less frequent, probably because the disease may affect subepicardial regions (Sen-Chowdhry et al., 2008). To the best of authors’ knowledge, the subtype of arrhythmogenic cardiomyopathy with pure left ventricular lesions has still not been described in veterinary medicine. Case DetailsA 2-year-old male Fila Brasileiro dog with a history of syncope episodes occurring during exercise was presented to the cardiology service. At clinical examination, auscultation revealed an irregular rhythm but no heart murmurs. A six-lead electrocardiogram was performed (Cardiotecnica PR 401, Argentina), which evidenced the presence of isolated premature ventricular complexes with right bundle branch block morphology. An echocardiography with a phased array transducer was performed (Esaote mylab30 goldVet Italia), which showed an increase of the left ventricle end-diastolic diameter normalized to body weight (LVIDdn=21.3 mm) with preserved fractional shortening (FS) and ejection fraction (EF) (FS and EF 41% and 71%, respectively). A complete hemogram was performed, which revealed no abnormalities. A 24-hour Holter monitoring was also performed on the following day, during which time the patient was at the house. Three-channel equipment (cardioscan 300-3A, China) was used. The electrodes were placed on the left and right hemithorax (two channels had the positive electrode on the left hemithorax and the other one had the positive electrode on the right hemithorax). The recording showed the presence of sinus arrhythmia as baseline rhythm during 95% of the study period. Maximum and minimum heart rates were 231 and 54 bpm, respectively. Eleven supraventricular tachycardia episodes of an average duration of 3 seconds were recorded. In addition, 93 ventricular complexes were detected, with approximately 50% corresponding to escape beats. All the escape ventricular complexes presented left bundle branch block morphology, whereas the premature ventricular complexes exhibited right bundle branch block morphology (Figs. 1 and 2). During the study, an increase in the corrected QT interval by the Bazzett’s formula (QT/RR) was observed (Table 1). After 24 Holter monitoring, the patient died suddenly during physical activity. Permission for conducting necropsy was obtained from the animal owners. Postmortem examination showed marked cardiomegaly (heart weight bigger than 13 g/kg body weight). No lung edema, pleural effusion or ascites were observed. The heart was explanted and preserved in 10% formalin and then sent for examination to the pathology service of the Hospital Santojani (Buenos Aires), where fragments from both ventricles and the septum were processed with the routine technique to make paraffin-embedded blocks. 4 μm thick sections were cut and stained with hematoxylin-eosin (H&E). Adipose and fibroadipose replacement of the right ventricle free wall of up to 50% of its thickness was observed. These lesions had a predominantly subepicardial distribution. Subepicardial fibroadipose replacement of the left ventricle with involvement of the septum was also observed (Fig. 3). In addition, adipose tissue was also observed in the left ventricle, affecting the base of implantation of the papillary muscles at the septum (Fig. 4). In areas of the left ventricle, co-existence of perivascular fibrosis, myocardial fibrosis suggestive of previous necrosis, and interstitial fibrosis were detected (Fig. 5). In addition, wavy fibers in the myocardium in both ventricles were observed. Clusters of myocardial fibers were also observed within all areas of adipose tissue deposition. DiscussionIn this article, we report findings compatible with left-dominant arrhythmogenic cardiomyopathy in a Fila Brasileiro. The case was characterized by the lack of signs of congestive heart failure and the presence of arrhythmias detected by auscultation, electrocardiography, and 24-hour Holter monitoring. Echocardiographic findings indicated a preserved systolic function of the left ventricle, but an increase of LVIDdn was observed. The arrhythmias were complex since they were characterized by ventricular premature complexes with right bundle branch block, conduction disorders with escape beats and rhythm, and isolated episodes of focal supraventricular tachycardia. In addition, a more prolonged corrected QT interval was observed. The patient experienced sudden death during physical activity, possibly related to the arrhythmias, with no clinical signs suggestive of the presence of left congestive heart failure. Histological findings indicate affection of the left ventricular free wall and the left interventricular septum with adipose and fibroadipose tissue replacement, and with adipose tissue replacement at the base de implantation of papillary muscles, with no evidence of myocarditis. An adipose tissue replacement of up to 50% of the free wall in some areas of the right ventricle was also found. The findings and the distribution of lesions (mainly the left septal lesion and the involvement of the base of implantation of papillary muscles), along with the clinical findings and the electrocardiographic records suggest a condition compatible with a left dominant arrhythmogenic cardiomyopathy. Physical activity may cause mechanical stress and cause arrhythmias and sudden death, triggering cardiac failure (Sen-Chowdhry et al., 2008; Xu et al., 2010; Lyon et al., 2014; Akdis et al., 2016; Corrado et al., 2017; Stadiotti et al., 2017).
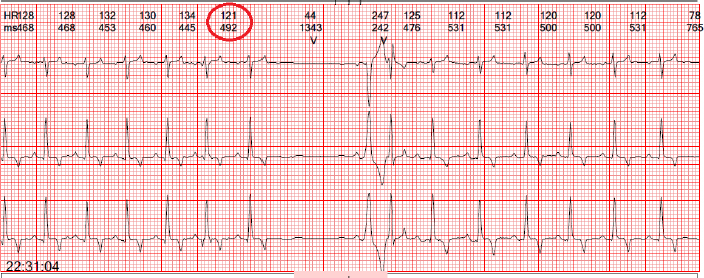
Fig. 1. An escape beat with left bundle branch block morphology is observed.
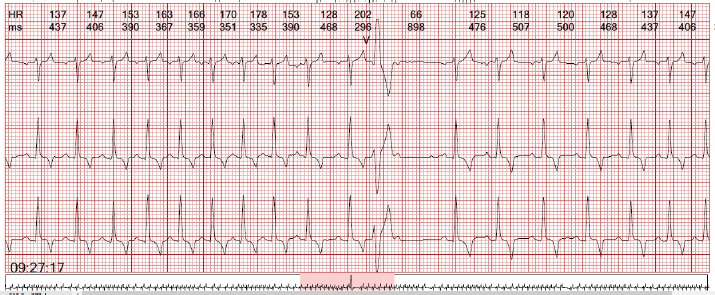
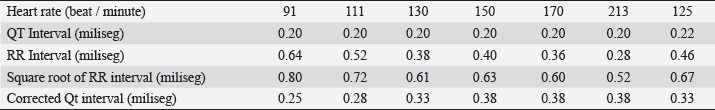
Fig. 2. Premature ventricular beats with right bundle branch block morphology suggestive of an origin in the left ventricle are observed. Table 1. Selected records of the variables obtained during the 24-hour Holter monitoring.
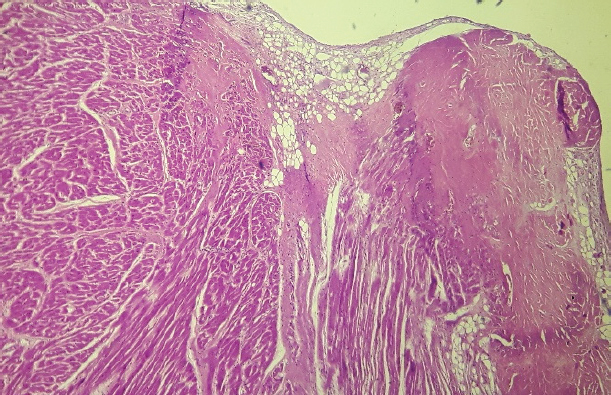
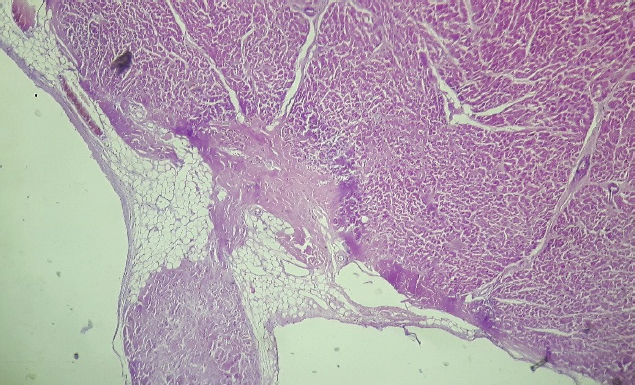
Fig. 3. Septum, basal level of the left ventricular side (H&E, 100×). Histopathological image showing subendocardial adipose tissue deposition with fibrosis and hyalinization.
Fig. 4. Base of the left ventricle papillary muscle (H&E, 100×). Histopathological image showing presence of adipose tissue at the base of the papillary muscle.
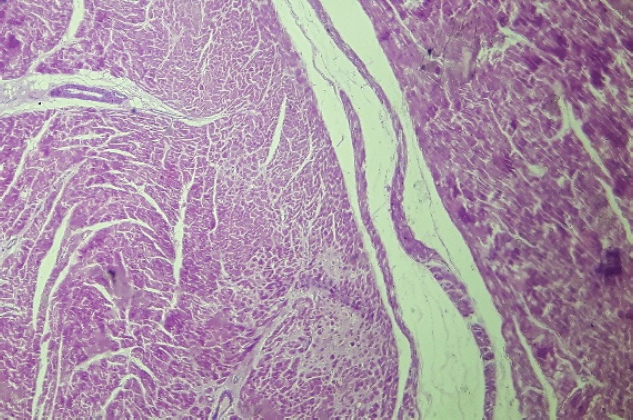
Fig. 5. Free wall of the left ventricle (H&E, 100×). Histopathological image showing connective tissue and adipose tissue deposition, with perivascular fibrosis and interstitial fibrosis. In the typical form of arrhythmogenic right ventricular dysplasia, currently known as arrhythmogenic right ventricular cardiomyopathy, the global function of the left ventricle is usually not involved (Xu et al., 2010; Corrado et al., 2017). The involvement of both ventricles usually occurs in the outer third of the myocardium and generally on the right side of the interventricular septum (Xu et al., 2010; Corrado et al., 2017). In the present case, the structural anomalies detected in the echocardiography correspond with an increase of the LVIDdn, with preservation of the FS, without evident alterations of the thickness of the left ventricle free wall or the septum. In the left-dominant arrhythmogenic cardiomyopathy, fibrosis distribution usually prevails in the outer third of the myocardium, starting subepicardial, and it may progress toward the endocardium (Xu et al., 2010; Nakao et al., 2011; Lyon et al., 2014; Berte et al., 2015; Gandjbakhch et al., 2018). This lesion may be circumferential or have a patchy distribution (Xu et al., 2010; Yoo et al., 2010; Nakao et al., 2011; Stadiotti et al., 2017; Shen et al., 2019; Protonotarios and Elliott, 2020). The left-dominant arrhythmogenic cardiomyopathy, may or may not be associated with dilation of the right ventricle (Berte et al., 2015). In the classic disease form, ventricular tachycardia with left bundle branch block morphology is usually observed, whereas, in the left ventricular form, ventricular tachycardia with right branch block morphology is observed (Sen-Chowdhry et al., 2008; Sen-Chowdhry et al., 2010; Berte et al., 2015; Calkins and Tandri, 2015; Akdis et al., 2016; Shen et al., 2019). Previous reports of left-dominant arrhythmogenic cardiomyopathy in humans showed more than 50% of involvement of the interventricular septum (Sen-Chowdhry et al., 2008; Sen-Chowdhry et al., 2010; Berte et al., 2015; Calkins and Tandri, 2015; Shen et al., 2019). Those findings are consistent with those observed in this case. Furthermore, unlike our results, in the biventricular form, dilation of both ventricles is usually observed. In human medicine, it is difficult to differentiate the left form of arrhythmogenic cardiomyopathy from the biventricular presentation at early disease stages (Berte et al., 2015; Calkins and Tandri, 2015). To differentiate dilated cardiomyopathy from the left form of arrhythmogenic cardiomyopathy, it is necessary to consider the subepicardial distribution of the lesion, among other aspects, since it is more frequent in the left form of the disease than in dilated cardiomyopathy (Akdis et al., 2016). Another differential characteristic that supports the diagnosis of left-dominant arrhythmogenic cardiomyopathy over dilated cardiomyopathy is the presence of severe ventricular arrhythmias that are often disproportionately high for the degree of systolic involvement of the left ventricle (Sen-Chowdhry et al., 2008; Sen-Chowdhry et al., 2010; Groeneweg et al., 2014; Corrado et al., 2017; Gandjbakhch et al., 2018; Protonotarios and Elliott, 2020). The cases of classic arrhythmogenic cardiomyopathy usually present arrhythmias, but with the normal systolic function of the left ventricle, whereas congestive heart failure is a late event in the final biventricular condition of the classic right form of arrhythmogenic cardiomyopathy, and on the other hand, usually is an early event of dilated cardiomyopathy (Sen-Chowdhry et al., 2008; Groeneweg et al., 2014; Corrado et al., 2017; Gandjbakhch et al., 2018; Protonotarios and Elliott, 2020). Moreover, in right arrhythmogenic cardiomyopathy, the septum on the left side is usually less affected (Sen-Chowdhry et al., 2008; Nakao et al., 2011; Stadiotti et al., 2017; Shen et al., 2019; Protonotarios and Elliott, 2020). In the present case, the septal condition of our patient was a distinctive characteristic suggestive of a left form of the disease. Another differential diagnosis of the present findings is ischemic cardiopathy. Patients affected by such disease usually exhibit fibrosis of the affected area; however, fibrosis usually has subendocardial distribution and can progress to become transmural (Nakao et al., 2011; Stadiotti et al., 2017; Shen et al., 2019). In this work, the mid-ventricular lesion of the left ventricle was present without dysfunction or global dilation of the left ventricle. These findings show the low sensitivity of the echocardiogram to detect this type of lesion. The use of more advanced image tools, such as magnetic resonance imaging (MRI) with gadolinium, may be more useful for the diagnosis of this type of lesion (Yoo et al., 2010; Tavano et al., 2015). Mutations of genes coding for desmosomal and extradesmosomal proteins found in humans and animals show an overlap between dilated cardiomyopathy, arrhythmogenic cardiomyopathy, and channelopathies, with an expression of different phenotypes (Xu et al., 2010; Akdis et al., 2016; Protonotarios and Elliott, 2020). At present, mutations affecting genes encoding proteins of the composite area of adhering junctions are important in the pathogenesis of these diseases, which could represent a continuum from a purely arrhythmogenic phenotype to one with left ventricular dilatation and congestive failure (Sheikh et al., 2009; Delmar and McKenna, 2010; Fernández-Falgueras et al., 2017; Protonotarios and Elliott, 2020). The patient of this report exhibited characteristics that might suggest the presence of a channelopathy (prolonged corrected QT interval), structural alterations suggestive of arrhythmogenic cardiomyopathy (connective and adipose tissue deposition), and findings frequently observed in dilated cardiomyopathy (presence of wavy myocardial fibers). Further studies are necessary to confirm this hypothesis. Conflict of interestThe authors declare that there is no conflict of interest. ReferencesAkdis, D., Medeiros-Domingo, A., Gaertner-Rommel, A., Kast, J.I., Enseleit, F., Bode, P., Klingel, K., Kandolf, R., Renois, F., Andreoletti, L., Akdis, C.A., Milting, H., Lüscher, T.F., Brunckhorst, C., Saguner, A.M. and Duru, F. 2016. Myocardial expression profiles of candidate molecules in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia compared to those with dilated cardiomyopathy and healthy controls. Heart. Rhythm. 13(3), 731–741. Basso, C., Fox, P.R., Meurs, K.M., Towbin, J.A., Spier, A.W., Calabrese, F., Maron, B.J. and Thiene, G. 2004. Arrhythmogenic right ventricular cardiomyopathy causing sudden cardiac death in boxer dogs: a new animal model of human disease. Circulation 109(9), 1180–1185. Berte, B., Denis, A., Amraoui, S., Yamashita, S., Komatsu, Y., Pillois, X., Sacher, F., Mahida, S., Wielandts, J.Y., Sellal, J.M., Frontera, A., Jefairi, N.Al., Derval, N., Montaudon, M., Laurent, F., Hocini, M., Haïssaguerre, M., Jaïs, P. and Cochet, H. 2015. Characterization of the left-sided substrate in Arrhythmogenic right ventricular cardiomyopathy. Circ. Arrhythm. Electrophysiol. 8(6), 1403–1412. Calkins, H. and Tandri, H. 2015. Left ventricular involvement in ARVD/C: is it time to readjust our views? Circ. Arrhythm. Electrophysiol. 8(6), 1311–1312. Corrado, D., Link, M.S. and Calkins, H. 2017. Arrhythmogenic right ventricular cardiomyopathy. N. Engl. J. Med. 376(1), 61–72. Delmar, M. and McKenna, W.J. 2010. The cardiac desmosome and arrhythmogenic cardiomyopathies: from gene to disease. Circ. Res. 107(6), 700–714. Fernández-Falgueras, A., Sarquella-Brugada, G., Brugada, J., Brugada, R. and Campuzano, O. 2017. Cardiac channelopathies and sudden death: recent clinical and genetic advances. Biology (Basel) 6(1), 7. Gandjbakhch, E., Redheuil, A., Pousset, F., Charron, P. and Frank, R. 2018. Clinical diagnosis, imaging, and genetics of arrhythmogenic right ventricular cardiomyopathy/dysplasia: JACC State-of-the-art review. J. Am. Coll. Cardiol. 72(7), 784–804. Groeneweg, J.A., van der Heijden, J.F., Dooijes, D., van Veen, T.A., van Tintelen, J.P. and Hauer, R.N. 2014. Arrhythmogenic cardiomyopathy: diagnosis, genetic background, and risk management. Neth. Heart J. 22(7–8), 316–325. Hariu, C.D. and Carpenter, D.H.,Jr. 2010. Arrhythmogenic right ventricular cardiomyopathy in boxers. Compend. Contin. Educ. Vet. 32(12), e3. Harpster, N.K. 1991. Boxer cardiomyopathy. A review of the long-term benefits of antiarrhythmic therapy. Vet. Clin. North Am. Small Anim. Pract. 21(5), 989–1004. Lyon, R.C., Mezzano, V., Wright, A.T., Pfeiffer, E., Chuang, J., Banares, K., Castaneda, A., Ouyang, K., Cui, L., Contu, R., Gu, Y., Evans, S.M., Omens, J.H., Peterson, K.L., McCulloch, A.D. and Sheikh, F. 2014. Connexin defects underlie arrhythmogenic right ventricular cardiomyopathy in a novel mouse model. Hum. Mol. Genet. 23(5), 1134–1150. Mu, J., Zhang, G., Xue, D., Xi, M., Qi, J. and Dong, H. 2017. Sudden cardiac death owing to arrhythmogenic right ventricular cardiomyopathy: two case reports and systematic literature review. Medicine (Baltimore) 96(47), e8808. Nakao, S., Hirakawa, A., Yamamoto, S., Kobayashi, M. and Machida, N. 2011. Pathological features of arrhythmogenic right ventricular cardiomyopathy in middle-aged dogs. J. Vet. Med. Sci. 73(8), 1031–1036. Orgeron, G.M. and Crosson, J.E. 2017. Arrhythmogenic right ventricular dysplasia/cardiomyopathy. Cardiol. Young. 27(S1), S57–S61. Palermo, V., Stafford Johnson, M.J., Sala, E., Brambilla, P.G. and Martin, M.W.S. 2011. Cardiomyopathy in Boxer dogs: a retrospective study of the clinical presentation, diagnostic findings and survival. J. Vet. Cardiol. 13(1), 45–55. Protonotarios, A. and Elliott, P.M. 2020. Arrhythmogenic cardiomyopathy: a disease or merely a phenotype? Eur. Cardiol. 15, 1–5. Sen-Chowdhry, S., Morgan, R.D., Chambers, J.C. and McKenna, W.J. 2010. Arrhythmogenic cardiomyopathy: etiology, diagnosis, and treatment. Annu. Rev. Med. 61, 233–253. Sen-Chowdhry, S., Syrris, P., Prasad, S.K., Hughes, S.E., Merrifield, R., Ward, D., Pennell, D.J. and McKenna, W.J. 2008. Left-dominant arrhythmogenic cardiomyopathy: an under-recognized clinical entity. J. Am. Coll. Cardiol. 52(25), 2175–2187. Sheikh, F., Ross, R.S. and Chen, J. 2009. Cell-cell connection to cardiac disease. Trends. Cardiovasc. Med. 19(6), 182–190. Shen, M.T., Yang, Z.G., Diao, K.Y., Jiang, L., Zhang, Y., Liu, X., Gao, Y., Hu, B.Y., Huang, S. and Guo, Y.K. 2019. Left ventricular involvement in arrhythmogenic right ventricular dysplasia/cardiomyopathy predicts adverse clinical outcomes: a cardiovascular magnetic resonance feature tracking study. Sci. Rep. 9(1), 14235. Stadiotti, I., Catto, V., Casella, M., Tondo, C., Pompilio, G. and Sommariva, E. 2017. Arrhythmogenic cardiomyopathy: the guilty party in adipogenesis. J. Cardiovasc. Transl. Res. 10(5–6), 446–454. Tavano, A., Maurel, B., Gaubert, J.Y., Varoquaux, A., Cassagneau, P., Vidal, V., Bartoli, J.M., Moulin, G. and Jacquier, A. 2015. MR imaging of arrhythmogenic right ventricular dysplasia: what the radiologist needs to know. Diagn. Interv. Imaging. 96(5), 449–460. Xu, T., Yang, Z., Vatta, M., Rampazzo, A., Beffagna, G., Pillichou, K., Scherer, S.E., Saffitz, J., Kravitz, J., Zareba, W., Danieli, G.A., Lorenzon, A., Nava, A., Bauce, B., Thiene, G., Basso, C., Calkins, H., Gear, K., Marcus, F., Towbin, J.A and Multidisciplinary Study of Right Ventricular Dysplasia Investigators. 2010. Compound and digenic heterozygosity contributes to arrhythmogenic right ventricular cardiomyopathy. J. Am. Coll. Cardiol. 55(6), 587–597. Yoo, S.J., Grosse-Wortmann, L. and Hamilton, R.M. 2010. Magnetic resonance imaging assessment of arrhythmogenic right ventricular cardiomyopathy/dysplasia in children. Korean Circ. J. 40(8), 357–367. | ||
| How to Cite this Article |
| Pubmed Style Belerenian G, Donati PA, Rodríguez CD, Castillo V, Guevara JM, Olivares RWI, . Left-dominant arrhythmogenic cardiomyopathy in a Fila Brasileiro dog. Open Vet J. 2022; 12(4): 495-501. doi:10.5455/OVJ.2022.v12.i4.11 Web Style Belerenian G, Donati PA, Rodríguez CD, Castillo V, Guevara JM, Olivares RWI, . Left-dominant arrhythmogenic cardiomyopathy in a Fila Brasileiro dog. https://www.openveterinaryjournal.com/?mno=468 [Access: April 20, 2024]. doi:10.5455/OVJ.2022.v12.i4.11 AMA (American Medical Association) Style Belerenian G, Donati PA, Rodríguez CD, Castillo V, Guevara JM, Olivares RWI, . Left-dominant arrhythmogenic cardiomyopathy in a Fila Brasileiro dog. Open Vet J. 2022; 12(4): 495-501. doi:10.5455/OVJ.2022.v12.i4.11 Vancouver/ICMJE Style Belerenian G, Donati PA, Rodríguez CD, Castillo V, Guevara JM, Olivares RWI, . Left-dominant arrhythmogenic cardiomyopathy in a Fila Brasileiro dog. Open Vet J. (2022), [cited April 20, 2024]; 12(4): 495-501. doi:10.5455/OVJ.2022.v12.i4.11 Harvard Style Belerenian, G., Donati, P. A., Rodríguez, C. D., Castillo, V., Guevara, J. M., Olivares, R. W. I. & (2022) Left-dominant arrhythmogenic cardiomyopathy in a Fila Brasileiro dog. Open Vet J, 12 (4), 495-501. doi:10.5455/OVJ.2022.v12.i4.11 Turabian Style Belerenian, Guillermo, Pablo Alejandro Donati, Cristian Daniel Rodríguez, Víctor Castillo, Juan Manuel Guevara, Roberto Walter Israel Olivares, and . 2022. Left-dominant arrhythmogenic cardiomyopathy in a Fila Brasileiro dog. Open Veterinary Journal, 12 (4), 495-501. doi:10.5455/OVJ.2022.v12.i4.11 Chicago Style Belerenian, Guillermo, Pablo Alejandro Donati, Cristian Daniel Rodríguez, Víctor Castillo, Juan Manuel Guevara, Roberto Walter Israel Olivares, and . "Left-dominant arrhythmogenic cardiomyopathy in a Fila Brasileiro dog." Open Veterinary Journal 12 (2022), 495-501. doi:10.5455/OVJ.2022.v12.i4.11 MLA (The Modern Language Association) Style Belerenian, Guillermo, Pablo Alejandro Donati, Cristian Daniel Rodríguez, Víctor Castillo, Juan Manuel Guevara, Roberto Walter Israel Olivares, and . "Left-dominant arrhythmogenic cardiomyopathy in a Fila Brasileiro dog." Open Veterinary Journal 12.4 (2022), 495-501. Print. doi:10.5455/OVJ.2022.v12.i4.11 APA (American Psychological Association) Style Belerenian, G., Donati, P. A., Rodríguez, C. D., Castillo, V., Guevara, J. M., Olivares, R. W. I. & (2022) Left-dominant arrhythmogenic cardiomyopathy in a Fila Brasileiro dog. Open Veterinary Journal, 12 (4), 495-501. doi:10.5455/OVJ.2022.v12.i4.11 |