| Short Communication | ||
Open Vet J. 2021; 11(3): 337-341 Open Veterinary Journal, (2021), Vol. 11(3): 337–341 Short Communication Serological evidence of Rift Valley fever in domestic ruminants in Tunisia underlines the need for effective surveillanceJihene Hellal1,2†, Selma Mejri1†*, Sandra Lacote3, Soufien Sghaier1, Abderrazek Dkhil1, Elena Arsevska4, Didier Calavas4, Viviane Hénaux4, Philippe Marianneau3 and Salah Hammami51Tunisian Institute of Veterinary Research, Rue Djebel Lakhdhar, University of Tunis El Manar, Tunis, Tunisia 2Faculty of Sciences of Bizerte, Carthage University, Tunis, Tunisia 3Virology Unit, French Agency for Food, Environmental and Occupational Health & Safety (ANSES), Laboratory of Lyon, Lyon, France 4Epidemiology and Support to Surveillance Unit, French Agency for Food, Environmental and Occupational Health & Safety (ANSES), Laboratory of Lyon, Lyon, France 5National School of Veterinary Medicine Sidi-Thabet, University of Manouba, Ariana, Tunisia †These authors contributed equally to this work *Corresponding Author: Selma Mejri. Laboratoire de Virologie, Institut de la Recherche Vétérinaire, Rue Djebel Lakhdhar, Tunis, Tunisia. Email: selma_mejri [at] yahoo.fr Submitted: 24/03/2021 Accepted: 09/06/2021 Published: 09/07/2021 © 2021 Open Veterinary Journal
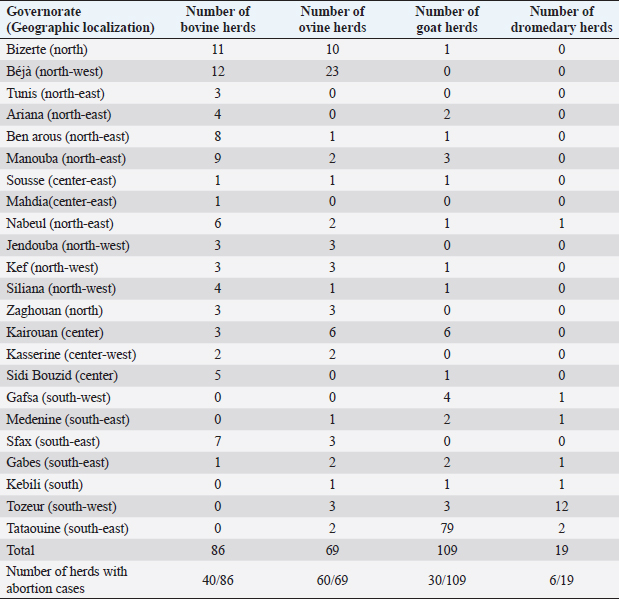
AbstractBackground: Rift Valley fever (RVF) is an infectious zoonotic disease infecting, mainly, domestic ruminants and causing significant economic and public health problems. RVF is a vector-borne disease transmitted by mosquitoes. Aim: In this work, we tried to seek any RVF virus circulation in Tunisia. Methods: Thus, we investigated 1,723 sera from different parts of Tunisia, collected in 2009 and 2013–2015 from sheep, goats, cattle, and dromedaries. All sera were assessed using enzyme-linked immunosorbent assay techniques. Results: Eighty-seven sera were detected positive and 11 doubtful. All of them were investigated by the virus-neutralization technique (VNT), which confirmed the positivity of three sera. Conclusion: This is the first case of RVF seropositive confirmed by the VNT in Tunisian ruminants. Such a result was expected considering the climate, entomology, and geographic location of the country. Further investigations must enhance our findings to understand the RVF epidemiologic situation better and implement risk-based surveillance programs and effective control strategies. Keywords: Rift Valley fever, Tunisia, Epidemiology, ELISA, Virus-neutralization technique. IntroductionRift Valley fever (RVF) is an acute infectious disease transmitted by various mosquitoes, particularly those of the genera Aedes and Culex. It is caused by the RVF virus (RVFV) that belongs to the genus Phlebovirus of the Phenuiviridae family. The RVFV affects mainly domestic ruminants; however, other mammalian species can be infected, such as buffalo. Humans are considered dead-end hosts (Gerdes, 2004; Bird et al., 2009; Jourdain et al., 2019). RVF was thought to be restricted to sub-Saharan African countries until massive outbreaks were declared in Egypt, Saudi Arabia, and Yemen [Centre for Disease Control (CDC), 2000a, 2000b; Al-Afaleq and Hussein, 2011]. This expansion of RVF was probably related to geopolitical, human behavior, and socio-economic and climate changes, dramatically affecting several countries in the Middle East and North Africa region (Hotez et al., 2012; Zakham et al., 2018). However, due to their geographical location and borders with the Sahel region, the North African countries are vulnerable to transboundary animal diseases, such as RVF (Kardjadj, 2017, 2018). Accordingly, it has been demonstrated that camels moving across the Sahara in Morocco have contact with RVFV (El-Harrak et al., 2011). RVF is considered among the most dangerous emerging diseases because of the ability of the etiologic agent to adapt to different ecosystems and many vector species (Métras et al., 2011; Cito et al., 2013). Few studies on RVF have been conducted in Tunisia. The first one demonstrated no evidence of RVFV presence in livestock (sheep, goats, cattle, and camels) (Ayari-Fakhfakh et al., 2011). Two other studies were conducted on Tunisian dromedaries: Ben Hassine et al. (2017) found no evidence of RVF circulation in two groups of dromedaries in the south of Tunisia. On the contrary, Selmi et al. (2020) showed that 34% of investigated dromedaries were positive in enzyme-linked immunosorbent assay (ELISA). However, these results were not confirmed by the virus-neutralization technique (VNT), which is considered the most specific diagnostic serological test (OIE, 2018). Another work showed serological evidence of RVF exposure in humans who had not left the country during the 2 months preceding the sampling (Bosworth et al., 2015). Also, in this mentioned study, ELISA results were not confirmed by VNT, and the detection of the viral genome using qRT-PCR turned out to be negative. Such results should be considered with caution. It is important to note that RVFV vectors are present in Tunisia, especially the mosquito Culex pipiens, which can disseminate the virus after experimental infection (Krida et al., 2011; Amraoui et al., 2012). A study published in 2016 demonstrated that northern and central eastern Tunisian regions are appropriate for an RVF epizootic (Arsevska et al., 2016). The same study showed that northern regions of the Maghreb are moderately suitable to enzootic RVF but are highly suitable for RVF epizootics, and those areas at-risk extend along the coasts and in the Atlas Mountains in Morocco, Algeria, and Tunisia. It is important to indicate that the movement of live animals remains the most likely way to introduce RVF to Tunisia from other African regions (Ayari-Fakhfakh et al., 2011). This particularly concerns neighboring countries: Algeria and Libya, where RVF cases have been recently (January 2020) reported in small ruminants (OIE, 2020). Materials and MethodsThis study aimed to detect any circulation or any trace of circulation of RVFV in Tunisian domestic ruminants. A total of 1,723 serum samples have been obtained from all the Tunisian territory with its different climatic characteristics (Fig. 1) in the context of systematic serological diagnosis of abortive diseases, which is regularly carried out at the Tunisian Veterinary Research Institute. Serum samples were distributed as follows: 450 bovine sera (from 86 herds), 630 ovine sera (from 69 herds), 316 goat sera (from 109 herds), and 327 dromedary sera (from 19 herds) (Table 1). This work began in 2012 as part of a project aiming to introduce diagnostic and research techniques on RVF at the Tunisian Veterinary Research Institute. Thus, the first part of the study was carried out on sera already available and well stored at the institute and dating from 2009. Then, investigations continued on samples from 2013 until 2015. All sera were tested to detect anti-RVFV IgG. Two ELISA tests were used: a homemade ELISA technique and a commercial kit “ID Screen® RVF competition multi-species ELISA kits (ID-Vet. InnovativeDiagnostics, Montpellier, France). A part of the investigations were carried out in the French National Reference Laboratory of RVF serologic analyses (ANSES – Lyon, France), which produces its own homemade ELISA kit detecting IgG-type antibodies. For the purposes of this technique, plates were coated with antigens extracted from the lysate of virus-infected cells. The used conjugate consists of anti-species antibodies combined with peroxidase. Considering the relatively high number of samples, the use of the homemade ELISA is less expensive. The other part of the ELISA tests was carried out in the Virology Lab of the Tunisian Veterinary Research Institute using the above-mentioned commercial kit. Comparing the two techniques, the homemade ELISA is more sensitive and less specific than the commercial one. All positive and doubtful samples detected by the ELISA technique were investigated by VNT, which was carried out to confirm ELISA-positive samples. The VNT was conducted on the culture system of vero cells (kidney cells from African green monkey) infected by the attenuated viral strain MP12. The inhibition of the virus cytopathic effect was assessed on different dilutions of the serum to be tested. If this inhibition is obtained, it will be concluded that the neutralizing antibodies are present in the serum. The VNT technique is highly specific and is generally accepted as the standard assay system for the quantitative determination for neutralization antibody activity in serum samples (OIE, 2018). Ethical approvalAll technical procedures of animal restraint and blood sampling were in accordance with the European legislation regarding ethics and animal welfare. Blood samples were professionally collected by veterinarians from restrained animals during their monitoring to avoid animal suffering. Results and DiscussionSixty-nine sera were detected positive, 11 were detected doubtful by the homemade ELISA technique, and 18 were detected positive by competition multi-species ELISA test. Among the ELISA-positive sera, three were confirmed positive by VNT. One of them was from a sheep sampled in 2009 in Kasserine (center-west). The two others were from cattle, one from Kairouan (center) sampled in 2009, and one from Siliana (north-west) sampled in 2013. Only the case from Siliana was from a herd where some abortions had been detected, and the serum was also detected positive to brucellosis. No further information was available on these cases. According to the VNT results, the global seroprevalence among tested animals was about 0.17% (3/1723). An attempt was made to detect the viral genome from positive sera using the PCR technique, but no amplification was obtained. Such a result probably indicates that the viremic phase was exceeded.
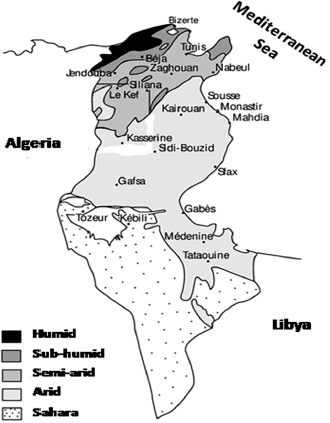
Fig. 1. Climatic map of Tunisia (Rekik et al., 2000). NB: Tunis includes the governorates of Tunis, Ben Arous, Ariana, and Manouba. Table 1. Sera repartition according to geographic localization and animal species.
The present work was initiated as part of a project which aims to introduce diagnostic techniques of RVF in the virology lab of the Tunisian Veterinary Research institute. In 2015, the serological diagnosis of RVF was introduced and is now carried out regularly on suspected cases. Apart from the RVF seropositive cases detected in this study, no other one was declared positive so far by serological techniques. Hence, the importance of our results, which show a trace of circulation of the virus in few animals in 2009 and 2013 and no outbreak of RVF or even seropositive cases since then. The presence of RVFV seropositive animals in Tunisia is probably due to the virus introduction via the movements of positive animals from a neighboring country. It can also be explained by undetected long-term circulation because of the absence of large-scale epidemiological study and without any surveillance system. However, this second hypothesis is less probable given the absence of confirmed positive RVF cases since the completion of this study. The occurrence of RVF in Tunisia is expected because climatic and entomologic conditions are in favor of the emergence of this disease (Arsevska et al., 2016). Furthermore, legal and illegal animal trade from neighboring countries strengthens the probability of RVF introduction in Tunisia, particularly in the current context where recent outbreaks of RVF were declared in Libya (OIE, 2020), a neighboring country with which Tunisia has a lot of exchanges. Regarding this present study, the three regions where the trace of the circulation of the virus was detected correspond to areas where competent vectors, such as the mosquito C. pipiens, are present (Moutailler et al., 2008; Krida et al., 2011; Amraoui et al., 2012). This is supported by the fact that regions where positive cases were detected present a high livestock density and are considered crossroads of livestock movements in Tunisia (Mohamed-Brahmi et al., 2010; Jemaa, 2016). Considering the results of this study and knowing the severe impact of RVFV infection on public and animal health, large-scale epidemiologic studies are needed to better estimate the RVF situation in Tunisia and identify risk factors. These findings will allow the development and implementation of effective epidemiologic surveillance and control programs, considering that generally, vector-borne diseases circulate at a low level before the surge of an epidemic. Conflict of interestThe authors declare that there is no conflict of interests Authors’ contributionHellal Jihene: acquisition, analysis, and interpretation of data. Mejri Selma: conception and design of the study and drafting of the article. Lacote Sandra: analysis and interpretation of data. Sghaier Soufien: acquisition of data. Dkhil Abderrazek: analysis of data. Arsevska Elena: review of the article. Calavas Didier: review of the article. Henaux Viviane: review of the article. Marianneau Philippe: conception and design of the study. Hammami Salah: review of the article. FundingThis work was supported by the PHC-Utique program funded by the Comité Mixte de Coopération Universitaire (CMCU: Tunisia–France) [Project Code 12G0815]. ReferencesAl-Afaleq, A.I. and Hussein, M.F. 2011. The status of Rift Valley fever in animals in Saudi Arabia: a mini review. Vector Borne Zoonotic Dis. 11(12), 1513–1520. Amraoui, F., Krida, G., Bouattour, A., Rhim, A., Daaboub, J., Harrat, Z., Boubidi, S.C., Tijane, M., Sarih, M. and Failloux, A.B. 2012. Culex pipiens, an experimental efficient vector of West Nile and Rift Valley fever viruses in the Maghreb region. PLoS One. 7(5), e36757. Arsevska, E., Hellal, J., Mejri, S., Hammami, S., Marianneau, P., Calavas, D. and Hénaux, V. 2016. Identifying areas suitable for the occurrence of Rift Valley fever in North Africa: implications for surveillance. Transbound. Emerg. Dis. 63, 658–674. Ayari-Fakhfakh, E., Ghram, A., Bouattour, A., Larbi, I., Gribâa-Dridi, L., Kwiatek, O., Bouloy, M., Libeau, G., Albina, E. and Cêtre-Sossah, C. 2011. First serological investigation of peste-des-petits-ruminants and Rift Valley fever in Tunisia. Vet. J. 187(3), 402–404. Ben Hassine, T., Amdouni, J., Monaco, F., Savini, G., Sghaier, S., Ben Selimen, I., Chandoul, W., Ben Hamida K. and Hammami, S. 2017. Emerging vector-borne diseases in dromedaries in Tunisia: West Nile, bluetongue, epizootic haemorrhagic disease and Rift Valley fever. Onderstepoort. J. Vet. Res. 84(1), 1316. Bird, B.H., Ksiazek, T.G., Nichol, S.T. and Mac Lachlan, N.J. 2009. Rift Valley fever virus. J. Am. Vet. Med. Assoc. 234, 883–893. Bosworth, A., Ghabbari, T., Dowall, S., Varghese, A., Fares, W., Hewson, R., Zhioua, E., Chakroun, M., Tiouiri, H., Ben Jemaa, M., Znazen, A. and Letaief, A. 2015. Serologic evidence of exposure to Rift Valley fever virus detected in Tunisia. New Microbes New Infect. 28(9), 1–7. Centre for Disease Control (CDC). 2000a. Outbreak of Rift Valley fever–Saudi Arabia, August–October, 2000. MMWR Morb Mortal Wkly Rep. 49, 905–908. Centre for Disease Control (CDC). 2000b. Outbreak of Rift Valley fever–Yemen, August–October. MMWR Morb Mortal Wkly Rep. 49, 1065–1066. Cito, F., Narcisi, V., Danzetta, M.L., Iannetti, S., Sabatino, D.D., Bruno, R., Carvelli, A., Atzeni, M., Sauro, F. and Calistri, P. 2013. Analysis of surveillance systems in place in European Mediterranean Countries for West Nile Virus (WNV) and RiftValley fever (RVF). Transbound. Emerg. Dis. 60(Suppl. 2), 40–44. El-Harrak, M., Martín-Folgar, R., Llorente, F., Fernández-Pacheco, P., Brun, A., Figuerola, J. and Ángel Jiménez-Clavero, M. 2011. Rift Valley and West Nile virus antibodies in camels, North Africa. Emerg. Infect. Dis. 17(12), 2372–2374. Gerdes, G.H. 2004. Rift Valley fever. Rev. Sci. Tech. 23, 613–623. Hotez, J., Savioli, L. and Fenwick, A. 2012. Neglected tropical diseases of the Middle East and North Africa: review of their prevalence, distribution, and opportunities for control. PlosNegl. Trop. Dis. 6(2), 1–8. Jemaa, T. 2016. Thèse de Doctorat “Stratégie d’adaptation des éleveurs et modalités d’utilisation des parcours en Tunisie Centrale” Montpellier, France: SupAgro. Spécialité: Zootechnie-Système, p: 194. Jourdain, F., Samy, A.M., Hamidi, A., Bouattour, A., Alten, B., Faraj, C., Roiz, D., Petrić, D., Pérez-Ramírez, E., Velo, E., Günay, F., Bosevska, G., Salem, I., Pajovic, I., Marić, J., Kanani, K., Paronyan, L., Dente, M.G., Picard, M., Zgomba, M., Sarih, M., Haddad, N., Gaidash, O., Sukhiasvili, R., Declich, S., Shaibi, T., Sulesco, T., Harrat, Z. and Robert, V. 2019. Towards harmonisation of entomological surveillance in the Mediterranean area. PLoS Negl. Trop. Dis. 13(6), e0007314. Kardjadj, M. 2017. An epidemiological overview of small ruminant diseases in Algeria. Rev. Sci. Tech. 36(3), 997–1006. Kardjadj, M. 2018. Epidemiological situation of transboundary animal diseases in North African countries-proposition of a regional control strategy. Trop. Anim. Health Prod. 50, 459–467. Krida, G., Diancourt, L., Bouattour, A., Rhim, A., Chermiti, B. and Failloux, A.B. 2011. Assessment of the risk of introduction to Tunisia of the Rift Valley fever virus by the mosquito Culex pipiens. Bull. Soc. Pathol. Exot. 104(4), 250–259. Métras, R., Collins, L.M., White, R.G., Alonso, S., Chevalier, V., Thuranira-McKeever, C. and Pfeiffer, D.U. 2011. Valley fever epidemiology, surveillance, and control: what have models contributed? Vector-Borne Zoonotic Dis. 11(6), 761–771. Mohamed-Brahmi, A., Khaldi, R. and Khaldi, G. 2010. L’Elevage ovin extensif enTunisie: Disponibilité alimentaire et innovations pour la valorisation des ressources fourragères locales. ISDA 2010, Montpellier, France, p 12. hal-00521129. Moutailler, S., Krida, G., Schaffner, F., Vazeille, M. and Failloux, A. 2008. Potential vectors of Rift Valley fever virus in the Mediterranean region. Vector Borne Zoonotic Dis. 6, 749–753. OIE. 2018. Rift Valley fever (Infection with Rift Valley Fever Virus). Terrestrial Manual 2018, Chapter 3.1.18. Paris, France: OIE, pp: 613–633. OIE. 2020. Available via https://rr-africa.oie.int/fr/projets/gf-tads-afrique/fievre-de-la-vallee-du-rift/ (Accessed 14 May 2021). Rekik, M., Mahouachi, M., Gharbi, M., Attia, W. and Medhioub, L. 2000. Le dilemme de l’élevage ovin extensif dans les régions élevées du nord-ouest semi-aride tunisien. Revue Élev. Méd. Vét. 53(4), 377–385. Selmi, R., Mamlouk, A., Ben Said, M., Ben Yahia, H., Abdelaali, H., Ben Chehida, F., Daaloul-Jedidi, M., Gritli, A. and Messadi, L. 2020. First serological evidence of the Rift valley fever Phlebovirus in Tunisian camels. Acta Trop. 207, 105462. Zakham, F., Alaoui A. and Vapalahti O. 2018. Rift Valley fever in the Middle East North Africa (MENA) region. Curr. Trop. Med. Rep. 5(4), 257–263. | ||
| How to Cite this Article |
| Pubmed Style JH, SM, SL, SS, DKHIL A, EA, CALAVAS D, HENAUX V, MARIANNEAU P, HAMMAMI S. Serological evidence of Rift Valley fever in domestic ruminants in Tunisia underlines the need for effective surveillance. Open Vet J. 2021; 11(3): 337-341. doi:10.5455/OVJ.2021.v11.i3.1 Web Style JH, SM, SL, SS, DKHIL A, EA, CALAVAS D, HENAUX V, MARIANNEAU P, HAMMAMI S. Serological evidence of Rift Valley fever in domestic ruminants in Tunisia underlines the need for effective surveillance. https://www.openveterinaryjournal.com/?mno=65393 [Access: August 31, 2024]. doi:10.5455/OVJ.2021.v11.i3.1 AMA (American Medical Association) Style JH, SM, SL, SS, DKHIL A, EA, CALAVAS D, HENAUX V, MARIANNEAU P, HAMMAMI S. Serological evidence of Rift Valley fever in domestic ruminants in Tunisia underlines the need for effective surveillance. Open Vet J. 2021; 11(3): 337-341. doi:10.5455/OVJ.2021.v11.i3.1 Vancouver/ICMJE Style JH, SM, SL, SS, DKHIL A, EA, CALAVAS D, HENAUX V, MARIANNEAU P, HAMMAMI S. Serological evidence of Rift Valley fever in domestic ruminants in Tunisia underlines the need for effective surveillance. Open Vet J. (2021), [cited August 31, 2024]; 11(3): 337-341. doi:10.5455/OVJ.2021.v11.i3.1 Harvard Style , J. H., , . S. M., , . S. L., , . S. S., DKHIL, . A., , . E. A., CALAVAS, . D., HENAUX, . V., MARIANNEAU, . P. & HAMMAMI, . S. (2021) Serological evidence of Rift Valley fever in domestic ruminants in Tunisia underlines the need for effective surveillance. Open Vet J, 11 (3), 337-341. doi:10.5455/OVJ.2021.v11.i3.1 Turabian Style , Jihene HELLAL, Selma MEJRI, Sandra LACOTE, Soufien SGHAIER, Abderrazek DKHIL, Elena ARSEVSKA, Didier CALAVAS, Viviane HENAUX, Philippe MARIANNEAU, and Salah HAMMAMI. 2021. Serological evidence of Rift Valley fever in domestic ruminants in Tunisia underlines the need for effective surveillance. Open Veterinary Journal, 11 (3), 337-341. doi:10.5455/OVJ.2021.v11.i3.1 Chicago Style , Jihene HELLAL, Selma MEJRI, Sandra LACOTE, Soufien SGHAIER, Abderrazek DKHIL, Elena ARSEVSKA, Didier CALAVAS, Viviane HENAUX, Philippe MARIANNEAU, and Salah HAMMAMI. "Serological evidence of Rift Valley fever in domestic ruminants in Tunisia underlines the need for effective surveillance." Open Veterinary Journal 11 (2021), 337-341. doi:10.5455/OVJ.2021.v11.i3.1 MLA (The Modern Language Association) Style , Jihene HELLAL, Selma MEJRI, Sandra LACOTE, Soufien SGHAIER, Abderrazek DKHIL, Elena ARSEVSKA, Didier CALAVAS, Viviane HENAUX, Philippe MARIANNEAU, and Salah HAMMAMI. "Serological evidence of Rift Valley fever in domestic ruminants in Tunisia underlines the need for effective surveillance." Open Veterinary Journal 11.3 (2021), 337-341. Print. doi:10.5455/OVJ.2021.v11.i3.1 APA (American Psychological Association) Style , J. H., , . S. M., , . S. L., , . S. S., DKHIL, . A., , . E. A., CALAVAS, . D., HENAUX, . V., MARIANNEAU, . P. & HAMMAMI, . S. (2021) Serological evidence of Rift Valley fever in domestic ruminants in Tunisia underlines the need for effective surveillance. Open Veterinary Journal, 11 (3), 337-341. doi:10.5455/OVJ.2021.v11.i3.1 |