Open Veterinary Journal, (2021), Vol. 11(3): 483–499
Original Article
10.5455/OVJ.2021.v11.i3.22
The efficiency of natural-ecofriendly clay filters on water purification for improving performance and immunity in broiler chickens
Essam S. Soliman1* , Rania A. Hassan2
, Rania A. Hassan2 and Doaa S. Farid3
and Doaa S. Farid3
1Animal, Poultry, and Environmental Hygiene Division, Department of Animal Hygiene, Zoonosis, and Animal Behavior, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt
2Animal Production Division, Department of Animal Wealth Development, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt
3Department of Environmental Protection, Faculty of Environmental Agricultural Sciences, Arish University, Arish, Egypt
*Corresponding Author: Essam S. Soliman. Department of Animal Hygiene, Zoonosis and Animal Behavior, Faculty of Veterinary Medicine, Suez Canal University, Ismailia, Egypt. Email: soliman.essam [at] vet.suez.edu.eg
Submitted: 14/06/2021 Accepted: 10/08/2021 Published: 10/09/2021
© 2021 Open Veterinary Journal
This is an Open Access article distributed under the terms of the Creative Commons Attribution-Non Commercial-No Derivatives License (http://creativecommons.org/licenses/by-nc-nd/4.0/), which permits non-commercial re-use, distribution, and reproduction in any medium, provided the original work is properly cited, and is not altered, transformed, or built upon in any way.
Abstract
Background: Innovative water treatments and purification processes have become a point of interest to provide solutions and meet the basic water requirements and demands. Clay plays a key role in environmental protection from pollutants through ion exchange and/or adsorption.
Aim: The study evaluated the adsorption and antimicrobial efficiency of clay in purifying polluted water, as well as the influence of clay-purified water on performance, immunity, and microbial counts.
Methods: The experimented 280 one-day-old Hubbard broilers were divided into seven groups on a deep litter system. Polluted water (lead nitrate; 500 mg/l, calcium sulfate; 80 mg/l, yeast extract 5%; 5 mg/l, diazinon; 2.5 ml/l, Salmonella Typhimurium; 1.5 × 106 CFU/ml, and Eimeria tenella; 1 × 105 OPG/ml) was filtered using plastic basins of 1 m3 supplied with 60 cm layer of clay. Broiler groups (G1 to G6) were supplied with clay-filtered and G7 with control tape water. A total of 2,182 samples, including 54 water samples, 266 sera, 266 duodenal swabs, 266 breast muscles, 266 fecal samples, and 1,064 organs including liver, spleen, heart, and bursa of Fabricius were collected.
Results: Weight gains, performance indices, water intakes, water/feed intake ratios, live body weights, carcasses weights, edible and immune organs’ weights, immunoglobulin G and M, total antioxidant capacity, lactate dehydrogenase, malondialdehyde, and superoxide dismutase revealed highly significant (p < 0.01) increases in all broiler groups supplemented with clay-filtered water compared to the control group. Meanwhile, total protein, alanine aminotransferase, creatinine, glucose, triglycerides, total cholesterol, cortisol hormone, total bacterial and Enterobacteriaceae counts, total Salmonella counts, and E. tenella counts revealed highly significant (p < 0.01) declines in all broiler groups supplemented with clay-filtered water compared to the control group.
Conclusion: Clay filters provided high filtration, adsorption, and antimicrobial efficiency against polluted water, enhanced water quality, and improved performance and immunity in broiler chickens.
Keywords: Antimicrobial, Broiler chickens, Natural clay, Environmental protection, Filtration, Immunity.
Introduction
Water in our environment is continually exposed to pollution by impurities such as pesticides, heavy metals, and pathogens. In the last few years, water worldwide witnessed critical challenges resulting from the rapid development of industrial and agricultural practices, as well as artificial and anthropogenic activities that contributed to increased pollution and contamination of water resources, and thus reduced the amount of clean water available for drinking and planting purposes (Fu and Wang, 2011). In addition, the problem of water scarcity in different regions of the world infers the need for searching alternatives to obtain water for drinking and other purposes. Water and wastewater treatments have become a point of interest to provide solutions and meet the basic requirements and water demands, as well as water hygiene, has been included in all the development, biosafety, and biosecurity plans in animal and poultry farms. Treatments methodology like storage and sedimentation, filtration, osmosis (Zhu et al., 2016), ion exchange (Szczepanik, 2017), electrodialysis, ultrafiltration, and photocatalytic degradation (Syafalni et al., 2014; Raval et al., 2016) have experimented and have proven to be highly efficient in treating water and wastewater but were associated with high cost, toxic byproducts, detrimental effects on the environment, and low efficiency on a large scale.
Adsorption has been considered later as an alternative for all other methods for its efficiency. The choice of a good adsorbent has been carried out based on its physical and chemical qualities. Adsorbent materials have been used in the field of water treatment like activated carbons (Ahmed, 2017; Singh et al., 2018), biochar (Premarathna et al., 2019; Palansooriya et al., 2020), agricultural waste (Ahmad and Danish, 2018; Shakoor et al., 2018), sludge materials (Anastopoulos et al., 2017), and clay material (Rusmin et al., 2015; Olu-Owolabi et al., 2017; Han et al., 2019). Therefore, applying Green Chemistry principles had become essential to defend sustainable, environmental, and social guidelines (Royal Society of Chemistry, 2007).
Clay and clay minerals are very important and play a key role in environmental protection from pollutants (contaminants) by their ability to take up cations and anions either through ion exchange, adsorption, or a combination of both (Foo and Hameed, 2011). Natural clay is an available and low-cost resource that is non-toxic to the ecosystem. Clay is a natural product composed of aluminum phyllosilicates with tetra and octahedral groups positioned on isomorph positions that generate the charges of clay (Mukhopadhyay et al., 2017). Clay has many characterizations like high surface area, firmness, decolorizing abilities, low permeability, hydration properties, changed to ceramics when fired, catalytic, and ions exchange capabilities (Kumararaja et al., 2018). Clay, as an end product of many environmental processes, has been involved in many uses as an adsorbent for water treatment and removal of heavy metals (Sarkar et al., 2019) either in its natural form or in the modified nanocomposites form (Yadav et al., 2019; Mukhopadhyay et al., 2020). Also, clay has proven its high efficiency as a disinfectant (Unuabonah et al., 2018) and antimicrobial agent against E. coli and Salmonella Typhimurium ( Mohamed et al., 2020).
The current study aims to evaluate the filtration efficiency, adsorption, and antimicrobial actions of natural clay in purifying water challenged with lead nitrate at a rate of 500 mg/l, calcium sulfate at a rate of 80 mg/l, yeast extract 5% at a rate of 5 mg/l, diazinon at a rate of 2.5 ml/l, Salmonella Typhimurium 1.5 × 106 CFU/ml, and Eimeria tenella 1 × 105 OPG/ml and the impact of the clay-filtered water as a form of drinking water on performance, immunity, and intestinal microbial load in broiler chickens.
Material and Methods
Study period and location
The study was conducted from October 1st, 2020, to November 7th, 2020, in the poultry experimental unit—Faculty of Veterinary Medicine—Suez Canal University—Ismailia. Water physicochemical and microbial analysis, performance indices, carcass characteristics, and bacteriological assessments were conducted in the Animal, Poultry, and Environmental Hygiene laboratory. Water electrolyte analysis, antioxidant, hormonal, and immunity assays were performed in the Clinical Pathology laboratories, Suez Canal University Hospital. Heavy metal water analysis was conducted in the Toxicology Laboratory—Chemistry Department—Faculty of Science—Suez Canal University—Ismailia.
Experimental birds’ housing microclimate
The experimental units were prepared before the arrival of broiler chickens, as well as provided with some biosecurity measures to maintain broiler chickens’ health as recommended and evaluated by Soliman and Abdallah (2020), like cleaning and disinfection properly using hypochlorite, sodium hydroxide 5% solution, and formaldehyde spray with 24 hours intervals, foot dip with commercial carbolic acid 5% at the entrance of the units, fly-proof nets, mechanical traps and rodenticides against rodents, prohibition for the introduction of wild birds, restricted access to the experimental units, restricted access to feed storage areas, and proper movement control inside the rooms during daily observation, feeding, and watering of broiler chickens.
The floor of the seven experimental units was covered with a thin layer of superphosphate at a rate of 0.5 g.m−2, then covered with hay litters as recommended by Soliman et al. (2018) to maintain proper litter abiotic conditions, reduce microbial development, and minimize ammonia volatilization. The ventilation in the experimental units was based on negative cross-ventilation using V-shaped inlet windows, and suction fans serving as air outlets, as well as, the units were supplied with extra ceiling fans to encourage air movement across the units. The experimental rooms were provided with monochromic white LED lights of 18 watts and 1,750 lumen each to serve in a continuous lighting regimen for 23 hours of lighting and 1 hour of darkness as recommended by Soliman and Hassan (2019).
Experimental birds’ management
A total of 280 one-day-old female classic Hubbard chicks were purchased from Ismailia-Egypt Company. Broiler chickens on their arrival were weighed to record the initial body weights and were then divided into seven groups (40 birds each, 4 replicates of 10 birds) on separate research rooms/units supplied with a deep litter system of hay as guided by Soliman and Hassan (2020). The units were supplied with halogen and oil heaters as recommended by Soliman et al. (2021) that were turned on before the arrival of broiler chickens until a microclimatic temperature of 34°C was obtained, optimized for brooding conditions during the first week of age. By the end of the first week, heaters’ working hours were reduced to get a gradual decline in the microclimatic temperature at a rate of 3.5°C weekly until achieving stable thermoneutral conditions at 25°C by the end of the 3rd week. Broiler chickens were given ad libitum access to dechlorinated water. Broilers were supplied with corn-soybean ration to fulfill their nutritional requirements according to National Research Council; NRC (1994) and Applegate and Angel (2014) modifications. The corn-soybean ration as reported by the manufacturer (El-Eman company, El-Sharkia governorate, Egypt) contained nutritive substances as follow: protein, energy, crude fiber, and fat by rates of 22.5%, 2,900 kcal, 3.89%, and 5.5%, respectively, in the starter ration that was supplied to broiler chickens for 1:14 days, and 21%, 3,100 kcal, 3.40%, and 2.75%, respectively, in the grower pellet ration that was supplied to broiler chickens from 15 days and ongoing for the end of the fattening cycle (25 days). The experiment was designed to last for 39 days.
Broilers were observed for the development of any abnormalities and morbidities, specific and non-specific mortalities, indoor and outdoor temperatures, and humidity percentages were recorded daily for 39 days. Broilers were immunized in a traditional pattern using mass vaccination act via clay-filtered water after water deprivation for 4 hours against infectious bronchitis using PESTIKAL B1 SPF H120 ≥ 103.5 live attenuated virus vaccine on the seventh day, infectious bursal disease using SER-VAC D78 strain VMG91 ≥ 103.0 live attenuated virus vaccine on the 14th and 21st days, and Newcastle virus disease using PESTIKAL Lasota ≥ 106.0 live lentogenic virus vaccine on the 16th and 26th days.
Salmonella and Eimeria propagation
Salmonella Typhimurium lyophilized vial 2.5 × 103 was purchased from Animal Health Research Institute - Dokki - Egypt. Salmonella Typhimurium was propagated as recommended by Soliman et al. (2018) and Fritz et al. (2015) using pre-enrichments in tetrathionate broth (Thermo Scientific™ Oxoid™ Tetrathionate Broth Base, CM0029, 500 g) at 37°C/24 hours. The pre-enrichments were repeated daily for 7 days. Ten microliters from the pre-enrichment tubes were dropped aseptically onto CHROMagarTM Salmonella (BD BBL™ CHROMagar™ Salmonella READY-TO-USE Plated Media) at 37°C/24 hours. Pink colonies of Salmonella Typhimurium were counted, picked up, and resuscitated in buffered peptone water (Thermo Scientific™ Oxoid™ Buffered Peptone Water, CM0509B, 500 g), providing 1.5 × 106 suspensions.
Sporulated E. tenella 1 × 102 OPG suspension was purchased from Animal Health Research Institute—Dokki—Egypt. Eimeria tenella oocyst suspension was centrifuged, resuscitated in deionized water, and propagated experimentally by oral gavage in six female classic Hubbard broilers of 14-days old and housed in a metal galvanized battery. Broilers were observed for the development of bloody diarrhea as a prominent sign of the successful experimental infection. The feces were collected for 7 days post-infection and examined microscopically for the presence of E. tenella oocysts. Sporulated E. tenella oocyst 1 ×105 OPG was collected in a screw-capped tube at 4°C until the challenge takes place.
Clay filters and water filtration
The experimental filters were designed as plastic basins of 1 m3 (1 m length × 1 m width × 1 m depth). The basins were perforated in their lower ventral aspects into holes of approximately 220 microns in diameter. The ventral inner aspect of the tanks was covered using a double-layered sterile gauze, and later a thick layer of 60 cm of natural clay was added to the tanks.
Clay was obtained from Ismailia lake—Egypt, and sterilized through dry heat using a hot air oven (Daihan® LabTech® Hot air Oven, LDO-080N) at 160°C/30 minutes to obtain clay free from any microbial contaminants. The clay was analyzed for its physical and chemical characteristics. The analysis revealed silty clay loam in texture, pH up to 7.73, electrical conductivity (EC) up to 2.49 dS/m, and composed of silt (61.3%), clay (27.0%), sand (11.7%), organic carbon (1.59%), organic matter (2.74%), calcium carbonate (3.60%), cations like calcium (13.0 meq/l), magnesium (4.0 meq/l), sodium (7.1 meq/l), and potassium (0.9 meq/l), and anions like chloride (7.0 meq/l), sulfates (8.5 meq/l), and aldehydes (9.5 meq/l).
Challenged water (lead nitrate, calcium sulfate, yeast extract 5%, diazinon, Salmonella Typhimurium, and E. tenella) was allowed to pass through the filter overnight. The filtered water was received in clean and sterile containers with output control.
Water challenge and supply
Broiler groups were supplied with clay-filtered water that was previously polluted in the laboratory as follow: the first group (G1) with clay-filtrate of lead nitrate polluted water at a rate of 500 mg/l, the second group (G2) with clay-filtrate of calcium sulfate polluted water at a rate of 80 mg/l, the third group (G3) with clay-filtrate of yeast extract 5% polluted water at a rate of 5 mg/l, the fourth group (G4) with clay-filtrate of diazinon polluted water at a rate of 2.5 ml/l, the fifth group (G5) with clay-filtrate of Salmonella Typhimurium contaminated water at a rate of 1.5 × 106 CFU/ml, the sixth group (G6) with clay-filtrate of E. tenella contaminated water at a rate of 1 × 105 OPG/ml, and the seventh group (G7) with control tape water. The filtered water was provided with ad libitum access to the broiler chickens for 8 hours daily and during the vaccination act, clean white tape water was supplied for the rest of the day.
Performance indices
Live body weights (LBW/g) of a representative sample from the broilers of each group (approximately 36 birds) were weighed and recorded weekly using a digital scale (WONHENG® Computing Electronic Digital Counting Weight Balance Scale, 220 V, 30 kg). The number of the representative samples was measured using a simple random sampling design concerning Thrusfield and Christley (2018) and Charan and Biswas (2013) with an expected error of 5%: n=(Z1-α/2)2.p (1−p)/ d2
where (n) was the number of samples, (Z1-α/2)2) was the standard normal, (p) was the expected proportion in the population, (d) was the absolute precision.
The amount of feed consumed per bird (Feed intakes, FI/g) was calculated by proportioning the total amount consumed in each group by the number of surviving broilers. Water intake (WI/ml) was calculated by proportioning the total amount consumed in each group by the number of surviving broilers. Bodyweight gains (WG/g) were calculated by subtracting the final broilers’ weight in each group by the end of the week from the initial weights at the beginning of the same week, feed conversion ratios (FCR) proportionate the FI to WG, and performance index (PI) proportionate LBW/kg to FCR according to Soliman and Hassan (2017).
Sampling
A total of 2,182 samples, including 54 water samples (18 samples per type of water), 266 sera, 266 duodenal swabs, 266 breast muscles, 266 fecal samples, and 1,064 organs, including liver, spleen, heart, and bursa of Fabricius, were collected. Duodenal swabs and fecal samples were collected on 9 ml buffered peptone water (Thermo Scientific™ Oxoid™ Buffered Peptone Water, CM0509B, 500 g) and transferred to the laboratory in a dry ice box within 2–3 hours to be examined.
A total of 266 broiler chickens (38 broiler chickens from each group) were sacrificed by the end of the experiment for collecting blood samples. The carcasses were de-feathered, weighed, and expressed by grams [carcass weight (CW/g)]. Representative samples of the breast muscles were incised from the corpses and kept frozen for the bacteriological examination. Some edible organs like the liver and heart and immune organs like the spleen and bursa of Fabricius were collected, weighed, and expressed per grams concerning the CW. Sacrificed birds were hygienically disposed of after sampling using a burial technique with a lime lining of the burial bits. Blood samples were held in a water bath (Thermo® water bath Precision series Standard, 20 l, 30 to 100°C, 392 mm, GP20) at 25°C for 20 minutes and then centrifuged at 3,000 rpm for 15 minutes. Clear sera were collected in 2.5 ml capacity Eppendorf tubes and stored at −20°C until tested for biochemical, stress markers, antioxidant markers, and immunological assay (Soliman et al., 2017).
Water analysis
Polluted and contaminated water, clean tape water, and clay-filtered water were sampled in two sets of 500: 1,000 ml, each for analysis as recommended by American Public Health Association; APHA (2012). The first set of samples were analyzed for physicochemical characters like pH, EC (μS/cm) using a conductivity meter (PCE Instruments pH-Meter PCE-PHD 1), total dissolved solids (TDS, mg/l) that calculated by multiplying EC values by a factor of 0.67, dissolved oxygen (DO, mg/l) using wrinkle method against standard sodium thiosulfate solution in the presence of alkali-iodide-azide, total alkalinity (mg/l) using potentiometric titration against sulfuric acid 0.1 N in the presence of phenolphthalein, total hardness (mg/l CaCO3) using titration against ethylene diamine tetraacetic acid in the presence of aerochrom back-T, phosphate (PO42−, mg/l) using spectrophotometric detection against ascorbic acid, sulfate (SO42−, mg/l) using turbidimetric method against barium chloride, and nitrate (NO32−, mg/l) using spectrophotometric detection against hydrochloric acid.
The samples were analyzed for electrolytes like sodium (Na+, mg/l), potassium (K+, mg/l), calcium (Ca+2, mg/l), magnesium (Mg+2, mg/l, and chloride (Cl−, mg/l) using an electrolyte analyzer (Roche Diagnostics® AVL 9180 Series Electrolyte Analyzers). Heavy metal levels like iron (Fe2+, mg/l), cobber (Cu2+, mg/l), zinc (Zn2+, mg/l), and lead (Pb2+, mg/l) were quantified using atomic absorption spectrophotometer. The second set of samples was directed for bacteriological assessments like total bacterial counts (TBC, CFU/ml), total Enterobacteriaceae counts (TEC, CFU/ml), total Salmonella count (TSC, CFU/ml), and total E. tenella oocyst counts (TEtC, OPG/ml).
Biochemical profile
Sera samples (266 samples) were collected via sacrificing 38 broiler chicken per group and examined for some biochemical parameters like total protein (TP, g/dl), alanine aminotransferase (ALT, IU/l), creatinine (CREAT, mg/dl), Glucose (GLUCO, mg/dl), total cholesterol (TC, mg/dl), and triglycerides (TG, mg/dl), as well as some antioxidant markers like total antioxidant capacity (TAC, mM/l), lactate dehydrogenase (LDH, IU/l), malondialdehyde (MDA, nmol/ml), and superoxide dismutase (SOD, U/ml) calorimetrically using ROCHE COBAS Integra 800 chemical analyzer. Cortisol hormone (CORT, mcg/dl), as well as immunoglobulin G and M concentrations (IgG and IgM, mg/dl), were measured by using ROCHE ELECSYS 1010 Immunoassay Analyzer (Wu et al., 2017).
Bacteriological and protozoal examination
Frozen breast muscles samples (266 samples collected via sacrificing 38 broiler chickens per group) were thawed and smashed in the stomacher (Seward® Lab blenders Stomacher®400 circulators). Water samples (54 samples, 18 samples per type of water) and duodenal swabs (266 samples collected after sacrificing 38 broiler chicken per each group) that were collected on 9 ml buffered peptone water (Thermo Scientific™ Oxoid™ Buffered Peptone Water, CM0509B, 500 g) were prepared as recommended by American Public Health Association; APHA (2017) with tenfold serial dilutions up to 10−6.
Total bacterial (TBC) and TEC of water samples, intestinal swabs, and breast muscles, as well as Salmonella Typhimurium count of water samples and intestinal swabs, were carried out using a drop plate as recommended by Soliman et al. (2016) and Kim and Lee (2016). TBC were performed onto Standard Plate count agar (Thermo Scientific™ Oxoid™ Plate Count Agar, CM0325, 500 g) at 37°C for 24–48 hours, TEC was conducted onto Eosin Methylene Blue Agar (Modified Levine Eosine Methylene Blue Thermo ScientificTM OxoidTM, CM0069B, 500 g) at 37°C for 24–48 hours, and total Salmonella Typhimurium counts were performed onto CHROMagarTM Salmonella (BD BBL™ CHROMagar™ Salmonella READY-TO-USE Plated Media) at 37°C for 24–48 hours. Plates were counted using the Dark-field colony counter (R164109 Reichert-Jung Quebec Darkfield 3325 Colony Counter) (Murray et al., 2015).
Water (54 samples, 18 per type of water) and fecal samples (266 samples from inside the duodenum collected after sacrificing 38 broiler chickens per group) were prepared as recommended by American Public Health Association; APHA (2017) with tenfold serial dilutions up to 10−6. The samples were examined using the sugar flotation technique as recommended by Sangster et al. (2016) and Ghoneim et al. (2017) for detecting the number of E. tenella oocyst using McMaster about Zajac and Conboy (2012) multiplied by the dilution factor (100×) and expressed by OPG.
Statistical analysis
Statistical analysis was carried out using the statistical package for social sciences (SPSS version 21.0) software package (SPSS, 2016). The initial data were analyzed statistically using multifactorial Analysis of Variance (Two-tailed ANOVA) to determine the overall influence of the water pollutants/contaminants and broiler’s age and their interactions. The statistical model empathized as follow:
Yijk=μ + αi + βj + (αβ)ij + Ɛijk
where Yijk was the measurement of dependent variables; μ was overall mean; αi was the fixed effect of the water pollutants/contaminants, βj was the fixed effect of broiler’s age, (αβ)ij was the interactions of the water pollutants/contaminants by broiler’s age, and Ɛijk was the random error.
Water assessments were analyzed using a one-tailed ANOVA to investigate the differences between the three categories of supplied water (polluted/contaminated, tape, and clay-filtered water). The ranges and overall means have been displayed in the tables. The statistical model empathized as follow:
Yij=μ + αj + Ɛij
Yij was the measurement of dependent variables; μ was the overall mean; αj was the fixed effect of the water type, and Ɛij was the random error.
TBC and Enterobacteriaceae counts in water samples, duodenal swabs, and breast muscles, Salmonella Typhimurium, counts in water samples and duodenal swabs, and E. tenella counts in the water. Fecal samples were transformed and expressed as logarithms (Log10) using Microsoft Excel 2016. The results were expressed as highly significant at (p < 0.01), significant at (p ≤ 0.05), and non-significant at (p > 0.05).
Ethical approval
The Scientific Research Ethics Committee on animal and poultry researches, Faculty of Veterinary Medicine, Suez Canal University, Egypt, approved the experimental design, materials used, and protocol of the current study with approval number (2021013).
Results
Water analysis
Physicochemical characters of water samples revealed in Table 1 highly significant (p < 0.01) declines, as well as enhancement of all measured parameters in clay-filtered water compared to the polluted water, control water, and maximum permissible limits adjusted by the world health organization (WHO). Electrolytes analysis (Table 1) revealed highly significant (p < 0.01) improvements in the clay-filtered water. Heavy metals revealed highly significant (p < 0.01) declines (Table 1) in clay-filtered water up to levels that can be described as extremely far behind the maximum permissible limits.
TBC and Enterobacteriaceae counts revealed in Table 1 highly significant (p < 0.01) declines in clay-filtered water compared to that in contaminated and tape water, as well as clay-filtered water, exhibited highly significant (p < 0.01) disappearance of both Salmonella Typhimurium and E. tenella.
Performance indices
The crude mortality rate showed a total of 4.28% nonspecific mortalities (12 out of 280 broiler chickens) among the broiler groups. A total of 75% (9 out of 12 dead birds) were recorded during the first 2 weeks of age, while the other 25% (3 out of 12 dead birds) were recorded at the fourth and fifth week of age. The deceased birds were exposed to general postmortem examination. They revealed a moderate degree of hyperemia in the intestinal wall, congested liver, and spleen, and the digested ration was filling all over the intestine.
Weight gains revealed in Figure 1A highly significant (p < 0.01) increases in all supplemented broiler groups compared to the control group with no significant differences between G6 (E. tenella) and G5 (Salmonella Typhimurium). Broilers of the fifth group (G5) revealed no significant differences with G3 (yeast extract 5%), G2 (calcium sulfate), G1 (lead nitrate), and G4 (diazinon), chronologically. On the time scale (Fig. 1B), the highest weight gains were recorded with high significant (p < 0.01) differences at the fourth week and the least at the first week.
FCR (Fig. 2A) revealed the highest significant (p < 0.01) value in the control group (1.66) and the lowest value in G1 (1.20, lead nitrate). No significant differences were recorded between G2 (calcium sulfate), G6 (E. tenella), G3 (yeast extract 5%), and G5 (Salmonella Typhimurium), as well as no significant differences were recorded between G3, G5, and G4 (diazinon). The feed conversions concerning broiler’s age revealed in Figure 2B, highly significant (p < 0.01) lowest FCR at the 1st (1.10) and the fourth weeks (1.07) with no significant differences between the two values at these two timelines, while the highest highly significant (p < 0.01) FCR was recorded at the fifth week (2.30).
Calculated performance indices in Figure 2A revealed highly significant (p < 0.01) increases in G1 (lead nitrate) with no significant differences compared to G6 (E. tenella) and G5 (Salmonella Typhimurium). Concerning the broiler’s age, PI revealed in Figure 2B the highest significant values (p < 0.01) at the fourth week (13.52).
Table 1. Water quality analysis (range & mean ± SE) before and after the clay filtration process.
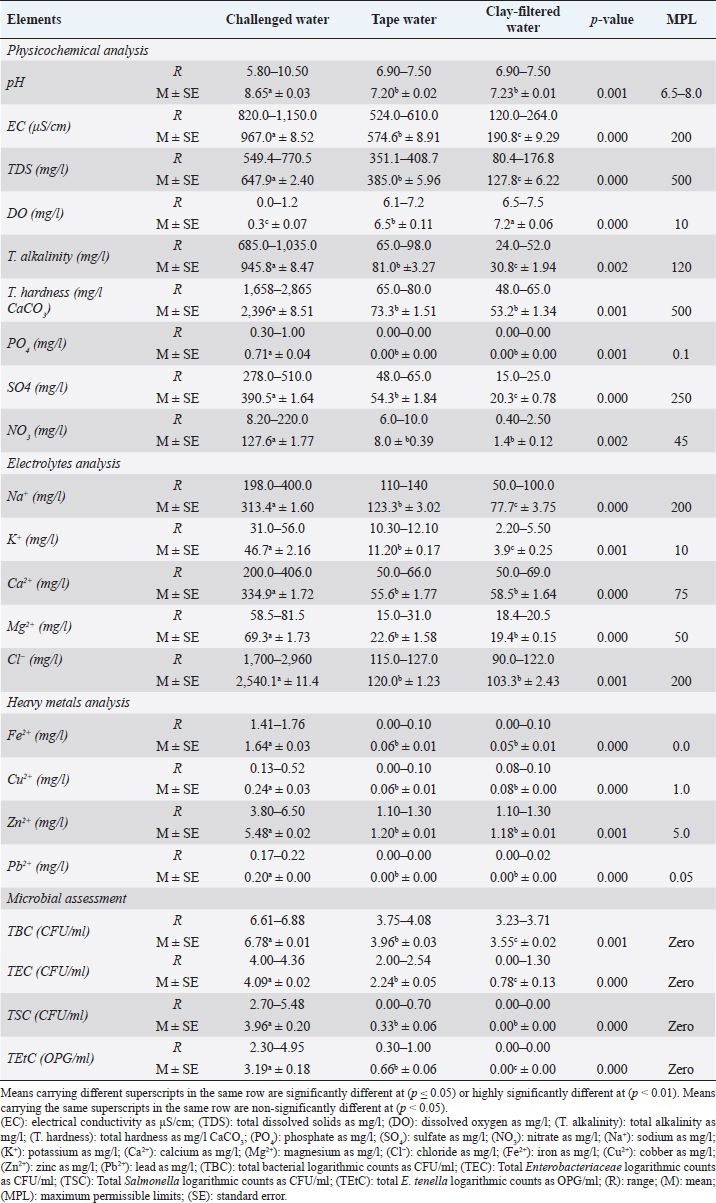
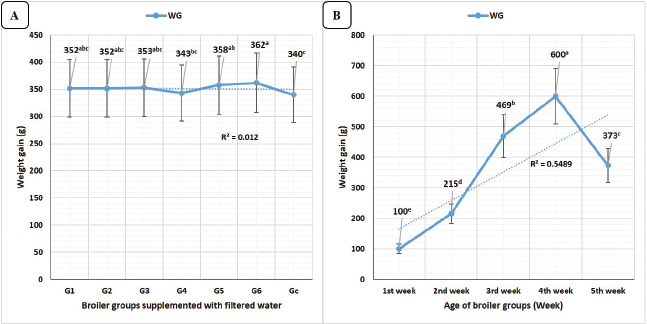
Fig. 1. Weight gain (g) in different broiler groups supplemented with clay-filtered water. A) Weight gain (g) overall means concerning different broiler groups. B) Weight gain (g) overall means concerning broilers’ age (week).
FI revealed highly significant (p < 0.01) declines in Figure 3A in all groups supplemented with clay-filtered water compared to G7 (control group). WIs showed highly significant (p < 0.01) increases in Figure 3A in G4 (diazinon) with no significant differences compared to G6 (E. tenella) and G5 (Salmonella Typhimurium). The time scale revealed in Figure 3B highly significant (p < 0.01) increases in feed and WIs as broiler’s age was increased with the highest significant values at the fifth week (809 g and 1,805 ml, respectively).
Water/FI ratios in Figure 4A revealed highly significant (p < 0.01) increases in G6 (E. tenella) with no significant differences compared to G5 (Salmonella Typhimurium) and G4 (diazinon). Concerning the broiler’s age, WI/FI ratios revealed in Figure 4B highly significant (p < 0.01) increases at the fifth week of age.
Live, carcasses, and organs weights
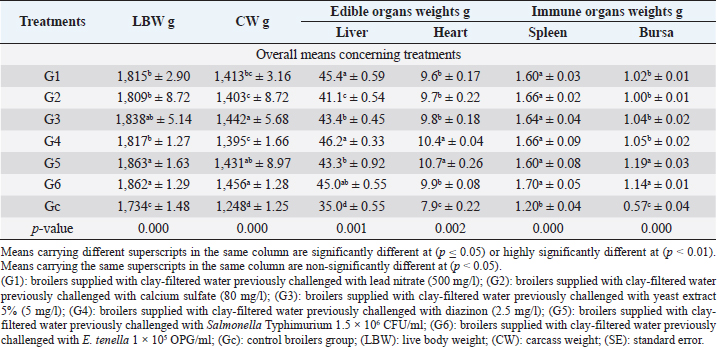
Live body, carcasses, liver, heart, spleen, and bursa of Fabricius weights in Table 2 revealed highly significant (p < 0.01) increases in all broiler groups supplied with clay-filtered water compared to the control group. The highest significant (p < 0.01) live weights were recorded in G5 (Salmonella Typhimurium) and G6 (E. tenella) with no significant differences between the two groups. While the highest significant (p < 0.01) CWs were recorded in G6 (E. tenella) and G3 (yeast extract 5%) with no significant differences between the two groups.
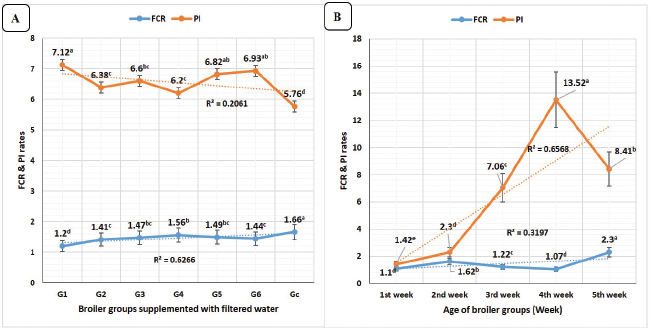
Fig. 2. FCR and PI rates in different broiler groups supplemented with clay-filtered water. A) FCR and PI rates overall means concerning different broiler groups. B) FCR and PI rates overall mean concerning broilers’ age (week).
Fig. 3. FI (g) and WI (ml) in different broiler groups supplemented with clay-filtered water. A) FI (g) and WI (ml) overall means concerning different broiler groups. B) FI (g) and WI (ml) overall means concerning broilers’ age (week).
The highest significant (p < 0.01) liver weights (Table 2) were recorded in G4 (diazinon) and G1 (lead nitrate) with no significant differences between the two groups. The highest significant (p < 0.01) heart weights were recorded in G5 (Salmonella Typhimurium) and G4 (diazinon) with no significant differences between the two groups. The highest significant (p < 0.01) spleen weights were recorded in the six groups supplied with clay-filtered water, with no significant differences between the six groups. The highest significant (p < 0.01) bursa of Fabricius weights were recorded in G5 (Salmonella Typhimurium) and G6 (E. tenella) with no significant differences between the two groups.
Biochemical profile
TP revealed in Table 3 highly significant (p < 0.01) decreases in all broiler groups supplied with clay-filtered water compared to the control group with no significant differences between G4 (diazinon) and G5 (Salmonella Typhimurium) and between G5 and G6 (E. tenella). ALT revealed highly significant (p < 0.01) decreases in all broiler groups supplied with clay-filtered water compared to the control group (Table 2) with no significant differences between G5 (Salmonella Typhimurium) and G6 (E. tenella), and between G1 (lead nitrate), G2 (calcium sulfate), G3 (yeast extract 5%), and G4 (diazinon).
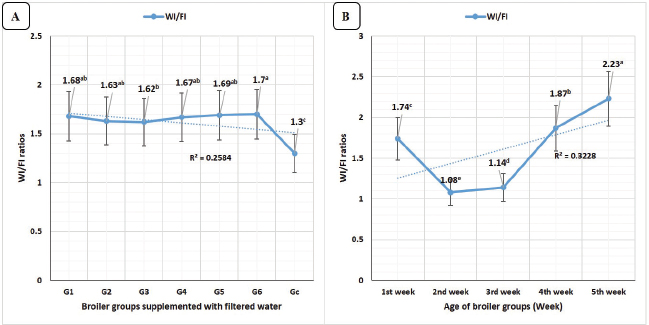
Fig. 4. FI/WI ratios in different broiler groups supplemented with clay-filtered water. A) FI/WI ratios overall means concerning different broiler groups. B) FI/WI ratios overall means concerning broilers’ age (week).
Table 2. Live, carcasses, and organs weights (mean ± SE) in different broiler groups supplied with clay-filtered water.
CREAT revealed highly significant (p < 0.01) decreases, as shown in Table 3, in all broiler groups supplied with clay-filtered water compared to the control group with no significant differences between G4 (diazinon), G5 (Salmonella Typhimurium), and G6 (E. tenella), and between G3 (yeast extract 5) and G5 (Salmonella Typhimurium). GLUCO revealed in Table 3 highly significant (p < 0.01) decreases in all broiler groups supplied with clay-filtered water compared to the control group with no significant differences between the six groups supplied with clay-filtered water.
Table 3. The biochemical profile (mean ± SE) in different broiler groups supplied with clay-filtered water.
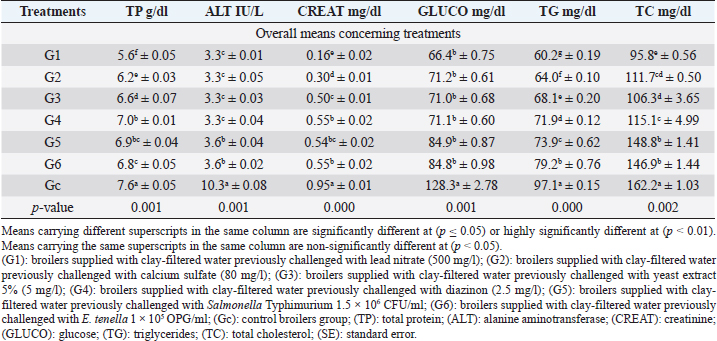
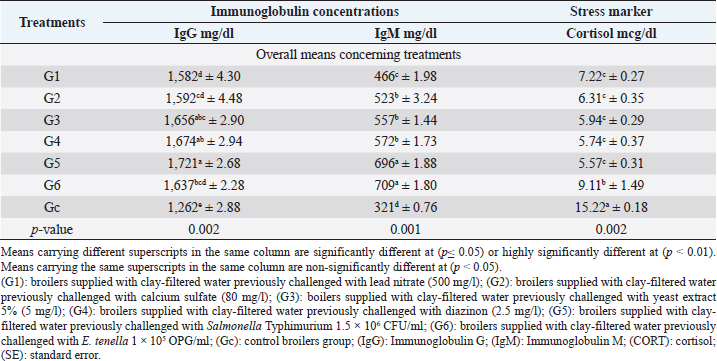
Table 4. Immunoglobulin concentrations and CORT levels (mean ±SE) in different broiler groups supplied with clay-filtered water.
TG revealed in Table 3 highly significant (p < 0.01) decreases in all broiler groups supplied with clay-filtered water compared to the control group. TC (Table 3) revealed significant (p < 0.01) decreases in all broiler groups supplied with clay-filtered water compared to the control group with no significant differences between G5 (Salmonella Typhimurium) and G6 (E. tenella), G2 (calcium sulfate), and G4 (diazinon), and G2 and G3 (yeast extract 5%).
Stress marker and immunoglobulin concentrations
Cortisol levels revealed in Table 4 highly significant (p < 0.01) declines in all broiler groups supplied with clay-filtered water compared to the control group with no significant differences between G1 (lead nitrate), G2 (calcium sulfate), G3 (yeast extract 5%), G4 (diazinon), and G5 (Salmonella Typhimurium).
Immunoglobulin G and M (Table 4) revealed highly significant (p < 0.01) increases in all groups supplied with clay-filtered water compared with the control group. The highest significant (p < 0.01, Table 4) IgG level was recorded in G5 (Salmonella Typhimurium), while the highest significant (p < 0.01 Table 4) IgM level was recorded in G5 (Salmonella Typhimurium) and G6 (E. tenella) with no significant differences between the two groups.
Table 5. Antioxidants levels (Mean ±SE) in different broiler groups were supplied with clay-filtered water.
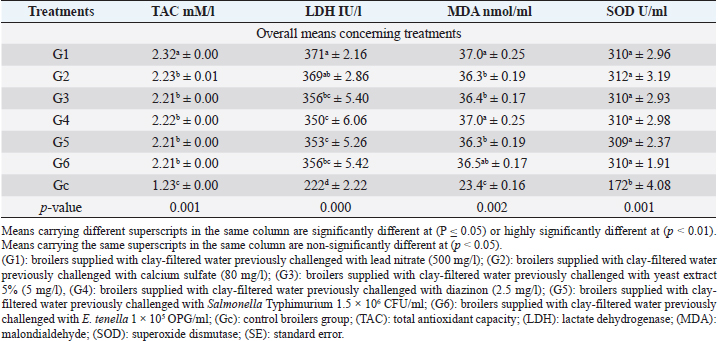
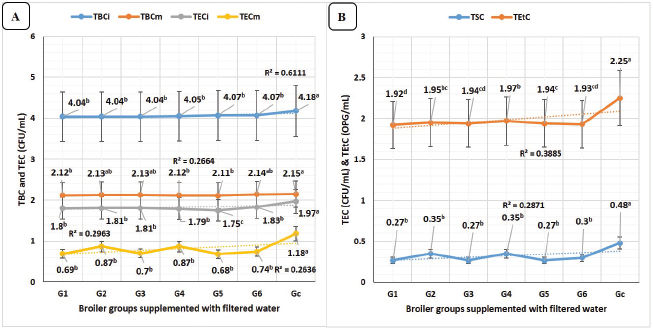
Fig. 5. Microbial counts in different broiler groups supplemented with clay-filtered water. A) TBC in intestinal swabs (TBCi) and breast muscles (TBCm), and TEC in intestinal swabs (TECi) and breast muscles (TECm) overall means (CFU/ml) concerning different broiler groups. B) Total E. tenella count (TEtC) overall means (OPG/ml) and total Salmonella Typhimurium count in intestinal swabs (TSC) overall means (CFU/ml) concerning different broiler groups.
Antioxidant levels
Antioxidant sera levels revealed in Table 5, highly significant (p < 0.01) increases in all groups supplied with clay-filtered water compared to the control group. TAC, LDH, MDA, SOD enzymes revealed the highest significant (p < 0.01, Table 5) values in G1 (lead nitrate), G1 (lead nitrate) with no significant difference with G2 (calcium sulfate), G1 (lead nitrate) with no significant differences with G4 (diazinon), and G6 (E. tenella), and G1 (lead nitrate) with no significant differences with all other groups supplied with clay-filtered water, respectively.
The microbial load of intestinal swabs and breast muscles
TBC of intestinal swabs revealed in Figure 5A, highly significant (p < 0.01) declines in all groups supplied with clay-filtered water compared to the control group with no significant differences between the six groups. TBC of breast muscles revealed in Figure 5A, highly significant (p < 0.01) decreases in all groups supplied with clay-filtered water compared to the control group with no significant differences between G7 (control group) and G6 (E. tenella), G2 (calcium sulfate), and G3 (yeast extract 5%).
TEC of intestinal swabs revealed in Figure 5A, highly significant (p < 0.01) declines in all groups supplied with clay-filtered water compared to the control group with no significant differences between G6 (E. tenella), G2 (calcium sulfate), G3 (yeast extract), G1 (lead nitrate), and G4 (diazinon), respectively. TEC of breast muscles revealed in Figure 5A, highly significant (p < 0.01) declines in all groups supplied with clay-filtered water compared to the control group with no significant differences between the six groups.
TSCs of intestinal swabs revealed in Figure 5B, highly significant (p < 0.01) declines in all groups supplied with clay-filtered water compared to the control group with no significant differences between the six supplied groups. Eimeria tenella counts of the fecal samples revealed in Figure 5B, highly significant (p < 0.01) decreases in all supplied groups compared to the control group with no significant differences between G4 (diazinon) and G2 (calcium sulfate), between G2, G5 (Salmonella Typhimurium), G3 (yeast extract 5%), G6 (E. tenella), and G1 (lead nitrate).
Discussion
Adsorption efficiency of clay-filtered water
Water as a natural element of life can be exposed daily to a large number of overwhelming challenges that might contribute to infectious diseases in poultry farms, especially when these farms depend on water from surface sources that might be exposed to pollution or contamination from numerous sources. Clay is a natural product that resulted from the weathering action, and upon chemical analysis, it’s found to be composed of minerals like smectite, kaolinite, chlorite, illite, and halloysite (Erdoğan, 2015). Clay as a natural product is known for its wide availability (Xing et al., 2017) and low toxic action to the environment (Xiang et al., 2020).
Water pollution resulted from the industrial effluent usually contained high levels of heavy metals, including copper, iron, zinc, cadmium, chromium, arsenic, thallium, and aluminum. These heavy metals usually presented life threats to broilers when polluted water served as drinking one (Li et al., 2015; Bel Hadjltaief et al., 2019). The current results revealed a significant reduction of lead nitrate (500 mg/l) in the clay-filtered water. The high affinity of clay to adsorb lead (Pb2+) was attributed to the ability of clay to act as a catalyst (photo-Fenton) via the oxidation abilities of the iron content and the production of the hydroxyl group. The results were consistent with those of Sundaram and Dharmalingam (2018), who used clay in removing chromium ions (Cr4+) via adsorption up to 113 mg from an aqueous system per gram of the clay used in the experiment. Wang et al. (2016) reported that clay was able to adsorb and significantly reduce the levels of cobber (Cu2+) up to 96.0% and lead (Pb2+) up to 99.5% at 6.5 pH. Kanchana et al. (2012) also recorded that clay-modified materials could adsorb lead (Pb2+) up to 85% with a dose of 7–8 g at an optimum pH of 6.0. Also, Msaadi et al. (2017) modified clay via photo-polymerization and exposed it to lead (Pb2+) in water, which contributed to the adsorption of 301 mg lead/g of the modified structure.
The current results retrieved high binding affinity and a significant reduction in the clay-filtered water analysis of the calcium salt (CaCO3), contributing to water hardness. The reduction action can be attributed to clay’s hydrophilic nature, that when comes in contact with the water surface, produced high ion-exchange properties and attracted cations as calcium (Ca2+) and sodium (Na+) from the water. The results were compatible with those reported by Shayesteh et al. (2016) and Dutta and Singh (2015), who revealed that clay could enhance water quality by increasing positive charges, polar-nonpolar method, and packing of pollutants contributing high binding affinity to the other cations as calcium compounds that might contribute to water hardness. Lijalem (2015) reported the high affinity of clay to adsorb calcium and magnesium salts that might contribute to water hardness. Aveen and Kafia (2014) reported the ability of clay to produce cations exchange with the release of the hydroxyl group, thus reducing the levels of insoluble salts contributing hardness.
Water and wastewater might be polluted with high levels of organic materials that have a common character as non-biodegradable elements and alter the water physicochemical characteristics and render the water unfit for consumption. These organic materials like phenol, human and animal excreta, pesticides, dyes, and organic acids. The clay-filtered water analysis in the current study showed that natural clays filters exhibited high adsorption capabilities against organic materials used in the experiment like yeast extract 5% (5 mg/l) and diazinon (2.5 ml/l). The adsorption affinity of clay to heavy metals and organic materials was attributed to the presence of the hydrophilic affinity of clay. Bentahar et al. (2017) reported the high affinity of clay to adsorb heavy metals like chromium, lead, and dyes. This might be attributed to the increased surface area, small particle size, and molecular stability. Also, the results were consistent with those reported by Wang et al. (2020), Shahadat et al. (2018), and Ghorbel-Abid and Trabelsi-Ayadi (2015), who reported the high affinity of clay to adsorb efficiently many organic pollutants at optimized pH. The higher the affinity of adsorption, the molecules start to shift the charges from the positive to the negative for the presence of the pollutant on the clay surface, indicating the product’s satisfaction.
Antimicrobial actions of clay-filtered water
Clay has been known in ancient history as an antimicrobial agent, as well as in the treatment of many symptoms like tape and hookworm infestation, abscesses, diarrhea, inflammation, and wound healing processes (Sinha Ray and Okamoto, 2003). The current study revealed strong antibacterial actions of clay against Salmonella Typhimurium, as well as antiprotozoal action against E. tenella in the water samples and broiler’s tissue, thus reduced the total and selective bacterial counts. The antimicrobial actions of natural clay were attributed to the chemical composition of clay that constitutes iron (Fe2+) and phosphorus (P) ions. Iron ions increased in the intracellular fluid after its oxidation into ferric form (Fe3+) and produce hydroxyl groups that interfere with the normal cell membrane functions, while phosphorus (P) ions interfere with the structure of the triple phospholipid of the cell membrane and the cell permeability contributing the inability of the cell to reproduce and finally death. The results were consistent with Williams et al. (2011), who reported the contribution of clay in shifting in the pH and oxidation processes contributing to the deaths of E. coli. Also, Behroozian et al. (2016) revealed that natural clay exhibited bactericidal actions against some gram-positive bacteria like Staphylococcus aureus and Acinetobacter baumannii, and some gram-negative bacteria like Enterococcus faecium, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Otto and Haydel (2013) recorded that the high zinc, copper, and iron concentrations in clay particles exhibited high antimicrobial actions against Escherichia coli and Staphylococcus aureus.
Waterborne protozoan has been categorized as the cause of many infectious diseases in animal and poultry farms and human communities (Efstratiou et al., 2017). Eimeria oocyst could be washed away from infected farms or litter using the farm’s water resources, exposing the other nearby farms to the coccidiosis if they shared a common water supply (Karanis et al., 2007). The current study recorded a significant reduction in E. tenella oocyst count in clay-filtered water and collected fecal samples. The significant reduction might be attributed to the shifting of pH produced by clay (6.5), modifying the water to unfavorable media for the survival of Eimeria oocyst, as well as clay might interfere with the sporocyst wall structure via the production of the hydroxyl group that destroys their walls, and thus the reduction in their count. The results might be consistent with those recorded by Cotruva et al. (2004), who revealed that filtration is the most effective water treatment procedure for protozoan oocysts. Omarova et al. (2018) and Shields et al. (2015) reported that different filtration methods could remove all the protozoans from water sources. Since the removal of protozoan oocyst from water depends mainly on management factors, disinfection to some extent, and strict hygienic measures (Betancourt and Rose, 2004), so the proposal of using a green clay filter according to the current results might provide a solution not only for the control of E. tenella in broiler farms but also for the control of a large number of zoonotic protozoan that might enter in the water resources and contribute to diseases (Graham and Polizzotto, 2013).
Broiler’s performance and carcasses characteristics
Clay (Kaolin or bentonite) has been used as a dietary supplement for broilers and laying hens for many years. The aim of using clay as a dietary supplement was to improve digestion and nutrients absorption and reduce the absorption of toxic substances that might contribute to intestinal injuries (Safaeikatouli et al., 2011).
The current study revealed significant improvements in weight gains, performance indices, WIs, water/FI ratios, as well as significant increases in the live body, carcasses, edible organs (liver and heart), and immune organs (spleen and bursa of Fabricius) weights in broiler groups supplemented with clay-filtered water. These actions might be attributed to the influence of clay to increase nutrients absorption from the intestine, improving intestinal mucosa conditions, increase nutrients bioavailability in blood, and enhance protein utilization, causing increases to feed conversions and muscle mass, and thus the final weights. The results were consistent with those of Ouachem et al. (2015), who reported that using clay as a feed supplement contributed to improve nutrient digestibility, live weights, weight gains, and feed conversions by increasing the feed retention time and improve the hygienic status of the digestive tract in poultry.
Owen et al. (2012) also recorded significant improvement in FI and efficiency and on growth performance of 120 one-day-old Hubbard broilers supplemented with 10, 20, and 30 g clay (Kaolin)/kg basal diet. They also recorded significant differences in liver, spleen, heart, kidney, and gall bladder weights of the supplemented broilers compared to the control group. Khanedar et al. (2012) experimented with sodium bentonite (1% and 1.5%) and calcium bentonite (1% and 1.5%) in 260 one-day-old male Ross®308 broilers. They recorded that using 1% bentonite was able to improve carcass characteristics and tibia ash.
Biochemical, antioxidant, immune, and hormonal profiles
The ion exchange capabilities of clay contributed to improved membrane actions and altered enzymatic activities. These alterations in enzymatic activities control the humeral white blood cells' activity against some pathogenic micro-organisms and increase their lytic enzymes secretions that improve the biochemical and immune profiles (Lemos et al., 2015).
The recorded results revealed significant improvement of immunoglobulin G and M, TAC, LDH, MDA, and SOD levels, as well as maintaining the levels of TP, ALT, CREAT, GLUCO , TG, TC, and CORT in all broiler groups supplemented with clay-filtered water compared to the control group. Clay structure revealed the presence of numerous anions and cations that produce ionic exchange actions and participate in the cell excitation conditions, as well as enhanced the net weight of some immune organs like the spleen and bursa of Fabricius. Thus, clay can activate the proliferation of B-lymphocytes for the production of immunoglobulin.
The results were in agreement with those of Bhatti et al. (2016), who recorded partial ameliorations in serum TP, albumin, and CREAT, as well as complete and significant ameliorations in ALT enzyme and urea concentrations in broilers supplemented with 3.7 and 7.5 g clay (bentonite) /kg ration. Indresh et al. (2013) reported a highly protective influence of high-grade bentonite in broiler chickens, as well as they recorded significant improvements in broiler’s immunity. Gul et al. (2016) and Saçakli et al. (2015) reported significantly improved protein utilization, maintained antioxidant levels, significantly increased intestinal localized patches and morphology, and reduced egg deformities in 45 white leghorn chickens supplemented with 1.5% clay bentonite. Solis-Cruz et al. (2019) reported significant improvement of immunoglobulin levels in 150 one-day-old male broiler chicks supplemented with clay bentonite and cellulosic polymers.
Conclusion
Clay filters in the current study revealed high efficiency to purify polluted water with high adsorption power to neutralize the influence of the challenging pollutants as lead nitrate (500 mg/l), calcium sulfate (80 mg/l), yeast extract 5% (5 mg/l), and diazinon (2.5 ml/l). Clay filters also revealed high efficiency to purify contaminated water with high antibacterial actions against Salmonella Typhimurium (1.5 × 106 CFU/ml) and antiprotozoal action against E. tenella (1 × 105 OPG/ml).
Clay filters that purify/treat polluted and contaminated water were able to optimize water quality compared to the tap water, and thus enhanced the performance, CWs, immune and edible organs’ weights, some biochemical parameters, bacterial load of breast muscle and intestine, and intestinal Salmonella and E. tenella count.
Clay as a natural product can be considered a green compound used in the treatment and purification of water and wastewater without any drawbacks on the surrounding environment. Therefore, green eco-friendly natural clay was advantageous by low-cost material, eco-friendly efficient adsorbent, and have the ability to remove a variety of contaminants/pollutants such as inorganic, organic, and pathogenic from drinking water and its sources.
Acknowledgments
Sincere and honorable thanking should be provided to Prof. MA Sobieh for his guidance and help in polymerizing the work design, and to Dr. OF Mohamed for her help in the bacteriological examination of intestinal swabs and breast muscles. The current study received no grant from any funding agency in the public and commercial fields and not-for-profit sectors. The authors funded the study.
Conflict of interest
The authors declare that they have no financial or personal conflicts which may have inappropriately influenced them in writing this manuscript.
Authors’ contributions
ESS designed the experimental design, executed the experiment, conducted water samples analysis, Eimeria count, and bacteriological examination. He also supervised the sera samples analysis and took a part in writing the manuscript. RHA participated in the estimation and calculation of performance indices during the experiment, carcass quality determination, and took a part in writing the manuscript. DSF participated in the water samples analysis, bacteriological examination, and took a part in writing the manuscript.
References
Ahmad, T. and Danish, M. 2018. Prospects of banana waste utilization in wastewater treatment: a review. J. Environ. Manage. 206, 330–348.
Ahmed, M.J. 2017. Adsorption of quinolone, tetracycline, and penicillin antibiotics from aqueous solution using activated carbons: a review. Environ. Toxicol. Pharmacol. 50, 1–10.
American Public Health Association, American Water Works Association, Water Environment Federation. 2012. Standard methods for the examination of water and wastewater, 22nd ed. Washington, DC.: American Water Work Association Publications.
American Public Health Association, American Water Works Association, Water Environment Federation. 2017. Standard methods for the examination of water and wastewater, 23rd ed. InEds., Rice, E.W., Baird, R.B. and Eaton, A.D. Washington, DC: American Water Work Association Publications.
Anastopoulos, I., Karamesouti, M., Mitropoulos, A.C. and Kyzas, G.Z. 2017. A review for coffee adsorbents. J. Mol. Liq. 229, 555–565.
Applegate, T.J. and Angel, R. 2014. Nutrient requirements of poultry publication: history and need for an update. Appl. Poult. Res. 23(3), 567–575.
Aveen, H.M. and Kafia, M.S. 2014. Kinetics of cation exchange capacity of homoionic sodium from NaY zeolite. Inter. J. Inno. Res. in Sci., Eng. Technol. 3(6), 13137–13137.
Behroozian, S., Svensson, S.L. and Davies, J. 2016. Kisameet clay exhibits potent antibacterial activity against the ESKAPE pathogens. mBio. 7(1), e01842-15.
Bel Hadjltaief, H., Galvez, M.E., Ben Zina, M. and Da Costa, P. 2019. TiO2/clay as a heterogeneous catalyst in photocatalytic/photochemical oxidation of anionic reactive blue 19. Arabian. J. Chem. 12(7), 1454–1462.
Bentahar, S., Dbik, A., El Khomri, M., El Messaoudi, N. and Lacherai, A. 2017. Adsorption of methylene blue, crystal violet and congo red from binary andternary systems with natural clay: kinetic, isotherm, and thermodynamic. J. Environ. Chem. Eng. 5, 5921–5932.
Betancourt, W.Q. and Rose J.B. 2004. Drinking water treatment processes for removal of Cryptosporidium and Giardia. Vet. Parasitol. 126, 219–234.
Bhatti, S.A., Khan, M.Z., Saleemi, M.K. and Saqib, M. 2016. Aflatoxicosis and ochratoxicosis in broiler chicks and their amelioration with locally available bentoinite clay. Pak. Vete. J. 36(1), 68–72.
Charan, J. and Biswas, T. 2013. How to calculate sample size for different study designs in medical research? Indian. J. Psychol. Med. 35(2), 121–126.
Cotruva, J.A., Durfour, A., Rees, G., Bartram, J., Carr, R., Cliver, D.O., Craun, G.F., Fayer, R. and Gannon, V.P.J. 2004. Waterborne zoonoses: identification causes and control. World Health Organization. London, UK: IWA Publishing, pp: 255–282.
Dutta, A. and Singh, N. 2015. Surfactant-modified bentonite clays: preparation, characterization, and atrazine removal. Environ. Sci. Pollut. Res. 22, 3876–3885.
Efstratiou, A., Ongerth, J.E. and Karanis, P. 2017. Waterborne transmission of protozoan parasites: Review of worldwide outbreaks - an update 2011-2016. Water. Res. 11, 14–22.
Erdoğan, Y. 2015. Physicochemical properties of handere clays and their use as a building material. J. Chem. 2015, 6, article ID 374245; doi:10.1155/2015/374245.
Foo, K.Y. and Hameed, B.H. 2011. The environmental applications of activated carbon/zeolite composite materials. Adv. Coll. Interface. Sci. 162, 22–28.
Fritz, B.G., Walker, D.K., Goveida, D.E., Parker, A.E. and Goeres, D.M. 2015. Evaluation of petrifilmTM aerobic count plates as an equivalent alternative to drop plating on R2A agar plates in a biofilm disinfectant efficacy test. Curr. Microbiol. 70, 450–456.
Fu, F. and Wang, Q. 2011. Removal of heavy metal ions from wastewaters: a review. J. Environ. Manage. 92(3), 407–418.
Ghoneim, N.H., Hassanain, M.A., Hamza, D.A., Shaapan, R.M. and Draz, S.H. 2017. Prevalence and molecular epidemiology of cryptosporidium infection in calves and hospitalized children in Egypt. Res. J. Parasitol. 12, 19–26.
Ghorbel-Abid, I. and Trabelsi-Ayadi, M. 2015. Competitive adsorption of heavy metals on local landfill clay. Rev. Arab. J. Chem. 8, 25–31.
Graham, J.P. and Polizzotto, M.L. 2013. Pit latrines and their impacts on groundwater quality: a systematic review. Environ. Health. Perspect. 121, 521–530.
Gul, H., Khan, S., Shah, Z., Ahmed S., Israr, M. and Hussain, M. 2016. Effects of local sodium bentonite as aflatoxins binder and its effects on production performance of laying hens. Kafkas Üniv. Vet. Fak. Derg. 23(1), 31–37.
Han, H., Rafiq, M.K., Zhou, T., Xu, R., Mašek, O. and Li, X. 2019. A critical review of clay-based composites with enhanced adsorption performance for metal and organic pollutants. J. Hazard. Mater. 369, 780–796.
Indresh, H.C., Devegowda, G., Ruban, S.W. and Shivakumar, M.C. 2013. Effects of high-grade bentonite on performance, organ weights and serum biochemistry during aflatoxicosis in broilers. Vet. World. 6(6), 313–317.
Kanchana, V., Gomathi, T., Geetha, V. and Sudha, P.N. 2012. Adsorption analysis of Pb(II) by nanocomposites of chitosan with methyl cellulose and clay. Pharm. Lett. 4, 1071–1079.
Karanis, P., Kourenti, C. and Smith H. 2007. Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J. Water. Health. 5, 1–38.
Khanedar, F., Vakili, R. and Zakizadeh, S. 2012. Effects of two kinds of bentonite on the performance, blood biochemical parameters, carcass characteristics and tibia ash of broiler chicks. Iran. J. Appl. Anim. Sci. 3(3), 577–581.
Kim, S.K. and Lee, J.H. 2016. Biofilm modeling systems. Korean. J. Microbiol. 52(2), 125–139.
Kumararaja, P., Manjaiah, K.M., Datta, S.C., Ahammed Shabeer, T.P. and Sarkar, B. 2018. Chitosan-g-poly (acrylic acid)-bentonite composite: a potential immobilizing agent of heavy metals in soil. Cellulose. 25, 3985–3999.
Lemos, M.J.D, Calixto, L.F.L., Alves, O.D.S., Souza, D.S.D., Moura, B.B. and Reis, T.L. 2015. Kaolin in the diet and its effects on performance, litter moisture and intestinal morphology of broiler chickens. Cienc. Rural. 45(10), 1835–1840.
Li, G., Duan, H., Wang, X., Meng, X. and Qin, D. 2015. Fabrication of porous resins via solubility differences for adsorption of cadmium (II). Chem. Eng. J. 262, 250–259.
Lijalem, A.J. 2015. Synthesis of zeolite a from ethiopian kaolin. Microporous and mesoporous materials. Thesis, Chemistry Department, Addis Ababa University, Addis Ababa, Ethiopia, pp: 29–30.
Mohamed, O.F., Hussein, M.M. and Soliman, E.S. 2020. In-vitro antimicrobial activity and adsorption adequacy of natural clay against some air and water pollutants. Adv. Anim. Vet. Sci. 8(12), 1367–1379.
Msaadi, R., Ammar, S., Chehimi, M.M. and Yagci, Y. 2017. Diazonium-based ion-imprinted polymer/clay nanocomposite for the selective extraction of lead (II) ions in aqueous media. Eur. Polym. J. 89, 367–380.
Mukhopadhyay, R., Bhaduri, D., Sarkar, B., Rusmin, R., Hou, D., Khanam, R., Sarkar, S., Biswas, J.K., Vithanage, M., Bhatnagar, A. and Ok, Y.S. 2020. Clay–polymer nanocomposites: progress and challenges for use in sustainable water treatment. J. Hazardous. Mater. 383, 121125.
Mukhopadhyay, R., Manjaiah, K.M., Datta, S.C., Yadav, R.K. and Sarkar, B. 2017. Inorganically modified clay minerals: preparation, characterization, and arsenic adsorption in contaminated water and soil. Appl. Clay. Sci. 147, 1–10.
Murray, P.R., Rosenthal, K.S. and Pfaller, M.A. 2015. Medical microbiology, 8th ed. Philadelphia, PA: Elsevier Health Sciences.
National Research Council (NRC). 1994. Nutrient requirements for poultry, 9th ed. New York, NY: National Research Council.
Olu-Owolabi, B.I., Alabi, A.H., Diagboya, P.N., Unuabonah, E.I. and Düring, R.A. 2017. Adsorptive removal of 2, 4, 6-trichlorophenol in aqueous solution using calcined kaolinite-biomass composites. J. Environ. Manage. 192, 94–99.
Omarova, A., Tusupova, K., Berndtsson, R., Kalishey, M. and Sharapatova, K. 2018. Protozoan parasites in drinking water: a system approach for improved water, sanitation and hygiene in developing countries. Int. J. Environ Res. Public. Health. 15(3), 495.
Otto, C.C. and Haydel, S.E. 2013. Exchangeable ions are responsible for the in vitro antibacterial properties of natural clay mixtures. PLoS. One. 8(5), e64068; doi:10.1371/journal.pone.0064068.
Ouachem, D., Kaboul, N., Meredef, A., Abdessemed, F. and Ahmed Gaid, Z. 2015. Effects of clay on performance, moisture of droppings and health status of poultry: an overview. World. Poult. Sci. J. 71(1), 184–189.
Owen, O.J., Nodu, M.B., Dike, U.A. and Ideozu, H.M. 2012. The effects of dietary kaolin (clay) as feed additive on the growth performance of broiler chickens. Greener. J. Agric. Sci. 2(6), 233–236.
Palansooriya, K.N., Yang, Y., Tsang, Y.F., Sarkar, B., Hou, D., Cao, X., Meers, E., Rinklebe, J., Kim, K.H. and Ok, Y.S. 2020. Occurrence of contaminants in drinking water sources and the potential of biochar for water quality improvement: a review. Crit. Rev. Environ. Sci. Technol. 50(6), 549–611.
Premarathna, K.S.D., Rajapaksha, A.U., Sarkar, B., Kwon, E.E., Bhatnagar, A., Ok, Y.S. and Vithanage, M. 2019. Biochar-based engineered composites for sorptive decontamination of water: a review. Chem. Eng. J. 372, 536–550.
Raval, N.P., Shah, P.U. and Shah, N.K. 2016. Adsorptive removal of nickel (II) ions from aqueous environment: a review. J. Environ. Manage. 179, 1–20.
Royal Society of Chemistry. 2007. Sustainable water: chemical science priorities. London, UK: Royal Society of Chemistry, Summary Report, 29 p. RCN 207890.
Rusmin, R., Sarkar, B., Liu, Y., McClure, S. and Naidu, R. 2015. Structural evolution of chitosan–palygorskite composites and removal of aqueous lead by composite beads. Appl. Surf. Sci. 353, 363–375.
Saçakli, P., Calik A., Bayraktaroğlu, A.G., Ergün, A., Şahan, Ö. and Özaydin, S. 2015. Effect of clinoptilolite and/or phytase on broiler growth performance, carcass characteristics, intestinal histomorphology and tibia calcium and phosphorus levels. Kafkas. Üniv. Vet. Fak. Der. 21(5), 729–737.
Safaeikatouli, M., Jafariahangari, Y. and Baharlouei, A. 2011. An evaluation on the effects of dietary kaolin and zeolite on broilers blood parameters, T4, TSH and growth hormones. Pak. J. Nutri. 10(3), 233–237.
Sangster, L., Blake, D.P., Robinson, G., Hopkins, T.C., Sa, R.C.C., Cunningham, A.A., Chalmers, R.M. and Lawson, B. 2016. Detection and molecular characterization of Cryptosporidium parvum in British European hedgehogs (Erinaceus europaeus). Vet. Parasitol. 217, 39–44.
Shahadat, M. and Isamil, S. 2018. Regeneration performance of clay-based adsorbents for the removal of industrial dyes: a review. RSC Adv. 8, 24571–24587.
Sarkar, B., Rusmin, R., Ugochukwu, U.C., Mukhopadhyay, R. and Manjaiah, K.M. 2019. Modified clay minerals for environmental application. In Modified clay and zeolite nanocomposite materials, environmental and pharmaceutical applications, micro and nano technologies. Eds., Mercurio, M., Sarkar, B. and Langella, A. pp: 113–127.
Shakoor, M.B., Niazi, N.K., Bibi, I., Shahid, M., Sharif, F., Bashir, S., Shaheen, S.M., Wang, H, Tsang, D.C.W., Ok, Y.S. and Rinklebe, J. 2018. Arsenic removal by natural and chemically modified water melon rind in aqueous solutions and groundwater. Sci. Total. Environ. 645, 1444–1455.
Shayesteh, H., Rahbar-Kelishami, A. and Norouzbeigi, R. 2016. Evaluation of natural and cationic surfactant modified pumice for congo red removal in batch mode: kinetic, equilibrium, and thermodynamic studies. J. Mol. Liq. 221, 1–11.
Shields, K.F., Bain, R.E., Cronk, R., Wright, J.A. and Bartram J. 2015. Association of supply type with fecal contamination of source water and household stored drinking water in developing countries: a bivariate meta-analysis. Environ. Health. Perspect. 123, 1222–1231.
Singh, N.B., Nagpal, G., Agrawal, S. and Rachna. 2018. Water purification by using adsorbents: a review. Environ. Technol. Innov. 11, 187–240.
Sinha Ray, S. and Okamoto, M. 2003. Polymer/layered silicate nanocomposites: a review from preparation to processing. Prog. Polym. Sci. 28, 1539–1641.
Soliman, E.S. and Abdallah, M.S. 2020. Assessment of biosecurity measures in broiler’s farms in the Suez Canal area – Egypt using a seasonal prevalence of Salmonellosis. Vet. World. 13(4), 622–632.
Soliman, E.S. and Hassan, R.A. 2019. Impact of lighting color and duration on productive performance and ewcastle disease vaccination efficiency in broiler chickens. Vet. World. 12(7), 1052–1059.
Soliman, E.S. and Hassan, R.A. 2020. Influence of housing floor on air quality, growth traits, and immunity in broiler chicken farms. Adv. Anim. Vet. Sci. 8(9), 997–1008.
Soliman, E.S. and Hassan, R.A., 2017. Evaluation of superphosphate and meta-bisulfide efficiency in litter treatment on productive performance and immunity of broilers exposed to ammonia stress. Adv. Anim. Vet. Sci. 5(6), 253–259.
Soliman, E.S., Ali, A.A. and Gafaar, R.E.M. 2021. Impact of heating systems on air and litter quality in broiler houses, performance, behavior, and immunity in broiler chickens. Adv. Anim. Vet. Sci. 9(2), 301–314.
Soliman, E.S., Hamad, R.T. and Ahmed, A. 2017. Prophylactic and immune modulatory influences of Nigella sativa Linn. in broilers exposed to biological challenge. Vet. World. 10(12), 1447–1455.
Soliman, E.S., Moawed, S.A. and Ziaan, A.M.G. 2016. Assessing cleaning and disinfection regime in a slaughterhouse against carcasses contamination. Adv. Anim. Vet. Sci. 4(9), 449–457.
Soliman, E.S., Sallam, N.H. and Abouelhassan, E.M. 2018. Effectiveness of poultry litter amendments on bacterial survival and Eimeria oocyst sporulation. Vet. World. 11(8), 1064–1073.
Solis-Cruz, B., Hernandez-Patlan, D., Petrone, V.M., Pontin, K.P., Latorre, J.D., Beyssac, E., Hernandez-Velasco, X., Merino-Guzman, R., Owens, C., Hargis, B.M., Lopez-Arellano, R. and Tellez-Isaias, G. 2019. Evaluation of cellulosic polymers and curcumin to reduce aflatoxin B1 toxic effects on performance, biochemical, and immunological parameters of broiler chickens. Toxins. 11(2), 121.
SPSS. 2016. Statistical packages of social sciences. Version 21 for windows. Chicago, Illinois: SPSS. Inc.
Sundaram, E.J.S. and Dharmalingam, P. 2018. Synthesis and characterization of magnetized clay polymer nanocomposites and its adsorptive behaviour in removal of Cr(VI) from aqueous phase. Asian J. Chem. 30, 667–672.
Syafalni, S., Singh, S.R.B. and Zawawi, M.H. 2014. Sorption of dye wastewater by using natural zeolite, anionic-cationic surfactant modified zeolite and cationic surfactant modified zeolite. World. Appl. Sci. J. 32 (5), 818–824.
Szczepanik, B. 2017. Photocatalytic degradation of organic contaminants over clay-TiO2 nanocomposites: a review. Appl. Clay. Sci. 141, 227–239.
Thrusfield, M. and Christley, R. 2018. Veterinary epidemiology, 4th ed. London, UK: Blackwell Science Ltd, pp: 214–256.
Unuabonah, E.I., Ugwuja, C.G., Omorogie, M.O., Adewuyi, A. and Oladoja, N.A. 2018. Clays for efficient disinfection of bacteria in water. Appl. Clay. Sci. 151, 211–223.
Wang, W., Wang, J., Zhao, Y., Bai, H., Huang, M., Zhang, T. and Song, S. 2020. High-performance two-dimensional montmorillonite supported-poly (acrylamide-co-acrylic acid) hydrogel for dye removal. Environ. Poll. 257, 113574.
Wang, Y.M., Duan, L., Sun, Y., Hu, N., Gao, J.Y., Wang, H. and Xie, X.M. 2016. Adsorptive removal of Cr (VI) from aqueous solutions with an effective adsorbent: cross-linked chitosan/montmorillonite nanocomposites in the presence of hydroxy-aluminum oligomeric cations. Desalin. Water. Treat. 57, 10767–10775.
Williams, L.B., Metge, D.W., Eberl, D.D., Harvey, R.W., Turner, A.G., Prapaipong, P. and Poret-Peterson, A.T. 2011. What makes a natural clay antibacterial? Environ. Sci. Technol. 45(8), 3768–3773.
Wu, Y.N., Yan, F.F., Hu, J.Y., Chen, H., Tucker, C.M., Green, A.R. and Cheng, H.W. 2017. The effect of chronic ammonia exposure on acute-phase proteins, immunoglobulin, and cytokines in laying hens. Poult. Sci. 96(6), 1524–1530.
Xiang, W., Zhang, X., Chen, J., Zou, W., He, F., Hu, X., Tsang, D.C.W., Ok, Y.S. and Gao, B. 2020. Biochar technology in wastewater treatment: a critical review. Chemosphere. 252, 126539.
Xing, Y., Xu, X., Gui, X., Cao, Y. and Xu, M. 2017. Effect of kaolinite and montmorillonite on fine coal flotation. Fuel. 195, 284–289.
Yadav, V.B., Gadi, R. and Kalra, S. 2019. Clay based nanocomposites for removal of heavy metals from water: a review. J. Environ. Manage. 232, 803–817.
Zajac, A.Z. and Conboy, G.A. 2012. Veterinary clinical parasitology, 8th ed. Hoboken, NJ: Wiley Blackwell, pp: 8–11.
Zhu, R., Chen, Q., Zhou, Q., Xi, Y., Zhu, J. and He, H. 2016. Adsorbents based on montmorillonite for contaminant removal from water: a review. Appl. Clay. Sci. 123, 239–258.


















