| Research Article | ||
Open Vet J. 2023; 13(5): 550-557 Open Veterinary Journal, (2023), Vol. 13(5): 550–557 Original Research Effect of age, season, and gender on bronchoalveolar lavage fluid cytology in camelsMohamed Ali Al-Ali and Turke Shawaf*Department of Clinical Sciences, College of Veterinary Medicine, King Faisal University, Al-Ahsa, Saudi Arabia *Corresponding Author: Turke Shawaf. Department of Clinical Sciences, College of Veterinary Medicine, King Faisal University, Al-Ahsa, Saudi Arabia. Email: tshawaf [at] kfu.edu.sa. Submitted: 26/12/2022 Accepted: 09/04/2023 Published: 08/05/2023 © 2023 Open Veterinary Journal
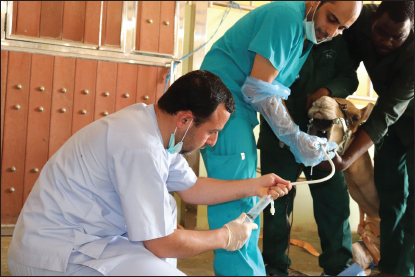
AbstractBackground: Bronchoalveolar lavage fluid (BALF) samples are valued mirrors of different parts of the airway and can be used with other approaches to the diagnosis of the lower respiratory tract. Several previous studies on various animal species showed the effect of the season, gender, and age on the percentage of cells in the BALF samples. Aim: The main aim of this study was to determine the impact of gender, age, and season on the cytological analysis in BALF of dromedary camels. Methods: Thirteen healthy camels were involved in this study. Camels were selected based on general respiratory clinical scoring. BALF was done using a special BALF catheter. BALF samples were analyzed from dromedary camels by microscopic examination of prepared smears. Results: The results of the BALF cytology percentage revealed that there was no variation between winter and summer in most cell types. Only the mean value of neutrophil cell percentage in BALF in winter increased significantly (10.75 ± 1.31) compared to summer (4.60 ± 0.81). The range of eosinophils was in summer (0–13) wider than in winter (0–2). A significant difference was recorded in lymphocytes, eosinophils, and epithelial cells percentage among adult and young camels. There was a high mean value of epithelial cells percentage in adult camels (10.17 ± 1.64) compared to young animals (3.0 ± 0.58). The results of the BALF cytology among males and camels showed no significant difference. Conclusion: The present study revealed significant differences in the BALF cytology regarding age and season, but no impact on gender. Keywords: Age, Airways, Bronchoalveolar, Camel, Season. IntroductionReference ranges of biochemical and cytological values, which are widely used in veterinary medicine, may differ according to season, age, sex, type of feeding, season, and environmental circumstances of the area (Hostetter et al., 2017; Shawaf et al., 2018; Davis and Sheats, 2019). The dromedary camel (Camelus dromedarius) belongs to the most important domestic animal species adapted to dry and hot regions in Asia and Africa (Kebedi, 2010). However, camel respiratory examination has received minor consideration, and more effort is still needed for the definitive etiology and identification of causative agents (Bekele, 1999; Kebedi, 2010). The early diagnosis of respiratory infections is a key factor for selecting the exact treatment to prevent disease progression and chronic respiratory disorders (Intisar et al., 2009, 2010a,b). Bronchoalveolar lavage fluid (BALF) is widely used as a source of specimens for cytological, microbiological, and immunological investigation of the respiratory tract (Couetil et al., 2007; Hoffman, 2008; Abutarbush et al., 2019). BALF samples are valued mirrors of different areas of the airway and can be used in combination with other approaches for the diagnosis of the deep respiratory tract (Malikides et al., 2003). BALF samples are also useful in giving insight into the severity and stage of inflammatory reactions in the lung and in the detection of subclinical pulmonary diseases (Van Driessche et al., 2019; Shawaf et al., 2021; Kang et al., 2022). Recently, two studies reported the percentage of cytological BALF in dromedary camels with and without respiratory diseases (Shawaf et al., 2021, 2022). However, several previous studies on different animal species indicated the effect of the season, gender, and age on the percentage of cells in the BALF samples (Pacheco et al., 2014; Secombe et al., 2015; Hostetter et al., 2017; Davis and Sheats, 2019). To our best knowledge, there is a lack of information about the impact of season, age, and gender on cytological analysis in apparently healthy dromedary camels. The aim of the present study was, therefore, to determine the impact of gender, age, and season on the cytological analysis in BALF of dromedary camels. Our hypothesis in this study was that the airway cells population might be varied among seasons, gender, and age in camels. Materials and MethodsAnimalsThirteen camels (Camelus dromedaries; five males and eight females) of ages ranged 1–14 years breeds were involved in this study. The camels were selected from animals maintained at the Camel Research Center, King Faisal University, Al-Ahsa, Saudi Arabia. The median age of camels was (3 ± 5.5). The cut-off age is up to two years for calf’s camel. The breed of studied animals was from the Magaheem breed. Camels were selected based on clinical scoring, physical and laboratory evaluation (no fever or signs of abnormal respiratory signs like cough, nasal discharge, dyspnea, or abnormal lung sounds, as well as normal white blood cell counts and biochemistry panel). All experimental procedures used in this study were approved by the Ethics Committee at King Faisal University, Saudi Arabia (Permission number DSR). Clinical examinationThe clinical history, general examination data, and special examination of the respiratory tract, including dyspnea, cough, nasal discharge, lung sounds, and rate of breathing for all animals, were registered. For clinical scoring of control, a scoring system designed for horses was adapted with some modifications. In addition, the animal’s rectal temperature was considered in the scoring procedure. Camels that showed respiratory symptoms or general pathological symptoms were excluded. Bronchoscopy and BALF collectionAfter clinical examination, camels were fixed for endoscopy in the sternal recumbency position. Sedation of xylazine (2%) (0.1 mg/kg bodyweight; Rompun, Bayer Health Care) was done before endoscopy and BALF sampling. A mouth gauge designed for camels was used to the expansion of the oral cavity during the insertion of the endoscope to protect it from the teeth and for easy entrance of the endoscope. Endoscopy of the lower respiratory tract was performed using a flexible 12 mm tip diameter bronchoscope 3.2 m long (EVIS Olympus, Vienna, OLYMPUS AUSTRIA GmbH) to ensure the absence of lower airways from mucous accumulation or congestion, showing respiratory disorders. The respiratory endoscope was also used to guide the BALF catheter to be inserted through the larynx, as described by (Shawaf et al., 2021). BALF catheter using a commercial tube (EQUIVET B.A.L. catheter 240 cm, KRUUSE, Denmark) was inserted in the lower airways via the oral cavity. The amount of 20– 40 ml of Lidocaine (1%) was infused via BALF catheter as local anesthesia into the deep airway to reduce coughing during the procedure. As soon as the BALF tube reached the part of the fluid injection, the cuff was then lightly inflated using 20 ml of air to stop the back flowing of infused fluid. The amount of 300 ml warm (approximately 37°C) sterile isotonic saline was placed using syringes (each of 100 ml) and was then installed via the BALF catheter. BALF was aspirated directly after injection and the fluid was instantly placed on ice and submitted to the laboratory within 20 minutes of collection. BALF was confirmed acceptable when they contained a surfactant foam layer. Cytological examination on BALF samples was done within 1 hour after sample collection. Cytological analysis of BALF samplesBALF samples were centrifuged at 1,500 rpm for 15 minutes for differential cell counting. After centrifugation, the supernatant was discarded from the samples. Cell pellets were used for smears, dried with room air, and then stained using Giemsa (Merck KGaA, Darmstadt, Germany). Using a magnification of 1,000 × and a counting protocol (De Brauwer et al., 2000; De Brauwer et al., 2002), the percentage of lymphocytes, macrophages, neutrophils, eosinophils, mast cells, and epithelial cells was calculated after counting 400 cells. Statistical analysisThe differences between the means were analyzed using Student’s t-test, for the comparison among two groups using the statistical software Graph Pad Prism 7. The normal distribution was assessed by D’Agostino & Pearson omnibus normality test. Ethical approvalThe experimental procedures in this study were approved by the Ethics Committee at King Faisal University, Saudi Arabia (Permission number KFU-REC / 2019 –10 - 01). All applicable rules for the care and use of animals were followed. ResultsNo differences were observed during the BALF collection procedures in camels in summer or winter. However, the collection of samples in female camels was more accessible than in adult male camels because of male ferocity during the sample collection process and the presence of dulla in adult male camels. The BALF collecting procedures were in young camels easier compared to adult camels. Because of collecting BALF samples through the oral cavity (Fig. 1) because of anatomical difficulties in the nasal cavity in camels (Shawaf et al., 2021), a presence of squamous epithelial cells appeared in BALF cytology because of contamination during the analysis. The presence of these cells in the BALF samples can be explained by the hypothesis that it contaminated with BALF during the insertion of the catheter through the oral cavity. A moderate amount of mucus was consistently detected as a cloudy network of light blue to purple material (Fig. 2). In general, alveolar macrophage and lymphocytes were the predominant cell type to be identified in the lavage fluid samples (Fig. 2). The percentage of recovered BALF collected ranged between 48%-58% of the total normal saline administrated in the present study (Table 1). The present study showed a variable percentage of epithelial cells on most slides and are a portion of the normal collected cells in lower airways cytology of healthy camels. The mean percentage, SEM, and range of differential nucleate cell count in summer and winter groups are presented in Table 2. The cytological differential counts analysis in BALF between young and adult camels is shown in Table 3, While differential cell counts parameters in BALF between male and female camels are presented in Table 4.
Fig. 1. BALF collection procedure using BALF catheter from dromedary camel.
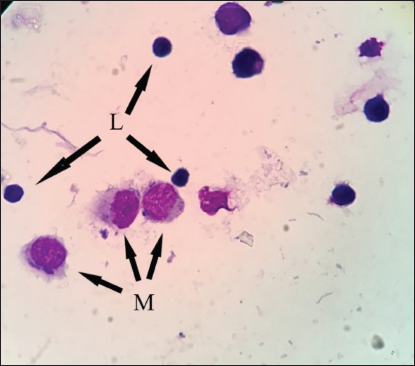
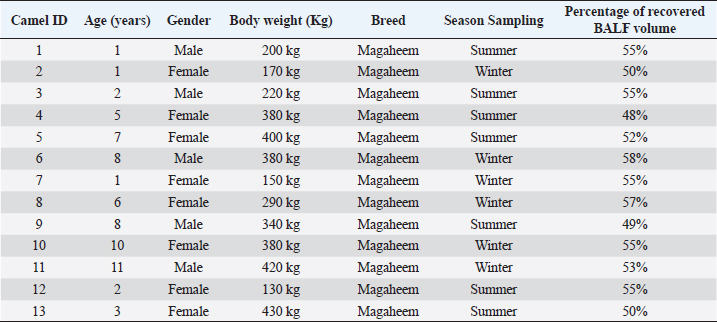
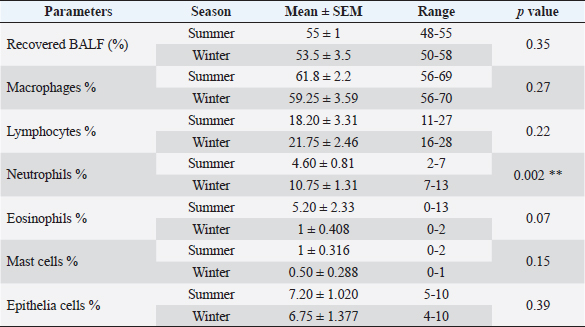
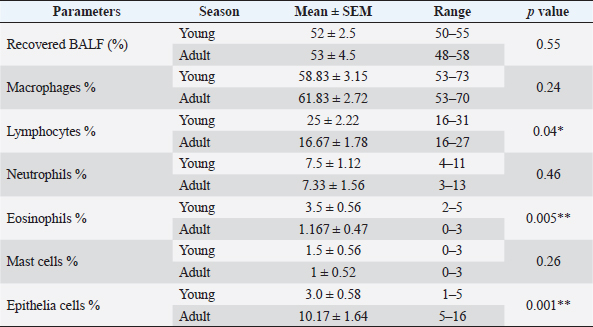
Fig. 2. BALF cytology from healthy dromedary camel showing macrophages (M) and lymphocytes (L). The results of BALF cytology percentage showed no variation between winter and summer in most cell types, while there was a significant difference in neutrophils (Table 2). The mean value of neutrophil cell percentage in winter increased significantly (10.75 ± 1.31) compared to summer (4.60 ± 0.81). The range of eosinophils was in summer (0–13) wider than in winter (0–2). Similar results for macrophages, lymphocytes, and epithelial cell percentages were recorded in summer and winter (Table 2). A significant difference was recorded in lymphocytes, eosinophils, and epithelial cells percentage among adult and young camels (Table 3). However, the range of lymphocytes was wider on young camels (16–31) compared to adult camels (16–27). There was a high mean value of epithelial cells percentage in adult camels (10.17 ± 1.64) compared to young animals (3.0 ± 0.58). On the other hand, the range of epithelial cells percentage was greater in adults (5–16) compared to young camels (1–5). Table 1. The data about age, gender, body weight, breed, sample collection season, and percentage of recovered BALF from each camel in the present study.
Table 2. Effect of season on BALF cytology in camels.
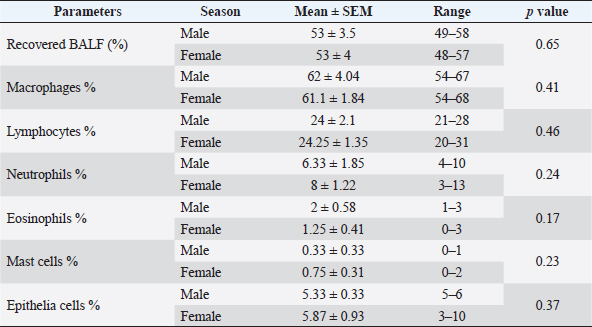
The results of BALF cytology among male and female camels showed no significant difference (Table 4). Neutrophils and mast cell percentages were slightly higher in female camels compared to males. DiscussionTo our best knowledge, this is the first study to investigate the changes in BALF cytology among seasons, gender, and age in camels. However, the physiological and pathological of respiratory tract values, including cytological parameters of BALF, may vary according to many factors as breed, gender, age, seasonal, and environmental circumstances (Olsen et al., 2012; Kohler et al., 2013; Pacheco et al., 2014). Collecting BALF samples in camels was a challenge compared to other animal species because of the typical anatomical structures in camels with the narrow nasal cavity, sharp teeth, and characteristic larynx movement in camels (Shawaf et al., 2021). In contrast to other studies, which reported that the BALF collection in foals was more difficult than in adult animals (Sponseller and Sponseller, 2017), we found that the BALF collection was in young animals easier than in adults. However, to compare our results regarding the challenge of BALF collection in male camels compared to females, we did not find previous studies that compared the ease of BALF collection among gender in veterinary medicine. Table 3. Effect of age on BALF cytology in camels.
Table 4. Effect of gender on BALF cytology in camels.
The higher percentage of neutrophils and lymphocytes in apparently healthy camels in the current study was in agreement with (Olsen et al., 2012; Secombe et al., 2015; Padalino et al., 2021), who reported that the exposure of animals to weather changes such as the cold air currents in winter may cause a change in the level of the immune response in the respiratory system, which stimulates a moderate level of inflammation, which may cause an increase in mucous secretions in the airways and the release of quantities of inflammatory cells in the lumen of the small airways like neutrophils. However, it may be difficult to evaluate and compare the effect of the annual season between camels and other animal species because of the way free camels are raised in the desert and their exposure to weather factors directly (Abdelhadi et al., 2012). It is known that camels live outdoors in a desert environment all year round and are exposed to sharp changes in weather between summer and winter, which may have a significant impact on the response of the respiratory system (Ibrahim and Al-Kheraije, 2021; Farsi et al., 2022). On the other hand, most animal species are arranged indoors during harsh seasons, which makes a comparison of the effect of weather on respiratory response and the percentage of cells in alveolar wash samples challenging to compare. Moreover, the sharp changes in weather changes among summer and winter in Saudi Arabia (Al-Bouwarthan et al., 2019) may affect the cellular immune response of the respiratory tract in camels. Interestingly, an increased percentage of eosinophils in summer compared to winter in the current study was in agreement with (Pacheco et al., 2014; Yasokawa et al., 2020), who indicated a high percentage of eosinophil cells in the BALF could be explained by the exposure of animals to allergens in the summer, such as dust and dry plant residues. Several studies have evaluated the influence of age on the cytological analysis of BALF in humans and animals (Umstead et al., 2009; Pacheco et al., 2014; Hostetter et al., 2017; Hansen et al., 2019). In contrast to a previous report (Sad et al., 2013; Pacheco et al., 2014), our study showed a higher percentage of lymphocyte cells in young camels compared to adults. Sad et al. (2013) reported lower percentages of lymphocytes (40.0 ± 14.4) and eosinophil (0.3 ± 0.3) in the BALF of healthy young horses compared to adults (35.3 ± 14.0) and (0.2 ± 0.4) respectively, which was in our present study for lymphocytes (25 ± 2.22) and eosinophil (3.5 ± 0.56) for young camels compared to adult camels (16.67 ± 1.78) and (1.167 ± 0.47), respectively. The difference in the BALF cytology between young and old camels, and the difference in their concentration compared to other animal species, maybe because of the difference in the composition of the immune system and the immune reaction in camels (Hussen and Schuberth, 2020; Hussen et al., 2022). Our study found a higher percentage of epithelial cells in adult camels compared to young’s, which was in arrangement with a previous study (Finotto et al., 1993), which reported that the higher epithelial cells in the BALF could be explained by injury of lower airways in the bronchial epithelium layer, which may occur after the inhalation of allergens and toxic materials, and through virus infections. However, another reason for the high percentage of epithelial cells percentage in BALF of adult camels compared to young animals in the current study could also be explained by the fact that the process of obtaining BALF, as previously mentioned, was more difficult and collected longer time in adult camels, which may be the reason for the release of larger amounts of epithelial cells in BALF. There is a lake of studies in veterinary medicine that discussed the difference between BALF cytology based on gender, while there were studies in humans and rats that found some changes and the cellular composition of BALF among gender because of the different styles of life and work, and the exposure to different environmental influences (Lopez et al., 1986; Mund et al., 2001). In agreement with our results, Frye et al. (2020) reported no difference in BALF cytology in healthy humans among gender. ConclusionIn conclusion, the current study provides the first comparison report that analysis BALF cytological cells in Dromedary camels among seasons, genders, and ages. Our work revealed significant differences in BALF cytology regarding age and season but no impact on gender. Additional studies, including a larger number of animals within a wider age range, breeds, and geographical areas, are necessary to confirm these conclusions. AcknowledgementsThe authors are also thankful to the Research Center of Camels, King Faisal University, Saudi Arabia for providing the camels. Conflict of interestThe authors have no conflict of interest in declaring Author’s contributionTS conceived and designed the study. MA and TS collected the samples, data interpretation, manuscript preparation, and revision of the final version. ReferencesAbdelhadi, O.M., Babiker, S.A., Picard, B., Jurie, C., Jailler, R., Hocquette, J.F. and Faye, B. 2012. Effect of season on contractile and metabolic properties of desert camel muscle (Camelus dromedarius). Meat. Sci. 90, 139–144. Abutarbush, S.M., Al-Rukibat, R.K., Quran, W. and Hananeh, W. 2019. Laboratory findings of tracheal wash and bronchoalveolar lavage in normal adult dairy cattle. J. Appl. Anim. Res. 47, 46–53. Al-Bouwarthan, M., Quinn, M.M., Kriebel, D. and Wegman, D.H. 2019. Assessment of heat stress exposure among construction workers in the hot desert climate of Saudi Arabia. Ann. Work. Expo. Health. 63, 505–520. Bekele, T. 1999. Studies on the respiratory disease ‘sonbobe’ in camels in the eastern lowlands of Ethiopia. Trop. Anim. Health. Prod. 31, 333–345. Couetil, L.L., Hoffman, A.M., Hodgson, J., Buechner-Maxwell, V., Viel, L., Wood, J.L. and Lavoie, J.P. 2007. Inflammatory airway disease of horses. J. Vet. Intern. Med. 21, 356–361. Davis, K.U. and Sheats, M.K. 2019. Bronchoalveolar lavage cytology characteristics and seasonal changes in a herd of pastured teaching horses. Front. Vet. Sci. 6, 74. De Brauwer, E.I., Drent, M., Mulder, P.G., Bruggeman, C.A., Wagenaar, S.S. and Jacobs, J.A. 2000. Differential cell analysis of cytocentrifuged bronchoalveolar fluid samples affected by the area counted. Anal. Quant. Cytol. Histol. 22, 143–149. De Brauwer, E.I., Jacobs, J.A., Nieman, F., Bruggeman, C.A. and Drent, M. 2002. Bronchoalveolar lavage fluid differential cell count. How many cells should be counted? Anal. Quant. Cytol. Histol. 24, 337-341. Farsi, H., Harti, D., Rachid Achaaban, M., Piro, M., Ouassat, M., Challet, E., Pevet, P. and El Allali, K. 2022. Seasonal variations in locomotor activity rhythm and diurnal activity in the dromedary camel (Camelus dromedarius) under mesic semi-natural conditions. Chronobiol. Int. 39, 129–150. Finotto, S., Rado, V., Dal Vecchio, A., Milani, G., Fabbri, L.M. and Maestrelli, P. 1993. Identification of epithelial cells in bronchoalveolar lavage. Hum. Exp. Toxicol. 12, 43–46. Frye, B.C., Schupp, J.C., Rothe, M.E., Kohler, T.C., Prasse, A., Zissel, G., Vach, W. and Muller-Quernheim, J. 2020. The value of bronchoalveolar lavage for discrimination between healthy and diseased individuals. J. Intern. Med. 287, 54–65. Hansen, S., Otten, N.D., Fjeldborg, J., Baptiste, K.E. and Horohov, D.W. 2019. Age-related dynamics of pro-inflammatory cytokines in equine bronchoalveolar lavage (BAL) fluid and peripheral blood from horses managed on pasture. Exp. Gerontol. 124, 110634. Hoffman, A.M. 2008. Bronchoalveolar lavage: sampling technique and guidelines for cytologic preparation and interpretation. Vet. Clin. North Am. Equine Pract. 24, 423–435. Hostetter, S.J., Clark, S.K., Gilbertie, J.M., Wiechert, S.A., Jones, D.E. and Sponseller, B.A. 2017. Age-related variation in the cellular composition of equine bronchoalveolar lavage fluid. Vet. Clin. Pathol. 46, 344–353. Hussen, J. and Schuberth, H.J. 2020. Recent advances in camel immunology. Front. Immunol. 11, 614150. Hussen, J., Shawaf, T., Al Humam, N.A., Alhojaily, S.M., Al-Sukruwah, M.A., Almathen, F. and Grandoni, F. 2022. Flow cytometric analysis of leukocyte populations in the lung tissue of dromedary camels. Vet. Sci. 9(6), 287. Ibrahim, Z.H. and Al-Kheraije, K.A. 2021. Seasonal morphology and immunoreactivity of cytokeratin and atrial natriuretic peptide in dromedary camel poll glands. Anat. Histol. Embryol. 50, 307–315. Intisar, K.S., Ali, Y.H., Khalafalla, A.I., Mahasin, E.A. and Amin, A.S. 2009. Natural exposure of dromedary camels in Sudan to infectious bovine rhinotracheitis virus (bovine herpes virus-1). Acta Trop. 111, 243–246. Intisar, K.S., Ali, Y.H., Khalafalla, A.I., Rahman, M.E. and Amin, A.S. 2010a. Respiratory infection of camels associated with parainfluenza virus 3 in Sudan. J. Virol. Methods. 163, 82–86. Intisar, K.S., Ali, Y.H., Khalafalla, A.I., Rahman, M.E. and Amin, A.S. 2010b. Respiratory syncytial virus infection of camels (Camelus dromedaries). Acta Trop. 113, 129–133. Kang, H., Bienzle, D., Lee, G.K.C., Piche, E., Viel, L., Odemuyiwa, S.O. and Beeler-Marfisi, J. 2022. Flow cytometric analysis of equine bronchoalveolar lavage fluid cells in horses with and without severe equine asthma. Vet. Pathol. 59, 91–99. Kebedi, F.G. 2010. Studies on major respiratory diseases of camel (Camelus dromedarius) in Northeastern Ethiopia. African J. Microbiol. Res. 4, 1560–1564. Kohler, M., Sandberg, A., Kjellqvist, S., Thomas, A., Karimi, R., Nyren, S., Eklund, A., Thevis, M., Skold, C.M. and Wheelock, A.M. 2013. Gender differences in the bronchoalveolar lavage cell proteome of patients with chronic obstructive pulmonary disease. J. Allergy. Clin. Immunol. 131, 743–751. Lopez, A., Yong, S., Sharma, A. and Bailey, D. 1986. Effect of sex, age, number of bronchoalveolar lavages and quantitation methods on the bronchoalveolar cell counts in rats. Can. J. Vet. Res. 50, 101–105. Malikides, N., Hughes, K.J., Hodgson, D.R. and Hodgson, J.L. 2003. Comparison of tracheal aspirates and bronchoalveolar lavage in racehorses. 2. Evaluation of the diagnostic significance of neutrophil percentage. Aust. Vet. J. 81, 685–687. Mund, E., Christensson, B., Larsson, K. and Gronneberg, R. 2001. Sex dependent differences in physiological ageing in the immune system of lower airways in healthy non-smoking volunteers: study of lymphocyte subsets in bronchoalveolar lavage fluid and blood. Thorax. 56, 450–455. Olsen, H.H., Grunewald, J., Tornling, G., Skold, C.M. and Eklund, A. 2012. Bronchoalveolar lavage results are independent of season, age, gender and collection site. PLoS One. 7, e43644. Pacheco, A.P., Paradis, M.R., Hoffman, A.M., Hermida, P., Sanchez, A., Nadeau, J.A., Tufts, M. and Mazan, M.R. 2014. Age effects on blood gas, spirometry, airway reactivity, and bronchoalveolar lavage fluid cytology in clinically healthy horses. J. Vet. Intern. Med. 28, 603–608. Padalino, B., Cirone, F., Zappaterra, M., Tullio, D., Ficco, G., Giustino, A., Ndiana, L.A. and Pratelli, A. 2021. Factors affecting the development of bovine respiratory disease: a cross-sectional study in beef steers shipped from france to Italy. Front. Vet. Sci. 8, 627894. Sad, E.P., Alencar. N.X., Viscardi, V., Costa, M., Hess, T.M. and LessaI, D.A. 2013. Cytology profile and age influence in the equine bronchoalveolar lavage in healthy and asymptomatic inflammatory airway disease. Ciência Rural, Santa Maria. 43, 452–455. Secombe, C.J., Lester, G.D., Robertson, I.D. and Cullimore, A.M. 2015. Retrospective survey of bronchoalveolar lavage fluid cytology in western australian horses presented for evaluation of the respiratory tract: effect of season on relative cell percentages. Aust. Vet. J. 93, 152–156. Shawaf, T., Almubarak, A., Alhumam, N., Almathen, F. and Hussen, J. 2021. Cytological analysis of tracheal wash and bronchoalveolar lavage fluid in health and respiratory disease in dromedary camels. PeerJ. 9, e11723. Shawaf, T., Hussen, J., Al-Zoubi, M., Hamaash, H. and Al-Busadah, K. 2018. Impact of season, age and gender on some clinical, haematological and serum parameters in Shetland ponies in east province, Saudi Arabia. Int. J. Vet. Sci. Med. 6, 61–64. Shawaf, T., Schuberth, H.J. and Hussen, J. 2022. Immune cell composition of the bronchoalveolar lavage fluid in healthy and respiratory diseased dromedary camels. BMC Vet. Res. 18, 353. Sponseller, B. and Sponseller, B., 2017. Bronchoalveolar lavage of adults and foals, manual of clinical procedures in the horse, pp: 247–254. Umstead, T.M., Freeman, W.M., Chinchilli, V.M. and Phelps, D.S. 2009. Age-related changes in the expression and oxidation of bronchoalveolar lavage proteins in the rat. Am. J. Physiol. Lung Cell Mol. Physiol. 296, L14–29. Van Driessche, L., Bokma, J., Deprez, P., Haesebrouck, F., Boyen, F. and Pardon, B. 2019. Rapid identification of respiratory bacterial pathogens from bronchoalveolar lavage fluid in cattle by MALDI-TOF MS. Sci. Rep. 9, 18381. Yasokawa, N., Kurose, K., Abe, M., Tanaka, H., Irei, I., Kato, S. and Oga, T. 2020. An unusual but unmissable link between summer-type hypersensitivity pneumonitis and asthma in an old house. Respir. Med. Case Rep. 31, 101145. | ||
| How to Cite this Article |
| Pubmed Style Al-Ali MA, Shawaf T. Effect of age, season, and gender on bronchoalveolar lavage fluid cytology in camels. Open Vet J. 2023; 13(5): 550-557. doi:10.5455/OVJ.2023.v13.i5.7 Web Style Al-Ali MA, Shawaf T. Effect of age, season, and gender on bronchoalveolar lavage fluid cytology in camels. https://www.openveterinaryjournal.com/?mno=116735 [Access: October 06, 2024]. doi:10.5455/OVJ.2023.v13.i5.7 AMA (American Medical Association) Style Al-Ali MA, Shawaf T. Effect of age, season, and gender on bronchoalveolar lavage fluid cytology in camels. Open Vet J. 2023; 13(5): 550-557. doi:10.5455/OVJ.2023.v13.i5.7 Vancouver/ICMJE Style Al-Ali MA, Shawaf T. Effect of age, season, and gender on bronchoalveolar lavage fluid cytology in camels. Open Vet J. (2023), [cited October 06, 2024]; 13(5): 550-557. doi:10.5455/OVJ.2023.v13.i5.7 Harvard Style Al-Ali, M. A. & Shawaf, . T. (2023) Effect of age, season, and gender on bronchoalveolar lavage fluid cytology in camels. Open Vet J, 13 (5), 550-557. doi:10.5455/OVJ.2023.v13.i5.7 Turabian Style Al-Ali, Mohamed Ali, and Turke Shawaf. 2023. Effect of age, season, and gender on bronchoalveolar lavage fluid cytology in camels. Open Veterinary Journal, 13 (5), 550-557. doi:10.5455/OVJ.2023.v13.i5.7 Chicago Style Al-Ali, Mohamed Ali, and Turke Shawaf. "Effect of age, season, and gender on bronchoalveolar lavage fluid cytology in camels." Open Veterinary Journal 13 (2023), 550-557. doi:10.5455/OVJ.2023.v13.i5.7 MLA (The Modern Language Association) Style Al-Ali, Mohamed Ali, and Turke Shawaf. "Effect of age, season, and gender on bronchoalveolar lavage fluid cytology in camels." Open Veterinary Journal 13.5 (2023), 550-557. Print. doi:10.5455/OVJ.2023.v13.i5.7 APA (American Psychological Association) Style Al-Ali, M. A. & Shawaf, . T. (2023) Effect of age, season, and gender on bronchoalveolar lavage fluid cytology in camels. Open Veterinary Journal, 13 (5), 550-557. doi:10.5455/OVJ.2023.v13.i5.7 |