| Original Article | ||
Open Vet J. 2023; 13(2): 206-217 Open Veterinary Journal, (2023), Vol. 13(2): 206–217 Original Research Cytodifferentiation of pinealocytes (I and II) and astrocyte types of mature male sheep epiphysis cerebri with special emphasis on the presence of neuronal and pigmented-like cellsNaief Dahran1 and Wael A. M. Ghonimi2*1Department of Anatomy, Faculty of Medicine, University of Jeddah, Jeddah, Saudi Arabia 2Department of Histology and Cytology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt *Corresponding Author: Wael A. M. Ghonimi. Department of Histology and Cytology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. Email: WAGhonemy [at] vet.zu.edu.eg Submitted: 23/12/2022 Accepted: 20/01/2023 Published: 16/02/2023 © 2023 Open Veterinary Journal
AbstractBackground: The epiphysis cerebri (pineal gland) is a small-sized, photo neuroendocrine organ in the brain of most vertebrates. Their effect is through secretion of melatonin, a serotonin-derived hormone which is stimulated by darkness and inhibited by light and modulates the circadian rhythm; light and dark cycle like a biological clock, sleep patterns (sleep-wake cycle), and sexual development. Aim: This study aimed to identify and differentiate the different cell types filling the pineal gland parenchyma of mature male sheep. Methods: Pineal glands were collected and sliced parasagitally then processed histologically for light and electron microscopic examinations. Results: Two main cell types; pinealocytes and astrocytes were recognized within the gland parenchyma. Pinealocytes were the chief parenchymatus cells that occupied the largest volume of the gland and were classified according to the nuclear pictures (activity status) into two subtypes; pinealocytes I (pale subtype, active) and II (dark subtype, inactive). Astrocyte neuroglial cells had cytoplasmic processes which form a huge supportive framework between the pinealocytes and clarified two types; type I were elongated cells with elongated snake shaped nucleus and type II were smaller in size, with oval nuclei. Another marginal cell type was identified as a neuron-like cell which appeared larger in size than others and distributed sporadically, has eccentric oval nucleus with prominent nucleoli and single, long cytoplasmic process that branched at its terminal forming T-shaped process looks like pseudo unipolar neuron. Moreover, aggregations of pigment granules were markedly observed in the intercellular spaces and also near the blood capillaries. With transmission electron microscope (TEM) a special characteristic feature of pinealocytes; synaptic ribbons were recognized that appeared as bands of electron-dense material with several synaptic spherules; vesicles adjacent to its surface helping in the multivesicular release. Conclusion: The gland parenchyma revealed two main cell types; pinealocytes and astrocytes. Each one was subdivided into two subtypes; I and II. The first one was classified according to their nuclear pictures (activity status) and the second one was according to their shape, size, and cytoplasmic processes. Other cell types were also identified as neuronal and pigmented-like cells in the pineal matrix. Keywords: Astrocyte, Epiphysis cerebri, Neuronal-like cell, Pinealocyte, Synaptic ribbons. IntroductionThe pineal gland or epiphysis cerebri, is a small-sized, photo neuroendocrine organ in the midline of the brain (Gheban et al., 2022). The pineal gland is shaped like a tiny pinecone, which gives it its name (“pine”-al gland) (Getty, 1975). This gland is a neuroendocrine organ which regulates the changes in the endocrine system as well as the functions of many other systems according to light and dark (Arendt, 1995; Cagnacci, 1996). So, the pineal gland is considered as the pacemaker of the circadian system regulating the light and dark cycle like a biological clock (Hardeland, 2012). Their effect is through secretion of melatonin, a serotonin-derived hormone which is stimulated by darkness and inhibited by light so, modulates sleep patterns in both circadian and seasonal cycles. In addition, melatonin hormone has an important role in controlling the functions of gonads in both males and females (Arendt, 1995; Cagnacci, 1996). Melatonin has a role on adjustment of sexual development, sleep-wake cycle, seasonal adaptation, and ovulation in addition to its antioxidant activity (Uyar and Alan, 2008; Pandi-Perumal et al., 2008). This hormone is produced by the pineal gland and by other organs such as gonads and is involved in the regulation of the hypothalamic–pituitary–gonadal axis in photoperiodic species (El-Raey et al., 2011). Melatonin blocks the secretion of gonadotropins (luteinizing hormone and follicle-stimulating hormone) from the anterior pituitary gland. So, these hormones are important for gonadal development and function (Lahmam et al., 2008). Melatonin secretion was increased at night (darkness) and decreases during the day light to control the circadian rhythm (Arendt, 1995; Gu¨ndu¨z, 2002; Mays et al., 2018). Histologically, the pineal gland was covered externally with fibrous connective tissue capsule from which incomplete septa extended within the parenchyma dividing it into incomplete lobules (Gaikwad et al., 2007). The glandular parenchyma is composed mainly of pinealocytes which were the most former types of parenchymatous cells that responsible for melatonin secretion, glial cells serve as supporting cells and they are fewer in number than pinealocytes and other cells as connective tissue cells and neuron-like cells (Arendt, 1995; Kus et al., 2004). Pinealocytes, also known as photoreceptor cells, constitute the majority of parenchymal cells. Pinealocytes are rounded cells with basophilic cytoplasm and large spherical nuclei with prominent nucleoli (Ekström and Meissl, 1997; Beheiry and Moselhy, 2016). Pinealocytes had two types including the light and dark cells (Lucy et al., 2011). Chords and islands of pinealocytes constitute the secretory parenchyma, while glial tissue and calcifications represent degenerative changes (Gheban et al., 2022). Furthermore, cells with a secretory function have numerous mitochondria and a well-developed Golgi apparatus (Tan et al., 2018). Glial cells, also known as pinealocyte supporting cells, are interstitial cells forming a network surrounding the pinealocytes (Prabhavathi et al., 2010). Due to their high chromatin density, they stain darkly and can be easily distinguished from pinealocytes (Ekström and Meissl, 1997). And also, calcium concretions and some cysts of different size are a distinct feature in the pineal gland (Ebada, 2012). Ultra structurally, pinealocytes have oval, indented nuclei and expansive endoplasmic reticulums, their cytoplasmic processes are reinforced by microtubules and their distended terminals contain groups of synaptic vesicles. Other type of cells was identified as interstitial cells with heterochromatic oval nuclei exhibiting extensively branching cytoplasmic processes containing bundles of intermediate filaments (Liebich, 2009). Our study aims to identify and differentiate the various cell types that make up the parenchyma of the pineal gland in mature male Egyptian Baladi sheep; Ovis aries with a focus on the presence of neuronal and pigmented-like cells. Material and MethodsCollection of samplesThis study was conducted on 10 apparently healthy, freshly slaughtered mature male Egyptian Baladi sheep; Ovis aries, over 1 year age, taken from Zagazig abattoir, Sharkia Governorate, Egypt. Pineal glands were dissected from the healthy animals immediately after the slaughter. Tissue preparation for the histological investigationThe glands were fixed in 10% buffered neutral formalin, dehydrated in ascending grades of ethanol, cleared in Xylol, and embedded in melted paraffin forming paraffin blocks. Pineal glands were sliced parasagitally, where 5 µm thick sections were obtained and stained with Harris’s Hematoxylin and Eosin (HandE) stain for general structure, Blue Masson’s trichrome stain for collagen fibers, Gomori’s reticulin stain for reticular fibers, Bielschowsky silver impregnation for neuronal processes including dendrites, axons, and neurofibrils and Alcian blue pH (2.5) for acidic mucopolysaccharides according to Suvarna et al. (2018). All the stained sections were examined with a standard light microscope (Olympus BX 21) and photographed by a digital Dsc-W 800 super steady cyper shot camera (Sony-Japan) at the Department of Histology and Cytology, Zagazig University). Tissue preparation for transmission electron microscopic (TEM) examinationApproximately 1-mm³-sized pieces of pineal glands were immediately fixed in a buffered glutaraldehyde-formaldehyde fixative (GA/FA) for 2 hours and then washed with cacodylate buffer. Subsequently, the pieces were post-fixed in 1% osmium tetroxide (OsO4) in 0.1 M cacodylate buffer (pH 7.2), dehydrated through a graded ethanol series, and embedded in epoxy resin (Glauert and Lewis, 2014). Semi-thin sections were cut on an MT2 Sorvall microtome and stained with toluidine blue. Ultrathin sections (50–90 nm) were cut on an RMC MT6000_XL ultramicrotome, mounted on mesh copper grids, and stained with uranyl acetate and lead citrate. The ready ultrathin sections were examined and photographed with a JEOL electron microscope (JEM 1200 EX II) operating at 80KV. Ethical approvalNot applicable. ResultsHistologically, pineal gland of sheep was covered externally with a thin fibrous highly vascularized connective tissue capsule (Fig. 1a and b) that was mainly composed of collagen fibers (Fig. 1c) and reticular fibers (Fig. 1d and e). From the capsule, a short, incomplete, highly branched septa were extended, dividing the gland parenchyma into incomplete compartments, however, very few number of long septa were also observed (Figs. 1f and 2a and b). Furthermore, these septa were highly vascularized (Fig. 2b–d), and composed mainly of reticular fibers (Fig. 2e) and collagen fibers (Fig. 2f).
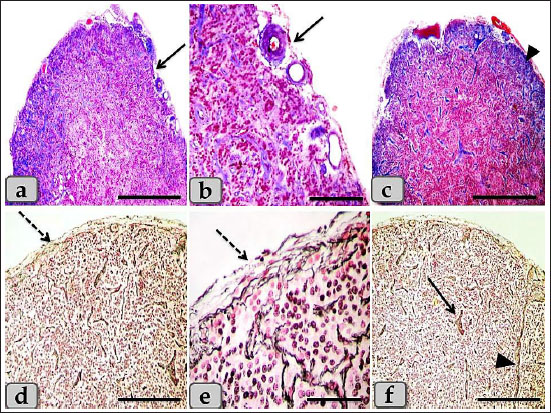
Fig. 1. A photomicrographs of the mature male sheep pineal gland, a and b) showing the thin, fibrous and highly vascularized connective tissue capsule (arrow). c) Showing the capsule collagen fibers (arrow head). d and e) Showing the capsule reticular fibers (dashed arrow). f) Showing short branched septa (arrow), long septa (arrow head). Stain: a–c) Blue Masson’s Trichrome, d–f) Gomori’s reticulin. Scale bars: a, c, d, f=600 µm, and b, e=200 µm. The gland parenchyma was composed of two main types of cells; the first one was the pinealocytes and the second type was the astrocytes (glial cells). The pinealocytes were the chief parenchymatous cells that occupied the largest volume of the gland and aggregated in large populations in a form of cords or clumps and had two subtypes according to the nuclear pictures; pinealocytes I (pale subtype) and pinealocytes II (dark subtype) (Fig. 3a and b). The pinealocytes I (light pinealocytes) represent the majority of pinealocytes within the sheep pineal gland and also, were the most common cell type distributed within the gland. They were appeared rounded or oval cells, with large round and remarkable vesicular euchromatic nucleus with a slight condensation of the chromatin material at the inner surface of the nuclear envelope and also, two or more prominent nucleoli were observed. Its cytoplasm is clear, abundant and slightly acidophilic with few granules (Fig. 3a–c). They were also possessed processes terminating close to the blood capillaries (Fig. 3a) and trabeculae (Fig. 3c and d) and also, anastomosed with each other. Meanwhile, pinealocytes II (dark pinealocytes) were fewer in number, smaller in size than pinealocytes I, appeared rounded or oval shaped, with scanty cytoplasm, and had darkly stained heterochromatic nucleus with indistinct nucleoli but the nuclear envelope appeared more darker due to aggregation of chromatin materials (Fig. 3a–c). The processes of these cells were extended into the blood capillaries and hard to be seen by the routine staining. But, with Gomori’s reticulin and also with Bielschowsky impregnation techniques, one or two extensive processes extended and ended with terminal expansions were observed (Fig. 3d). In general, the cell boundaries of both pinealocytes I and II were indistinct (Fig. 3a and b). In addition, individual lymphocytes were also observed in between the pineal parenchymatus cell populations (Fig. 3a). The second type of cells was the astrocyte glial cell; interstitial neuroglial supporting cells forming supportive framework to the pinealocytes. They were distributed in the interstitial tissue between the pinealocytes or around the blood capillaries. Two types of interstitial glia cells were recognized; type I were irregular in shape, small in size, with small, oval, or ovoid nuclei with densely stained chromatin materials and indistinct nucleoli. Its cytoplasm was darkly stained and had two or more cytoplasmic processes that were distributed in between the pinealocytes and blood capillaries and also, terminate in blood capillaries (perivascular feet; PVF) (Fig. 4a–c). Meanwhile, type II were elongated smooth muscle-like cells with elongated snake-shaped nucleus in addition, short, thin, and branched cytoplasmic processes were extended and terminated in the blood capillaries (PVF) (Fig. 4d).
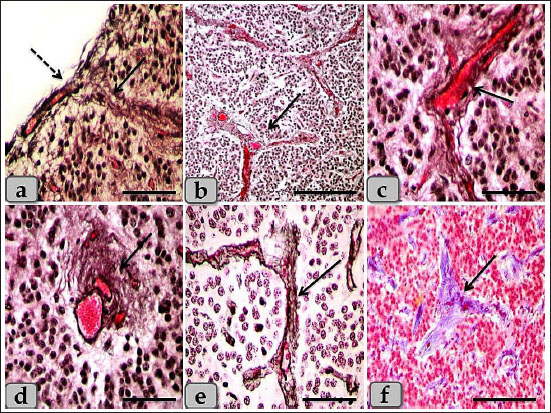
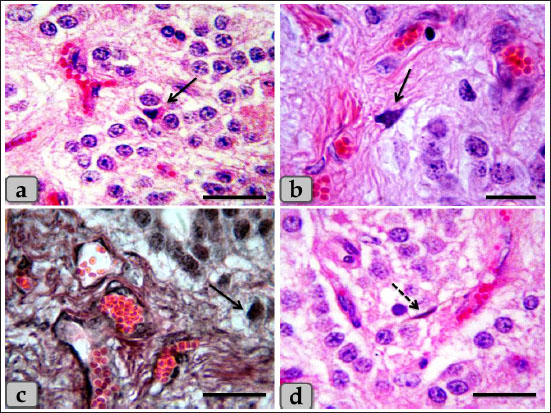
Fig. 2. a) Showing the pineal gland capsule (dashed arrow), thick, short septa (arrow). b) Showing thick, short, branched septa or trabeculae (arrow). c and d) Showing highly vascularized septa (arrow). e) Showing the septa reticular fibers (arrow). f) Showing the septa collagen fibers (arrow). Stain: a–d) Bielschowsky silver impregnation, e) Gomori’s reticulin, f) Blue Masson’s Trichrome. Scale bars: a, c, d, e=200 µm and b, f=300 µm. The melanin pigment was randomly scattered all over the cellular elements of pineal parenchyma. They were more frequent in the periphery of the pineal and around the blood vessels. Some pinealocytes, glial cells, and even fibroblasts were so packed with the pigment granules that appeared as numerous brownish to blackish granules which may mask the nucleus and cytoplasm of the pinealocytes (Fig. 5a). With the Bielschowsky impregnation techniques, aggregations of pigment granules were markedly observed in the intercellular spaces and also near the blood capillaries; they may be for degenerated pinealocytes (Fig. 5b). Heretofore no functional role has been assigned to the pineal pigment, so it may indicate non-specialization of pinealocytes or a function regression. Only one small patch of brain sand (corpora arenacea) of calcium concretions with irregular shape was recognized in all examined sections between the glands parenchymal cells (Fig. 5c and d). The brain sand or calcium deposit started by formation of small calcium granules that with aging increased gradually in size and number and coalesced with each other forming large patches of calcified concretions Numerous fibroblasts were also recognized adjacent to the blood capillaries with heavy collagen fibers. These cells were appeared elongated in shape with spindle, euchromatic nucleus with prominent nucleoli, and some cytoplasmic processes were extended (Fig. 6a–d). Another marginal cell type was identified as a neuron-like cell which appeared larger in size than others and distributed sporadically. They have eccentric oval-shaped nucleus with one or two prominent nucleoli. Its cytoplasm was darkly stained with single, long cytoplasmic process that branched at its terminal forming T-shaped process looks like pseudo unipolar neuron-like cells (Fig. 7a–c). In addition, the pineal gland revealed several connective tissue cells as plasma cells with cartwheel appearance for their nuclear chromatin (Fig. 8a). Other cell types; white blood cells were recognized between the pinealocytes such as eosinophil, monocyte, and lymphocyte. With Alcian blue stain, the pineal gland matrix was appeared lightly stained containing little acidic mucopolysaccharides (Fig. 8b and c). TEM examination revealed the presence of different pinealocytes types. These pinealocytes were classified according to the activity state into subtype I pinealocytes; larger in size, electron-lucent cytoplasm, with pale active euchromatic large nuclei and subtype II pinealocytes; smaller in size, electron-dense cytoplasm, with darker inactive heterochromatic small nuclei (Fig. 9a).
Fig. 3. A photomicrographs of the pineal gland parenchyma especially pinealocytes type I and II. a–c) showing the pinealocytes I populations (arrow), pinealocytes II (arrow head), individual lymphocytes (dashed arrow), in addition, the pinealocytes were possessed processes terminating close to the blood capillaries (Curved arrow). d) showing pinealocytes with one or two extensive processes extended (arrow head) and ended with terminal expansions (wavy arrow). Stain: a, b) H & E, c, d) Gomori's reticulin. Scale bars: a, b=20 µm and c, d=50 µm. The subtype I pinealocytes appeared polygonal in shape with some cytoplasmic processes. The nuclei appeared large, oval, or polygonal in shape and exhibited shallow invagination in their nuclear envelope. These nuclei were euchromatic with few amount of heterochromatin that was mainly located under the nuclear envelope and associated to their nucleoli. One or two eccentric nucleoli were seen in each nucleus. Its cytoplasm was less electron dense with numerous elongated mitochondria with tubular cristae and with numerous distributed free ribosomes throughout the cytoplasm. Rough endoplasmic reticulum with many ribosomal granules was being formed from stacked or isolated cisternae. Furthermore, numerous isolated and dilated cisternae covered with ribosomal granules were also observed in the periphery of the cytoplasm near to the cell membranes. Moreover, numerous electron dense secretory granules with variable shapes and sizes were observed in the cytoplasm of pinealocytes I. In addition, numerous scattered microtubules, microfilaments, and lysosomal bodies were also observed within the cytoplasm (Fig. 9b and c). The subtype II pinealocytes were smaller in size than pinealocytes I and characterized by their nuclear and cytoplasmic density. Its nuclei were small, oval, or ovoid in shape with more densification of chromatin distributed all over the nucleus especially under the nuclear envelope. Its cytoplasm was highly electron dense and darker than pinealocytes I with few free ribosomes and limited number of mitochondria with different shapes and sizes (Fig. 9d). These mitochondria were giving a sponge-like appearance to the pinealocytes II. These cells also showed some small cisternae of the rough endoplasmic reticulum (Fig. 9e). A special character of the pineal gland was the presence of a rod-like synaptic ribbons; a type of neuronal synapse that appeared as bands of electron-dense material with several sphere-like synaptic spherules; vesicles adjacent to its surface (Fig. 9b and f). The ultra-structural studies also showed some glial astrocyte cells; the glial astrocyte type I; (fibrous astrocyte) was ovoid in shape with irregular heterochromatic nucleus and an electron dense cytoplasm containing some mitochondria and some cytoplasmic processes (Fig. 10a). These cells also showed some lipid droplet and few vacuoles of variable shape and size (Fig. 10b). The glial astrocyte type II; (protoplasmic astrocytes) were elongated in shape with ovoid heterochromatic nuclei and little cytoplasm containing some mitochondria with several cytoplasmic extensions (Fig. 10c). Furthermore, large electron-dense secretory granules, lysosomes, scattered elongated and narrow cisternae of sarcoplasmic reticulum covered with ribosomal granules were also observed in addition, several cytoplasmic extensions (Fig. 10d). These cells were neighbored by numerous unmyelinated nerve fibers (Fig. 10b–d).
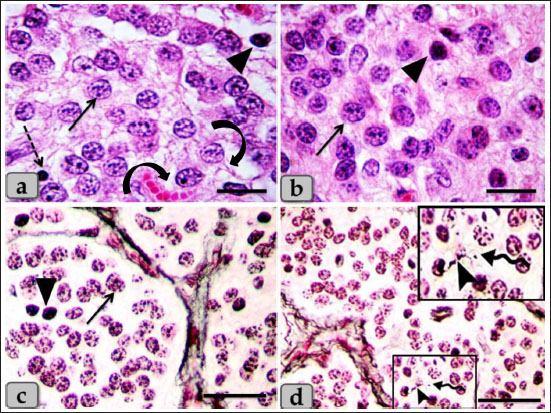
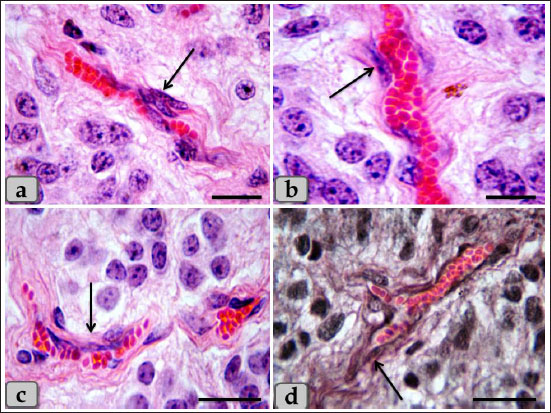
Fig. 4. A photomicrographs of the pineal gland parenchyma especially glial astrocytes type I and II. a–c) showing the astrocytes type I (arrow). d) showing the astrocytes type II (dashed arrow). Stain: a, b, d) H&E, c) Bielschowsky silver impregnation. Scale bars: a, c, d=50 µm and b=20 µm.
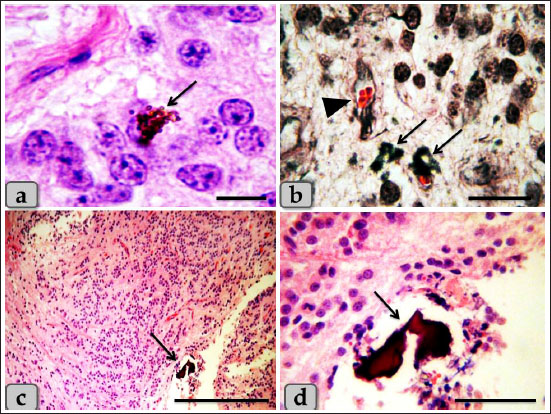
Fig. 5. a) Showing numerous brownish to blackish pigment granules which mask the nucleus and cytoplasm of the pinealocytes (arrow). b) showing aggregations of pigment granules in the intercellular spaces and also near the blood capillaries (arrow), blood capillary (arrow head). c, d) showing brain sands (corpora arenacea) of calcium concretions forming patches (arrow). Stain: a, c, d) H&E, b) Bielschowsky silver impregnation. Scale bars: a=20 µm, b=50 µm, c=600 µm, d=200 µm.
Fig. 6. a–d) Showing numerous fibroblasts adjacent to the blood capillaries with heavy collagen fibers (arrow). Stain: a–c) H&E, d) Bielschowsky silver impregnation. Scale bars: a, b=20 µm, c, d=50 µm.
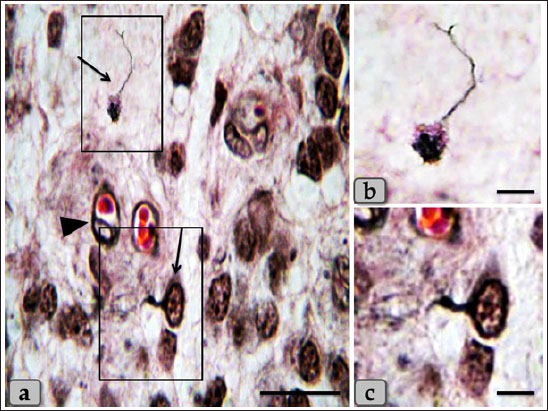
Fig. 7. a) Showing individual neuron-like cell (arrow), blood capillaries with pericytes (arrow head). b, c) higher magnification of neuron-like cell. Stain: all) Bielschowsky silver impregnation. Scale bars: a=50 µm, b,c=20 µm.
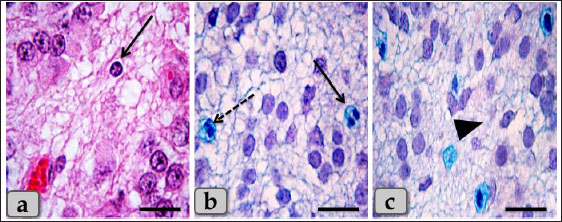
Fig. 8. a) Showing individual plasma cell with cartwheel appearance for their nucleus (arrow). b) showing eosinophil (arrow), monocyte (dashed arrow). c) showing slightly alcian blue positive reactivity of the pineal gland matrix (arrow head). Stain: a) H&E, b, c) Alcian blue. Scale bars: all=50 µm.
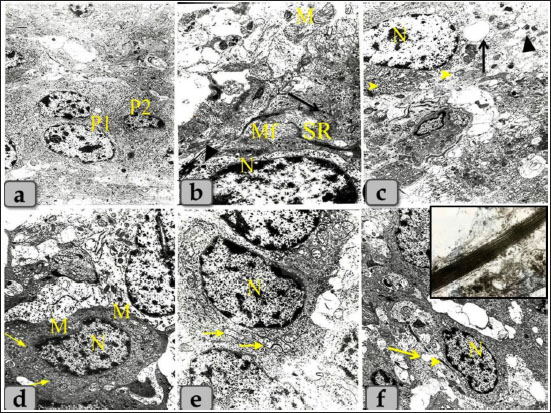
Fig. 9. Transmission electron micrographs of the pineal gland parenchyma showing different types of pinealocytes, a) showing pinealocytes I with large euchromatic nucleus and distinct nucleoli (P1) and pinealocytes II with smaller and darker nucleus (P2). b) Higher magnificated pinealocytes I showing the euchromatic nucleus with few amount of heterochromatin located under the nuclear envelop (N), mitochondria with tubular cristae (M), numerous distributed free ribosomes (arrow), microfilaments (Mf), electron dense secretory granules (arrow head) and synaptic ribbon (SR). c) Higher magnificated pinealocytes I showing the euchromatic nucleus with distinct nucleoli (N), mitochondria (arrow head), secretory granules (arrow head) and wide isolated and dilated cisternae covered with ribosomal granules (arrow). d) showing pinealocytes II with high nuclear and cytoplasmic density, heterochromatic nucleus (N), few free ribosomes (arrow), few and small sized mitochondria (M). e) showing two adjacent pinealocytes II with heterochromatic nucleus (N), mitochondria with different shape and size which giving a sponge-like appearance (arrow). f) showing pinealocytes II with synaptic ribbon (arrow head), synaptic spherules or vesicles (arrow) and heterochromatic nucleus (N). Inset box: showing magnified rods-like synaptic ribbons (bands of electron-dense material). Magnification power, all=×5,000 except figure a=×3,000.
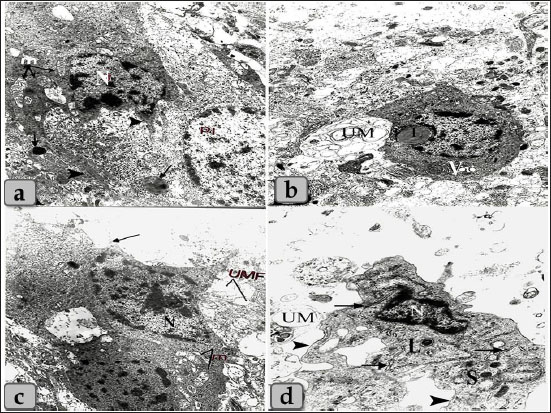
Fig. 10. Transmission electron micrographs of the pineal gland parenchyma showing different types of the glial astrocytes, a) showing astrocytes type I (fibrous astrocytes) with ovoid shape or irregular heterochromatic nucleus (N), an electron dense cytoplasm containing some mitochondria (m), some cytoplasmic processes (arrow head) and fat globules; liposomes (arrow). b) showing astrocytes type I with some large lipid droplet (L) and few vacuoles of variable shape and size (V), and beside this cells numerous unmyelinated nerve fibers were present (UM). c) showing astrocytes type II (protoplasmic astrocytes) with elongated shape, ovoid heterochromatic nuclei (N) and little cytoplasm containing some mitochondria (M) with several cytoplasmic extensions (arrow), beside unmyelinated nerve fibers (UMF). d) showing astrocytes type II with ovoid heterochromatic nuclei (N), electron dense secretory granules (S), lysosomes (L), scattered elongated and narrow cisternae of sarcoplasmic reticulum covered with ribosomal granules (arrow) and several cytoplasmic extensions (arrow head). Magnification power, all=×6,000 except figure b=×5,000. Pineal gland revealed an ovoid shape neural-like cell with euchromatic nucleus with distinct nucleoli occupying most of the cell. Its cytoplasm had some mitochondria with some cytoplasmic processes. TEM also showed some myelinated nerve fibers enveloped by schwann cell, these structures were found adjacent to pinealocytes (Fig. 11a). Irregular shape pigmented cells were found with heterochromatic nucleus (Fig. 11b). Spindle shaped fibroblast cells were also seen with elongated slightly heterochromatic nuclei and little cytoplasm containing some mitochondria, few rough endoplasmic reticulum and slender shape cytoplasmic processes (Fig. 11c and d). DiscussionThe pineal gland was considered one of the most crucial organs in an animal’s body. It secretes melatonin, which controls and regulates the circadian rhythm, seasonal breeding, sexual development, and ovulation process (Pandi-Perumal et al., 2008). Pineal gland was surrounded by a thin fibrous connective tissue capsule with short, highly vascularized branched septa. This result goes hand to hand with Prabhavathi et al. (2010) in sheep who clarified that the pineal gland was covered with a fibrous capsule. Our results also showed the presence of two cell types; pinealocytes and astrocyte glial cells. The pinealocytes were round in shape with acidophilic cytoplasm while the second type; astrocytes were randomly distributed between the pinealocytes; these cells were considered as supporting cells to the pinealocytes where they form a network through their processes. These results were similar to that mentioned by Gaikwad et al. (2007) in goat who revealed that the pineal gland parenchyma consisted of two types of cells; pinealocytes or chief cells and glial cells. Pinealocytes were rounded to oval in shape and were arranged in groups or in irregular cords.
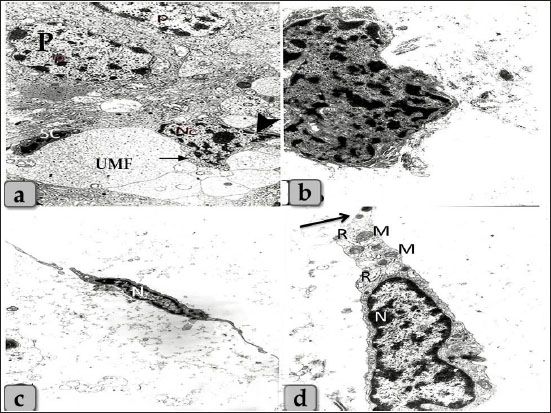
Fig. 11. Transmission electron micrographs showing a) an ovoid shaped neuron-like cell with euchromatic nucleus with distinct nucleoli occupying most of the cell (Nc), with some mitochondria (arrow), cytoplasmic processes (arrow head) and also some unmyelinated nerve fibers (UMF) enveloped by Schwann cell (SC) were present adjacent to pinealocytes (p) and neuron-like cell. b) showing irregular shaped pigmented cells with irregular patches of electron dense materials. c) showing spindle shaped fibroblast cells with elongated slightly heterochromatic nuclei (N). d) Higher magnificated fibroblast showing little cytoplasm, some mitochondria (M), few rough endoplasmic reticulum (R) and slender shape cytoplasmic processes (arrow). Magnification power, a=×5,000, b=×6,000, c=×3,000, d=×6,000. Pinealocytes were also possessed processes terminating close to the blood capillaries. These findings were supported by Robertis and Iraldi (1961) who clarified that the main morphological characteristic of the pinealocytes was represented by club-shaped perivascular expansions connected to the cell by thin pedicles. They were found lying in a large, clear space surrounding the blood capillaries. The name plurivesicular secretory processes was proposed, to claim the main structural feature and the probable secretory function of these cellular expansions. Moreover, astrocytes had short, thin, branched cytoplasmic processes that extended and terminated in the blood capillaries forming PVFs. Astrocytes extend polarized cellular processes that ensheath either neuronal processes or blood vessels (Abbott et al. 2006). The endfeet of the basal process almost completely ensheath the vascular tube. Astrocytes provide a cellular link between the neuronal circuitry and blood vessels (Gordon et al. 2011). Brain sand of calcium concretion was observed in the gland parenchyma between their cells and appeared as a small area of hyalinization which also found in the pineal gland of donkey described after Ebada (2012). It was thought that these structures were increased in size and number with aging. Where, Abou-Easa et al. (2009) claimed that the pineal glands were exhibited a moderate degree of hyalinization in the middle-aged group, and higher degree of hyalinization in old-aged group. And added that, the calcium deposits (brain sands) were appeared in the form of patches in the gland parenchyma and along courses of blood vessels, respectively Some melanin pigment scattered all over the cellular elements of pineal parenchyma. These pigment granules were appeared as numerous brownish to blackish granules which may mask the nucleus and cytoplasm of pinealocytes and glial cells. In addition, aggregations of pigment granules were markedly observed in the intercellular spaces and also near the blood capillaries; they may be for degenerated pinealocytes. Heretofore no functional role has been assigned to the pineal melanin pigment. We suggested that it may indicate non-specialization of pinealocytes or a function regression. These findings were completely supported by Abou-Easa et al. (2009) in camel who described that the melanin pigments were scattered all over the cellular elements of pineal tissue. They were more frequent in the periphery of the pineal gland and around the blood vessels. Most of the pinealocytes of all age groups had melanin pigment, the pigment-containing cells increased in number with increasing age. Some pinealocytes, glial cells, and even fibroblasts were intensively packed with the pigment granules that obscured the nuclei. Aggregations of pigment granules were sometimes observed in the intercellular spaces. Moreover, Busolini et al. (2017) suggested that the melanin pigment is not a constant characteristic in the pineal gland of mammals. And also, the biological significance of these pigments in the pineal gland has not yet been clearly determined. In the present work, the ultra-structural studies showed two main types of cells including pinealocytes and interstitial glial cells. These pinealocytes classified according to the activity state into pale active subtype with euchromatic nuclei and darker inactive subtype with heterochromatic nuclei and electron-dense cytoplasm. These findings were stated in mammals by Liebich (2009). A special character of the pineal gland of synaptic ribbons was identified, which appeared as bands of an electron-dense material with several synaptic vesicles adjacent to its surface. It is suggested that this structure is important to regulate and facilitate the multivesicular release and display a circadian rhythm with higher levels at night paralleling melatonin synthesis but regulated differently (Reuss, 2010) and also, Struwe and Vollrath (1990) in cow, sheep, and pig were supported our findings by distinguished the presence of few rod-like (synaptic ribbons) and few sphere-like (synaptic spherules) in cow and sheep pineal gland. In the pig, small numbers of both “synaptic” ribbons and “synaptic” spherules (SS) were found. Our study revealed that the pineal gland cells were associated with several nerve fibers which present adjacent to the pinealocytes. These results go hand to hand with those stated previously by Liebich (2009) who added that the perikarya of these nerve fibers are located in the cranial cervical ganglion. Eurell and Frabier (2006) stated that astrocytes interdigitate between the pinealocytes and capillaries. These astrocyte glia cells were also recognized in the pineal parenchyma which forms a massive network through its cytoplasmic processes, supporting the pinealocytes. ConclusionWe can conclude that the parenchyma of sheep pineal gland was composed of two main cell types; pinealocytes and astrocytes glial cells. Pinealocytes were the chief parenchymatous cells that occupied the largest volume of the gland and classified according to the nuclear pictures (activity status) into two subtypes; pinealocytes I (pale subtype) and pinealocytes II (dark subtype). The astrocyte; interstitial neuroglial supporting cells had long cytoplasmic processes which forms a huge supportive framework between the pinealocytes. Other cells were also identified as neuronal and pigmented-like cells in the pineal matrix. TEM revealed a rod-like synaptic ribbons, a type of neuronal synapse that appeared as bands of electron-dense material in the pinealocytes with several synaptic spherules; vesicles which suggested to have a role in the multivesicular release. Author contributionConceptualization: Wael A. M. Ghonimi. Data Curation: Wael A. M. Ghonimi. Methodology: Wael A. M. Ghonimi and Naief Dahran. Histological examination - Images analyze: Wael A. M. Ghonimi. Writing -Original draft: Wael A. M. Ghonimi and Naief Dahran. Review and Editing: Wael A. M. Ghonimi and Naief Dahran Data availabilityAll data generated during this study are included in this manuscript. Conflict of interestThe authors declare that there is no conflict of interest. Funding informationThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. ReferencesAbbott, N.J., Ronnback, L. and Hansson, E. 2006. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 7, 41–53. Abou-Easa, K., Tousson, E. and Abd-El-Gawad, M. 2009. Involution signs during the postnatal life in the pineal tissue of buffalo and camel. Nature Sci. 7(9), 35–44. Arendt, J. 1995. Melatonin and the mammalian pineal gland. London, UK: Chapman and Hall, pp: 6–49. Beheiry, R.R. and Moselhy, A.A.A. 2016. Macro and microscopical studies on the pineal gland of camel with immunohistochemical localization to pinealocytes and glia cells markers. Int. J. Adv. Res. 4(8), 1154–1163. Busolini, F.I., Rodríguez, G.B., Filippa, V.P. and Mohamed, F.H. 2017. Pigmented cells in the pineal gland of female Viscacha (Lagostomus maximus maximus): a histochemical and ultrastructural study. Int. J. Endocrinol. 2017, 7492960. Cagnacci, A. 1996. Melatonin in relation to physiology in adult humans. J. Pineal Res. 21, 200–213. Ebada, S. 2012. Morphological and immunohistochemical studies on the pineal gland of the donkey (Equus asinus). J. Vet. Anat. 5(1), 47–74. Ekström, P. and Meissl, H. 1997. The pineal organ of teleost fishes. Rev. Fish Biol. Fish. 7(2), 199–284 El-Raey, M., Geshi, M., Somfai, T., Kaneda, M., Hirako, M., Abdel-Ghaffar, A.E., Sosa, G.A., El-Roos, M.E. and Nagai, T. 2011. Evidence of melatonin synthesis in the cumulus oocyte complexes and its role in enhancing oocyte maturation in vitro in cattle. Mol. Reprod. Dev. 78(4), 250–262. Eurell, J. and Frappier, B.l. 2006. Dellmann's textbook of veterinary histology, 6th ed. New York and London: Blackwell, pp: 31. Gaikwad, N.L., Mainde, U.P., Nadeshwar, N.C., Dalvi, R.S. and Mshram, B.N. 2007. Pineal gland of goat: A histomorphological study. RVGL. 3(1), 37–38. Getty, R. 1975. The anatomy of the domestic animals, Vol. 1, 5th ed. Toronto, Canada: W.B. Saunders company, pp: 955–956. Gheban, B.A., Colosi, H.A., Gheban-Roșca, I.A., Georgiu, C., Gheban, D., Crișan, D. and Crișan, M. 2022. Digital histological morphometry of the human pineal gland in a postmortem study, with endocrine and neurological clinical implications. Anat. Histol. Embryol. 52(1), 12–20. Glauert, A.M. and Lewis, P.R. 2014. Biological specimen preparation for transmission electron microscopy. Princeton, NJ: Princeton University Press, pp: 80. Gordon, G.R., Howarth, C. and MacVicar, B.A. 2011. Bidirectional control of arteriole diameter by astrocytes. Exp. Physiol. 96, 393–399. Gu¨ndu¨z, B. 2002. Daily rhythm in serum melatonin and leptin levels in the Syrian hamster (Mesocricetus auratus). Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 132(2), 393–401. Hardeland, R. 2012. Melatonin in aging and disease-multiple consequences of reduced secretion, options and limits of treatment. Aging Dis. 3(2), 194–225. Kus, I., Sarsilmaz, M., Ozen, O.A., Turkoglu, A.O., Pekmez, H., Songur, A. and Kelestimur, H. 2004. Light and electron microscopic examination of pineal gland in rats exposed to constant light and constant darkness. Neuroendocrinol. 25, 102–108. Lahmam, M., El M'rabet, A., Ouarour, A., Pévet, P., Challet, E. and Vuillez, P. 2008. Daily behavioral rhythmicity and organization of the suprachiasmatic nuclei in the diurnal rodent, Lemniscomys barbarus. Chronobiol. Int. 25(6), 882–904. Liebich, H. 2009. Veterinary histology of domestic mammals and birds. a textbook and color atlas, 5th ed., 5M Publishing, Sheffield, UK, pp: 166. Lucy, K.M., Harshan, k.R., Chungath, J. and Ashok, N. 2011. Histomorphogenesis of pineal gland in goat fetuses. J. Vet. Anim. Sci. 42, 16–19. Mays, J.C., Kelly, M.C., Coon, S.L., Holtzclaw, L., Rath, M.F. and Kelley, M.W. 2018. Single-cell RNA sequencing of the mammalian pineal gland identifies two pinealocyte subtypes and cell type specific daily patterns of gene expression. PLoS One 13(10), e0205883. Pandi-Perumal, S.R., Trakht, I., Srinivasan, V., Spence, D.W., Maestroni, G.J., Zisapel, N. and Cardinali, D.P. 2008. Physiological effects of melatonin: role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 85(3), 335–353. Prabhavathi, M., Basha, S.H., Paramasivan, S., Venkatesan, S. and Ramesh, G. 2010. Histomorphology of the pineal gland in sheep. Indian Vet. J. 87, 698–700. Reuss, R. 2010. Pineal ribbon synapses: regulated by the gland’s central innervation. Neuroendocrinol. Lett. 31(6), 761–765. Robertis, E. and Iraldi, A.P. 1961. Plurivesicular secretory processes and nerve endings in the pineal gland of the rat. J. Biophys. Biochem. Cytol. 10(3), 361–372. Struwe, M.C.W. and Vollrath, L. 1990. ‘Synaptic’ bodies in the pineal glands of the cow, sheep and pig. Cells Tissues Organs 139, 335–340. Suvarna, K.S., Layton, C. and John, D. 2018. Bancroft’s theory and practice of histological techniques, 8th ed. New York, NY and London, UK: Churchill Livingstone, pp: 83–92. Tan, D.X., Xu, B., Zhou, X. and Reiter, R.J. 2018. Pineal calcification, melatonin production, aging, associated health consequences and rejuvenation of the pineal gland. Molecules 23(2), 301. Uyar, A. and Alan, M. 2008. Koyunlarda erken anostrus doneminde melatonin uygulamalarinin ovulasyon ve gebe likuzerine etkisi. Yyu. Vet. Fak. Derg. 19(1), 47–54. | ||
| How to Cite this Article |
| Pubmed Style Dahran N, Ghonimi WAM. Cytodifferentiation of pinealocytes (I and II) and astrocyte types of mature male sheep epiphysis cerebri with special emphasis on the presence of neuronal and pigmented like cells. Open Vet J. 2023; 13(2): 206-217. doi:10.5455/OVJ.2023.v13.i2.9 Web Style Dahran N, Ghonimi WAM. Cytodifferentiation of pinealocytes (I and II) and astrocyte types of mature male sheep epiphysis cerebri with special emphasis on the presence of neuronal and pigmented like cells. https://www.openveterinaryjournal.com/?mno=133671 [Access: September 01, 2024]. doi:10.5455/OVJ.2023.v13.i2.9 AMA (American Medical Association) Style Dahran N, Ghonimi WAM. Cytodifferentiation of pinealocytes (I and II) and astrocyte types of mature male sheep epiphysis cerebri with special emphasis on the presence of neuronal and pigmented like cells. Open Vet J. 2023; 13(2): 206-217. doi:10.5455/OVJ.2023.v13.i2.9 Vancouver/ICMJE Style Dahran N, Ghonimi WAM. Cytodifferentiation of pinealocytes (I and II) and astrocyte types of mature male sheep epiphysis cerebri with special emphasis on the presence of neuronal and pigmented like cells. Open Vet J. (2023), [cited September 01, 2024]; 13(2): 206-217. doi:10.5455/OVJ.2023.v13.i2.9 Harvard Style Dahran, N. & Ghonimi, . W. A. M. (2023) Cytodifferentiation of pinealocytes (I and II) and astrocyte types of mature male sheep epiphysis cerebri with special emphasis on the presence of neuronal and pigmented like cells. Open Vet J, 13 (2), 206-217. doi:10.5455/OVJ.2023.v13.i2.9 Turabian Style Dahran, Naief, and Wael A. M. Ghonimi. 2023. Cytodifferentiation of pinealocytes (I and II) and astrocyte types of mature male sheep epiphysis cerebri with special emphasis on the presence of neuronal and pigmented like cells. Open Veterinary Journal, 13 (2), 206-217. doi:10.5455/OVJ.2023.v13.i2.9 Chicago Style Dahran, Naief, and Wael A. M. Ghonimi. "Cytodifferentiation of pinealocytes (I and II) and astrocyte types of mature male sheep epiphysis cerebri with special emphasis on the presence of neuronal and pigmented like cells." Open Veterinary Journal 13 (2023), 206-217. doi:10.5455/OVJ.2023.v13.i2.9 MLA (The Modern Language Association) Style Dahran, Naief, and Wael A. M. Ghonimi. "Cytodifferentiation of pinealocytes (I and II) and astrocyte types of mature male sheep epiphysis cerebri with special emphasis on the presence of neuronal and pigmented like cells." Open Veterinary Journal 13.2 (2023), 206-217. Print. doi:10.5455/OVJ.2023.v13.i2.9 APA (American Psychological Association) Style Dahran, N. & Ghonimi, . W. A. M. (2023) Cytodifferentiation of pinealocytes (I and II) and astrocyte types of mature male sheep epiphysis cerebri with special emphasis on the presence of neuronal and pigmented like cells. Open Veterinary Journal, 13 (2), 206-217. doi:10.5455/OVJ.2023.v13.i2.9 |