| Short Communication | ||
Open Vet J. 2023; 13(4): 485-494 Open Veterinary Journal, (2023), Vol. 13(4): 485–494 Short Communication Study of the saiga helminth fauna and Ural sheep in the western region of KazakhstanKaissar Kushaliyev1*, Zhangeldi Ussenov2, Serik Alimbekov3, Orazali Millakaev1, Aigerim Kozhayeva1 and Artur Khairushev11Higher School of Veterinary Clinical Sciences, Institute of Veterinary Medicine and Animal Husbandry, Zhangir Khan West Kazakhstan Agrarian Technical University, Uralsk, Republic of Kazakhstan 2Higher School of Veterinary and Biological Safety, Institute of Veterinary Medicine and Animal Husbandry, Zhangir Khan West Kazakhstan Agrarian Technical University, Uralsk, Republic of Kazakhstan 3Technological Department, Higher Engineering-Technological College, Uralsk, Republic of Kazakhstan *Corresponding Author: Kaissar Kushaliyev. Higher School of Veterinary Clinical Sciences, Institute of Veterinary Medicine and Animal Husbandry, Zhangir Khan West Kazakhstan Agrarian Technical University, Uralsk, Republic of Kazakhstan. Email: kushaliyevkaissar [at] yahoo.com. Submitted: 07/01/2023 Accepted: 20/03/2023 Published: 23/04/2023 © 2023 Open Veterinary Journal
AbstractBackground: Contact between wild (saiga) and domestic (sheep) animals on pastures results in a composite community of helminths. Wild animals like saigas are vulnerable to parasites and the diseases they transmit are fatal. Adults may be less susceptible to infection than their offspring but remain a dangerous source of parasite spread. Aim: The aim of the article is to determine the environmental factors influencing the spread of helminthiasis (echinococcosis, coenurosis, and moniziosis) in animals. Methods: Epizootological indicators of the saigas helminth fauna have been studied to assess the epizootic state of the area, and the causes of invasive foci appearance (caenurosis, moniziosis, and echinococcosis) in farms in the Kaztalov and Zhanybekov districts of the Western Kazakhstan region. The diagnosis of saiga helminth infections was confirmed by well-grounded helminthological and pathological anatomical examinations of dead saigas. Results: Climatic, natural, and anthropogenic factors of the seasonality of infestation are considered. The climatic factors of helminth infestation in animals were described, based on the influence of environmental factors, which include favourable conditions for the survival of helminth larvae. The main source of helminth infestation is the animals’ watering places; therefore, it is necessary to green and create a large number of watering places, which will reduce the disease rate and improve the health of the animals from infestation. Conclusion: Regular helminthological and ecological monitoring in animal populations is necessary to ensure and preserve natural biocenoses. Keywords: Helminthiasis, Saigas, Moniziosis, Wildlife, Echinococcus. IntroductionParasitic diseases are widespread in domestic and wild mammals (Balgimbayeva et al., 2019). These agents, by penetrating the body of animals, take root in it, injure its cells and organs, feed at its expense, and become pathogens of various diseases. The study of helminth infestations as a factor affecting the state of the wild animal population is one aspect of ecological research (Goltsman, 2011). The aim of the article is to study the environmental factors of animal helminth infection spread, clarify the main sources of invasiveness, and adopt preventive measures to reduce morbidity and mortality (Zharov et al., 1982; Zharov and Kunakov, 1983) of wild and domestic animals. All environmental factors are known to affect helminths to a greater or lesser extent. Geohelminths (because their development cycle is associated with soil) and helminths (biohelminths) are directly affected by climatic factors such as temperature, humidity, and insolation; their development cycle involves intermediate hosts such as oribatid mites, mollusks, ants, and other insects, whose vital activity begins in the warm season (Zharov et al., 1982; Zharov and Kunakov, 1983). Frosts, large temperature changes at night, thaws, constant dry winds, and high temperatures of up to +50°C on the soil surface contribute to the death of nematode larvae and such cestodes as echinococcus, multiceps, hydatigenic taeniasis, and many others. There are climatic, environmental, and anthropogenic seasonality factors that influence the helminth cycle. Climatic factors such as relatively high soil humidity, low insolation, and rich vegetation are favorable for the survival of nematode larvae and mature forms of cestodes. All the above factors are fundamental for the seasonality of helminthiasis infestations and the degree of infestation of animals and their immune system (Kosminkov, 2004; Bulegenova et al., 2019). To detect the process and cycle of infestation (Kosminkov et al., 1995) among saigas and sheep (Manjiev et al., 2002), comprehensive epizootological monitoring and helminthological and pathomorphological studies are necessary. At present, it is necessary to study the parasite fauna of saigas and farm animals in their range and infestation with cestodes and nematodes, and to study parasitic diseases such as coenurosis, moniziosis, echinococcosis, and other infestations in studies on other helminth infestations circulating in the saiga population. Exchanges of pathogens can occur between wild and domestic animals. Saigas are known to migrate long distances throughout the year, including crossing borders into neighboring countries and coming into contact with domestic animals on their way. The scientific novelty lies because in summer 9.5% of saigas were infested with helminths, Moniezia expansa—4.28%, Trichocephalus skrjabini—4.28%, Eimeria elegans—88.57%, and in autumn: Nematodirus spathiger—11.76%, M. expansa—12.34%, Strongyloides papillosus—11.76%, E. elegans—76.47%, Eimeria tekenovi—76.47%. All the above factors are major in the manifestation of the seasonality of animal helminth infestations. Materials and MethodsMaterials on the modern parasite fauna of the saiga are very scarce, as, for about half a century, there has been virtually no scientific research in this area. There are data from systematic studies carried out in the 50–70s by scientists from the Institute of Zoology, in particular by Pryadko et al. (1964). The latter works were carried out in the 1990s by Baitursinov (2005) as assisting with theriological research. Diagnosis of sheep and saigas for helminth infestation (moniziosis and echinococcosis) in a comparative aspect was determined by studying the species composition, degree of invasiveness, seasonal and age dynamics of the main helminthiasis of the gastrointestinal tract of animals. The diagnosis and carrier of sheep for helminth infections (coenurosis, moniziosis, and echinococcosis) were determined on farms in the Kaztalov and Zhanibek districts of the Western Kazakhstan region; these districts are located in the migration routes of wild saigas of the Ural population. The method was agreed upon with the management of the Committee for Forestry and Wildlife. Based on clinical signs of animal disease, sick saigas were removed from the core herd on a commission basis (the method of capturing saigas was tested). Fresh saiga carcasses were autopsied for helminth infestation studies, and autopsies were carried out on the carcasses of poached saigas for forensic biological examinations of animal deaths. In addition, helminthological studies were conducted on saigas kept in the breeding centre of the Zhangir Khan West Kazakhstan Agrarian and Technical University (Kushaliev and Uazirov, 2018). The team of scientists has experience in keeping and breeding captive saigas in Kazakhstan. During the period from 2012 to the present day, they have carried out studies aimed at the safety of young saigas, developed, and successfully tested various diets for captive saiga breeding, minimized traumatic injuries to animals, and developed a set of preventive measures against infectious and invasive diseases in saigas. Diagnostic technique for coenurosis in sheep and saigas (Lazarev and Durdusov, 1997). When diagnosing coenurosis, besides the clinical signs described above, that coenurosis peaks in February and March, and that coenurotic bullae can occur in the cerebral lobes, spinal cord, and cerebellum must be considered. When the cranium is palpated and tapped at the sites where the parasites are localized, the sound is blunted and the bones are felt to be thin. Coenurosis of animals is diagnosed operatively by trepanation of the cranium. At the site of the blunted sound or softened bones of the skull, an operation is performed, and an arched incision of the skin and tissues to the periosteum is made. The periosteum is peeled away from the incision on both sides with the diameter of the cutter, 5–7 holes are made with a hand-held trepan, using a lance or spherical cutter so as not to “fall through” into the cranial cavity, and then the skull is opened. Diagnosis of sheep and saiga for moniziosis and echinococcosis in a comparative perspective was determined by studying the species composition, degree of invasiveness, seasonal, and age dynamics of the main helminthiasis of the gastrointestinal tract of animals (Skryabin, 1976). Studies of fecal excrement samples from sheep and saigas were carried out using the Fülleborn method. The seasonal dynamics of infection of sheep and saigas with the main types of gastrointestinal helminths were studied using helminthological pyptic and larvicoscopic methods of examination of feces. Surveys were conducted quarterly, in different seasons. The invasiveness was investigated in different age groups of sheep and saigas, i.e., under 1 year, from 1 to 3 years, from 4 to 5 years, and from 6 to 8 years. The genus of helminths was determined by invasive larvae using the Polyakov (1953) method. The physiological state of cysts in sheep of different ages suggests that sheep of 4 years and older play a major role in the epizootiology of echinococcosis. In the research, it was necessary to find out the age-specific infestation of saigas with echinococcosis. Helminth identification was carried out using determinants by Skryabin et al. (1952). Parenchymatous organs (liver and lungs) were examined to study larval dynamics. Echinococcosis in animals was determined by the detection of echinococcal blisters in organs (lungs, liver). Coenurosis must be differentiated from oestrosis (false spinning), caused by sheep gadfly larvae parasitising in the nasal and other head cavities, and also from moniziosis, accompanied by nerve phenomena (moniziosis segments are found in the feces of lambs). Pathological and anatomical autopsies were carried out by complete evisceration of the organs using the Shore method (Zharov et al., 2000). Results and DiscussionIn the saiga migration routes in Western Kazakhstan, 61 farms are operating in the Kaztalov district and 16 farms in the Zhanibek district. In both districts, the main direction of agriculture is meat cattle breeding. The number of cattle as of January 1, 2021 in Zhanibek district includes large cattle (LC)–46,537 animals, small cattle (SC)—53,964 animals, goats—6,341 animals, horses—16,713 animals, and camels—103 animals. The number of cattle as of January 1, 2021 in Kaztalov district: LG—100,187 animals, SC—216,524 animals, goats—33,6691 animals, horses—27,793 animals, camels—215 animals. The research studies epizootological indicators of the helminth fauna of saigas in order to assess the epizootic state of the area, and the causes of outbreaks of infestations (coenurosis, moniziosis, and echinococcosis) in farms in Kaztalov and Zhanibek districts of West Kazakhstan region, since these districts are the migration routes of the Ural saiga population. The study identified the main helminth species that have been identified in saigas in Western Kazakhstan, which are also parasitized in sheep and other agricultural ruminants. In a comparative study, there were distinctive differences in some of the individual helminth infestations between saigas and sheep. Both groups of animals are equally involved in the circulation of parasites in nature, and dogs play an active role in the spread of these parasites. In recent years, the number of domestic animals has decreased significantly and saiga numbers are not constant. The Ural saiga population during calving mainly migrates from the south-western part of Western Kazakhstan region to the North-Western part, to the territory of Zhanybek district, the reason for this migration is the rich forage base and abundance of water in the area. Epizootic situation of the region in terms of infectious diseases in Zhanibek district is good, and in Kaztalov district a case of rabies of one head of LC was registered in 2020. Since January 2021, no cases of animal mortality caused by infectious diseases have been registered. Tuberculinization of LC and camels is carried out annually, there are no positive reactions. Studies are conducted for the glanders of horses and the results for Kaztalov and Zhanibek districts are negative. Vaccinations against rabies, anthrax, emcarbies, pasteurellosis, and plague of camels are carried out on the general population of farm animals, as the territories of the two districts are not free from these diseases. For the successful prevention of parasitic diseases, regional studies of the epizootic process of infestation are of particular importance, which allows studying the peculiarities of their manifestation in a particular territory, with subsequent forecasting. For the given period, 35 helminth species of trematode, cestode, and nematode classes registered in animals in Western Kazakhstan are known. In recent years, the number of Ural saigas (Saiga tatarica) populations in the Western Kazakhstan region has been unstable; according to observations, clinical signs, and pathological anatomical changes in animal organs in parasitic diseases such as echinococcosis, coenurosis, and moniziosis are clear among saigas. The surveys of 61 farms in 15 farms of the Kaztalov region and 14 in 8 farms of the Zhanibek region which raise SC, showed that mortality of sheep with the diagnosis “coenurosis” was 15%–17%, among young sheep with “moniziosis” 7%–9%, and among sheep aged over 4 years—echinococcosis with an annual incidence of 25%–30%. Analysis of epizootic situation on echinococcosis in the Western Kazakhstan region in 2020 among animals in two districts shows that the percentage of infection among LC in Kaztalov district is 22.2%, and in Zhanibek district—6.8%. Among SM, the percentage of infection in the Kaztalov district is 21.7%, and in the Zhanibek district—19.6%. The specific composition of sheep helminths may have changed significantly in recent years because of various factors. One such factor is the wild animals inhabiting the territory of the Western Kazakhstan region (Yespembetov et al., 2019b). Saigas are the most numerous of these animals. The Ural saiga population mainly inhabits the southern districts of Kaztalov and Zhanibek districts. In recent years, the Ural saiga population (S. tatarica) in the Western region has been unstable and the observations show clinical signs and pathological changes in parasitic diseases among saigas. For laboratory tests, water and soil samples were taken for general chemical analysis, and pathological materials from dead saigas were collected for the presence or exclusion of infectious and parasitic animal diseases (Fig. 1). During an analysis of mortality among wildlife—saigas, data from the West Kazakhstan regional inspectorate of forestry and wildlife for the last 2 months of 2021 (March–April) shows that there were 115 saiga deaths of various etiologies. Of these, 14 saigas (12%) showed clinical signs of coenurosis, and in the grazing period, the deaths of young saigas with clinical signs of moniziosis in 2021 were 25%. Epizootological monitoring of farm animals in the saiga range has been carried out, and the boundaries of the hot spot and threatened zone have been defined. Together with the Kaztalov and Zhanibek rural districts, the organization of burial of carcasses of all species of animals, fencing of burial sites with marking of burial coordinates, disinfection of sick, and fallen animals were taken under control. An additional helminthological survey was conducted on saigas kept in the nursery of the Zhangir Khan West Kazakhstan Agrarian and Technical University, for which preliminary scientific research was carried out on comprehensive diagnosis for the presence of helminths.
Fig. 1. Taking a water and soil sample for general chemical analysis. During the period of nursery functioning from 2012 to the present day, studies have been conducted on the safety of young saigas, different diets have been developed and successfully tested in captive breeding, traumatic injuries to animals have been minimized, and a set of preventive measures against infectious and invasive diseases in saigas has been developed. The Zhangir Khan ZKATU breeding center (Sarsenova et al., 2013) was established in 2012 and is in Taskalin district, in the northwest of the region, bordering Zelenov district, Akzhaik and Kaztalov districts, and Saratov region of Russia. The nursery holds about 19 saigas of the Ural population, 10 adults and 9 juveniles. The climate in the west is moderately continental, with the highest average monthly rainfall in July (340 mm), in October (320 mm), and the lowest in February (140 mm). The average annual precipitation is 290 mm. In the south, the climate is moderately continental, insufficiently humid with moderately mild winters; the average monthly temperature in January is −14.0°C, in July +23.4°C, and the average annual precipitation is 248 mm. Adult saigas in the breeding center were kept in enclosures, and insulated enclosures were provided for young saigas for a certain period (Petrischev et al., 1998; Minoransky and Tolcheeva, 2010). The total area of the aviary complex is 1,050 m2, and each aviary (section) is 150 m2. A special feature of the aviary is its compactness and good conditions for working with a small group of animals. Each aviary has special sections for keeping adults. When keeping animals in a confined area, one must take into consideration that they are skittish, and their immediate reaction to humans, dogs, and other living objects. When keeping easily excitable and fearful saigas in captivity, the size, shape, and nature of enclosures are among the most important aspects of their success in captivity. Animal care, housing, feeding, and several zoohygienic requirements are important for the diagnosis and prevention of parasitic diseases in saigas. Figures 2 and 3 show saiga feeding under captive breeding conditions. Among the diversity of grasses, only a few families form the main forage for saigas: cereals, Compositae, Chenopodiaceae, and crucifers. The grasses of the study area were based on herbs (Artemisia) and cereals. In terms of nutritional value related to biochemical composition, plants are arranged in the following order. Crucifers are the crucifers, followed by Compositae, and in the last place are cereals. In general, most of the vegetation available in the pasture is of the required quality. Additional fecal and blood samples were also taken from saigas kept under nursery conditions (Fig. 4) for comparative helminthological and hematological testing. To carry out therapeutic and prophylactic measures (Shodmonov and Vasiliev, 2005; Shabdarbayeva et al., 2019) against parasitic diseases in captive saigas, measures were taken to determine the body weight of saigas by weighing, measuring, and thermometry (Figs. 5–7). Conducted researches in peasant farms of the Western Kazakhstan region, Kaztalov and Zhanibek districts engaged in breeding and growing of small ruminants reveal daily all-year-round mortality of sheep with the diagnosis: “coenurosis,” among young sheep “moniziosis,” and sheep older than 4 years with echinococcosis. Repeated observations of clinical signs of coenurosis (Chubaryan et al., 1996) in many wild saigas have manifested in the following form: sick animals lag behind the herd, do not cross roads, run large circular movements around the axis, then fall down with signs of nervous seizures, and maneuvring movements of fore and hind limbs of a swimming nature are clear. Because of signs of purulent hemorrhagic conjunctivitis, saigas lose their vision and, because of metabolic disorders (dystrophy) and signs of malnutrition, there is dehydration with obvious signs of moulting. They die with clinical signs of “Cachexia”—exhaustion and intracranial pressure. Death and mortality of saigas of different ages occur throughout the territory, in all habitats, without an established diagnosis.
Fig. 2. Feeding saiga calves.
Fig. 3. Saiga calves in an aviary. A pattern of mortality in wild saigas was observed in young saigas showing signs of moniziosis, rarely the nervous form, with the disease mostly showing signs during the grazing period, with saigas becoming sluggish, thin, and lagging behind the herd. Sick saiga calves aged 5–6 months become diarrhoeic, weak, and often lie down; clear signs of diarrhea lead to dehydration and exhaustion. Signs of intestinal congestion with moniezia appeared as intestinal colic. The young saigas would start to twist, fall to the ground, and kick their limbs. The diagnosis of saiga moniziosis was confirmed by well-grounded coprological and pathological anatomical examinations of the dead saigas, and it was possible to isolate moniziosis from the saigas by giving antihelminthic drugs in the saiga nurseries environment (Fig. 8). Echinococcosis of saigas was common among adult saigas. The results of comprehensive studies have made it possible to objectively assess the epizootological situation regarding sheep and saiga helminths in the ecosystems of the region, and the adoption of veterinary and sanitary measures has made it possible to halt the further spread of animal helminth infections. In the summer in the steppes of the Western Kazakhstan region, with the onset of heat, by the end of June months the pastures burnout, and the specific weight of the grass vegetation decreases, which contributes to a certain extent to the “purification” of pastures from infestation elements (Biyashev et al., 2019). Studies have established that saiga echinococcosis occurs frequently in adults; the physiological condition of cysts in sheep of different ages shows that the main role in the epizootiology of echinococcosis is played by sheep aged 4 years and over, while in wild saiga the age of 2-year-old animals was found to be predominantly affected by echinococcosis. The parenchymatous organs (liver and lungs) were examined to study larval dynamics.
Fig. 4. Saigas kept in the Zhangir Khan WKATU nursery.
Fig. 5. Determining saiga body weight by weighing and measuring.
Fig. 6. Performing thermometry on a sick saiga calves. The results of saiga helminthological sampling (Yakov et al., 1963; Jatusevitch, 2000) for the summer period showed the following parameters: an infestation of saigas by M. expansa—83.34%, Echinococcus granulosis larvae—16.66%, Coenurus cerebralis—16.66%, N. spathiger—100%, Trichocephalus ovis—66.66%. The infestation rate of saigas by coprological survey during the summer period was Oesophagostomum venulosum—5.94%, N. spathiger—5.94%, Nematodirella sp. —5.94%, M. expansa—4.28%, T. skrjabini—4.28%, E. elegans—88.57%, for autumn period: N. spathiger—11.76%, M. expansa—12.34% (Fig. 1), S. papillosus—11.76%, E. elegans—76.47%, E. tekenovi—76.47% (Fig. 8).
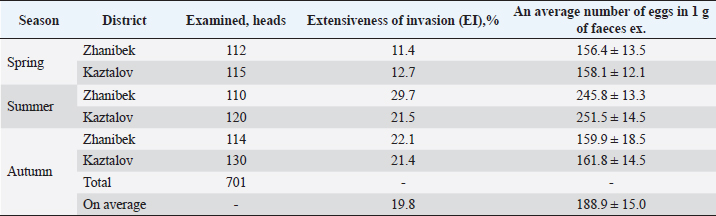
Fig. 7. Weighing a saiga calves. The infestation of SC (sheep) with helminths of moniezia in the West Kazakhstan region is demonstrated in Table 1, depending on environmental factors (Umitzhanov et al., 2022). The links between wild and domestic ruminants in grazing areas lead to a composite commonality of helminths detectable in autopsy and coprological studies. Wildlife animals are susceptible to various parasitic diseases that are often fatal. Although adult animals may be less infected than young animals, they are an important source of the infestation and contribute to its spread. The threat of transmission from wild animals to domestic animals, humans, and vice versa, is possible when parasitic diseases are widespread (Kukhtyn et al., 2020). One of the epizootologically important features of the study region is that in semi-desert climate zones (southern Kaztalov region) because of climate change (environmental factor) almost all year round sheep grazing is practised, which is one of the indicators of high infestation of both domestic and wild animals. Also important in the geography of helminth distribution is the fact that the study areas (Zhanibek and Kaztalov districts) are the main areas where saigas live. In summer, they are in the steppe zone (Kaztalov and Zhanibek districts of Western Kazakhstan region), where it is cool and there is enough water and forage. In autumn, they move to the semi-desert zone (Zhangalin, Bokeyordin districts of Western Kazakhstan region) and in winter to the desert on the border with the Atyrau region. Table 1. An infection rate of SC with Moniezia in the West Kazakhstan region depending on the geographical location of the area and climatic conditions.
As the feeding areas are shared, large numbers of livestock and wild ungulates are concentrated in limited areas, which contributes both to the formation of common parasites among domestic and wild animals and to an increase in the population of parasitic pathogens in the external environment (Fig. 9). Such a position of anthropogenic factor influences the epizootic situation as a whole. It has been established that in spring, summer, and early autumn the infestation of animals with many types of helminths increases, while in winter the indicators decrease (Yespembetov et al., 2019a). The findings confirm the conclusions of other scientists who have studied the epizootic situation of saiga parasitic diseases, that their seasonality is directly related to climatic, environmental, and anthropogenic factors in each region. Clinical signs of coenurosis in many wild saigas were as follows, with sick animals lagging behind the herd and making circular movements around their axis. An autopsy was carried out on a saiga corpse (Shishkov and Naletov, 1996), and the brain was examined for coenurosis (Fig. 10). Fecal samples were taken for helminthological and coprological examination, and saiga carcasses were disposed of by incineration (Fig. 11). On saiga migration routes in the farms of Kaztalov and Zhanibek districts during the breeding season (May–June, Kuralay period), clinical signs of moniziosis in saiga calves were manifested by acute colic and mortality among young animals (Fig. 12).
Fig. 8. Intravital diagnosis of moniziosis in saigas. Saiga moniziosis was confirmed by sound helminthological, coprological, and pathologist examinations of dead saigas. In the period from April to May 2021, saiga mortality because of difficult births was observed in the Kaztalov district during the breeding season, where animal carcasses were also autopsied and gastrointestinal contents were taken for helminthological and parenchymal organs for pathomorphological examination (Fig. 13). The helminth fauna research will make it possible to identify helminth species dangerous for saigas, the devastation of which is the primary task in the conservation of the wild fauna, which is the heritage of the Republic. Measures to reduce contact between saigas and farm animals and the elimination of outbreaks of infestation will make it possible to halt the seasonal continuing mortality of saigas. Epizootological and environmental monitoring of farm animals in the saiga range should be carried out, and the boundary of the hot spot and endangered zone defined (Nguyen et al., 2020; Issimov et al., 2021). The probability of contact between farm animals and saigas is highest in the Western Kazakhstan region, as saigas migrate long distances throughout the year, including across the borders of neighboring countries, and come into contact with domestic animals on their way. Pathogens can be exchanged between wild and domestic animals because of contact. Circulation of helminths of domestic and wild animals occurs in mixed natural foci of the Western Kazakhstan region.
Fig. 9. Common feeding areas of wild and domestic animals.
Fig. 10. Autopsy and postmortem examination of the saiga brain with coenurosis.
Fig. 10. Disposal of a saiga carcass by incineration.
Fig. 11. Mortality of saiga calves showing signs of intestinal blockage.
Fig. 12. Difficult saiga childbearing during the breeding season in the Kaztalov district. ConclusionThe results of the research confirm the necessity to develop urgent measures for the devastation of detected parasites, first, among sheep on peasant farms in the Western Kazakhstan region. The organization of burial of corpses, fencing of burial places with marking of burial coordinates, disinfection of places where sick and fallen animals are found. The development of preventive measures for helminth infections of animals, to ensure epizootic welfare for invasive diseases, is a very complex but high-priority task of veterinary science and practice. Further studies of the saiga helminth fauna using molecular genetic diagnostic techniques should be conducted, which will provide new data on the species composition of the parasites and enable scientifically based recommendations for the conservation of the saiga population in Kazakhstan to be prepared. The main source of invasiveness remains at the animals’ watering points. Ecologization of watering places is necessary in order to reduce the pressure on them, which leads to a reduction in disease and recovery of wild and domestic animals. There is a need for further development of new scientifically based, effective implementation programs, recommendations on comprehensive diagnosis, and urgent preventive measures in saigas diagnosed with helminth infections (coenurosis, moniziosis, and echinococcosis), as these diseases could become enzootic shortly in the Western region. Developing and implementing measures to reduce contact between saigas and farm animals, eradicate outbreaks of infestation, and halts ongoing seasonal mortalities in saigas and sheep. Addressing aspects of the problems of helminths in sheep and saigas will allow a scientifically validated integrated system of helminth disease control measures to be put in place. Hence, regular helminthological and environmental monitoring in wild and domestic animal populations is necessary to control parasitic infestations and invasion levels. ReferencesBaitursinov, K.K. 2005. Saiga antelope helminths in Kazakhstan. Bull. Toraigyrov Univ. 2, 67–81. Balgimbayeva, A., Shabdarbaeva, G., Zhanteliyeva, L., Ibazhanova, A. and Khussainov, D. 2019. Diagnostics and treatment of dioctophymosis in dogs. Izvest. Nac. Akad. Nauk Resp. Kazah. 6(54), 5–12. Biyashev, K.B., Kirkimbaeva, Z.S., Biyashev, B.K., Makbuz, A.Z. and Bulegenova, M.D. 2019. Determination of the level of resistance of probiotic strain Escherichia coli 64g to hydrochloric acid, bile and antimicrobial agents. Ecol. Environ. Conserv. 25(4), 1930–1933. Bulegenova, M., Biyashev, K., Kirkimbaeva, Z., Biyashev, B., Ermagambetova, S., Oryntayev, K. and Altenov, A. 2019. The effect of the drug enterocol on the humoral factors of calf body resistance. Adv. Anim. Vet. Sci. 7(8), 674–680. Chubaryan, F.A., Movsesyan, R.O. and Kurbet, A.V. 1996. Biomorphological features of multiceps phytomorphological. experiments on laboratory animals. Vet. Med. 2(6), 67–72. Goltsman, M.E. 2011. Behavior. Ecology and conservation biology. Moscow, Russia: IPEE RAS. Issimov, A., Taylor, D.B., Shalmenov, M., Nurgaliyev, B., Zhubantayev, I., Abekeshev, N., Kushaliyev, K., Kereyev, A., Kutumbetov, L., Zhanabayev, A., Zhakiyanova, Y. and White, P.J. 2021. Retention of lumpy skin disease virus in Stomoxys spp. (Stomoxys calcitrans, Stomoxys sitiens, Stomoxys indica) following intrathoracic inoculation, Diptera: Muscidae. PLoS One 16(2), e0238210; doi:10.1371/journal.pone.0238210 Jatusevitch, A.I. 2000. Little-studied and new parasitic diseases of animals. Ed. Method Manual Vet. 2, 67–68. Kosminkov, N.E. 2004. Prospects for immunoprophylaxis of helminthiases in animals. Actual problems of veterinary medicine, veterinary and sanitary control and biological safety of agricultural products. Moscow, Russia: Biotechnology State University. Kosminkov, N.E., Laipanov, B.K. and Verkhovskaya, G.L. 1995. New in the prevention of coenurosis in sheep. Actual Probl. Vet. San. Contr. Agric. Prod. 1, 87–88. Kukhtyn, M., Salata, V., Berhilevych, O., Malimon, Z., Tsvihun, A., Gutyj, B. and Horiuk, Y. 2020. Evaluation of storage methods of beef by microbiological and chemical indicators. Potrav. Slov. J. Food Sci. 14, 602–611. Kushaliev, K.Z. and Uazirov, M.S. 2018. The state of the immune system of saigas in captivity. Qual. Manag. Search Sol. 27, 409–415. Lazarev, G.M. and Durdusov, S.D. 1997. Kalmyk research institute of agriculture. Elista. Epizootology of sheep coenurosis in the arid zone. Topic. Issues Theor. Appl. Trematodol. Cestodol. 7, 85–87. Manjiev, B.A., Sisoeva, N.U. and Verkhovskaya, G.L. 2002. Vaccination of lambs against parasitic diseases in the farms of the Maloderbetovsky district of the Republic of Kalmykia. Actual Prob. Young Dis. Mod. Cond. 3, 392–395. Minoransky, V.A. and Tolcheeva, S.V. 2010. Aviary keeping of the saiga (Saiga tatarica L.). Rostov-on-Don, Russia: Kovcheg. Nguyen, T.B., Paul, R.C., Okuda, Y., Pham, P., Kaissar, K.J., Kazhmurat, A., Bibigul, S., Bakhtin, M., Kazymbet, P., Maratbek, S.Z., Meldebekov, A., Nishibori, M., Ibi, T., Tsuji, T. and Kunieda, T. 2020. Genetic characterization of Kushum horses in Kazakhstan based on haplotypes of mtDNA and Y chromosome, and genes associated with important traits of the horses. J. Equine Sci. 31(3), 35–43. Petrischev, B.I., Maksimuk, A.V. and Abaturov, B.D. 1998. Breeding keeping saigas in captivity (method of capturing and keeping young saigas). Moscow, Russia: Kolos. Polyakov, P.A. 1953. Lifetime differential diagnostics of digestive tract strongylatosis of ruminants on invasive larvae. Moscow, Russia: Moscow State University. Pryadko, E.I., Shol, V.A., Beisova, T.K., Teterin, V.I. and Boev, S.N. 1964. Helminths of Cervus elaphus sibiricus and C. nippon and their distribution on deer farms in the Altay region of the Kazakh SSR. Almaty, Kazakhstan: Nauka. Sarsenova, B.B., Sergaliev, N.H. and Usenov, Z.T. 2013. Organization and creation of a nursery for saigas in Kazakhstan. Saiga Conserv. Alliance 3, 72–76. Shabdarbayeva, G.S., Ibazhanova, A.S. and Ivanov, N.P. 2019. Providing veterinary care for parasitic diseases of farm animals in Bayserke-Agro LLP. Bull. Nat. Acad. Sci. Rep. Kazakh. 50(2), 99–104. Shishkov, V.P. and Naletov, N.A. 1996. Autopsy technique and pathomorphological diagnostics of animal diseases. Moscow, Russia: Kolos. Shodmonov, I.V. and Vasiliev, F.I. 2005. Specific prevention of helminthiases in sheep semi-finished vaccine. Vet. Med. 3(4), 16–18. Skryabin, K.I., Shikhobalova, N.P. and Schultz, R.S. 1952. Strongyloides. Series determinant of parasitic nematodes. Moscow, Russia: Publishing House of Academy of Sciences of the USSR. Skryabin, K.I. 1976. Veterinarian encyclopedia. Moscow, Russia: Sovetskaya Entsiklopediya. Umitzhanov, M., Musaeva, A.K., Abishov, A.A., Zhamansarin, T.M., Omarbekova, U.Z., Turyspayeva, S.Z. and Siyabekov, S.T. 2022. Approaches to reducing the toxic exposure hazard on the sheep population. Cell Tissue Bank. 23(4), 753–765. Yakov, T.A., Boev, S.N., Sokolova, I.B. and Panin, V.Y. 1963. Helminths of ungulates of Kazakhstan. Almaty, Kazakhstan: Nauka. Yespembetov, B.A., Syrym, N.S., Syzdykov, M.S., Kuznetsov, A.N., Koshemetov, Zh.K., Mussayeva, A.K., Basybekov, S.Z., Kanatbayev, S.G., Mankibaev, A.T. and Romashev, C.M. 2019a. Impact of geographical factors on the spread of animal brucellosis in the Republic of Kazakhstan. Comp. Immunol. Microbiol. Infect. Dis. 67, 101349. Yespembetov, B.A., Syrym, N.S., Zinina, N.N., Sarmykova, M.K., Konbayeva, G.M., Basybekov, S.Z., Mussayeva, A.K., Kanatbayev, S.G., Bazarbayev, M. and Siyabekov, S.T. 2019b. Phenotypic and genotypic characteristics of Brucella isolates from the Republic of Kazakhstan. Trop. Anim. Health Prod. 51(8), 2361–2370. Zharov, A.V., Ivanov, I.V. and Kunakov, N.A. 1982. Autopsy and pathological diagnosis of diseases of farm animals. Moscow, Russia: Kolos. Zharov, A.V., Kunakov, N.A. 1983. Pathological anatomy of farm animals. Moscow, Russia: Kolos. Zharov, A.V., Ivanov, I.V. and Strelnikov, A.P. 2000. Autopsy and pathomorphological diagnostics of animal diseases. Moscow, Russia: Kolos. | ||
| How to Cite this Article |
| Pubmed Style Kushaliyev K, Ussenov Z, Alimbekov S, Mullakaev O, Kozhayeva A, Khairushev A. Study of the saiga helminth fauna and Ural sheep in the western region of Kazakhstan. Open Vet J. 2023; 13(4): 485-494. doi:10.5455/OVJ.2023.v13.i4.11 Web Style Kushaliyev K, Ussenov Z, Alimbekov S, Mullakaev O, Kozhayeva A, Khairushev A. Study of the saiga helminth fauna and Ural sheep in the western region of Kazakhstan. https://www.openveterinaryjournal.com/?mno=137526 [Access: November 08, 2024]. doi:10.5455/OVJ.2023.v13.i4.11 AMA (American Medical Association) Style Kushaliyev K, Ussenov Z, Alimbekov S, Mullakaev O, Kozhayeva A, Khairushev A. Study of the saiga helminth fauna and Ural sheep in the western region of Kazakhstan. Open Vet J. 2023; 13(4): 485-494. doi:10.5455/OVJ.2023.v13.i4.11 Vancouver/ICMJE Style Kushaliyev K, Ussenov Z, Alimbekov S, Mullakaev O, Kozhayeva A, Khairushev A. Study of the saiga helminth fauna and Ural sheep in the western region of Kazakhstan. Open Vet J. (2023), [cited November 08, 2024]; 13(4): 485-494. doi:10.5455/OVJ.2023.v13.i4.11 Harvard Style Kushaliyev, K., Ussenov, . Z., Alimbekov, . S., Mullakaev, . O., Kozhayeva, . A. & Khairushev, . A. (2023) Study of the saiga helminth fauna and Ural sheep in the western region of Kazakhstan. Open Vet J, 13 (4), 485-494. doi:10.5455/OVJ.2023.v13.i4.11 Turabian Style Kushaliyev, Kaissar, Zhangeldi Ussenov, Serik Alimbekov, Orazali Mullakaev, Aigerim Kozhayeva, and Artur Khairushev. 2023. Study of the saiga helminth fauna and Ural sheep in the western region of Kazakhstan. Open Veterinary Journal, 13 (4), 485-494. doi:10.5455/OVJ.2023.v13.i4.11 Chicago Style Kushaliyev, Kaissar, Zhangeldi Ussenov, Serik Alimbekov, Orazali Mullakaev, Aigerim Kozhayeva, and Artur Khairushev. "Study of the saiga helminth fauna and Ural sheep in the western region of Kazakhstan." Open Veterinary Journal 13 (2023), 485-494. doi:10.5455/OVJ.2023.v13.i4.11 MLA (The Modern Language Association) Style Kushaliyev, Kaissar, Zhangeldi Ussenov, Serik Alimbekov, Orazali Mullakaev, Aigerim Kozhayeva, and Artur Khairushev. "Study of the saiga helminth fauna and Ural sheep in the western region of Kazakhstan." Open Veterinary Journal 13.4 (2023), 485-494. Print. doi:10.5455/OVJ.2023.v13.i4.11 APA (American Psychological Association) Style Kushaliyev, K., Ussenov, . Z., Alimbekov, . S., Mullakaev, . O., Kozhayeva, . A. & Khairushev, . A. (2023) Study of the saiga helminth fauna and Ural sheep in the western region of Kazakhstan. Open Veterinary Journal, 13 (4), 485-494. doi:10.5455/OVJ.2023.v13.i4.11 |