| Research Article | ||
Open Vet J. 2023; 13(5): 541-549 Open Veterinary Journal, (2023), Vol. 13(5): 541–549 Original Research Ultrasonographic appearance and possible clinical relevance of hyperechoic foci of mineralization in the canine intrahepatic biliary treeFederico Puccini Leoni1*, Caterina Puccinelli1, Tina Pelligra1, Eleonora Gori1, Veronica Marchetti1, Alessia Diana2, Nikolina Linta2 and Simonetta Citi11Veterinary Teaching Hospital “Mario Modenato,” Department of Veterinary Sciences, University of Pisa, Pisa, Italy 2Department of Veterinary Medical Science, Alma Mater Studiorum, University of Bologna, Bologna, Italy *Corresponding Author: Federico Puccini Leoni. Veterinary Teaching Hospital “Mario Modenato,” Department of Veterinary Sciences, University of Pisa, Pisa, Italy. Email: federico.puccini33 [at] gmail.com. Submitted: 18/01/2023 Accepted: 08/04/2023 Published: 07/05/2023 © 2023 Open Veterinary Journal
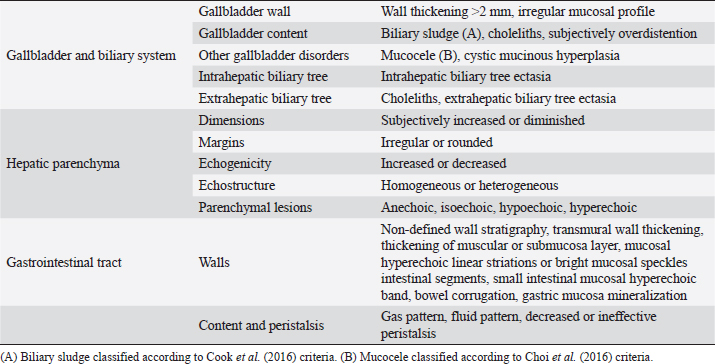
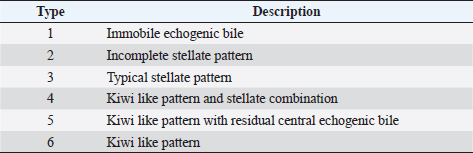
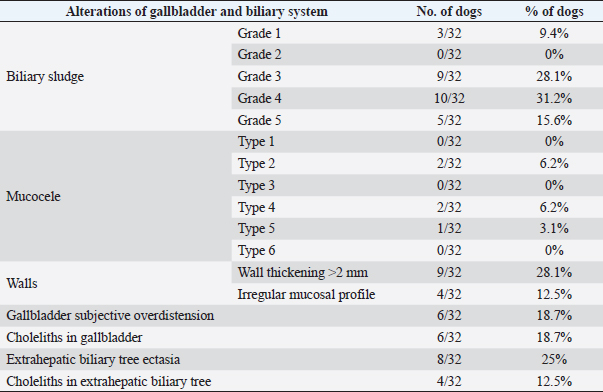
AbstractBackground: The evidence of mineralizations in the canine liver is usually considered an incidental finding of unclear clinical significance, frequently observed in small-size old dogs. Aim: To describe the ultrasound features of intrahepatic biliary tree foci of mineralization, to assess their clinical relevance and their possible relationship with other gastrointestinal pathological disorders. Methods: A retrospective analysis evaluating the database of canine patients admitted to two referral veterinary centers we carried out. All dogs under study underwent an abdominal ultrasound examination in which intrahepatic biliary tree mineralization was found. Clinical and anamnestic data of the included dogs were reviewed. Results: Approximatively 90% of the patients showed ultrasonographic abnormalities regarding the biliary system, and over 85% presented ultrasonographic abnormalities of the hepatic parenchyma. In 81.2% of dogs, ultrasonographic anomalies in the digestive tract were observed. In approximately half of our patients, we evidenced increased liver enzymes (alkaline phosphatase, alanine aminotransferase, and gamma-glutamyl transferase). At the clinical evaluation, 84.4% (23 out of 32 dogs) of patients showed signs of gastrointestinal disease that persisted for over 3 months. Conclusion: The presence of intrahepatic biliary tree mineralizations is an unusual and frequently incidental finding that could be related to a bile stasis condition, a chronic inflammatory disease involving the biliary system and the hepatic parenchyma, and it could be associated with a liver-gut axis alteration. Keywords: Intrahepatic biliary ducts, Cholelithiasis, Liver, Abdominal ultrasound, Dog. IntroductionIn dogs, ultrasonographic and radiographic detection of mineralized structures in the gall bladder or choledochus lumen is much more frequent than the detection of intrahepatic bile ducts and hepatic parenchymal mineralizations (Cantwell et al., 1983; Lamb et al., 1991; Moon Larson, 2013; Nyland et al., 2019). Based on previous literature, the prevalence of hepatic mineralization in dogs is very low, frequently observed in small-size old dogs, and usually associated with scarce or no clinical significance (Wisner and Zwingenberger, 2015; Genain et al., 2018). A predisposition in Cavalier King Charles Spaniel is reported, although the prevalence of histopathologically-confirmed primary hepatic disease at post-mortem examination in this breed is very similar to the general canine population (Kent et al., 2016; Genain et al., 2018). Hepatic mineralizations are often discovered as incidental findings during abdominal ultrasound (AU) examination and, if radiopaque, they can be confirmed by radiographs (Nyland and Park, 1983; Nyland and Hager, 1985) or computed tomography (CT) images (Bertolini, 2017). In small animal diagnostic imaging, AU is the most used imaging modality for patients with suspected hepatobiliary disease (Washabau, 2013a, 2013b). In healthy dogs, normal intrahepatic bile ducts are not ultrasonographically observed (Nyland et al., 2019). Otherwise, they can be visualized if there is an ectasia of the intrahepatic biliary tree because of a complete extrahepatic obstruction that persists for over 5–7 days (Nyland et al., 2019), or if mineralizations with linear branching appearance are present in the intrahepatic biliary ducts (Genain et al., 2018). On ultrasound, choleliths in the biliary ducts typically appear as well-defined, hyperechoic shadowing foci, and are generally differentiated from parenchymal lesions because of their gravitational mobility (Center, 2009). At AU, distal acoustic shadowing is usually present and becomes more evident as the mineralization size and calcium content increase, although distal acoustic shadowing can be occasionally absent (Center; 2009; Nyland et al., 2019). Some authors assume intrahepatic choleliths could result from bile flow stasis, chronic biliary tree duct inflammation, or a consequence of congenital malformations (Center, 2009; Bertolini, 2017). Many pathological alterations, such as granulomas, abscesses, neoplasms, hematomas, gas, and hepatic necrosis, can be related to the presence of shadowing lesions with dystrophic calcification, and abdominal radiography or CT may be necessary for a final determination (Washabau, 2013a, 2013b; Nyland et al., 2019). According to other authors, mineralization of the biliary tree may be also associated with bile duct carcinoma, as reported in a dog (Thamm, 2001). In cats, mineralized choleliths observed at abdominal radiographs can be referrable to dystrophic mineralizations of small biliary ducts secondary to chronic cholangitis (Center, 2009). In humans, CT-perceived liver mineralizations in the intrahepatic biliary tree can be primarily caused by bile stasis, bile infection, malnutrition, parasitic infections, and inflammatory or infectious liver diseases such as cholangiohepatitis (Paley and Ros, 1998; Araujo Bezerra et al., 2003; Tazuma, 2006). To date, in dogs, the clinical significance of ultrasonographic-detectable intrahepatic biliary tree foci of mineralization (IBTM) remains unclear. The aim of the present study is to describe IBTM ultrasound features, to evaluate the clinical relevance and a possible association between IBTM and hepatic, biliary, and/or gastrointestinal disorders. We also investigated a possible involvement of gastrointestinal tract diseases based on ultrasonographic and clinical data. Materials and MethodsA retrospective analysis of the medical database searching for dogs admitted to two referral centers from January 2012 to February 2022 was performed. To be included, dogs had to meet the following criteria: (1) complete AU examination with the presence of intrahepatic ultrasonographic-detectable IBTM with AU features referrable to an intrahepatic biliary duct localization and complete clinical evaluation, including signalment, physical examination, clinical signs, and biochemical profile. Dogs that presented exclusively randomly distributed intrahepatic foci of mineralization, and for which it was not possible to be certain of their biliary duct localization, were excluded from the study population. Ultrasonographic-detectable intrahepatic foci of mineralizationIBTM consists of hyperechoic structures or interfaces of different sizes, with or without distal acoustic shadowing, localized in the lumen of the intrahepatic biliary ducts, seeming to reproduce the shape of the intrahepatic biliary tree. For each dog, we evaluated the IBTM shape, size, localization, distribution pattern, and the presence or absence of artifacts. Various localizations were then distinguished: left hepatic lobes (i.e., left medial lobe, left lateral lobe), central hepatic lobes (i.e., quadrate lobe, papillary process of caudate lobe), right hepatic lobes (i.e., right medial lobe, right lateral lobe, caudate process of caudate lobe), or widespread distribution, if IBTM were distributed throughout the liver. Complete AU examinationAll included dogs underwent a full AU, including a systematic description of the digestive tract, hepatic parenchyma, and gallbladder, intrahepatic and extrahepatic bile ducts. All AUs were performed by experienced radiologists. All ultrasonographic images and video files were stored in DICOM format and retrospectively blindly reviewed by the same third operator. The AU images were obtained using a Toshiba Aplio 400 (Canon Medical Systems Europe B.V., Zoetermeer, The Netherlands) and an iU22 ultrasound system (Philips Healthcare, Monza, Italy) with a 7.5 MHz micro-convex probe and a 12 MHz linear probe. For each patient, we evaluated the presence of the ultrasonographic-observed alterations, summarized in the following Table 1. Referring to the presence of biliary sludge we followed the classification proposed by Cook et al. (2016) as reported in Table 2, while we referred to Choi et al. (2014) classification to define different grades of mucocele, as synthesized in Table 3. Referring to the ultrasonographic abnormalities, we grouped gastrointestinal alterations for each part of the digestive tract (i.e., stomach, small intestine, and large intestine) and divided them into three main groups (i.e., alterations of content, walls, and peristalsis). Clinical evaluationComplete recent and remote clinical history, with particular focus on gastrointestinal signs and physical examination, were performed by two experienced clinicians, and recorded for each dog. The biochemical profile was carried out in certificated veterinary laboratories of clinical pathology using an automated biochemistry analyzer (Liasys SAT 450, Assel, Rome, Italy) in both centers. The biochemical profile included the following parameters: alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), aspartate aminotransferase (AST), alanine aminotransferase (ALT), cholesterol, triglycerides, total bilirubin, total protein, and albumin. Statistical analysisStatistical analysis was performed using a commercial statistical software (GraphPad Prism 7.0, GraphPad Software Inc, San Diego, CA). Normality for quantitative variables was assessed by the Shapiro-Wilk test. Data are reported as mean and standard deviation or median and range for normally and non-normally distributed variables, respectively. Ethical approvalNot needed as this was a retrospective study. Table 1. Summary of all ultrasonographic features evaluated during AU.
Table 2. Ultrasonographic gallbladder scoring by Cook et al. (2016).
Table 3. Ultrasonographic mucocele scoring by Choi et al. (2013).
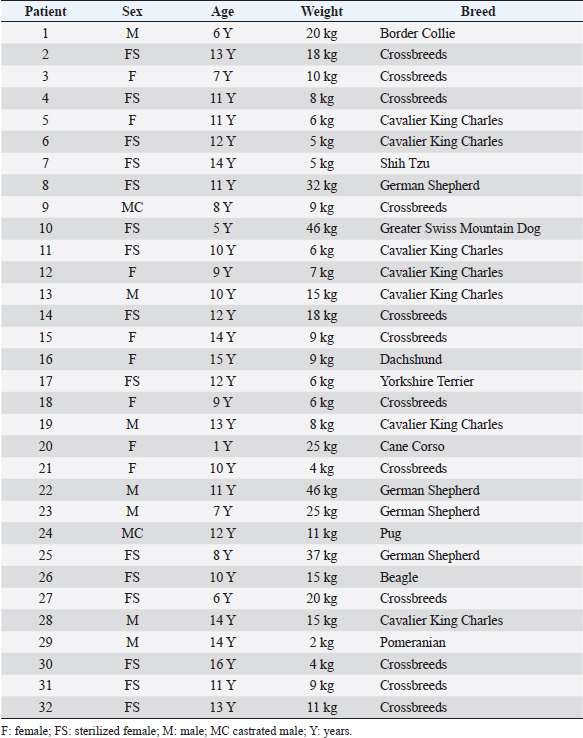
ResultsThe study population was composed of 32 dogs. There were 23 females (71.9%), 15 of which were sterilized, and 9 males (28.1%), 2 of which were castrated. Twelve dogs were crossbreeds, while the other subjects belonged to the following breeds: Cavalier King Charles (n =7), German Shepherd (n=4), Border Collie (n =1), Shih Tzu (n =1), Dachshund (n =1), Beagle (n=1), Greater Swiss Mountain Dog (n=1), Pug (n=1), Cane Corso (n=1), Yorkshire Terrier (n=1), and Pomeranian (n=1). The dogs’ mean age was 10.5 years, with a standard deviation of 3.3 years (range 1.0–16.0 years). The median weight was 9.5 kg (range 2.0–46.0 kg). Information concerning the sex, age, weight, and breed of all dogs included in the study is summarized in Table 4. Ultrasonographic-detectable foci of mineralizationFrom the retrospective review of the ultrasound images and based on IBTM size, they were divided into two groups: millimetric IBTM and sub-centimetric or centimetric IBTM. Millimetric foci of mineralization (if the dimension was less than or equal to 3 mm and with/without distal acoustic shadow) were observed in 14 out of 32 dogs (43.7%). In each dog with millimetric IBTM, mineralizations were all round-shaped, and they seemed to be positioned in the smaller intrahepatic biliary ducts. Sub-centimetric or centimetric foci of mineralization (if dimensions were from 4 mm to over 10 mm with a distal acoustic shadow) were observed in 18 out of 32 dogs (56.2%). They had an elongated shape and were positioned in larger intrahepatic bile ducts. Two mineralization patterns were observed: aligned and branched patterns. Table 4. Summary of the main data for each patient of the population study.
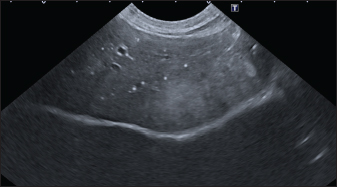
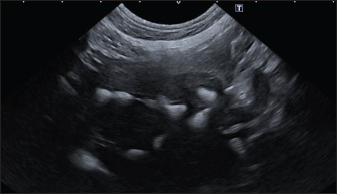
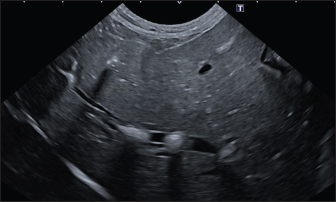
In the aligned pattern, mineralization foci are often millimetric, rarely sub-centimetric, forming isolated linearities in the hepatic parenchyma, without a real branching pattern, seeming to follow the course of some intrahepatic biliary ducts (Fig. 2). Sub-centimetric and centimetric IBTM determined a focal dilatation of the biliary ducts in 34.4% of dogs (11/32). In eight of these subjects (25%), we also found millimetric IBTM randomly distributed in the liver. In the branched pattern, mineralizations were usually sub-centimetric or centimetric and they replicated the shape of the intrahepatic biliary tree (Fig. 3). Also in this case, sub-centimetric and centimetric IBTM determined a focal dilatation of the biliary ducts observed in 65.6% of dogs (21/32) (Fig. 4). In six of them (18.7%), we found millimetric IBTM randomly distributed in the liver.
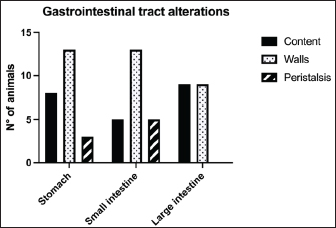
Fig. 1. Different kinds of ultrasonographic alteration affecting the digestive tract.
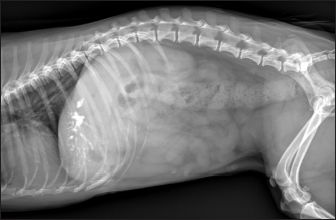
Fig. 2. Ultrasound image of the liver showing millimetric IBTM not generating acoustic shadow, with an aligned pattern. As shown in Table 5, the most represented localization was the widespread one, while we noted a prevalent localization on the right lobes compared to the central and the left lobes. In two dogs (6.2%) that showed sub-centimetric or centimetric foci of mineralization distributed in a branched pattern, we found diffuse intrahepatic biliary tree ectasia. Only two dogs underwent a radiographic examination of the abdomen: in both cases, the site of IBTM observed ultrasonographically was confirmed by radiographs, and in particular, they were localized on the ventral portion of the right lobes with a branched mineralization pattern. In both cases, IBTM was sub-centimetric or centimetric (Fig. 5). Ultrasound of gallbladder and biliary systemOnly 3 dogs out of 32 (9.4%) presented uniformly hypoechoic bile without sediment (biliary sludge grade 1), and the remaining subjects (90.6%) showed moderate-to-severe gallbladder or biliary system disorders. Table 6 summarizes all ultrasonographic findings of the gallbladder and the biliary tree. Two dogs had undergone a previous cholecystectomy to treat type 6 mucocele.
Fig. 3. Ultrasound image of the liver showing sub-centimetric and centimetric IBM, generating acoustic shadow, with a branched pattern.
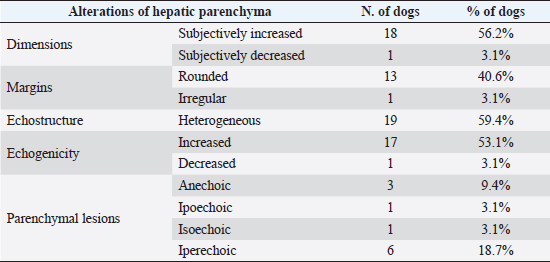
Fig. 4. Ultrasound image of the liver showing centimetric IBTM, generating acoustic shadow, with an aligned pattern. In the image, we can observe a focal dilation of the larger biliary duct, upstream and downstream to the calculi, because of the presence of the choleliths themselves. Ultrasound of hepatic parenchymaAbnormalities of the hepatic parenchyma were present in 28/32 dogs (87.5%). In six of them (18.7%), IBTM was present as the only AU abnormality, without the presence of other ultrasonographic signs of liver disease. In more than half of these 28 subjects, there was a subjective increase in liver size (56.2%), an inhomogeneous echo structure (59.4%), and a diffuse increase in echogenicity (53.1%). Multifocal parenchymal lesions were found in 11 dogs (34.4%): in 3 cases (9.4%), they were anechoic and considered compatible with cysts; in 8 cases (25%), they were ipoechoic, isoechoic, or iperechoic, and classified as regenerative lesions. All observed abnormalities are reported in Table 7. Ultrasound of stomach, small and large intestineWe found ultrasonographic abnormalities of the digestive tract in 26/32 dogs (81.2%). Figure 1 summarizes all ultrasonographic gastro-intestinal abnormalities divided for each part of the digestive tract. The most frequently observed abnormalities were those affecting the walls of the digestive tract (71.9%), followed by those of content (50.0%) and peristalsis (25.0%). In 43.7%, 50.0%, and 37.5% of dogs, we found ultrasonographic signs of gastric, small intestinal, and colic disorders, respectively. On AU, the most common gastric, small intestinal, and colic abnormalities were transmural walls thickening (25.0% of dogs), increased echogenicity of the mucosa layer (25.0% of dogs) and presence of fluid content in the lumen (28.1% of dogs), respectively. Table 5. Sites of mineralization in the overall population.
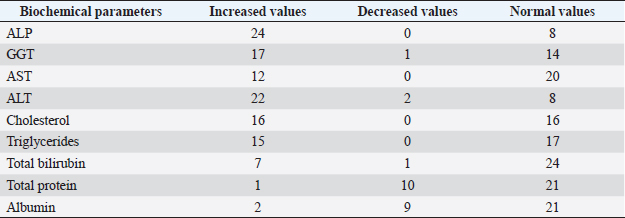
Fig. 5. Right lateral abdomen radiograph. Sub-centimetric and centimetric IBTM are visualized as radiopaque structures superimposed on the hepatic silhouette in a dog. Clinical signsFive (15.6%) dogs showed no clinical signs, while the most common clinical signs in the symptomatic animals (84.4%) were: diarrhea (68.7%), vomiting (62.5%), abdominal pain (21.8%), dysorexia (18.7%), and anorexia (9.4%). These symptoms had persisted for less than 3 months in 9 dogs (28.1%), for a period of between 3 and 12 months in 20 patients (62.5%), and for longer than 12 months in 3 cases (9.4%). Table 8 summarizes the results of biochemical analyzes. Most biochemical parameters were within normal limits. However, we observed an increase in ALP and ALT in 75.0% and 68.7% of dogs, respectively, and an increase in GGT values in over half of the cases (53.1%). Hypercholesterolemia and hypertriglyceridemia were present in 50.0% and 46.9% of dogs, respectively. Total proteins were diminished in 31.2% of dogs, whereas total bilirubin was increased in seven subjects (21.9%). DiscussionBased on our data, our study confirmed that the presence of IBTM is a rare finding in dogs, since their incidence was 32/34.874 dogs (0.092%). The result agrees with Genain et al.’s (2018) study, in which only 17 dogs have been radiographically and/or ultrasonographically evaluated for 30 years. Similarly, the presence of IBTM on the AU in only 32 dogs in a 10-year-long period from two referral centers confirms that this finding is uncommon. In our research, in accordance with previous studies, IBTM were more frequent in small-size (median weight 9.5 kg) adult/old (mean age 10.5 years) dogs and Cavalier King Charles Spaniel was the most represented breed (Kent et al., 2016; Genain et al., 2018). In Table 9, we grouped the data concerning the weight of the patients into three ranges; 53.1% of them weighed less than 10 kg. In the same way, in Table 10, the patients’ age was divided into three main groups and 68.8% of the dogs were over 10 years old. In our study, two AU-main-evidences allow us to affirm that IBTM consists of intrahepatic choleliths. First of all, their intrahepatic aligned or branched distribution pattern, reproducing the shape of the intrahepatic biliary tree. Second, in the case of centimetric or sub-centimetric IBTM, the frequent presence of a focal or multifocal dilation of the larger biliary ducts, upstream and downstream to the calculus, because of the presence of the choleliths themselves. In literature, it is reported that biliary calculi are moveable depending on gravity (Center, 2009). However, in the presence of mineralizations in the lumen of the smaller biliary ducts, it is not possible to show their mobility because of dog positioning in different recumbencies. When intrahepatic foci of mineralization were millimetric and randomly distributed in the liver, it was difficult to evaluate their specific localization because they could be in the lumen of the smaller intrahepatic biliary ducts or they could consist of dystrophic mineralizations of the hepatic parenchyma. For this reason, dogs that presented only this kind of mineralization were excluded from the study population. In the same way, in the absence of a clear acoustic shadow, we supposed that millimetric hyperechoic randomly distributed foci could indicate the presence of gas in the intrahepatic biliary tree or the context of the hepatic parenchyma. The presence of a distal acoustic shadow was mostly related to IBTM size and in IBTM less than or equal to 3 mm, usually distal acoustic shadow was not observed. On the contrary, a bigger IBTM showed a posterior acoustic shadowing in each case. This finding is supported by human literature, in which one study conducted on 81 patients with an ultrasonographic diagnosis of choledocolitiasis demonstrated that the presence of the shadow could be conditioned by the stone dimensions. This latter research, indeed, showed that 64 out of 81 patients had stones with acoustic shadowing in 14 patients, choleliths did not create acoustic shadow and in 3 out of 81 cases, the shadow was equivocal. In 13 out of the above-mentioned 14 subjects, the stones were 5 mm or less (Parulekar and McNamara, 1983). Table 6. Ultrasonographic gallbladder and biliary system findings observed in the study population.
Table 7. Abnormalities of hepatic parenchyma observed at AU in 28 dogs.
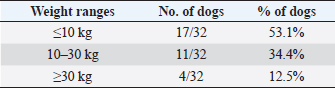
In the present research, IBTM measuring three mm or less rarely showed a mild posterior shadow with a non-defined pattern, while sometimes they produced a neat distal shadow. We must also consider the potential role of different chemical compositions of choleliths referring to the presence or the absence of a posterior acoustic shadow, as reported in the literature (Bertolini, 2017). Regarding IBTM localization, the vast majority of patients (84.4%) presented a widespread distribution, suggesting a possible pathological phenomenon involving the entire liver. In the clinical scenario, bigger intrahepatic choleliths were ultrasonographically easy to identify, especially if they presented a branched pattern and a neat acoustic shadow. On the other hand, millimetric randomly distributed foci of mineralization without distal acoustic shadow were more difficult to evidence and their specific localization could remain equivocal. In veterinary medicine, some authors considered the detection of intrahepatic choleliths as an incidental finding without clinical significance (Wisner and Zwingenberger, 2015; Genain et al., 2018), while others assumed that IBTM could be a consequence of bile stasis or chronic inflammation of the biliary tree (Center, 2009; Bertolini, 2017). Regarding this second assumption, choleliths themselves may predispose to a stasis of bile flow and a consequent inflammatory or infectious phenomenon resulting in a perpetuation of the pathologic condition (Bertolini, 2017). In humans, it is reported that the presence of intrahepatic choleliths is related to an inflammatory, infectious, or parasitic phenomenon and to a bile stasis condition (Paley and Ros, 1998; Araujo Bezerra et al., 2003; Tazuma, 2006). Based on our data, all dogs included in the study presented clinical and ultrasonographic signs referrable to hepatic and/or biliary disease. The hepatic parenchyma was ultrasonographically abnormal in most dogs, suggesting in a first hypothesis a degenerative or chronic inflammatory hepatopathy. Approximately 90% of dogs showed ultrasonographic alterations regarding the biliary tree, reflecting different grades of bile stasis and/or biliary system inflammation/infection. All five asymptomatic dogs showed ultrasonographic signs of hepatic and biliary disease, and two of them presented also AU-perceived alterations in the gastrointestinal tract. This evidence was supported by biochemical results, which in most of the patients consisted of an increase both in biliary and parenchymal enzymatic values. A large part of the canine study population was affected by a chronic hepatobiliary disorder associated with gastrointestinal signs, chronically persistent for over 3 months in over 70% of the patients. Cholestasis determines a maldigestion of dietary lipids, which could lead to intestinal inflammation and malabsorption (Washabau, 2013a, 2013b). In human medicine, it is also known that chronic inflammation of the biliary system could be related to a dysbiotic inflammatory intestinal condition; therefore, to date, we refer to this linkage as the liver-gut axis (Schnabl and Brenner, 2014; Albillos et al., 2020; Blesl and Stadlbauer, 2021; Zheng and Wang, 2021). In addition, some studies in veterinary medicine highlight an alteration in the metabolism of biliary acids in dogs diagnosed with chronic enteropathies (Giaretta et al., 2018; Blake et al., 2019). For these reasons, we hypothesize that a chronic concomitant inflammatory condition of the liver and digestive tract could be a trigger element for the development of IBTM. The main limitations of our study include the lack of cyto-histopathological examinations to confirm the suspected diagnosis and to characterize the nature of the potential hepatopathy. Even if the clinical aspect does not agree with infectious etiology, no bile culture was executed to point out any infectious disease involving the biliary system. In conclusion, the presence of IBTM on the AU is in our opinion an unusual and frequently incidental finding that must be taken into consideration as a potential sign of bile stasis and chronic inflammatory hepatobiliary illness, and it could be associated with a liver-gut axis alteration. Table 8. Results of biochemical laboratory parameters.
Table 9. Differentiation of the population study based on three main weight ranges.
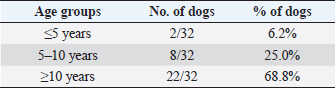
Table 10. Differentiation of the population study based on three main age groups.
Conflict of interestThe Authors declare that there is no conflict of interest. Author contributionsFederico Puccini Leoni: study design, collected the data, drafted, revised, and approved the submitted manuscript; Caterina Puccinelli: study design, collected the data, drafted, revised, and approved the submitted manuscript; Tina Pelligra: study design, collected the data, revised, and approved the submitted manuscript; Eleonora Gori: collected the data and revised and approved the submitted manuscript; Veronica Marchetti: conception of the study, collected the data, and revised and approved the submitted manuscript; Alessia Diana: conception of the study, study design, collected the data, drafted, revised, and approved the submitted manuscript; Nikolina Linta: collected the data and revised and approved the submitted manuscript; Simonetta Citi: conception of the study, study design, collected the data, drafted, revised, and approved the submitted manuscript. ReferencesAlbillos, A., de Gottardi, A. and Rescigno, M. 2020. The gut-liver axis in liver disease: pathophysiological basis for therapy. J. Hepatol. 72(3), 558–577. Araujo Bezerra, A.S., D′Ippolito, G., Martelli, P., Pinto, G.A.D.H., Filho, M.M.G. and Szejnfeld, J. 2003. Liver calcifications: frequency and significance. Radiol. Bras. 36, 199–205. Bertolini, G. 2017. The gallbladder and biliary system. In: Body MDCT in small animals, 1st ed. Eds., Bertolini, G. Cham, Switzerland: Springer, pp: 127–141. Blake, A.B., Guard, C.B., Honneffer, B.J., Lidbury, J.A., Steiner, J.M. and Suchodolski, J.S. 2019. Altered microbiota, fecal lactate, and fecal bile acids in dogs with gastrointestinal disease. PLoS One 14(10), e0224454. Blesl, A. and Stadlbauer, V. 2021. The gut-liver axis in cholestatic liver diseases. Nutrients 13, 1018. Cantwell, H.D., Blevins, W.E., Hanika-Rebar, C. and Godshalk, C.P. 1983. Radiopaque hepatic and lobar duct choleliths in a dog. Am. Anim. Hosp. Assoc. 19, 373–375. Center, S.A. 2009. Diseases of the gallbladder and biliary tree. Vet. Clin. North Am. Small Anim. Pract. 39(3), 543–598. Choi, J., Kim, A., Keh, S., Oh, J., Kim, H. and Yoon, J. 2014. Comparison between ultrasonographic and clinical findings in 43 dogs with gallbladder mucoceles. Vet. Radiol. Ultrasound. 55(2), 202–207. Cook, A.K., Jambhekar, A.V. and Dylewski, A.M. 2016. Gallbladder sludge in dogs: ultrasonographic and clinical findings in 200 patients. J. Am. Anim. Hosp. Assoc. 52(3), 125–131. Genain, M.A., Barbosa, A., Herrtage, M. and Watson, P. 2018. Clinical relevance of radiographic linear branching mineral opacities in the canine liver. J. Small. Anim. Pract. 59(7), 432–437. Giaretta, P.R., Rech, R.R., Guard, B.C., Blake, A.B., Blick, A.K., Steiner, J.M., Lidbury, J.A., Cook, A.K., Hanifeh, M., Spillman, T., Kilpinen, S., Syrja, P. and Suchodolski, J.S. 2018. Comparison of intestinal expression of the apical sodium-dependent bile acid transporter between dogs with and without chronic inflammatory enteropathy. J. Vet. Intern. Med. 32(6), 1918–1926. Kent, A.C., Constantino-Casas, F., Rusbridge, C., Corcoran, B.M., Carter, M., Ledger, T. and Watson, P.J. 2016. Prevalence of pancreatic, hepatic and renal microscopic lesions in post-mortem samples from Cavalier King Charles spaniels. J. Small Anim. Pract. 57(4), 188–193. Lamb, C.R., Kleine, L.J. and McMillan, M.C. 1991. Diagnosis of calcification on abdominal radiographs. Vet. Radiol. 32(5), 211–220. Moon Larson, M. 2013. The liver and spleen. In: Textbook of veterinary diagnostic radiology, 6th ed. Eds., Thrall, D. St. Louis, MO: Saunders, pp: 679–704. Nyland, T.G. and Hager, D.A. 1985. Sonography of the liver, gallbladder, and spleen. Vet. Clin. North Am. Small. Anim. Pract. 15, 1123–1148. Nyland, T.G., Moon Larson, M. and Mattoon, J.S. 2019. Fegato. In: Trattato di Ecografia del Cane e del Gatto, 3rd ed. Eds., Mattoon, J.S., Nyland, T.G. and Spattini, G. St. Louis, MO: Saunders, pp: 348–417. Nyland, T.G. and Park, R.D. 1983. Hepatic ultrasonography in the dog. Vet. Radiol. 24, 74–84. Paley, M.R. and Ros, P.R. 1998. Hepatic calcification. Radiol. Clin. North Am. 36(2), 391–398. Parulekar, S.G. and McNamara, M.P. Jr. 1983. Ultrasonography of choledocholithiasis. J. Ultrasound Med. 2(9), 395–400. Schnabl, B. and Brenner, D.A. 2014. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 146(6), 1513–1524. Tazuma, S. 2006. Epidemiology, pathogenesis, and classification of biliary stones (common bile duct and intrahepatic). Best. Pract. Res. Clin. Gastroenterol. 20(6), 1075–1083. Thamm, D.H. 2001. Hepatobiliary tumors. In: Small animal clinical oncology, 3rd ed. Eds., Withrowand, S.J. and MacEwen, E.G. Philadelphia, PA: Saunders, pp: 327–334. Zheng, Z. and Wang, B. 2021. The gut-liver axis in health and disease: the role of gut microbiota-derived signals in liver injury and regeneration. Front. Immunol. 12, 775526. Washabau, R.J. 2013a. Antidiarrheal agents. In: Canine and feline gastroenterology, 1st ed. Eds., Washabau, R.J. and Day, M.J. St. Louis, MO: Saunders, pp: 445–449. Washabau, R.J. 2013b. Liver. In: Canine and feline gastroenterology, 1st ed. Eds., Washabau, R.J. and Day, M.J. St. Louis, MO: Saunders, pp: 849–9570. Wisner, E.R. and Zwingenberger, A.L. 2015. Hepatobiliary disorders. In: Atlas of small animal CT and MRI, 1st ed. Eds., Wisner, E.R. and Zwingenberger, A.L. Ames, IA: John Wiley & Sons, Inc., pp: 522–537. | ||
| How to Cite this Article |
| Pubmed Style Puccini-Leoni F, Puccinelli C, Pelligra T, Gori E, Marchetti V, Diana A, Linta N, Citi S. Ultrasonographic appearance and possible clinical relevance of hyperechoic foci of mineralization in the canine intrahepatic biliary tree. Open Vet J. 2023; 13(5): 541-549. doi:10.5455/OVJ.2023.v13.i5.6 Web Style Puccini-Leoni F, Puccinelli C, Pelligra T, Gori E, Marchetti V, Diana A, Linta N, Citi S. Ultrasonographic appearance and possible clinical relevance of hyperechoic foci of mineralization in the canine intrahepatic biliary tree. https://www.openveterinaryjournal.com/?mno=140742 [Access: July 27, 2024]. doi:10.5455/OVJ.2023.v13.i5.6 AMA (American Medical Association) Style Puccini-Leoni F, Puccinelli C, Pelligra T, Gori E, Marchetti V, Diana A, Linta N, Citi S. Ultrasonographic appearance and possible clinical relevance of hyperechoic foci of mineralization in the canine intrahepatic biliary tree. Open Vet J. 2023; 13(5): 541-549. doi:10.5455/OVJ.2023.v13.i5.6 Vancouver/ICMJE Style Puccini-Leoni F, Puccinelli C, Pelligra T, Gori E, Marchetti V, Diana A, Linta N, Citi S. Ultrasonographic appearance and possible clinical relevance of hyperechoic foci of mineralization in the canine intrahepatic biliary tree. Open Vet J. (2023), [cited July 27, 2024]; 13(5): 541-549. doi:10.5455/OVJ.2023.v13.i5.6 Harvard Style Puccini-Leoni, F., Puccinelli, . C., Pelligra, . T., Gori, . E., Marchetti, . V., Diana, . A., Linta, . N. & Citi, . S. (2023) Ultrasonographic appearance and possible clinical relevance of hyperechoic foci of mineralization in the canine intrahepatic biliary tree. Open Vet J, 13 (5), 541-549. doi:10.5455/OVJ.2023.v13.i5.6 Turabian Style Puccini-Leoni, Federico, Caterina Puccinelli, Tina Pelligra, Eleonora Gori, Veronica Marchetti, Alessia Diana, Nikolina Linta, and Simonetta Citi. 2023. Ultrasonographic appearance and possible clinical relevance of hyperechoic foci of mineralization in the canine intrahepatic biliary tree. Open Veterinary Journal, 13 (5), 541-549. doi:10.5455/OVJ.2023.v13.i5.6 Chicago Style Puccini-Leoni, Federico, Caterina Puccinelli, Tina Pelligra, Eleonora Gori, Veronica Marchetti, Alessia Diana, Nikolina Linta, and Simonetta Citi. "Ultrasonographic appearance and possible clinical relevance of hyperechoic foci of mineralization in the canine intrahepatic biliary tree." Open Veterinary Journal 13 (2023), 541-549. doi:10.5455/OVJ.2023.v13.i5.6 MLA (The Modern Language Association) Style Puccini-Leoni, Federico, Caterina Puccinelli, Tina Pelligra, Eleonora Gori, Veronica Marchetti, Alessia Diana, Nikolina Linta, and Simonetta Citi. "Ultrasonographic appearance and possible clinical relevance of hyperechoic foci of mineralization in the canine intrahepatic biliary tree." Open Veterinary Journal 13.5 (2023), 541-549. Print. doi:10.5455/OVJ.2023.v13.i5.6 APA (American Psychological Association) Style Puccini-Leoni, F., Puccinelli, . C., Pelligra, . T., Gori, . E., Marchetti, . V., Diana, . A., Linta, . N. & Citi, . S. (2023) Ultrasonographic appearance and possible clinical relevance of hyperechoic foci of mineralization in the canine intrahepatic biliary tree. Open Veterinary Journal, 13 (5), 541-549. doi:10.5455/OVJ.2023.v13.i5.6 |