| Research Article | ||
Open Vet J. 2023; 13(7): 864-872 Open Veterinary Journal, (2023), Vol. 13(7): 864-872 Original Research The development and use of an inactivated vaccine against animals trichophytosisMynbay Umitzhanov1*, Aitkali Imanbaev2, Gulmira Janabekova1, Ainur Dzhangabulova1 and Symbat Usmangaliyeva11Department of Biological Safety, Kazakh National Agrarian Research University, Almaty, Republic of Kazakhstan 2Department of Obstetrics, Surgery and Biotechnology of Reproduction of Animals, Kazakh National Agrarian Research University, Almaty, Republic of Kazakhstan *Corresponding Author: Mynbay Umitzhanov. Department of Biological Safety, Kazakh National Agrarian Research University, Almaty, Republic of Kazakhstan. Email: mynbayumitzhanov [at] yahoo.com Submitted: 01/02/2023 Accepted: 19/06/2023 Published: 31/07/2023 © 2023 Open Veterinary Journal
AbstractBackground: The annual increase in the number of camels entails a parallel increase in the incidence of trichophytosis, which poses a great threat to the health and life of both this species of animals and other organisms that contact and surround them. Aim: The aim of the study was to develop and establish the quality of vaccines inactivated by ultrasonic exposure for the prevention and treatment of trichophytosis in camels, and to compare them with chemically deactivated vaccines. Methods: The peculiarity of the technology of production of these vaccines was the use of an innovative method of inactivation of fungal strains by ultrasonic waves, which allowed to achieve high positive results in theory, and was subsequently confirmed in practice by immunizing sick and healthy animals. The first tests of the obtained vaccines were conducted in laboratory conditions on experimental rabbits. Results: The results of prophylactic and therapeutic vaccinations were one hundred percent positive, which made it possible to conduct further tests directly on camels of industrial farms, the expected result of which was also positively confirmed at the end of the research. Conclusion: As a result of this experiment, the effectiveness, stability, and safety of the manufactured vaccines were established, which made it possible to approve the regulatory and technical documentation and patent them as an innovative and effective development for the prevention and treatment of camel trichophytosis, which will reduce the growth of infection and further overcome the mass spread of the disease both among camels and among the surrounding organisms to which it is transmitted. Keywords: Monovaccine, Pentavalent vaccine, Trichophytosis, Trichophyton, Strain. IntroductionDermatomycoses is a group of diseases caused by pathogens—deuteromycetes (dermatophytes), which in turn are divided into three genera: Trichophyton—causing the disease known as trichomycosis (-phythia, -phytosis, ringworm); Microsporum—leading to microsporia (microsporosis) and Achorion—the clinical manifestation of which is favus (scabies). The most common cause of the lesion is Trichophyton. Among the different species, Trichophyton verrucosum and Trichophyton faviforme affect cattle and horses, whereas Trichophyton equinum and Trichophyton mentagrophytes (rarely) affect horses. On the other hand, carnivores (cats and dogs) and pigs are mostly affected by Trichophyton gypseum and T. mentagrophytes, while other species that are similar in morphology are detected much less frequently. However, the impact of the latter should not be underestimated, and it is important to pay attention to them, looking for methods of their suppression (Klymnyuk et al., 2004; Skibitskyi et al., 2012; Mohammadpour et al., 2020). According to the latest data, there are about 338,000 camels in the Republic of Kazakhstan, most of which are in Mangistau (41,500), Atyrau (17,300) and South Kazakhstan (16,900) regions. In addition to the number, there is also a great species diversity: compared to other countries of the world, where only two species of camels are common, in Kazakhstan there are about 32 existing hybrid species, which is due to two factors: firstly, the growing demand for camel milk, which is fermented to obtain the drink shubat and secondly, the fact that keeping camels in both large and small quantities is economically profitable for both small entrepreneurs and the country as a whole (Shoman, 2019). The complexity of the disease lies in the fact that the pathogen is easily transmitted by contact of a healthy animal with a sick one, and therefore the scale of spread is quite fast and large (Mel’nychuk and Hryshchenko, 2014; Yespembetov et al., 2019). There are two types of measures for combating camel trichophytosis: preventive measures and treatment measures. Preventive measures include sanitary and hygienic measures, climatic measures, and ecotrophological measures that ensure environmentally safe and rational nutrition. These measures are necessary because the causative agents of dermatomycoses are highly stable outside the body and can be transferred through various means, such as air, dust, water, household items, soil, and manure. However, these preventive measures do not guarantee full protection against infection with the pathogen. As a result, there is a threat of further spread of the disease, especially among young animals who are more sensitive to the disease and may experience a follicular or deep course of the disease (Gryshchenko and Lytvynenko, 2007; Vashchyk et al., 2020). In addition, preventive measures may not be effective during cold seasons when there is no solar radiation. The economic losses of the livestock sector due to this disease are also significant, not only in terms of animal mortality but also in terms of reduced growth, development, and productivity. On the other hand, treatment measures are needed to address the disease once it has occurred. Similarly, treatment, which is most often conducted by the use of antibiotics internally, does not always help in the treatment of pathogenic fungi with high contagiousness (Saiduldin, 2009; Levinson, 2014; Parker et al., 2021; Vakhidova, 2021; McVey et al., 2022). Although the issue of external treatment of trichophytosis in animals has been more or less solved by the use of ointments and solutions for the treatment of dermatomycotic skin lesions, as well as disinfectants to prevent the disease (Romich and Wagner, 2020; Mohamed et al., 2022). However, the annually increasing number of camels further complicates the situation and requires urgent attention and action, in the form of the creation and production of a safe and effective vaccine as the most effective way to combat trichophytosis, which is being worked on by scientists in Kazakhstan and other countries of the world, and which is also the purpose of this study. The aim of the study was to develop and establish the quality of vaccines inactivated by ultrasonic exposure for the prevention and treatment of trichophytosis in camels, and to compare them with chemically deactivated vaccines. Materials and MethodsThe largest animal breeding farms in the Mangistau, Atyrau, South Kazakhstan, and Kyzylorda regions (Fig. 1) were surveyed to investigate the epizootic situation of camel trichophytosis. The investigation involved collecting anamnesis, performing clinical examinations, and gathering pathological material from untreated wounds, crusts, and wool. Additionally, the research took into account the potential causes and modes of infection transmission, as well as various animal-related factors, including age, sex, and individual characteristics, along with climatic features of the environment (Basybekov et al., 2018; Khelifi-Ouchene et al., 2020).
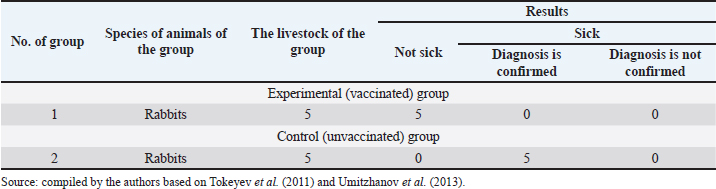
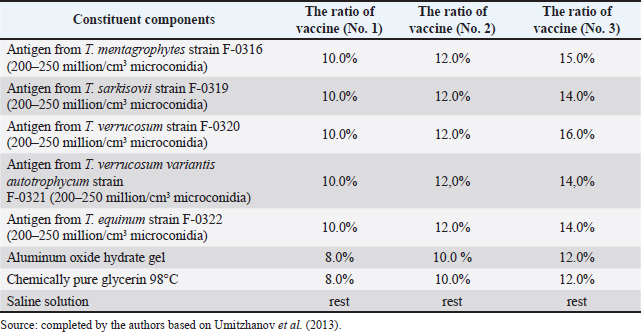
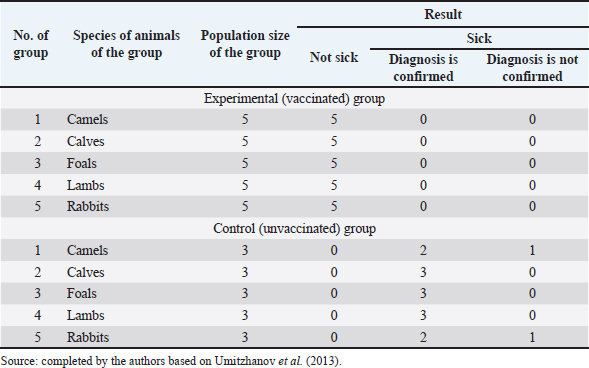
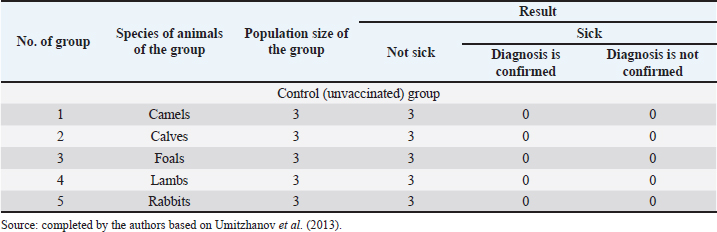
Fig. 1. Map of the study regions. Samples of pathological material were placed in special paper bags, which were sealed with an indication of the place of sampling (region, district, exact place of the farm) and date, information about the sick animal (its registration number, age, sex, severity of the condition) and sent for further research to the laboratory of the Kazakh Research Veterinary Institute. In the laboratory, for further microscopy, the examined pathological material was placed with sterile forceps in Petri dishes made according to GOST 1770-74, which in turn were placed on a black background. An eye scalpel was used to cut and remove the thickening of some areas of the white root coat and skin scales, which were then transferred to a slide (GOST 9284-75) in an amount of about 8–10, on which 10%–15% potassium or sodium solution of your choice (GOST 4328-77) was added dropwise. They were heated with a burner flame until a white halo appeared around the drop of the solution, after which a 50% aqueous solution of glycerol (GOST 6259-75) was added to it and then the slide was covered with a cover glass, proceeding to microscopy of the slides with a ×10 magnification, later with a ×40 lens. However, in order to accurately identify the pathogen species, it was necessary to obtain a pure culture of the fungus on nutrient medium (Sabouraud’s dextrose agar containing 2% agar, 1% peptone, and 4% dextrose) in a slightly acidic environment (pH=5.4–5.8), by inoculating wool and skin, as follows: pathological material up to 2 mm in size was transferred to a sterile Petri dish, from where the inoculum was collected using a bacteriological loop cooled after fire treatment. The material taken by the loop, according to the rules of asepsis, was transferred to 2–3 sections of the nutrient medium at a distance of 1–1.5 cm between each and sent to the thermostat (t=25°C–30°C). The appearance of fungal growth was noted already in the first days. However, the growth was also observed for 20–30 days, which is typical for fungi of the genus Trichophyton, and therefore the process of cultivation of fungi was conducted for a month. During the development of mycoses, they were examined by inspection, where they observed a wide growth of rounded colonies of fungi (maximum value was 25–40 mm), the transition of the shape of which was from flat to fluffy-bumpy, and the color—from greyish to white-beige. The reverse side of the fungal colony had a red-brown color, which was also released into the nutrient medium (Alhasan et al., 2022). The study was also carried out by microscopy, where the staining of the preparations showed Gram-positive staining of young fungal cells, the presence of mycelium of various shapes—from straight to sinuous, the size of hyphae was up to 9 mm, and their endings were spiral and ring-shaped, the size of chain arthrospores—6–18 μm, chlamydospores—6–17 μm (single or absent), micro- and macroconidia—2.5–5.0 × 3.0–10.0 μm, and 15–53 μm, respectively (Bulashev et al., 2014; Borysevych et al., 2020). At the end of the cultivation process, the obtained fungi were examined for a concentration of 1 cm3 by dilution with saline (9 cm3), followed by counting in a Goryaev chamber under a microscope. All the above actions allowed us to identify and confirm the presence of the causative agent of trichophytosis in camels (Tokeyev et al., 2011). The most immunogenic field homologous epizootic crops were used for control infectious studies. Ethical approvalAll procedures performed using animals were revised and approved by the Scientific Committee of Ethics of the laboratory of the Kazakh Research Veterinary Institute, Republic of Kazakhstan. ResultsIt is important to note that the production of a monovalent vaccine for camels is a complex process that requires specialized facilities and expertise. It typically involves collaboration between scientists, veterinarians, and manufacturers to ensure that the vaccine is safe and effective. The laboratory of mycology at the Kazakh Veterinary Research Institute developed inactivated monovalent and pentavalent vaccines against camel trichophytosis and trichophytosis of farm animals, respectively, based on the cultured fungi of the genus Trichophyton. To produce monovalent vaccines, fungi strains Trichophyton sarkisovii F-0080 and Trichophyton camel F-0174 were separately grown in mat flasks on nutrient medium for 18 days (wort agar, the acidity of which was from 7.2 to 7.4; temperature—unchanged, about 25°C–30°C). The resulting cultures were removed from the medium using glass scrapers and placed in sterile jars following aseptic techniques. The vaccine material was then added to sterile saline solution in a 1:1 ratio and homogenized with mixers. The manufacturing process of the monovalent and pentavalent vaccines did not involve the use of chemical inactivation agents to avoid negative effects on animal health after vaccination (Kondibaeva et al., 2021). Instead, an innovative method using ultrasonic disintegration of the biomass of trichophyte spore cultures was used. The process was carried out in an ultrasonic low-frequency disperser (ULDN-1) with a frequency of 22 kHz and a power of 100 W/cm2 until the corpuscular antigen, the spore elements of fungi, were completely destroyed. This process took approximately 1 hour of exposure. It is important to note that the innovative method of ultrasonic disintegration of trichophyte spore culture biomass involves the use of ultrasonic waves to destroy trichophyte fungal spores. This method is used to obtain pure antigens for the development of vaccines against ringworm, including trichophytosis. The method is considered innovative because it allows efficient extraction of antigens while maintaining their purity and integrity, which is necessary for the development of effective vaccines (Zholdasbekova et al., 2018; Dębowski et al., 2023). The resulting homogeneous mass was sent to the refrigerator for 1 day at a temperature of 4°C–8°C, after which a sample was taken for microscopic analysis, the purpose of which was to check the presence of undestroyed spores with their subsequent sowing on nutrient media, as well as three samples for bacteriological and mycological control. After these analyses, the finished substance was subjected to centrifugation at 6,000 rpm for 20 minutes. The upper layer formed—the supernatant, became the basis for further production of the vaccine, as it contains protein formed in the process, the concentration of which was determined using a photoelectrocolorimeter (1.0 cm3). Next, to the antigenic mixture in NaCI saline was added a gel of aluminum oxide hydrate (8%–12% by dry matter) and placed in a thermostat for up to 1 day, stirring the mixture from time to time. After that, 98°C chemically pure glycerin (8%–12% by volume of homogeneous mass) was added to the vaccine, bringing the product to a final concentration of 1.5 mg/cm3. After thoroughly mixing the mixture, it was poured 2.0 cm3 into sterile ampoules of 2.0–5.0 cm3, which were then sterile sealed and capped, or 10, 50, 100, 200, and 500 cm3 into vials of the appropriate volume, closed with rubber stoppers, rolled with aluminum, and labeled. The resulting protein product was injected into the experimental group of rabbits intramuscularly in the rump area, twice in a prophylactic dose of 1.0 cm3, with an interval of 14 days between both vaccinations. After 25–28 days, from the time of the second immunization, the animals of the experimental group, as well as the control group, the number of which is five in each, were experimentally infected with a homologous virulent epizootic pathogen of trichophytosis, where every 3–5 days, for 30 days, a therapeutic and clinical examination was carried out, as well as constant monitoring of the condition of the animals. As a final result of this study, in immunized experimental rabbits (group No. 1), clinical signs of trichophytosis were absent, and therefore they did not need additional preventive vaccination, while in the control group (No. 2) there were clearly expressed clinical signs of the disease, which were confirmed by selecting pathological material of animals, sowing on nutrient medium and its subsequent control experiments. The results of this study can be seen in Table 1. To determine the therapeutic effect of the vaccine, the following experiment was conducted on the control group No. 2, which was previously infected. To do this, the animals were administered 2–3 therapeutic doses of inactivated vaccine at intervals similar to preventive vaccination (14 days), where 25–30 days after the last vaccine was administered, the following results of the study were established (Table 2). To obtain a pentavalent inactivated vaccine against trichophytosis of farm animals, the following strains of fungi of the genus Trichophyton were taken as a basis: – Trichophyton verrucosum F-0320 (21–day); – Trichophyton verrucosum variantis autotrophycum F-0321 (21–day); – Trichophyton sarkisovii F0319 (18–day); – Trichophyton equinum F-0322 (16–day); – Trichophyton mentagrophytes F-0316 (18–day). These strains were grown and technologically involved in research similar to the processes that occurred with monovaccines and are described above. The only difference was that the preparation of monovalent vaccine was conducted in different component ratios (Table 3), which further allowed us to experimentally determine the most pharmacologically effective recipe for vaccine preparation. The obtained vaccine variants are a yellow-brown liquid, which was poured into sterile ampoules. Prior to immunization, the vaccine, in order to preserve its biological effect, had to be stored in a dark place at a temperature of 2°C–10°C, no more than 12 months from the date of its bottling, which in turn was observed. To study the immunological effect of the vaccine, five groups of animals were taken, each of which had five distinct species of animals under the age of 6 months, namely: camels, calves, foals, lambs, and rabbits. Each of the animals was twice injected with 1.0 cm3 of the preventive dose of the vaccine, with an interval of 14 days between them. After 25–28 days, from the time of the second immunization, all animals of the experimental group, the number of which was 25 animals, as well as animals of the control (unvaccinated) group, the number of which was 15 animals (5 groups, each of them 3 animals), were experimentally infected with homologous virulent epizootic pathogens by 5 species of Trichophyton genus cultures. Every 3–5 days, for 30–35 days, clinical examination of animals was conducted, as well as constant monitoring of their well-being and health. According to the results (Table 4) of this study, in the immunized animals of the experimental group, clinical signs of trichophytosis were absent, and therefore they did not require additional prophylactic or therapeutic vaccination, while in the control group, there were clearly expressed clinical signs of trichophytosis, which were largely confirmed by sampling pathological material of animals, sowing on nutrient medium and its subsequent control studies. Pathological material was taken from the animals of the control group, in which clinical signs of trichophytosis were detected, for accurate diagnosis, and examined. In case of confirmation of the diagnosis, the animals were injected with 2–3 therapeutic doses of the vaccine, in intervals identical to the preventive ones (14 days). The results (Table 5) of the study were established 25–30 days after the last dose of the vaccine. From the table can see that regardless of the group of animal species on which the experiment was conducted, in the conclusion the authors get a multifaceted and high effect of the inactivated pentavalent vaccine. At the same time, the inefficiency of the vaccine can be observed in a study comparing several vaccines, which were mainly developed for immunization of cattle but can also be used in so-called companion animals (e.g., cats, dogs, horses, camels). The first of them (Insol® Trichophyton, Germany) was developed on the basis of formalin-inactivated cells of T. verrucosum strain, while the second was a monovalent live attenuated vaccine (Bovilis® Ringvac, the Netherlands), which was developed on the basis of attenuation of the same strain. Table 1. Results of the study of the preventive effectiveness of inactivated vaccine against T. camel.
Table 2. Results of the study of therapeutic efficacy of inactivated vaccine against T. camel.
Table 3. Ratio of components of pentavalent inactivated vaccine against trichophytosis of farm animals.
Both vaccines were administered in a therapeutic dose to the species of animals for which they were primarily developed and prescribed, that is, cattle, namely 5–8 month-old calves, which is the most sensitive to exogenous and endogenous changes in age, because in case of success of research of such vaccines on this age category of this species of animals, in the future it would be possible to vaccinate for early prevention of the disease, or its treatment, which is more likely in young organisms, which in turn would give them immunity for their adult future. Table 4. Results of the study of the preventive effectiveness of pentavalent inactivated vaccine against trichophytosis of farm animals.
Table 5. Results of the study of therapeutic efficacy of inactivated vaccine against trichophytosis of farm animals.
As a result of this study, it was found that none of the calves that were immunized with the live vaccine had clinical signs of ringworm, or had a slight presence of spots with a diameter of about 1 mm and their peeling, which had no signs of development, that is, they were residual and still observed in some animals for up to 4 weeks after vaccination, and on the fifth week a complete recovery of all the studied calves was observed. Such results are quite successful, which meant the full effectiveness of the live vaccine. What the authors cannot observe in the results of the inactivated vaccine, because the calves in which it was administered for a long time had distinct clinical signs of trichophytosis, which differed slightly in the signs and timing of their manifestation after vaccination, from animals belonging to the control group, that is, the unvaccinated infected group (up to 8 weeks of clinical signs in the case of animals from the control group, up to 9 weeks—in the case of immunized animals of the group, the number of which in this case is insignificant (one out of five experimental animals), indicating the probable complications caused by the vaccine in the recovery of the infected animal) (Gryshchenko et al., 2016; Zafar, 2017). DiscussionSimilarly, comparing the results of tests of formalin-inactivated Insol® Trichophyton vaccine and the resulting pentavalent vaccine against trichophytosis of farm animals, which has undergone ultrasonic inactivation, see a significant advantage of the latter, as the recovery of the experimental animals, including calves, was guaranteed, including calves, which also belonged to one of several experimental groups, took place within 25–30 days, i.e., about 4 weeks, moreover, with a guaranteed positive result, which is similar to the results of the previous, above-described comparison of animal recovery by vaccination with live vaccine (Biyashev et al., 2016). But such cases are quite uncommon in the modern world of science, if compared with the developments and research of the late 20th century, because quite a lot has been studied and discovered by scientists in the field of medicine and veterinary medicine, including the issue of fungal infections, their prevention, and treatment with drugs, including those based on fungal strains (Mel’nychuk et al., 2014; Zhakupova et al., 2014). An example is the research of scientists of the Kazakh Veterinary Research Institute, which was conducted on a vaccine against trichophytosis of cattle, for the production of which the fungal strain of the trichophytic pathogen—T. verrucosum, and which was inactivated and preserved by using formalin and glycerin, and as a result the finished product obtained completely different results compared to the above example. The experimental animals of both preventive and therapeutic groups, in the amount of five animals each, were injected with the vaccine twice with an interval of 14 days between them. Already on the 30th–35th day after the last dose of the vaccine was administered, it was found that the animals in the number that was taken for testing were absolutely healthy. Obtaining such results allowed to expand the range of effectiveness of specific prevention and treatment of trichophytosis in cattle (Lund and DeBoer, 2008; Kirkimbayeva et al., 2015) As examples, can also cite other discoveries of some of the same authors, namely inactivated vaccines for animals such as horses and carnivores, which were developed similarly, based on formalin inactivation of other fungal strains, to improve the immune action of the vaccinated organism against infection with trichophytosis pathogens, because in the results of both prophylactic and therapeutic experiments on animals, 100% clinical indicators in favor of these vaccines were established (Umitzhanov et al., 2010; Boranbayeva and Umitzhanov, 2015; Bizhanov et al., 2015). However, all the same, the previously listed problems that arise with the chemical method of vaccine inactivation will still arise, and repeatedly, until there are developments of new, universal vaccines that will be used for many species of animals, moreover, with high efficiency and safety for all intended living organisms, which scientists are currently searching for. The method of ultrasonic inactivation is also not new, is quite common and is undoubtedly considered to be the basis among the methods of vaccine inactivation along with chemicals, and as it is already clear even prevails over it by some criteria, as it was found during the recent development of immunology, so it is quite successful in fulfilling the purpose of creating and applying vaccines to animals of different species, age, sex and other, already few important criteria (Gryshchenko et al., 2019). However, to obtain such high results, it is also quite important to find the ultrasonic parameters of power, frequency, and time of action on fungal elements and structures, as well as effective ratios of fungal strains and elements of their dilution and preservation, for long-term vaccine action and storage under conditions of temperature fluctuations that may occur during transportation of vaccines to production farms, which was performed and observed for the development and manufacture of innovative vaccines for camels in this study. ConclusionFrom the results obtained, as well as their discussions and comparisons with other inactivated vaccines, moreover, produced by different inactivation technologies, the authors understand that the obtained vaccines, the development and quality of which was established for the purpose of this scientific study, significantly exceed chemically deactivated vaccines, because the latter among the disadvantages are the risks of incomplete inactivation, which both theoretically and practically carries the threat of diseases of a certain percentage of animals, and accordingly the costs of both economic and population, as in some cases it can lead to death among immunized, that is, it speaks of both environmental and time losses. In addition, such vaccines have a rather substantial risk of allergic reactions, which are possible with vaccination in general, but in combination with chemicals are more likely. But, in the case of vaccines inactivated by ultrasonic exposure, such adverse reactions were not detected during tests both on experimental rabbits in the laboratory and directly on camels of industrial farms. Also, in addition to experimental studies, there is evidence of positive tests of monovalent and pentavalent vaccines directly in the production conditions of animal husbandry. However, despite the claimed success of the developed vaccines, their use should be extremely cautious, following all the instructions that are especially important for obtaining a positive result, namely: proper storage and use of vaccines, their exact dosage, depending on the purpose of immunization (prevention or treatment) and compliance with the intervals between vaccinations. In addition, for effective control of trichophytosis in camels, it is advisable to observe other preventive conditions, which, as it was said, are not highly effective alone, especially without vaccination of animals, but are also important, especially in complex application. From these studies and their results, can conclude that the purpose of the experiment is fully achieved, because the obtained vaccines will allow to conduct higher quality preventive and therapeutic measures in all spectrums, without possible threats and harm to the health of animals both at the time of immunization and after months and even years of their life. AcknowledgmentsNone. Authors contributionsMynbay Umitzhanov and Aitkali Imanbaev were involved in planning and supervised the work. Gulmira Janabekova performed the analysis and drafted the manuscript. Ainur Dzhangabulova helped in interpreting the results. All authors discussed the results, read and approved the final manuscript. Conflict of interestThe authors declare that there is no conflict of interest. FundingNone. Data availabilityThe data that support the findings of this study are available on request from the corresponding author. ReferencesAlhasan, D.A., Al-Abedi, H.F., Hussien, T.J. and Mohammad Ali, A.Q. 2022. Morphological detection of dermatophytes isolated from cattle in Wasit province. Iraqi J. Vet. Sci. 36(1), 167–172. Basybekov, S.Z., Bazarbayev, M.B., Yespembetov, B.A., Mussaeva, A., Kanatbayev, S.G., Romashev, K.M., Dossanova, A.K., Yelekeyev, T.A., Akmatova, E.K. and Syrym, N.S. 2018. Diagnostics of tuberculosis and differentiation of nonspecific tuberculin reactions in animals. Braz. J. Microbiol. 49(2), 329–335. Biyashev, K.B., Biyashev, B.K. and Saribayeva, D.A. 2016. Persistence of the Escherichia coli 64G-probiotic strain in the intestine of calves. Biol. Med. 8(2), 2–3. Bizhanov, B.R., Boranbayeva, R.S., Shalabayev, B.A. and Umitzhanov, M. 2015. Inactivated vaccine against trichophytosis of cattle. Innovation patent of the Republic of Kazakhstan No. 29588. Bulletin No. 3. Boranbayeva, R.S. and Umitzhanov, M. 2015. Inactivated bivalent vaccine against trichophytosis and microsporia in horses. Innovation patent of the Republic of Kazakhstan No. 29587. 2015. Bulletin No. 3. Borysevych, B.V., Lisova, V.V. and Chumakov, K.A. 2020. Pathological morphology of animals. Kyiv, Ukraine: Agrar Media Group. Bulashev, A., Taubayev, O., Suranshiev, J. and Mirzabayev, K. 2014. Microbiology. Astana, Kazakhstan: Folio. Dębowski, M., Kazimierowicz, J., Świca, I. and Zieliński, M. 2023. Ultrasonic disintegration to improve anaerobic digestion of microalgae with hard cell walls—Scenedesmus sp. and Pinnularia sp. Plants. 12(1), 1–13. Gryshchenko, V.A. and Lytvynenko, O.N. 2007. Peculiarities of the bilious acid spectrum of bile and duodenal content in mice at medicamentous hepatitis and use of correction therapy. Ukr. Biokhim. Zh. 79(4), 97–101. Gryshchenko, V.A., Chernyshenko, T.M., Gornitska, O.V. and Platonova, T.M. 2016. Evaluation of the functional state of liver and the efficiency of therapy for enteropathy of calves. Fiziol. Zh. 62(6), 102–109. Gryshchenko, V.A., Musiychuk, V.V., Chernyshenko, V.O., Gornytska, O.V. and Platonova, T.M. 2019. Evaluation of biochemical indicators in blood plasma of rats with tetracycline-induced hepatosis and their correction by milk phospholipids. Ukr. Biochem. J. 91(1), 92–99. Khelifi-Ouchene, A.N., Ouchene, N., Dahmani, A., El Aid Kaaboub, A.E., Ouchetati, I. and Haif, A. 2020. Investigation of internal and external parasites of the camels (Camelus Dromedarius) in algeria. Poland: Ann. Parasitol. 66(3). Kirkimbayeva, Z., Lozowicka, B., Biyashev, K., Sarsembaeva, N., Kuzembekova, G. and Paritova, A. 2015. Leptospirosis in cattle from markets of Almaty province, Kazakhstan. Bull. Vet. Inst. Pulawy. 59(1), 29–35. Klymnyuk, S.I., Sytnyk, I.O., Tvoriko, M.S. and Shyrobokov, V.P. 2004. Practical microbiology. Ternopil, Ukraine: Ukrmedkniga. Kondibaeva, Z.B., Yespembetov, B.A., Abeuov, K.B., Mussayeva, A.K., Siyabekov, S.T., Nussupova, S.T., Akmatova, E.K., Pazylov, Y.K., Maikhin, K.T. and Syrym, N.S. 2021. Inactivated vaccine against Aujeszky’s disease. Vet. World. 14(11), 2957–2963. Levinson, W. 2014. Review of medical microbiology and immunology. San Francisco, CA: McGraw-Hill Medical. Lund, A. and DeBoer, D. 2008. Immunoprophylaxis of dermatophytosis in animals. Berlin, Germany: Springer Science+Business Media. McVey, S., Kennedy, M., Chengappa, M.M. and Wilkes, R. 2022. Veterinary microbiology. Hoboken, NJ: John Wiley & Sons. Mel’nychuk, D.O. and Hryshchenko, V.A. 2014. Exchange of bile pigments under the action of ecopathogenic factors on organism. Ukr. Biochem. J. 86(5), 156. Mel’nychuk, D.O., Hryshchenko, V.A. and Vesel’skyǐ, S.P. 2014. Indicators of exchange of bile pigments under the action of ecopathogenic factors on the organism and correction with liposomes. Ukr. Biochem. . Zh. 86(3), 125–132. Mohamed, M.B., Rouby, S.R., and El Aziz, S.A. 2022. In-vitro evaluation of different commercial antimycotics and disinfectants against Trichophyton verrucosum isolated from beef farm in Beni Suef, Egypt. Egypt. J. Vet. Med. Res. 5, 37–45. Mohammadpour, R., Champour, M., Tuteja, F. and Mostafavi, E. 2020. Zoonotic implications of camel diseases in Iran. Hoboken, NJ: John Wiley & Sons. Parker, N., Schneegurt, M., Thi Tu, A., Forster, B.M. and Lister, P. 2021. Microbiology. Houston, TX: OpenStax. Romich, J.A. and Wagner, S. 2020. Fundamentals of pharmacology for veterinary technicians. Boston, MA: Cengage Learning. Saiduldin, T. 2009. Epidemiology and infectious diseases of animals. Almaty, Kazakhstan: Okulyk. Shoman, A.E. 2019. Development of a system of regulatory and technical documentation of quality management for veal meat processing enterprises. Almaty, Kazakhstan: Kazakh National Agrarian University. Skibitskyi, V.G., Vlasenko, V.V., Kozlovska, G.V. and Ibatulina, F.J. 2012. Veterinary microbiology. Kyiv, Ukraine: Nichlava. Tokeyev, Sh.O., Umitzhanov, M., Bizhanov, B.R., Boranbayeva, R.S. and Arisbekova, A.T. 2011. Method for obtaining an inactivated vaccine against trichophytosis of camels. Innovation patent of the Republic of Kazakhstan No. 23842. Bulletin No. 4. Umitzhanov, M., Boranbayeva, R.S., Shevtsova, O.A., Arybekova, A.T., Bizhanov, B.R. 2010. Inactivated vaccine against trichophytosis of carnivorous animals. Innovation patent of the Republic of Kazakhstan No. 22265. 2010. Bulletin No. 2. Umitzhanov, M., Tokeyev, S.O., Bizhanov, B.R., Boranbayeva, R.S. and Shalabayev, B.A. 2013. Method for obtaining an inactivated vaccine against trichophytosis of camels. Innovation patent of the Republic of Kazakhstan No. 27243. Bulletin No. 8. Vakhidova, D.S. 2021. Causes, clinical signs, and measures to combat trichophytosis. Tashkent, Uzbekistan: IQTISOD-MOLIYA. Vashchyk, Y., Shcherbyna, R., Parchenko, V., Bushueva, I., Gutyj, B., Fotina, H., Fotina, T. And Stronskyi, Y. 2020. Histological study of a corrective influence of a compound potassium 2-((4-amino-5-(morpholinomethyl)-4H-1,2,4-triazol-3-yl)thio)acetate (PKR-173) on the state of chicken’s liver under infection by Pseudomonas aeruginosa. Ankara .Univ. Eczacilik Fak. Derg. 44(1), 1–17. Yespembetov, B.A., Syrym, N.S., Syzdykov, M.S., Kuznetsov, A.N., Koshemetov, Zh.K., Mussayeva, A.K., Basybekov, S.Z., Kanatbayev, S.G., Mankibaev, A.T. and Romashev, C.M. 2019. Impact of geographical factors on the spread of animal brucellosis in the Republic of Kazakhstan. Comp. Immunol. Microbiol. Infect. Dis. 67, 101349. Zafar, A. 2017. Practical guide and atlas for the diagnosis of fungal infections. Pakistan, Karachi: The Aga Khan University. Zhakupova, A.A., Maulanov, A.Z., Biyashev, B.K., Biyashev, K.B. and Sarsembaeva, N.B. 2014. Histological study of the interaction of the Escherichia with epithelium of the small intestine of rats. Adv. Environ. Biol. 8(10), 553–555. Zholdasbekova, A., Biyashev, K.B., Biyashev, B.K., Sarybaeva, D.A. and Zhumanov, K.T. 2018. Method for producing attenuated Salmonella strain. J. Pharm. Sci. Res. 10(1), 162–163. | ||
| How to Cite this Article |
| Pubmed Style Umitzhanov M, Imanbaev A, Janabekova G, Dzhangabulova A, Usmangaliyeva S. The development and use of an inactivated vaccine against animals trichophytosis. Open Vet J. 2023; 13(7): 864-872. doi:10.5455/OVJ.2023.v13.i7.8 Web Style Umitzhanov M, Imanbaev A, Janabekova G, Dzhangabulova A, Usmangaliyeva S. The development and use of an inactivated vaccine against animals trichophytosis. https://www.openveterinaryjournal.com/?mno=141990 [Access: May 10, 2024]. doi:10.5455/OVJ.2023.v13.i7.8 AMA (American Medical Association) Style Umitzhanov M, Imanbaev A, Janabekova G, Dzhangabulova A, Usmangaliyeva S. The development and use of an inactivated vaccine against animals trichophytosis. Open Vet J. 2023; 13(7): 864-872. doi:10.5455/OVJ.2023.v13.i7.8 Vancouver/ICMJE Style Umitzhanov M, Imanbaev A, Janabekova G, Dzhangabulova A, Usmangaliyeva S. The development and use of an inactivated vaccine against animals trichophytosis. Open Vet J. (2023), [cited May 10, 2024]; 13(7): 864-872. doi:10.5455/OVJ.2023.v13.i7.8 Harvard Style Umitzhanov, M., Imanbaev, . A., Janabekova, . G., Dzhangabulova, . A. & Usmangaliyeva, . S. (2023) The development and use of an inactivated vaccine against animals trichophytosis. Open Vet J, 13 (7), 864-872. doi:10.5455/OVJ.2023.v13.i7.8 Turabian Style Umitzhanov, Mynbay, Aitkali Imanbaev, Gulmira Janabekova, Ainur Dzhangabulova, and Symbat Usmangaliyeva. 2023. The development and use of an inactivated vaccine against animals trichophytosis. Open Veterinary Journal, 13 (7), 864-872. doi:10.5455/OVJ.2023.v13.i7.8 Chicago Style Umitzhanov, Mynbay, Aitkali Imanbaev, Gulmira Janabekova, Ainur Dzhangabulova, and Symbat Usmangaliyeva. "The development and use of an inactivated vaccine against animals trichophytosis." Open Veterinary Journal 13 (2023), 864-872. doi:10.5455/OVJ.2023.v13.i7.8 MLA (The Modern Language Association) Style Umitzhanov, Mynbay, Aitkali Imanbaev, Gulmira Janabekova, Ainur Dzhangabulova, and Symbat Usmangaliyeva. "The development and use of an inactivated vaccine against animals trichophytosis." Open Veterinary Journal 13.7 (2023), 864-872. Print. doi:10.5455/OVJ.2023.v13.i7.8 APA (American Psychological Association) Style Umitzhanov, M., Imanbaev, . A., Janabekova, . G., Dzhangabulova, . A. & Usmangaliyeva, . S. (2023) The development and use of an inactivated vaccine against animals trichophytosis. Open Veterinary Journal, 13 (7), 864-872. doi:10.5455/OVJ.2023.v13.i7.8 |