| Research Article | ||
Open Vet J. 2023; 13(7): 839-845 Open Veterinary Journal, (2023), Vol. 13(7): 839-845 Original Research Effect of humic acid and probiotics on immunity of broiler chickensFouziyah Ahfeethah1, Altayeb Elazomi2 and Abdulwahab Kammon3,4*1Department of Zoology, Faculty of Science, University of Zawia, Zawia, Libya 2Department of Medical Laboratories, Faculty of Medical Technology, University of Zawia, Zawia, Libya 3Department of Poultry and Fish Diseases, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya 4National Research Center for Tropical and Transboundary Diseases, Alzintan, Libya *Corresponding Author: Abdulwahab Kammon. Department of Poultry and Fish Diseases, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya. Email: a.kammon [at] nrcttd.ly Submitted: 14/02/2023 Accepted: 09/06/2023 Published: 31/07/2023 © 2023 Open Veterinary Journal
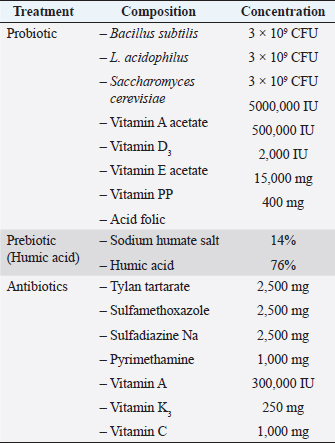
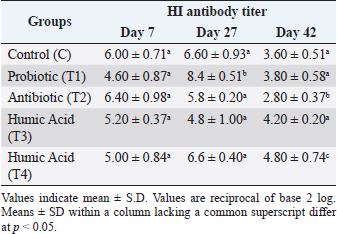
AbstractBackground: The immune system in chickens has a fundamental role in controlling many diseases based on vaccination, thus enhancement of the immune system response is a priority. Aim: The aim of this experiment was to study the effect of probiotics and humic acid on immunity of broiler chickens. Methods: Day-old 300 Ross broiler chicks were segregated into 5 groups of 60 chicks per group. Group C was considered as a control. Groups T1, T2, T3, and T4 were given probiotics, antibiotics, humic acid for the first 7 days and humic acid for 42 days, respectively. Samples were collected on days 27 and 42 to assess the humoral immunity, cellular immunity, lymphoid organs weight, and differential leucocyte count (DLC). Results: The results showed a significant increase (p < 0.05) in antibodies titer against Newcastle disease virus in chickens given humic acid (T4) daily for 42 days as compared to the control. There was also a significant increase in antibodies titer in the T1 group given probiotic for the first week lasting up to day 27 as compared to the control. The skin thickness of T4 group showed a significant increase as compared to T1 and T2 groups after 24 hours of DNCB challenge. After 48 hours, the thickness was still significantly higher in the T4 group as compared to other groups except for the control. There were no significant differences in Bursa of Fabricius/Body weight (%) between the groups. Spleen/Body weight (%) was significantly higher in the control group and T1 than the other groups on day 42. The DLC remains normal in all groups. Conclusion: It is concluded that the humic acid has a stimulant and strengthening effect on the humoral and cellular immune system when given daily to broiler chickens. Moreover, the use of humic acid and probiotics with good hygiene in the first week of age may alternate the use of antibiotics which could be toxic and raise bacterial resistance. Keywords: Humic acid, Probiotics, Broiler chickens, Humoral immunity, Cellular immunity. IntroductionThe avian immune system has a fundamental role in controlling many diseases based on vaccination and invaluable model for the study of basic immunology and fundamental contributions to the basic principles of this science, from the accidental invention of the attenuated avian cholera vaccine discovered by Louis Pasteur to the first description of the major histocompatibility complex and the discovery of differentiation of lymphocytes into B-cells and T-cells, the discovery of interferon, the first successful cancer vaccines and the first administration of vaccines into fertilized egg embryos (Silim and Abbassi, 2015). Poultry birds are vulnerable to many viral, bacterial, and parasitic diseases. The control of viral diseases is mainly depending on vaccines but bacterial diseases are treated with antibiotics which are also used as growth promoters. The misuse of antibiotics is one of the most important reasons for bacterial resistance to these antibiotics and the increase in their residues in poultry products such as meat and eggs. The use of antibiotics as growth promoters has been banned in many countries of the world, encouraging organic animal production, and searching for new alternatives to antibiotics, the most important of which are probiotics and prebiotics (Kammon, 2017). There is a great trend towards the use of vaccines to prevent bacterial, and parasitic diseases and to raise and improve the immunity of birds using organic materials. The use of probiotics has increased rapidly in recent years, which has improved the growth performance of broilers. These probiotics maintain the beneficial microflora present in the gastrointestinal tract and prevent harmful bacteria from growing. Therefore, these probiotics act as a selective regimen (Kizerwetter-Swida and Binek, 2009). Probiotics also alter metabolism by increasing the activity of digestive enzymes and decreasing the activity of bacterial enzymes and ammonia production (Yoon et al., 2004), improving digestion and nutritional intake (Awad et al., 2006), as well as stimulating the immune system (Brisbin et al., 2008). The term prebiotics generally refers to non-digestible feed ingredients that beneficially affect birds by selectively stimulating the growth and activity of beneficial bacteria in the gut, and some experiments have shown improvement in growth performance of broilers with reduced mortality or increased resistance to bacterial invasion using feed ingredients. Nutritional ingredients mainly include glucose, fructose, galactose, and mannose, in addition to some volatile oils and organic acids such as citric acid, orthophosphoric acid, and lactic acid (Kammon et al., 2019). Some organic acids such as humic acid have inhibition properties against acid-intolerant bacteria, including Escherichia coli, Salmonella, and Clostridium perfringens, and can therefore be used as alternatives to antibiotics (Fascina et al., 2012). Humic acids are not approved as food additives but as veterinary medicines in the European Union, although many reports note that they have a growth-promoting effect. Humate is a natural bioactive growth factor, primarily degraded from organic matter by live soil bacteria (MacCarthy, 2001). It is a naturally odorless dark brown or black powder, which are salts of humic acid. Several studies reported that humate is not toxic and does not contain carcinogens (Yasar et al., 2002). Various effects of adding humate to poultry diet have been observed in recent years. It was found that the addition of dietary humate to broilers had no effects on productive performance, while other studies indicated beneficial (Ceylan et al., 2003) and harmful (Hassan, 2014) effects. It is expected that humic acid and humate will have an important supportive effect on the immune system of birds. The mechanism may be due to the important role humic acid plays in the growth of immune organs, mainly the thymus and bursa of Fabricius, as major elements of the avian immune system (Disetlhe et al., 2017). However, more research is needed since it is hard to compare the actual impacts of humic acid products due to various preparations and sources, in addition to animal rearing in different areas of the world (Arif et al., 2019). The growth of chicks in the first week of life is an important condition and indicator for their performance in the future because physiological processes such as cell enlargement and increase in number, maturity of thermoregulation and the immune system, and growth and differentiation in the digestive system will significantly affect the body weight of meat birds at marketing age (Moraes et al., 2002; Ipek and Sozcu, 2015). There is not enough information about the importance of giving vital probiotics and humic acid to the broiler chicks in the first week, and should they continue to be given until the age of marketing, or is giving them in the first week sufficient for the purpose, and does it dispense with the use of antibiotics that are given to chicks in the first 5 days of life for prevention or treatment of early infection due to contamination of the hatchery with bacteria and its entry through the incompletely healed navel. Therefore, this experimental study was planned to investigate the effects of the probiotics (Bacillus subtilis, Lactobacillus acidophilus, and yeast Saccharomyces cerevisiae) and prebiotics (Humic acid, and Sodium humate) on the immune response and to study the possibility of using probiotics and humic acid as alternatives to antibiotics in the first week of life of broilers. Materials and MethodsExperimental animals300 Ross broiler day-old chicks were reared in a poultry house until the age of 42 days. The birds were provided with all the appropriate conditions for breeding, including temperature, ventilation and humidity according to their age throughout the experiment period. The chicks were given a commercial broiler starter diet until the age of 21 days, then a complementary diet until the end of the experiment. Chicks in each group were weighed at the age of 1 day, and weekly until the sixth week. Experimental designThe chicks were divided into 5 groups, each group containing 60 chicks. The first group was considered as a control group with which other groups are compared. The second group was given a commercial mixture of probiotics in drinking water containing two types of beneficial bacteria B. subtilis and L. acidophilus and a type of yeast S. cerevisiae at a concentration of 3 × 109 CFU and some vitamins during the first week of life only (Table 1). The dose is 1 g/l of drinking water for the first week of life. The third group was given a commercial mixture of antibiotics consisting of tylan, sulfa compounds, pyrimethamine, and some vitamins during the first week of life at a dose of 3 g/l of drinking water for the first week. The fourth group was given a commercial mixture of humic acid and humate at a dose of 1 g/l of drinking water during the first week of life. The fifth group was given the same treatment as the fourth group until the end of the experiment. All the birds were vaccinated according to the schedule followed and approved by the National Center for Animal Health. The birds were vaccinated against Newcastle disease at the age of 7 days with the Hechner B1 vaccine (HIPRA®HB1) in drinking water, then another dose was given at the age of 21 days with the LaSota vaccine (HIPRA®S) in the drinking water in order to study the immune response of birds. Measurements of immunityAssessment of humoral immunity Assessment of humoral immunity was carried out using hemagglutination and hemagglutination inhibition (HI) tests (n=5 chickens from each group) according to the method described by OIE (2012). Assessment of cellular immunityThe cell-mediated immunity was assessed by the contact hypersensitivity response of the chicken’s skin to dinitrochlorobenzene (DNCB) (n=5 chickens from each group) as per the method of Tiwary and Goel (1985). After 24, 48, and 72 hours post DNCB contact, the percentage of skin thickness increase was calculated as follows: Percentage of thickness increase (%)=[(skin thickness after challenge – skin thickness before challenge)/skin thickness before challenge] × 100 (Chowdhury et al., 2005). Lymphoid organs weightOn day 27 and 42, the bursa of Fabricius, spleen, and body weights were determined and the bursa of Fabricius/body weight and spleen/body weight ratios were calculated as per the method of Heckert et al. (2002) as follows (n=5 chickens from each group): Percentage (%) bursa of Fabricius/body weight=[bursa of Fabricius weight / body weight] × 100. Percentage (%) spleen/body weight=[spleen weight / body weight] × 100. Differential leukocyte countOn days 7, 27, and 42 of the age a fresh (without anticoagulant) drop from each blood sample (n=5 chickens from each group) was smeared on a clean glass slide and dried in the air before staining with Wright-Giemsa stain. One hundred white blood cells were counted under oil immersion and the results were expressed in percentage. Statistical analysisThe results of the current study were expressed as the mean ± SD and the statistical significance (p < 0.05) of the difference between the control and treated groups were analyzed by analysis of variance, with the Tukey HSD test for multiple comparisons. Statistical calculations were performed using the computer program (SPSS 26). Ethical approvalThis experiment was conducted following the animal welfare and ethical conditions based on the Scientific Research Ethics Document approved by the Scientific Committee of the National Research Center for Tropical and Transboundary Diseases. ResultsAssessment of humoral immunityThe effect of probiotics and humic acid on humoral immunity is shown in Table 2. There was no significant difference in HI antibodies titer between all groups at day 7 indicating homogeneity of maternal immunity against ND. There was a significant increase in HI antibodies titer of the probiotic group (T1) as compared to the control (C) and other groups on day 27. However, on day 42, the antibody titer was significantly higher in the humic acid group (T4) that given the treatment continually until the end of the experiment. The antibody titer was significantly very low in the antibiotic group (T2) as compared to all other groups. Table 1. Composition and concentration of the study treatments.
Table 2. Effect of probiotic and humic acid on HI antibody titer in broiler chickens on days 7, 27 and 42 (n=5).
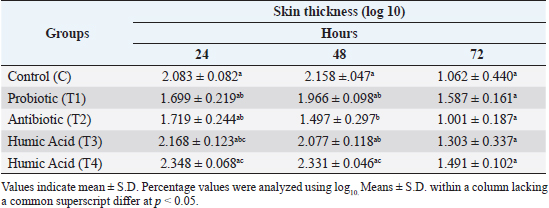
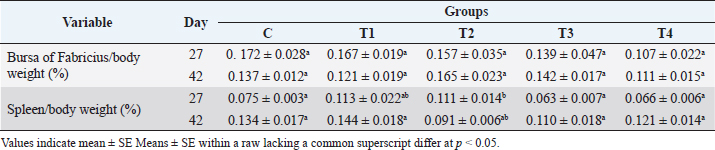
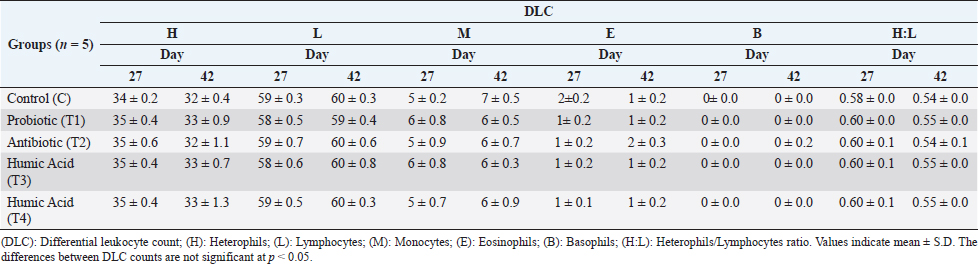
Assessment of cellular immunityThe effect of probiotics and humic acid on cellular immunity is summarized in Table 3. The skin thickness of the humic acid group (T4) showed a significant increase as compared to the probiotic (T1) and antibiotic (T2) groups after 24 hours of the DNCB challenge. After 48 hours, the thickness was still significantly higher in the humic acid group (T4) as compared to other groups except for the control. Moreover, the skin thickness of the antibiotic group (T2) was significantly lower than the control and T4. However, after 72 hours the skin thickness of all groups became equal. Lymphoid organs weightThe effect of probiotics and humic acid on lymphoid organs weight is shown in Table 4. There were no significant differences in bursa of Fabricius/Body weight (%) between all groups on day 27 and 42. Spleen/body weight (%) was significantly higher in the T2 group on day 27 as compared to the control. On day 42, spleen/body weight (%) was significantly lower in the T2 group as compared to the control. Differential leukocyte countThe effect of probiotics and humic acid on differential leukocyte count is shown in Table 5. There were no significant differences in various differential leukocyte counts between tested groups. DiscussionThe immune system of birds plays an important role in controlling viral, bacterial, and parasitic diseases via vaccination. Thus, the integrity of the immune system is vital for the good performance of poultry birds. The failure of the immune system leads to extensive use of antibiotics causing bacterial resistance. Many research studies were conducted to enhance the integrity of the immune system using prebiotics (Vahabi-Asil et al., 2017), probiotics (Aziz Mousavi et al., 2018), medicinal plants (Raza et al., 2015), and essential oils (Gharaibeh et al., 2021). In current study, we assessed the effect of humic acid and probiotics on humoral and cellular immunity, lymphoid organs weight, and DLC of broiler chickens. There was no significant difference of HI antibodies titer between all groups at day 7 indicating homogeneity of maternal immunity against Newcastle disease. Our data showed a significant increase in antibodies titer of the probiotic group (T1) as compared to the control (C) and other groups on day 27. Khaksefidi and Ghoorchi (2006) reported that antibody titer in a 50 mg/kg probiotic-supplemented group is significantly higher 5 and 10 days after immunization compared to the control group when sheep-red-blood cells (SRBC) is injected in 7 and 14 days of age. The highest levels of SRBC and G-type immunoglobulin were obtained in groups of broiler chickens receiving 0.45% sodium humate and 0.02% probiotic in their diet (Eivollahi et al., 2019). Giving probiotics for only the first week seems to be not enough to provide humoral immunity lasting for the 6 weeks of broilers’ life. Despite the booster dose of LaSota vaccine on day 21, the titer of antibodies against ND decreased in all groups but was significantly higher in the T4 group on day 42. The T4 group was given humic acid in drinking water daily until day 42. This result is in agreement with the findings of Mehdi and Hasan (2012) who demonstrated an increase in the antibody titers against ND in broilers fed diets containing humate. Some other studies also reported stimulation of immune response in broilers (Islam et al., 2005; Arif et al., 2019). In contrast, Nagaraju et al. (2014) reported no significant differences in antibody titer against ND on day-10 post vaccination in broilers given 0.1% humic acid in diet. However, stimulation of the broilers’ immune system by humic acid might be increased gradually by increase of age. Table 3. Effect of probiotics and humic acid on skin thickness in broiler chickens on hours 24, 48 and 72 post challenge with DNCB (n=5).
Table 4. Effect of probiotic and prebiotic on bursa/body weight (%) and spleen/body weight (%) on day 27 and 42.
Table 5. Effect of probiotic and humic acid on various differential leukocyte count in broiler chickens on days 27 and 42.
Beside the increase of antibodies against ND, increased concentration of antibodies against avian influenza subtype H9 (Tohid et al., 2010) and infectious bursal disease (Nagaraju et al., 2014) were also reported. The mechanism of action of humic acid and humate as an immune stimulator is not well defined. Elevated lymphoid tissue and uniformly distribution and defined density in the bursa of Fabricius and thymus may contribute to the mechanism of action (Disetlhe et al., 2017). However, our study found no significant differences in bursa of Fabricius/body weight (%) between all groups on days 27 and 42. Spleen/body weight (%) was also not altered due to probiotics and humic acid in our study. This result is in agreement with Fong et al. (2022) who found no significant effects of probiotics bacteria on the spleen and liver weights after the treatment as compared to the control group in mice. These probiotic bacteria increased the abundance of the natural killer cells, the percentage of both the hepatic and splenic T-helper 17 cells and the percentage of splenic regulatory T-cells indicating potential enhancement of cell-mediated responses and cytokine production in a strain- and organ/tissue-specific immunomodulatory effects. In contrast, our study showed no significant effect on cellular immunity since the skin thickness of group T4 which was given humic acid daily had no significant increase as compared to the control group. In other studies, supplementation of humic substances (HS) to laying hens, significantly increased the serum IgG and IgM level (Zhang et al., 2020), and greater lymphocytes and leukocytes counts, globulins (α, β, and γ), phagocytosis, and phagocytic index have been significantly increased in HS-added broilers indicating immune system stimulation (Salah et al., 2015). There were no significant differences in various differential leukocyte counts between tested groups. This result is in agreement with Rath et al. (2006) who stated that red blood cell, white blood cell, monocyte, and hematocrit values were not affected, but there was a decrease in blood heterophil counts and heterophil/lymphocyte ratio, which was significant in 4-week humic acid treated birds. However, there was no significant difference in heterophil/ lymphocyte ratio in our study. In our study, group T2 which given a commercial mixture of antibiotics and vitamins had significant decrease in antibodies titer against Newcastle disease virus on day 42, significant decrease in cell-mediated immunity 48 hours after sensitization with DNCB and a significant increase in Spleen/Body weight (%) on day 27. We also observed a significant decrease in the body weight in this group (unpublished data) which may indicate toxicity. The mixture of antibiotics consisted of Tylan tartarate, Sulfamethoxazole, Sulfadiazine Na, and Pyrimethamine. Pyrimethamine the folic acid and thiamine antagonist, was found to potentiate the activity of sulfanilamide, sulfamerazine, sulfadimidine, and sulfaquinoxaline through synergistic interaction against coccidia (Joyner and Kendal, 1956; Horton-Smith et al., 1960). Lucas (1958) reported toxic effects produced by pyrimethamine characterized by decreased growth-rate and anemia in Rhode Island × Light Sussex chickens fed on a diet containing 20 ppm of pyrimethamine for 28 days. Continuous administration of pyrimethamine alone or with sulphaguanidine to young chicks to combat cecal coccidiosis resulted in failure of growth, poor feathering and the development of macrocytic anemia (Stockdale, 1958). Besides these toxic effects, it seems that pyrimethamine has deleterious effects on the immune response of chickens (incidental findings of our study). However, the mechanism of action needs to be elucidated. ConclusionIn conclusion, humic acid has immunomodulation effects when given daily for broiler chickens until the marketing age. Humic acid has a stimulant and strengthening effect on the humoral and cellular immune system. Moreover, the use of humic acid and probiotics with good hygiene in the first week of age may alternate the use of antibiotics which could be toxic and raise bacterial resistance. Authors contributionFA and AK conceived the ideas of the study, performed the analysis and wrote the manuscript; AE conceived the ideas of the study, revised and commented on the manuscript. Conflict of interestThe authors declared that there is no conflict of interest. FundingThis research received no specific grant or funding. Data availabilityAll data supporting the findings of this study are available within the manuscript. Any extra data needed are available from the corresponding author upon reasonable request. ReferencesArif, M., Alagawany, M., Abd El-Hack, M.E., Saeed, M., Arain, M.A. and Elnesr, S.S. 2019. Humic acid as a feed additive in poultry diets: a review. Iran J. Vet. Res. 20(3), 167–172. Awad, W.A., Böhm, J., Razzazi-Fazeli, E., Ghareeb, K. and Zentek, J. 2006. Effect of addition of a probiotic microorganism to broiler diets contaminated with deoxynivalenol on performance and histological alterations of intestinal villi of broiler chickens. Poult. Sci. 85(6), 974–979. Aziz Mousavi, S.M.A., Mahmoodzadeh Hosseini, H. and Mirhosseini, S.A. 2018. A review of dietary probiotics in poultry. J. Appl. Biotechnol. Rep. 5(2), 48–54. Brisbin, J.T., Zhou, H., Gong, J., Sabour, P., Akbari, M.R., Haghighi, H.R., Yu, H., Clarke, A., Sarson, A.J. and Sharif, S. 2008. Gene expression profiling of chicken lymphoid cells after treatment with Lactobacillus acidophilus cellular components. Dev. Comp. Immunol. 32(5), 563–574. Ceylan, N., Ciftci, I. and Ilhan Z. 2003. The effects of some alternative feed additives for antibiotic growth promoters on the performance and gut microflora of broiler chicks. Turk. J. Vet. Anim. Sci. 27, 727–733. Chowdhury, S.R., Smith, T.K., Boermans, H.J. and Woodward, B. 2005. Effects of feedborne Fusarium mycotoxins on hematology and immunology of turkeys. Poult. Sci. 84, 1698–1706. Disetlhe, A.R.P., Marume, U., Mlambo, V. and Dinev, I. 2017. Humic acid and enzymes in canola-based broiler diets: effects on bone development, intestinal histomorphology and immune development. S. Afr. J. Anim. Sci. 47, 914–922. Eivollahi, L., Ahady, M.T. and Sahraei, M. 2019. The effect of sodium humate and probiotic on performance, carcass traits, immunological indices and gut morphology in broiler chickens. J. Vet. Res. 74(4), 528–544. Fascina, V., Sartori J., Gonzales, E., DeCarvalho, F., DeSouza, I., Polycarpo, G., Stradiotti, A. and Pel´ıcia, V. 2012. Phytogenic additives and organic acids in broiler chicken diets. Rev. Bras. Zootec. 41, 2189–2197. Fong, F.L.Y., El-Nezami, H., Mykkänen, O. and Kirjavainen, P.V. 2022. The effects of single strains and mixtures of probiotic bacteria on immune profile in liver, spleen, and peripheral blood. Front. Nutr. 9, 773298. Gharaibeh, M.H., Khalifeh, M.S., Nawasreh, A.N., Hananeh, W.M. and Awawdeh, M.S. 2021. Assessment of immune response and efficacy of essential oils application on controlling necrotic enteritis induced by Clostridium perfringens in broiler chickens. Molecules. 26(15), 4527. Hassan, M. 2014. Effect of adding dietary humate on productive performance of broiler chicks. Asian J. Poult. Sci. 8(2), 23–31. Heckert, R. A., Estevez, I., Russek-Cohen, E. and Pettit-Riley, R. 2002. Effects of density and perch availability on the immune status of broilers. Poult. Sci. 81, 451–457. Horton-Smith, C., Long, P.L. and Collier, H.O.J. 1960. Potentiation of sulfadimidine by 2,4-diamino-6,7-di-isopropylpteridine and other 6,7-disubstituted 2,4-diaminopteridines against eimeria infections of chicks Br. J. Pharmacol. 15, 298–303. Ipek, A. and Sozcu, A. 2015. The effects of broiler breeder age on intestinal development during hatch window, chick quality and first week broiler performance. J. Appl. Anim. Res. 43(4), 402–408. Islam, K.M.S., Schuhmacher, A. and Gropp, J.M. 2005. Humic acid substances in animal agriculture. Pak. J. Nutr. 4, 126–134. Joyner, L.P. and Kendall, S.B. 1956. The mode of action of a mixture of pyrimethamine and sulphadimidine on eimeria tenella. Brit. J. Pharmacol. 11, 454. Kammon, A.M. 2017. The future use of medicinal plants as alternatives to antibiotics in animal health and production. Appro. Poult. Dairy Vet. Sci. 1(1), 000503. Kammon, A., Alzentani, S., Tarhuni, O. and Asheg, A. 2019. Effect of some organic acids on body weight, immunity and cecal bacterial count of chicken during heat stress. Int. J. Poult. Sci. 18, 293–300. Khaksefidi, A. and Ghoorchi, T. 2006. Effect of probiotic on performance and immunocompetence in broiler chicks. J. Poult. Sci. 43(3), 296–300. Kizerwetter-Swid, M. and Binek, M. 2009. Protective effect of potentially probiotic Lactobacillus strain on infection with pathogenic bacteria in chickens. Pol. J. Vet. Sci. 12(1), 15–20. Lucas, J. 1958. Toxic effects produced by pyrimethamine in chickens and their antagonism by folic acid. Nature. 182, 1449. MacCarthy, P. 2001. The principles of humic substances. Soil. Sci. 166, 738–751. Mehdi, A. and Hasan, G. 2012. Immune response of broiler chicks fed yeast derived mannan oligosaccharides and humate against newcastle disease. World Appl. Sci. J. 18(6), 779–785. Moraes, V.M.B., Malheiros, R.D., Furlan, R.L., Bruno, L.D.G., Malheiros, E.B. and Macari, M. 2002. Effect of environmental temperature during the first week of brooding period on broiler chick body weight, viscera and bone development. Bra. J. Poult. Sci. 4(1), 1–8. Nagaraju, R., Reddy, B.S., Gloridoss, R., Suresh, B.N. and Ramesh, C. 2014. Effect of dietary supplementation of humic acids on performance of broilers. Ind. J. Anim. Sci. 84, 447–452. OIE terrestrial manual. 2012. Newcastle Disease (infection by Newcastle disease virus), Chapter 2.3.14, pp: 555–574. Rath, N.C., Huff, W.E. and Huff, G.R. 2006. Effects of humic acid on broiler chickens. Poult. Sci. 85(3), 410–414. Raza, A., Muhammad, F., Bashir, S, Anwar, M.I., Awais, M.M., Akhtar, M., Aslam, B. Khaliq, T. and Naseer, M.U. 2015. Antiviral and immune boosting activities of different medicinal plants against newcastle disease virus in poultry. World Poult. Sci. J. 71(3), 523–532. Salah, H., Masour, E.S., Reham, R.R. and El-Hamid E.S.A. 2015. Study on the effect of humic acid on growth performance, immunological, some blood parameters and control intestinal closterdium in broiler chickens. Vet. J. 43,102–109. Silim, A. and Abbassi, H. 2015. Avian immunology. In Manual of Poultry Diseases. Eds., Brugere-Picoux, J., Vaillancourt, J., Shivaprassad, H., Venne, D. and Bouzouaia M. pp: 102–109. Stockdale, H.G. 1958. The toxicity of pyrimethamine for the chick. Vet. Rec.70, 720–725. Tiwary, B.K. and Goel, M.C. 1985. Contact sensitivity to DNCB in normal and cell-mediated immunity deficient chickens: in vivo detection and correlation with lymphocyte transformation and graft-versus-host reaction. Vet. Immunol. Immunopathol. 8, 329–339. Tohid, T., Hasan, G. and Alireza, T. 2010. Efficacy of mannanoligosaccharides and humate on immune response to avian influenza (H9) disease vaccination in broiler chickens. Vet. Res. Commun. 34, 709–717. Vahabi-Asil, O., Bouyeh, M., Qotbi, A, Kadim, I. T., Seidavi A., Centoducati G., Laudadio V. and Tufarelli V. 2017. Effects of a prebiotic on growth performance, blood parameters and immunity response of turkeys fed low protein diets. Eur. Poult. Sci. 81, 196. Yasar, S., Gokcimen, A. Altuntas, I. Yonden, Z. and Petekkaya, E. 2002. Performance and ileal histomorphology of rats treated with humic acid preparations. J. Anim. Physiol. Anim. Nutr. 86, 257–264. Yoon, C., Na, C.S., Park, J.H., Han, S.K., Nam, Y.M. and Kwon, J.T. 2004. Effect of feeding multiple probiotics on performance and fecal noxious gas emission in broiler chicks. Korean J. Poult. Sci. 31(4), 229–235. Zhang, A.R, Pirzado, S.A., Liu, G.H., Chen, Z.M. Chang, W.H., Cai. Y.H., Brtden, W.L. and Zheng, A.J. 2020. Dietary supplementation with sodium humate improves egg quality and immune function of laying hens. J. Appl. Anim. Nut. 8(2), 93–99. | ||
| How to Cite this Article |
| Pubmed Style Ahfeethah F, Elazomi A, Kammon A. Effect of humic acid and probiotics on immunity of broiler chickens. Open Vet J. 2023; 13(7): 839-845. doi:10.5455/OVJ.2023.v13.i7.5 Web Style Ahfeethah F, Elazomi A, Kammon A. Effect of humic acid and probiotics on immunity of broiler chickens. https://www.openveterinaryjournal.com/?mno=143750 [Access: July 27, 2024]. doi:10.5455/OVJ.2023.v13.i7.5 AMA (American Medical Association) Style Ahfeethah F, Elazomi A, Kammon A. Effect of humic acid and probiotics on immunity of broiler chickens. Open Vet J. 2023; 13(7): 839-845. doi:10.5455/OVJ.2023.v13.i7.5 Vancouver/ICMJE Style Ahfeethah F, Elazomi A, Kammon A. Effect of humic acid and probiotics on immunity of broiler chickens. Open Vet J. (2023), [cited July 27, 2024]; 13(7): 839-845. doi:10.5455/OVJ.2023.v13.i7.5 Harvard Style Ahfeethah, F., Elazomi, . A. & Kammon, . A. (2023) Effect of humic acid and probiotics on immunity of broiler chickens. Open Vet J, 13 (7), 839-845. doi:10.5455/OVJ.2023.v13.i7.5 Turabian Style Ahfeethah, Fouziyah, Altayeb Elazomi, and Abdulwahab Kammon. 2023. Effect of humic acid and probiotics on immunity of broiler chickens. Open Veterinary Journal, 13 (7), 839-845. doi:10.5455/OVJ.2023.v13.i7.5 Chicago Style Ahfeethah, Fouziyah, Altayeb Elazomi, and Abdulwahab Kammon. "Effect of humic acid and probiotics on immunity of broiler chickens." Open Veterinary Journal 13 (2023), 839-845. doi:10.5455/OVJ.2023.v13.i7.5 MLA (The Modern Language Association) Style Ahfeethah, Fouziyah, Altayeb Elazomi, and Abdulwahab Kammon. "Effect of humic acid and probiotics on immunity of broiler chickens." Open Veterinary Journal 13.7 (2023), 839-845. Print. doi:10.5455/OVJ.2023.v13.i7.5 APA (American Psychological Association) Style Ahfeethah, F., Elazomi, . A. & Kammon, . A. (2023) Effect of humic acid and probiotics on immunity of broiler chickens. Open Veterinary Journal, 13 (7), 839-845. doi:10.5455/OVJ.2023.v13.i7.5 |