| Research Article | ||
Open Vet J. 2023; 13(6): 742-752 Open Veterinary Journal, (2023), Vol. 13(6): 742-752 Original Research Clinical findings using echocardiography and plasma cardiac troponin I and pathological findings in dogs with hypertrophic cardiomyopathy: A retrospective studyTakeki Ando1,2, Takeshi Izawa3, Hidetaka Nishida1 and Hideo Akiyoshi1*1Laboratory of Veterinary Surgery, Graduate School of Veterinary Science, Osaka Metropolitan University, Osaka, Japan 2Ando Animal Hospital, Awaji, Japan 3Laboratory of Veterinary Pathology, Graduate School of Veterinary Science, Osaka Metropolitan University, Osaka, Japan *Corresponding Author: Hideo Akiyoshi. Laboratory of Veterinary Surgery, Graduate School of Veterinary Science, Osaka Metropolitan University, Osaka, Japan. Email: h.akiyoshi [at] omu.ac.jp Submitted: 09/03/2023 Accepted: 14/05/2023 Published: 11/06/2023 © 2023 Open Veterinary Journal
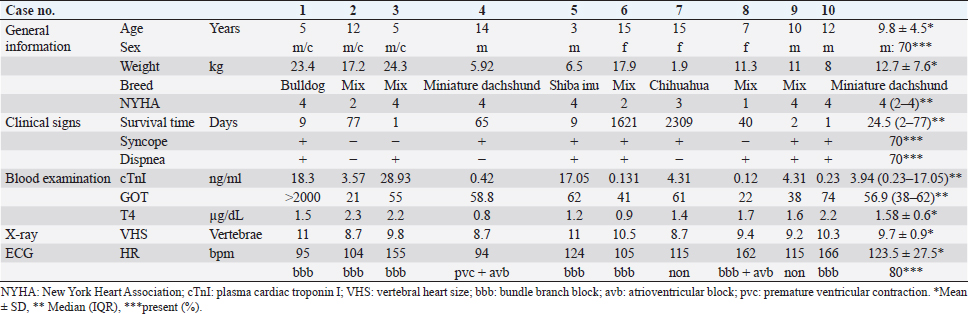
AbstractBackground: Hypertrophic cardiomyopathy (HCM) is considered rare in dogs, and there is a lack of clinical data. Cardiac troponin I (cTnI) is a biomarker of cardiomyocyte damage and necrosis and can be used to diagnose cat and human HCM. Aim: We investigated whether the presence of cTnI in clinical data can be used in conjunction with echocardiography to diagnose canine HCM. Methods: This study comprised client-owned dogs with clinical evidence of concentric hypertrophy on echocardiographic images, serum total thyroxine levels of ≤5 µg/dl, systolic blood pressure of ≤180 mmHg, and absence of aortic stenosis. All cases were necropsied. Results: Cardiomyocyte hypertrophy (mean diameter, 18.3 ± 1.8 µm), myocardial fiber disarray (70%), interstitial fibrosis (80%), and small vessel disease (100%) were assessed. In dogs with HCM, the left ventricles were concentric, almost symmetrical, and hypertrophied above the aortic diameter. The end-diastolic interventricular septum normalized to body weight [intraventricular septal thickness in diastole (IVSDN)] was 0.788 [interquartile range (IQR), 0.7–0.92], which exceeded the normal range (5%–95%, IQR: 0.33–0.52). In total, 70% of the dogs with HCM had syncope and dyspnea, and all dogs had high cTnI levels (median, 3.94 ng/ml), exceeding the upper limit of normal (0.11 ng/ml) and indicating cardiomyocyte damage. IVSDN and serum cTnI levels were correlated (ρ =0.839, p =0.01). Conclusion: Ventricular wall thickening and high serum cTnI levels can provide a presumptive diagnosis of HCM and prompt the initiation of treatment or additional diagnostic investigations. Keywords: Biomarker, Cardiac troponin I, Dog, End-diastolic thickness of the interventricular septum, Hypertrophic cardiomyopathy. IntroductionHypertrophic cardiomyopathy (HCM) is a common disease in humans and domestic cats, with genetic mutations being the most frequently identified underlying etiology (Freeman et al., 2017; Ueda and Stern, 2017; Luis Fuentes et al., 2020). Despite being a common cause of sudden death in humans (Richardson et al., 1996) and the leading cause of feline heart disease (Fox et al., 2018), there are limited reports of HCM in dogs (Washizu et al., 2003; Schober et al., 2022). However, HCM has been identified after anesthesia-related accidents or sudden cardiac death (SCD) in dogs (Liu et al., 1979b). HCM is characterized by concentric hypertrophy of the left ventricular wall with a normal or small left ventricular size. The diagnosis of HCM requires the exclusion of systemic hypertension, hyperthyroidism, congenital aortic stenosis, and less common causes of wall hypertrophy, such as multicentric lymphoma and pseudohypertrophy secondary to dehydration (Schober et al., 2022). HCM has been reported in male and female dogs aged 1–14 years (Liu et al., 1979a; Washizu et al., 2003; Pang et al., 2005; Schober et al., 2022). Previous studies have reported HCM in all dog sizes, including German shepherds, Doberman pinschers, Airedale terriers, Great Danes, Boston terriers, poodles, bulldogs (Liu et al., 1979b), corgis (Marks, 1993), Shih-Tzu, and terriers (Washizu et al., 2003; Schober et al., 2022). Typical historical and physical examination findings in dogs with HCM include cardiac murmur, exercise intolerance, and convulsive seizures (Schober et al., 2022). B-mode echocardiography in cases of HCM may demonstrate moderate enlargement of the left atrium, with M-mode echocardiography revealing the excess motion of the left ventricular free wall and ventricular septum (Washizu et al., 2003). Systolic anterior motion of the mitral valve and left ventricular diastolic dysfunction are frequently observed on echocardiography in dogs with HCM (Schober et al., 2022). Pathological findings, such as myocardial fiber hypertrophy, intricate myocardial alignment, plexiform fibrosis, and coronary arteriosclerosis have been reported in HCM in cats (Fox, 2003), dogs (Liu et al., 1979b; Washizu et al., 2003), and humans (Fujiwara et al., 1982). Cardiac troponin I (cTnI) has been used as a biomarker to assess damage and necrosis of cardiomyocytes (Adams et al., 1994; Fonfara et al., 2010; Kociol et al., 2010; White, 2011; Langhorn and Willesen, 2016). Normal cTnI concentrations in dogs have been reported as <0.1 (Porter et al., 2016), <0.07 (Sleeper et al., 2001), and <0.03 ng/ml (with an upper 95th percentile of 0.11 ng/ml) (Oyama and Sisson, 2004). In recent years, cTnI levels in HCM have been measured in humans (Cambronero et al., 2009; Kubo et al., 2010, 2011; McGorrian et al., 2013) and cats (Langhorn et al., 2014; Hori et al., 2018; Hertzsch et al., 2019; Luis et al., 2020) and are expected to have utility in diagnosis and prognosis. HCM is typically diagnosed via echocardiography (Anwar and TenCate, 2021; Schober et al., 2022). The cutoff value for left ventricular wall hypertrophy when diagnosing HCM is 36 mm in cats (Payne et al., 2013) and 315 mm in humans (Gersh et al., 2011; Enriquez and Goldman, 2014); however, there is currently no accepted cutoff value in dogs given the significant variations in body size between dog breed. Additionally, reference data for dogs are limited. Therefore, the assessment of concentric hypertrophy requires the use of standardized echocardiographic values (Schober et al., 2022). However, diagnosing HCM by evaluating left ventricular wall thickening alone can be challenging (Schober et al., 2022). Therefore, pathological diagnosis (Fox, 2003; Maron and Fox, 2015; Ueda and Stern, 2017) is still considered a common method of diagnosing HCM. Given the above, there is a need for novel diagnostic methods for HCM that can be used in conjunction with the use of echocardiography for assessing myocardial wall hypertrophy. This study included only cases with left ventricular wall hypertrophy that underwent necropsy. Dogs with confirmed HCM were evaluated for echocardiography and common clinical signs. In particular, we investigated whether clinical data cTnI can help diagnose HCM in dogs. Materials and MethodsAnimalsAll dogs included in this study were raised and cared for by their owners and screened for heart disease at Ando Animal Hospital (Hyogo Prefecture, Japan). Dogs with HCM (n =10) were examined between 2007 and 2017. HCM was suspected based on clinical examinations. All dogs were definitively diagnosed after death based on histopathological examinations. Clinical examination and diagnosisPresumptive diagnoses of HCM were based on the results of general physical examinations, blood examinations, electrocardiography (ECG), thoracic radiography, and ultrasonography. Holter monitoring and angiography were also performed in dogs requiring close monitoring for arrhythmias or close examination of the left heart chamber, respectively. Information on general conditions, including previous history of syncope, was obtained by interviewing the owners at the hospital. Physical examinations were performed to assess heart and respiratory symptoms, such as cough and heart murmurs, using the Levine scale (Silverman and Wooley, 2008). Blood testing was performed to determine serum thyroid hormone and cTnI levels. Additionally, ECG was performed to determine the presence of premature ventricular contractions and atrioventricular or bundle branch blocks. Thoracic radiography was performed to measure the vertebral heart size (VHS) in the lateral view (Buchanan, 2000). EchocardiographyA single veterinarian performed all echocardiographic evaluations. Three cardiac cycles were monitored in each measurement, with the mean of three cycles calculated. Relative myocardial wall thickness was measured using M-mode echocardiography in the right parasternal long-axis view. Measurements were calculated using the following formulas for standardization (Cornell et al., 2004; Schober et al., 2022): end-diastolic interventricular septum (IVSd) normalized for body weight (BW; IVSDN=IVSd, in cm/BW0.241) and end-diastolic left ventricular posterior wall normalized for body weight (LVPWDN=LVPWd, in cm/BW0.232). The end-diastolic left ventricular internal dimension normalized for body weight (LVIDDN=LVIDd, in cm /BW0.294) was calculated from measurements in the two-dimensional (2D) long-axis view of the outflow tract from the right parasternal location by applying an ultrasonic beam vertically to the left ventricular wall at the level of the tendinous cords in M-mode (Bodh et al., 2019). The type of concentric or eccentric left ventricular hypertrophy was determined using the following equation in long- and short-axis views: 0.53 < (IVSd + LVWd)/LVDd (Borgarelli et al., 2007; Schober et al., 2022). Left ventricular fractional shortening (FS) was calculated using the following formula: (end-diastolic left ventricular internal dimension—end-systolic left ventricular internal dimension) × 100. In addition, the left atrium-to-aorta (LA/Ao) ratio was calculated by comparing the left atrium’s internal diameter in maximum diastole to the aorta’s internal diameter (i.e., the measured length between the aortic leaflets) based on the 2D short-axis view from the right parasternal location at the level of the heart base during the diastolic phase (Rishniw and Erb, 2000). The ratio of the E wave to the A wave was calculated by measuring the early diastolic and atrial contraction velocities at the approximate center of the mitral valve where the mitral leaflets meet using pulsed-wave Doppler based on the 2D four-chamber view of the apex from the left parasternal location (Bodh et al., 2019). Survival duration was defined as the period from the day of clinical HCM diagnosis to that of death owing to heart failure. At the time of the clinical diagnosis of HCM, signs of heart failure in dogs were classified according to the modified New York Heart Association (NYHA) classification (Atkins et al., 2009) as follows: class I, asymptomatic heart disease; class II, heart disease displaying clinical signs only during strenuous exercise; class III, heart disease displaying clinical signs during routine activities or mild exercise; and class IV, heart disease showing severe clinical signs, even at rest. Histopathological examinationsHistopathological examinations were performed by a veterinary pathologist at the Laboratory of Pathology of Osaka Prefecture University. Death was confirmed at the hospital before pathological dissection. Hearts were cut vertically from the base to the apex to allow observation of the aorta and the four chambers of the heart. The thicknesses of the IVSd and left ventricular free wall were measured. The left ventricular outflow tract diameter was measured. Photography of the septum and left ventricular outflow tract was performed to obtain macroscopic images. Sections were fixed in 10% formalin before histopathological examinations. Morphological observation of the myocardium was performed by cross-sectioning (3 mm) the IVSd and left ventricular free wall from the endocardium to the pericardium at the base and apex while avoiding the papillary muscle. The sections were embedded in paraffin and stained with hematoxylin and eosin. Myocardial disarray and changes in cardiomyocytes and small-to-medium-sized arteries were subsequently evaluated. Myocardial fibrosis was assessed using paraffin-embedded sections stained with Azan. Myocyte hypertrophy was evaluated by calculating the mean short diameter of 40–100 myocytes measured at sites where the longitudinal section contained nuclei using micrographs in three fields of view at ×200 magnification (Hoshino et al., 1983). Myocyte disarray (Fox, 2003) was assessed and scored according to the degree of abnormality in cardiomyocyte arrangement. When hypertrophied, myocytes typically display a pinwheel configuration or herringbone pattern that can be scored using the following system: 0, none; 1+, mild; 2+, moderate; and 3+, severe. A comprehensive histopathological examination, including the thorough evaluation of cardiomyocyte hypertrophy (cutoff, >15 µm/100 cells) (Hoshino et al., 1983) and disarray, intimal thickening, cavity narrowing in small vessels, focal necrosis, and fibrosis was required for the definitive diagnosis of HCM. The pattern of left ventricular hypertrophy was determined based on visual estimation and was classified as symmetrical or asymmetrical according to the difference in wall thickness, with 50% considered the cutoff value. As Langhorn and Willesen (2016) reported that plasma cTnI levels may also be high in myocarditis, myocarditis was excluded by pathological examination in this study. Plasma cTnI measurementsBlood testing to measure cTnI levels was performed at the time of clinical diagnosis of HCM in all cases. Blood samples (1 ml) were collected from the saphenous or cephalic veins, placed in heparinized tubes, and centrifuged at 3,000 rpm for 5 minutes. Separated plasma was then measured using i-STATÒ1 (Abbott Laboratories, Princeton, NJ) equipped with a dedicated cartridge (i-STATÒ cTnI test cartridge) that uses a two-site enzyme-linked immunosorbent assay based on a monoclonal antibody. This method has a measurable range of serum cTnI values in dogs of 0.00–50.00 ng/ml (Porter et al., 2016) and a detection limit of 0.02 ng/ml in humans (Apple et al., 2004). Statistical analysesData distribution normality was assessed using the c2 goodness-of-fit test. Normally distributed data are presented as means ± standard deviations. Nonnormally distributed data are presented as medians and interquartile ranges (IQR). Additionally, Spearman’s rank correlation coefficient (r) was used to assess correlative relationships in dogs with HCM. The log-rank test was used to test bivariate associations between each prognostic factor and survival duration. The cutoff value of the clinical index used for the log-rank test was the mean or median in dogs with HCM. All statistical analyses were performed using statistical software (Statcel on Excel 3rd ed., OMS Ltd., Tokyo, Japan). p-values of <0.05 were considered statistically significant. Ethical approvalThis retrospective study complied with the Regulations for Animal Experiments and Related Activities at Osaka Prefecture University (Sakai, Japan). ResultsTable 1 presents the characteristics of dogs with HCM (n =10; mean age, 9.8 ± 4.5 years; mean BW, 12.7 ± 7.6 kg). Most dogs with HCM were male (n =7; 70%). Dogs with HCM were categorized by breed as follows: mixed breed (n =5; 50%), miniature dachshund (n =2; 20%), bulldog (n =1; 10%), Shiba inu (n =1; 10%), and Chihuahua (n =1; 10%). Most dogs (n =9; 90%) with HCM had clinical signs, including syncope (n =7) and dyspnea (n =7). Most dogs (n =8; 80%) had arrhythmia or abnormal wave morphologies on ECG. Arrhythmias were attributable to an atrioventricular block in two dogs and premature ventricular contractions in one dog. Abnormal wave morphology was attributable to a bundle branch block in seven dogs. The mean VHS on thoracic radiography was 9.7 ± 0.9 v, an almost normal value (Buchanan and Bücheler, 1995), except for two dogs (dogs one and five) with a mean VHS of 11.0 v. Table 1. Characteristics of dogs with HCM.
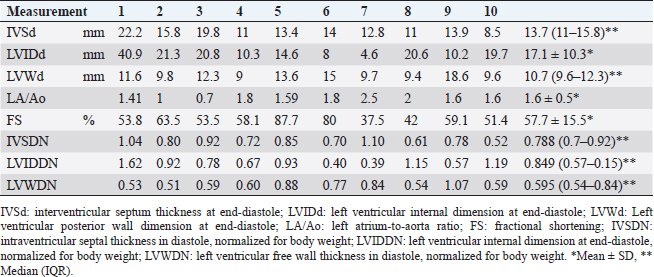
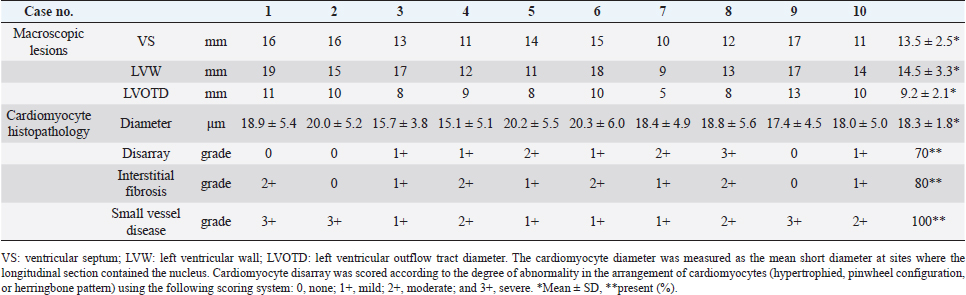
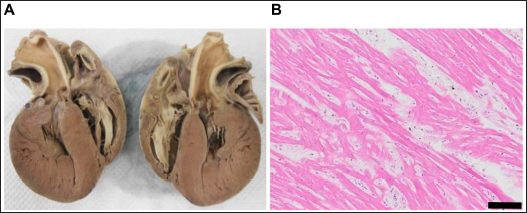
Dogs 3, 4, and 8 were treated with β-adrenergic blockers (4,8), angiotensin-converting enzyme inhibitors (3,4,8), furosemide (4,8), spironolactone (4,8), aspirin (4), clopidogrel (3,8), or cardiac glycosides (4,8) based on clinical signs. The other dogs were untreated. EchocardiographyEchocardiography has been used to evaluate abnormal ventricular wall thickening in previous studies (Morrison et al., 1992; Gonçalves et al., 2002; Cornell et al., 2004). As left ventricle morphological features are affected by BW, Cornell’s standardized formula was used (Cornell et al., 2004). Dogs with HCM had a median IVSd and IVSDN of 13.7 (IQR, 11–15.8 mm) and 0.788 (IQR, 0.7–0.92), respectively, which were higher than those observed in clinically normal dogs (a 95% prediction interval of 0.29–0.52 is typically used for IVSDN using M-mode variables) (Cornell et al., 2004). Moreover, dogs with HCM had a median LVIDd and LVIDDN of 17.1 ± 10.3 mm and 0.849 (IQR, 0.57–1.15), respectively, lower than those observed in clinically normal dogs (a 95% prediction interval of 1.35–1.73 is typically used for LVIDDN using M-mode variables) (Cornell et al., 2004). Dogs with HCM had a median LVWd and LVPWDN of 10.7 (IQR, 9.6–12.3 mm) and 0.595 (IQR, 0.54–0.84), respectively (Tabl 2), higher than those observed in clinically normal dogs (a 95% prediction interval of 0.29–0.53 is typically used for LVPWDN using M-mode variables) (Cornell et al., 2004). Echocardiography revealed concentric cardiac hypertrophy in all cases [(IVSd + LVWd)/LVDd > 0.53] (Tables 2 and 3). In dogs with HCM, the mean LA/Ao ratio (1.6 ± 0.5) and FS (57.7% ± 15.5%) were higher than those in clinically normal dogs (95% prediction intervals of 0.8–1.3 for LA/Ao and 25%–44% for FS are typically used for indexing M-mode variables) (Cornell et al., 2004). Pathology and histopathologyDogs with HCM demonstrated symmetrically hypertrophied ventricular septa (mean, 13.5 mm; range, 10–17 mm) and left ventricular free walls (mean, 14.5 mm; range, 9–19 mm). Greater concentric hypertrophy was observed compared with the ventricular cavity. The left ventricular wall was thickened compared with the aorta (9.2 mm) and protruded into the left ventricular outflow tract, which macroscopically appeared to be narrowed by >50% (Table 3, Figure 1A). Histopathological examination revealed hypertrophy of cardiomyocytes in all dogs (mean, 18.3 µm; range, 15.1–20.3 µm) compared with the normal range (≤13.0 ± 0.7 µm) (Fujiwara et al., 1982). The proportions of dogs with cardiomyocyte disarray (70%), interstitial fibrosis (80%), and small vessel disease (100%) are shown in Table 3 and Figure 1B. Table 2. Echocardiographic findings with HCM in dogs.
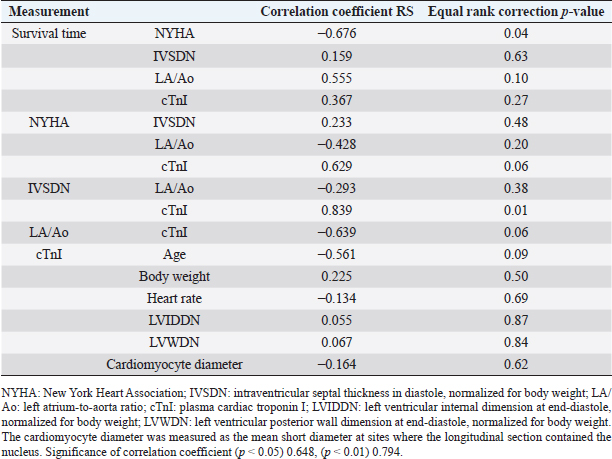
Statistical analysesTable 1 presents laboratory data from serum sampling. (Table 2 presents the echocardiography results. In dogs with HCM, the median plasma cTnI level (3.94 ng/ml; IQR, 0.23–17.05) was greater than that in healthy dogs (Langhorn and Willesen, 2016). Survival duration decreased as NYHA class increased (r =−0.676, p =0.04; Table 4). NYHA classification and serum cTnI levels were weakly correlated (r =0.629, p =0.06), IVSDN and serum cTnI levels were strongly correlated (r =0.839, p =0.01), and serum cTnI levels and the LA/Ao ratio were weakly correlated (r =−0.639, p =0.06; Table 4). There was no correlation between IVSDN and the LA/Ao ratio (Table 4). DiscussionOwing to the limited previous studies in dogs with HCM (Liu et al., 1979a; Marks, 1993; Washizu et al., 2003; Pang et al., 2005; Schober et al., 2022), there is a lack of information regarding the clinical signs of HCM in dogs compared with cats (Payne et al., 2013; Maron and Fox, 2015) and humans (Gersh et al., 2011; Enriquez and Goldman, 2014). The results of this study demonstrate correlations between clinical, echocardiographic, and serological biomarker findings. All dogs with HCM in this study had a thickened left ventricular wall [median IVSd, 13.7 mm (IQR, 11–15.8 mm)] and postmortem pathological findings confirming HCM. Echocardiography allows the measurement of wall hypertrophy for the diagnosis of HCM in cats (36 mm) (Payne et al., 2013) and humans (315 mm) (Gersh et al., 2011; Enriquez and Goldman, 2014). However, the results of the M-mode echocardiography in healthy dogs vary greatly according to BW. Therefore, a formula was devised to correct left ventricular wall measurements for BW (Cornell et al., 2004); however, these values cannot be used to differentiate between heart diseases (Cornell et al., 2004). Similarly, 2D echocardiography values can be weight-corrected (Schober et al., 2022). Dogs with HCM confirmed on postmortem pathology had IVSDN values [median, 0.788 (IQR, 0.7–0.92)] above the upper limit of normal (0.52, 95% prediction interval) (Cornell et al., 2004). Additional work to confirm or rule out HCM should be considered when echocardiography reveals ventricular wall hypertrophy in dogs (Schober et al., 2022). Previous studies have reported factors associated with secondary cardiac hypertrophy; however, there are limited studies describing factors that worsen HCM in dogs (Liu et al., 1979b). The pathological findings in this study of dogs with HCM revealed the greater thickness of the ventricular septum (mean, 13.5 ± 2.5 mm) compared to the left ventricular outflow tract diameter (mean, 9.2 mm ± 2.1), indicating narrowing of the outflow tract. When this occurs, environmental factors, such as intensive exercise, high temperatures, and high humidity, can further overload the heart, potentially worsening concentric cardiac hypertrophy over time. In some cases, mild mitral regurgitation can cause eccentric cardiac hypertrophy attributable to volume overload. Previous studies on clinical signs in cats with HCM (Payne et al., 2013) have reported syncope, dyspnea, SCD, and thrombosis. In this study, syncope (70%), dyspnea (70%), and SCD (30%) were observed. These results are similar to the previously reported findings in cats. Exercise intolerance and syncope were the most common clinical signs reported in previous studies of canine HCM (Schober et al., 2022). HCM reportedly causes atrial fibrillation and ventricular arrhythmia in humans based on electrocardiographic findings (Enriquez and Goldman, 2014). We observed ventricular arrhythmia (10%) but were unable to confirm atrial fibrillation in dogs with HCM. Additionally, abnormal waveforms (80%) were observed on ECG; however, these ECG findings were not associated with the incidence or severity of HCM in dogs. Table 3. Autopsy findings in dogs with HCM.
Histopathological examination is essential for diagnosing HCM in cats and humans. Characteristic histopathological findings include cardiomyocyte disarray (Fox, 2003) and myocyte hypertrophy (Hoshino et al., 1983). We performed necropsies on all dogs with HCM and observed histopathological findings characteristic of HCM, including cardiomyocyte hypertrophy (mean, 18.3 ± 1.8 µm), cardiomyocyte disarray (70%), interstitial fibrosis (80%), and small vessel disease (100%). These results demonstrate that dogs with HCM, similar to cats with HCM, had less cardiomyocyte disarray compared with humans with HCM (Lombardi and Betocchi, 2002). In this study, left ventricular hypertrophy was characteristically concentric with nearly symmetrical wall thickening between the IVSd and left posterior ventricular wall (70%) (Schober et al., 2022). Ventricular wall thickening beyond the aortic diameter was observed leading to obstruction of the outflow tract (Figure 1). Additionally, we observed cardiomyocyte hypertrophy as a histological feature of HCM; however, no association was identified between cardiomyocyte hypertrophy and myocardial wall thickening. Although cardiomyocyte hypertrophy is posited to result from pressure overload, myocardial wall thickening is strongly associated with increased fat and fibrotic connective tissue (Kociol et al., 2010; Kubo et al., 2011). Accordingly, cardiomyocyte hypertrophy may not be the only factor contributing to the pathogenesis of HCM. Numerous retrospective studies on cats and humans have reported risk factors for HCM. Many cats with HCM have an enlarged LA (Linney et al., 2014), which is believed to affect disease severity (Payne et al., 2013); however, other studies have reported that 34% of cats with HCM exhibit LA enlargement (Duler et al., 2019). LA enlargement was reported in only 22% of dogs with HCM (Schober et al., 2022); however, seven dogs in this study had apparent left atrium enlargement as indicated by an LA/Ao ratio of ≥1.6. However, no association was observed between left atrium enlargement (LA/Ao ratio ≥ 1.6) and prognostic factors. As this study’s cohort was relatively small and many dogs had severe disease, further studies are warranted to validate these findings. To the best of our knowledge, this is the first study to report serum cTnI levels in dogs with HCM. Serum cTnI levels were substantially higher (median, 3.94 ng/ml; IQR, 0.23–17.05) than those previously reported in healthy dogs (median, 0.03 ng/ml; range, 0.02–0.15 ng/ml) (Oyama and Sisson, 2004), dogs with dilated cardiomyopathy (median, 0.22 ng/ml) (Wess et al., 2017), dogs with mitral valve disease (median, 0.11 ng/ml) (Oyama and Sisson, 2004), dogs with congenital subaortic stenosis (median, 0.08 ng/ml) (Oyama and Sisson, 2004), and dogs with severe congestive heart failure (1–2 ng/ml) (Langhorn and Willesen, 2016). Various factors, including cardiac and noncardiac diseases, can increase serum cTnI levels (Kociol et al., 2010). Plasma cTnI levels have also been shown to be increased in cases of mild myocardial ischemia or stress due to leakage of free troponin (Hickman et al., 2010).
Fig. 1. Histopathological findings of HCM in dogs (A) 1 and (B) 5. (A) 2D long-axis view of the heart. Prominent left ventricular wall thickening, left ventricular cavity narrowing, and left ventricular outflow tract dysfunction are observed. (B) Staining of the IVSd myocardium with hematoxylin and eosin demonstrating cardiomyocyte disarray (black bar, 100 μm). Table 4. Spearman’s correlation coefficient between data with HCM in dogs.
The serum cTnI levels in dogs with HCM included in this study were highly variable (range, 0.12–28.93 ng/ml). The leakage of cTnI is attributable to myocardial injury and necrosis (Bertinchant et al., 1996). In this study, serum cTnI levels in dogs 1, 3, and 5 were extremely high (range, 17.05–28.93 ng/ml). This finding may be attributable to severe myocardial ischemia, injury, and necrosis in dogs with acute exacerbation of NYHA class IV HCM-induced heart failure. Conversely, reversible myocardial injury or stress without cardiomyocyte necrosis or severe damage is reportedly correlated with low serum cTnI levels (Kubo et al., 2010). Interestingly, serum cTnI levels in the other seven dogs with HCM in this study were relatively low (range, 0.12–4.31 ng/ml). These lower serum cTnI levels may indicate ischemia or excessive loading rather than cardiomyocyte necrosis. A correlation was observed between IVSDN and plasma cTnI levels in dogs with HCM (r =0.839, p =0.01) (Korraa et al., 2012). Elevated serum cTnI levels indicate myocardial ischemia, injury, or necrosis. Thus, elevated IVSDN levels in dogs with HCM may be attributable to myocardial injury or necrosis. The pathological analysis in this study revealed high rates of interstitial fibrosis (80%) and small vessel disease (100%) (Fox, 2003; Lombardi and Betocchi, 2004). These findings may indicate myocardial cell damage or ischemia (Pop et al., 2006; Belerenian et al., 2021), with myocardial cell damage known to cause cTnI leakage (Zhou et al., 2019). Our results should be considered within the context of several limitations. First, the statistical analysis may have decreased accuracy given the small sample size in this study. Further, dogs with HCM are rare in clinical practice and pathological examinations are infrequently performed. Thus, the cases included in this study may have been skewed toward particular breeds or dogs with specific pathological conditions. Second, this study included only clinical cases. Accordingly, enrolled dogs may have had varying levels of cardiomyocyte damage as dogs with asymptomatic, chronic, and terminal stages of heart failure were included. Similarly, some dogs were treatment-naive, whereas others had previously been treated for cardiac diseases. Thus, some dogs included in this study may have exhibited the side effects of medications. Dogs with acute exacerbations of heart failure may have experienced additional cardiomyocyte damage owing to myocardial ischemia and excess loading. These effects may explain the observed variability in the levels of serum cTnI. Additionally, we did not quantify the areas of myocardial ischemia or necrosis. Serum cTnI levels were used to identify factors associated with myocardial ischemia and necrosis; however, only qualitative evaluations were possible. Novel methods for quantifying the area of necrosis in myocardial injury or ischemia are required. Future prospective and well-powered studies are warranted to overcome the limitations of this study and increase the generalizability of its findings. In conclusion, IVSDN measurements have utility in assessing left ventricular wall hypertrophy in dogs. IVSDN values of >0.52 and increased serum cTnI levels are characteristic of HCM in dogs and may have utility in informing the clinical diagnosis of HCM or monitoring disease progression. AcknowledgmentsThe authors thank Enago (www.enago.jp) for the English language review. Conflict of interestThe authors declare that there is no conflict of interest. Author contributionsDr. Takeki Ando conceptualized the study, collected the data, analyzed the data, and wrote the manuscript. Dr. Takeshi Izawa performed the pathological diagnosis. Dr. Hidetaka Nishida proofread the manuscript. Dr. Hideo Akiyoshi provided general guidance on the structure of this research and the proofreading of the manuscript. ReferencesAdams 3rd, J.E., Schechtman, K.B., Landt, Y., Ladenson, J.H. and Jaffe, A.S. 1994. Comparable detection of acute myocardial infarction by creatine kinase MB isoenzyme and cardiac troponin I. Clin. Chem. 40, 1291–1295. Anwar, A.M. and TenCate, F.J. 2021. Echocardiographic evaluation of hypertrophic cardiomyopathy: a review of up-to-date knowledge and practical tips. Echocardiography 38, 1795–1808. Apple, F.S., Murakami, M.M., Christenson, R.H., Campbell, J.L., Miller, C.J., Hock, K.G. and Scott, M.G. 2004. Analytical performance of the i-STAT cardiac troponin I assay. Clin. Chim. Acta. 345, 123–127. Atkins, C., Bonagura, J., Ettinger, S., Fox, P., Gordon, S., Haggstrom, J., Hamlin, R., Keene, B., Luis-Fuentes, V. and Stepien, R. 2009. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J. Vet. Intern. Med. 23, 1142–1150. Belerenian, G., Donati, P.A., Rodriguez, C.D., Castillo, V., Guevara, J.M., Pucheta, C., Ferraris, S. and Olivares, R.W.I. 2021. Findings suggestive of coronary microvascular dysfunction in cats with myocardial ischemia. Open Vet. J. 11, 468–470. Bertinchant, J.P., Larue, C., Pernel, I., Ledermann, B., Fabbro-Peray, P., Beck, L., Calzolari, C., Trinquier, S., Nigond, J. and Pau, B. 1996. Release kinetics of serum cardiac troponin I in ischemic myocardial injury. Clin. Biochem. 29, 587–594. Bodh, D., Hoque, M. and Saxena, A.C. 2019. Echocardiographic study of healthy Indian Spitz dogs with normal reference ranges for the breed. Vet. World. 12, 740–747. Borgarelli, M., Tarducci, A., Zanatta, R. and Haggstrom, J. 2007. Decreased systolic function and inadequate hypertrophy in large and small breed dogs with chronic mitral valve insufficiency. J. Vet. Intern. Med. 21, 61–67. Buchanan, J.W. 2000. Vertebral scale system to measure heart size in radiographs. Vet. Clin. North Am. Small Anim. Pract. 30, 379–393, vii. Buchanan, J.W. and Bücheler, J. 1995. Vertebral scale system to measure canine heart size in radiographs. J. Am. Vet. Med. Assoc. 206, 194–199. Cambronero, F., Marín, F., Roldán, V., Hernández-Romero, D., Valdés, M. and Lip, G.Y. 2009. Biomarkers of pathophysiology in hypertrophic cardiomyopathy: implications for clinical management and prognosis. Eur. Heart J. 30, 139–151. Cornell, C.C., Kittleson, M.D., Della Torre, P., Häggström, J., Lombard, C.W., Pedersen, H.D., Vollmar, A. and Wey, A. 2004. Allometric scaling of M-mode cardiac measurements in normal adult dogs. J. Vet. Intern. Med. 18, 311–321. Duler, L., Scollan, K.F. and LeBlanc, N.L. 2019. Left atrial size and volume in cats with primary cardiomyopathy with and without congestive heart failure. J. Vet. Cardiol. 24, 36–47. Enriquez, A.D. and Goldman, M.E. 2014. Management of hypertrophic cardiomyopathy. Ann. Glob. Health 80, 35–45. Fonfara, S., Loureiro, J., Swift, S., James, R., Cripps, P. and Dukes-McEwan, J. 2010. Cardiac troponin I as a marker for severity and prognosis of cardiac disease in dogs. Vet. J. 184, 334-339. Fox, P.R. 2003. Hypertrophic cardiomyopathy. Clinical and pathologic correlates. J. Vet. Cardiol. 5, 39–45. Fox, P.R., Keene, B.W., Lamb, K., Schober, K.A., Chetboul, V., Luis Fuentes, V., Wess, G., Payne, J.R., Hogan, D.F., Motsinger-Reif, A., Häggström, J., Trehiou-Sechi, E., Fine-Ferreira, D.M., Nakamura, R.K., Lee, P.M., Singh, M.K., Ware, W.A., Abbott, J.A., Culshaw, G., Riesen, S., Borgarelli, M., Lesser, M.B., Van Israël, N., Côté, E., Rush, J.E., Bulmer, B., Santilli, R.A., Vollmar, A.C., Bossbaly, M.J., Quick, N., Bussadori, C., Bright, J.M., Estrada, A.H., Ohad, D.G., Fernández-Del Palacio, M.J., Lunney Brayley, J., Schwartz, D.S., Bové, C.M., Gordon, S.G., Jung, S.W., Brambilla, P., Moïse, N.S., Stauthammer, C.D., Stepien, R.L., Quintavalla, C., Amberger, C., Manczur, F., Hung, Y.W., Lobetti, R., De Swarte, M., Tamborini, A., Mooney, C.T., Oyama, M.A., Komolov, A., Fujii, Y., Pariaut, R., Uechi, M. and Tachika Ohara, V.Y. 2018. International collaborative study to assess cardiovascular risk and evaluate long-term health in cats with preclinical hypertrophic cardiomyopathy and apparently healthy cats: the reveal study. J. Vet. Intern. Med. 32, 930–943. Freeman, L.M., Rush, J.E., Stern, J.A., Huggins, G.S. and Maron, M.S. 2017. Feline hypertrophic cardiomyopathy: a spontaneous large animal model of human HCM. Cardiol. Res. 8, 139–142. Fujiwara, H., Hoshino, T., Fujiwara, T., Kawai, C. and Hamashima, Y. 1982. Classification and distribution of myocardial fascicle and fiber disarray in 14 hearts with hypertrophic cardiomyopathy in 25 mu thick sections. Jpn. Circ. J. 46, 225–234. Gersh, B.J., Maron, B.J., Bonow, R.O., Dearani, J.A., Fifer, M.A., Link, M.S., Naidu, S.S., Nishimura, R.A., Ommen, S.R., Rakowski, H., Seidman, C.E., Towbin, J.A., Udelson, J.E., Yancy, C.W. Gersh, B.J., Maron, B.J., Bonow, R.O., Dearani, J.A., Fifer, M.A., Link, M.S., Naidu, S.S., Nishimura, R.A., Ommen, S.R., Rakowski, H., Seidman, C.E., Towbin, J.A., Udelson, J.E., Yancy, C.W. and American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2011. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American college of cardiology foundation/American heart association task force on practice guidelines. Circulation 124, e783–e831. Gonçalves, A.C., Orton, E.C., Boon, J.A. and Salman, M.D. 2002. Linear, logarithmic, and polynomial models of M-mode echocardiographic measurements in dogs. Am. J. Vet. Res. 63, 994–999. Hertzsch, S., Roos, A. and Wess, G. 2019. Evaluation of a sensitive cardiac troponin I assay as a screening test for the diagnosis of hypertrophic cardiomyopathy in cats. J. Vet. Intern. Med. 33, 1242–1250. Hickman, P.E., Potter, J.M., Aroney, C., Koerbin, G., Southcott, E., Wu, A.H. and Roberts, M.S. 2010. Cardiac troponin may be released by ischemia alone, without necrosis. Clin. Chim. Acta. 411, 318–323. Hori, Y., Iguchi, M., Heishima, Y., Yamashita, Y., Nakamura, K., Hirakawa, A., Kitade, A., Ibaragi, T., Katagi, M., Sawada, T., Yuki, M., Kanno, N., Inaba, H., Isayama, N., Onodera, H., Iwasa, N., Kino, M., Narukawa, M. and Uchida, S. 2018. Diagnostic utility of cardiac troponin I in cats with hypertrophic cardiomyopathy. J. Vet. Intern. Med. 32, 922–929. Hoshino, T., Fujiwara, H., Kawai, C. and Hamashima, Y. 1983. Myocardial fiber diameter and regional distribution in the ventricular wall of normal adult hearts, hypertensive hearts and hearts with hypertrophic cardiomyopathy. Circulation. 67, 1109–1116. Kociol, R.D., Pang, P.S., Gheorghiade, M., Fonarow, G.C., O’Connor, C.M. and Felker, G.M. 2010. Troponin elevation in heart failure prevalence, mechanisms, and clinical implications. J. Am. Coll. Cardiol. 56, 1071–1078. Korraa, A., Ezzat, M.H., Bastawy, M., Aly, H., El-Mazary, A.A. and Abd El-Aziz, L. 2012. Cardiac troponin I levels and its relation to echocardiographic findings in infants of diabetic mothers. Ital. J. Pediatr. 38, 39. Kubo, T., Kitaoka, H., Okawa, M., Yamanaka, S., Hirota, T., Hoshikawa, E., Hayato, K., Yamasaki, N., Matsumura, Y., Yasuda, N., Sugiura, T. and Doi, Y.L. 2010. Serum cardiac troponin I is related to increased left ventricular wall thickness, left ventricular dysfunction, and male gender in hypertrophic cardiomyopathy. Clin. Cardiol. 33, E1–E7. Kubo, T., Kitaoka, H., Okawa, M., Yamanaka, S., Hirota, T., Baba, Y., Hayato, K., Yamasaki, N., Matsumura, Y., Yasuda, N., Sugiura, T. and Doi, Y.L. 2011. Combined measurements of cardiac troponin I and brain natriuretic peptide are useful for predicting adverse outcomes in hypertrophic cardiomyopathy. Circ. J. 75, 919–926. Langhorn, R. and Willesen, J.L. 2016. Cardiac troponins in dogs and cats. J. Vet. Intern. Med. 30, 36–50. Langhorn, R., Tarnow, I., Willesen, J.L., Kjelgaard-Hansen, M., Skovgaard, I.M. and Koch, J. 2014. Cardiac troponin I and T as prognostic markers in cats with hypertrophic cardiomyopathy. J. Vet. Intern. Med. 28, 1485–1491. Linney, C.J., Dukes-McEwan, J., Stephenson, H.M., López-Alvarez, J. and Fonfara, S. 2014. Left atrial size, atrial function and left ventricular diastolic function in cats with hypertrophic cardiomyopathy. J. Small Anim. Pract. 55, 198–206. Liu, S.K., Maron, B.J. and Tilley, L.P. 1979a. Canine hypertrophic cardiomyopathy. J. Am. Vet. Med. Assoc. 174, 708–713. Liu, S.K., Maron, B.J. and Tilley, L.P. 1979b. Hypertrophic cardiomyopathy in the dog. Am. J. Pathol. 94, 497–508. Lombardi, R. and Betocchi, S. 2002. Aetiology and pathogenesis of hypertrophic cardiomyopathy. Acta Paediatr. Suppl. 91, 10–14. Luis Fuentes, V., Abbott, J., Chetboul, V., Côté, E., Fox, P.R., Häggström, J., Kittleson, M.D., Schober, K. and Stern, J.A. 2020. ACVIM consensus statement guidelines for the classification, diagnosis, and management of cardiomyopathies in cats. J. Vet. Intern. Med. 34, 1062–1077. Marks, C.A. 1993. Hypertrophic cardiomyopathy in a dog. J. Am. Vet. Med. Assoc. 203, 1020–1022. Maron, B.J. and Fox, P.R. 2015. Hypertrophic cardiomyopathy in man and cats. J. Vet. Cardiol. 17, S6–S9. Maron, B.J., Gottdiener, J.S., Bonow, R.O. and Epstein, S.E. 1981. Hypertrophic cardiomyopathy with unusual locations of left ventricular hypertrophy undetectable by M-mode echocardiography. Identification by wide-angle two-dimensional echocardiography. Circulation 63, 409–418. McGorrian, C.M., Lyster, S., Roy, A., Tarrant, H., Codd, M., Doran, P., Fitzgibbon, M., Galvin, J. and Mahon, N.G. 2013. Use of a highly-sensitive cardiac troponin I assay in a screening population for hypertrophic cardiomyopathy: a case-referent study. BMC Cardiovasc. Disord. 13, 70. Morrison, S.A., Moise, N.S., Scarlett, J., Mohammed, H. and Yeager, A.E. 1992. Effect of breed and body weight on echocardiographic values in four breeds of dogs of differing somatotype. J. Vet. Intern. Med. 6, 220–224. Oyama, M.A. and Sisson, D.D. 2004. Cardiac troponin-I concentration in dogs with cardiac disease. J. Vet. Intern. Med. 18, 831–839. Pang, D., Rondenay, Y., Hélie, P., Cuvelliez, S.G. and Troncy, E. 2005. Sudden cardiac death associated with occult hypertrophic cardiomyopathy in a dog under anesthesia. Can. Vet. J. 46, 1122–1125. Payne, J.R., Borgeat, K., Connolly, D.J., Boswood, A., Dennis, S., Wagner, T., Menaut, P., Maerz, I., Evans, D., Simons, V.E., Brodbelt, D.C. and Luis Fuentes, V. 2013. Prognostic indicators in cats with hypertrophic cardiomyopathy. J. Vet. Intern. Med. 27, 1427–1436. Pop, G.A., Cramer, E., Timmermans, J., Bos, H. and Verheugt, F.W. 2006. Troponin I release at rest and after exercise in patients with hypertrophic cardiomyopathy and the effect of betablockade. Arch. Cardiol. Mex. 76, 415–418. Porter, A., Rozanski, E., Price, L.L. and Shaw, S. 2016. Evaluation of cardiac troponin I in dogs presenting to the emergency room using a point-of-care assay. Can. Vet. J. 57, 641–645. Richardson, P., McKenna, W., Bristow, M., Maisch, B., Mautner, B., O’Connell, J., Olsen, E., Thiene, G., Goodwin, J., Gyarfas, I., Martin, I. and Nordet, P. 1996. Report of the 1995 world health organization/international society and federation of cardiology task force on the definition and classification of cardiomyopathies. Circulation 93, 841–842. Rishniw, M. and Erb, H.N. 2000. Evaluation of four 2-dimensional echocardiographic methods of assessing left atrial size in dogs. J. Vet. Intern. Med. 14, 429–435. Schober, K.E., Fox, P.R., Abbott, J., Côté, E., Luis-Fuentes, V., Matos, J.N., Stern, J.A., Visser, L., Scollan, K.F., Chetboul, V., Schrope, D., Glaus, T., Santilli, R., Pariaut, R., Stepien, R., Arqued-Soubeyran, V., Toaldo, M.B., Estrada, A., MacDonald, K., Karlin, E.T. and Rush, J. 2022. Retrospective evaluation of hypertrophic cardiomyopathy in 68 dogs. J. Vet. Intern. Med. 36, 865–876. Silverman, M.E. and Wooley, C.F. 2008. Samuel A. Levine and the history of grading systolic murmurs. Am. J. Cardiol. 102, 1107–1110. Sleeper, M.M., Clifford, C.A. and Laster, L.L. 2001. Cardiac troponin I in the normal dog and cat. J. Vet. Intern. Med. 15, 501–503. Ueda, Y. and Stern, J.A. 2017. A one health approach to hypertrophic cardiomyopathy. Yale J. Biol. Med. 90, 433–448. Washizu, M., Takemura, N., Machida, N., Nawa, H., Yamamoto, T., Mitake, H. and Washizu, T. 2003. Hypertrophic cardiomyopathy in an aged dog. J. Vet. Med. Sci. 65, 753–756. Wess, G., Domenech, O., Dukes-McEwan, J., Häggström, J. and Gordon, S. 2017. European society of veterinary cardiology screening guidelines for dilated cardiomyopathy in doberman pinschers. J. Vet. Cardiol. 19, 405–415. White, H.D. 2011. Pathobiology of troponin elevations: do elevations occur with myocardial ischemia as well as necrosis? J. Am. Coll. Cardiol. 57, 2406–2408. Zhou, Y., Yuan, J., Wang, Y. and Qiao, S. 2019. Predictive values of apelin for myocardial fibrosis in hypertrophic cardiomyopathy. Int. Heart J. 60, 648–655. | ||
| How to Cite this Article |
| Pubmed Style Ando T, Izawa T, Nishida H, Akiyoshi H. Clinical findings using echocardiography and plasma cardiac troponin I and pathological findings in dogs with hypertrophic cardiomyopathy: A retrospective study. Open Vet J. 2023; 13(6): 742-752. doi:10.5455/OVJ.2023.v13.i6.9 Web Style Ando T, Izawa T, Nishida H, Akiyoshi H. Clinical findings using echocardiography and plasma cardiac troponin I and pathological findings in dogs with hypertrophic cardiomyopathy: A retrospective study. https://www.openveterinaryjournal.com/?mno=144798 [Access: August 31, 2024]. doi:10.5455/OVJ.2023.v13.i6.9 AMA (American Medical Association) Style Ando T, Izawa T, Nishida H, Akiyoshi H. Clinical findings using echocardiography and plasma cardiac troponin I and pathological findings in dogs with hypertrophic cardiomyopathy: A retrospective study. Open Vet J. 2023; 13(6): 742-752. doi:10.5455/OVJ.2023.v13.i6.9 Vancouver/ICMJE Style Ando T, Izawa T, Nishida H, Akiyoshi H. Clinical findings using echocardiography and plasma cardiac troponin I and pathological findings in dogs with hypertrophic cardiomyopathy: A retrospective study. Open Vet J. (2023), [cited August 31, 2024]; 13(6): 742-752. doi:10.5455/OVJ.2023.v13.i6.9 Harvard Style Ando, T., Izawa, . T., Nishida, . H. & Akiyoshi, . H. (2023) Clinical findings using echocardiography and plasma cardiac troponin I and pathological findings in dogs with hypertrophic cardiomyopathy: A retrospective study. Open Vet J, 13 (6), 742-752. doi:10.5455/OVJ.2023.v13.i6.9 Turabian Style Ando, Takeki, Takeshi Izawa, Hidetaka Nishida, and Hideo Akiyoshi. 2023. Clinical findings using echocardiography and plasma cardiac troponin I and pathological findings in dogs with hypertrophic cardiomyopathy: A retrospective study. Open Veterinary Journal, 13 (6), 742-752. doi:10.5455/OVJ.2023.v13.i6.9 Chicago Style Ando, Takeki, Takeshi Izawa, Hidetaka Nishida, and Hideo Akiyoshi. "Clinical findings using echocardiography and plasma cardiac troponin I and pathological findings in dogs with hypertrophic cardiomyopathy: A retrospective study." Open Veterinary Journal 13 (2023), 742-752. doi:10.5455/OVJ.2023.v13.i6.9 MLA (The Modern Language Association) Style Ando, Takeki, Takeshi Izawa, Hidetaka Nishida, and Hideo Akiyoshi. "Clinical findings using echocardiography and plasma cardiac troponin I and pathological findings in dogs with hypertrophic cardiomyopathy: A retrospective study." Open Veterinary Journal 13.6 (2023), 742-752. Print. doi:10.5455/OVJ.2023.v13.i6.9 APA (American Psychological Association) Style Ando, T., Izawa, . T., Nishida, . H. & Akiyoshi, . H. (2023) Clinical findings using echocardiography and plasma cardiac troponin I and pathological findings in dogs with hypertrophic cardiomyopathy: A retrospective study. Open Veterinary Journal, 13 (6), 742-752. doi:10.5455/OVJ.2023.v13.i6.9 |