| Case Report | ||
Open Vet J. 2023; 13(8): 1044-1055 Open Veterinary Journal, (2023), Vol. 13(8): 1044-1055 Case Report Linguatula serrata (Pentastomida: Linguatulidae) infection in a paucisymptomatic greyhound imported from Romania to Italy: A case report and literature overviewVeronica Marchetti1†, Fabio Macchioni1*†, Eleonora Gori1, Luigi Venco2 and Roberto Amerigo Papini11Department of Veterinary Sciences, University of Pisa, Pisa, Italy 2Veterinary Hospital Città di Pavia Viale Cremona, Pavia, Italy †These two authors contributed equally to this work. *Corresponding Author: Fabio Macchioni. Department of Veterinary Sciences, University of Pisa, Pisa, Italy. Email: fabio.macchioni [at] unipi.it Submitted: 15/03/2023 Accepted: 24/07/2023 Published: 31/08/2023 © 2023 Open Veterinary Journal
AbstractBackground: Linguatula serrata is a pentastomid zoonotic parasite with worldwide distribution. Although some cases of L. serrata infection have been reported in dogs, the epidemiology of this parasite remains largely unknown in developed countries. In recent years, canine linguatulosis has been repeatedly linked to cases of imported infections. This study aims to focus attention on this uncommon parasite through the presentation of a case report and an overview of the literature. Case Description: A 1-year-old intact female Borzoi imported from Romania to Italy sneezed spontaneously a worm-like parasite specimen. Morphological and molecular diagnosis identified the parasite as a female of the zoonotic pentastomid L. serrata (so-called European tongue worm) that lives in the nasopharyngeal tract of canids. Eggs of Linguatula were detected in the feces. Molecular identification (99%–100% homology) was based on DNA extraction, polymerase chain reaction of a 700-bp fragment of the mitochondrial cytochrome c oxidase subunit I gene, and alignment with BLAST analysis. Due to the possibility of other adult or juvenile specimens of the parasite still occurring in the dog, a treatment attempt with a combination of febantel/pyrantel/praziquantel was made. No parasite eggs were detected in fecal samples after the drug was administered. Endoscopy confirmed the absence of adult parasites and slight pathological changes. A follow-up examination conducted 3 months after the treatment did not reveal any clinical and laboratory abnormalities. Conclusion: Linguatula serrata appears to be currently prevalent in some European countries, but there are no recent extensive studies on the prevalence of canine linguatulosis, so the parasite frequently remains undetected and unreported in dogs as the diagnosis is often overlooked. Parasites not commonly found such as L. serrata can become increasingly prevalent and may be detected in imported dogs. Therefore, veterinarians must be aware of the possible presence of uncommon and exotic pathogens in these dogs, be able to recognize the relevant clinical signs, and diagnose the infection quickly. This will improve the prognosis in individual dogs, reduces the risk of possible public health implications, and reduces the risk of uncommon and exotic pathogens establishing new endemic foci. Keywords: Linguatula serrata, Linguatulosis, Imported dogs, Romania, Italy. IntroductionLinguatula serrata Frölich, 1789 is a cosmopolitan, zoonotic, and food-borne obligate parasite belonging to the subclass of Pentastomida within the phylum Arthropoda. Despite this, it is commonly called “tongue worm” because of the dorsoventrally flattened shape (Berberich et al., 2022). This parasite has an indirect life cycle. Dogs, wild canids (foxes, wolves, golden jackals, dingoes), cats, and many other carnivorous mammals can be definitive hosts while a wide range of ruminants (cattle, sheep, goats, buffaloes, camelids), rabbits (Barton et al., 2020), hares (Diakou et al., 2014), rats (Tavassoli et al., 2018), red-necked wallabies (Barton et al., 2019), and steppe field mice (Mohtasebi et al., 2021) are known intermediate hosts. Linguatula serrata is a zoonotic parasite. Humans can act as accidental intermediate hosts when they ingest water or vegetables contaminated by embryonated eggs of the parasite (Hajipour and Tavassoli, 2019), or as definitive hosts when they feed on raw or undercooked viscera containing infectious nymphs from a parasitized intermediate host, commonly sheep and goats (Hajipour and Tavassoli, 2019). Commonly, females measure up to 13 cm in length and males are up to 2 cm long (Melhorn, 2008). However, some Australian female specimens showed great morphological variation in total body length and were up to 15 cm long (Shamsi et al., 2020). The adult parasites live attaching to the mucous membranes of the nasal cavity, turbinates, and frontal sinuses of the definitive hosts, primarily dogs, by using their hooks. After mating, females lay up to 5,000,000 embryonated eggs per day (Melhorn, 2008). Eggs are passed into the environment via the nasal mucus (by coughing and sneezing) or are swallowed and then expulsed by the fecal route. The intermediate hosts become infested when they accidentally ingest larvated eggs from contaminated environments. After ingestion, the first instar larva hatches in the intestinal tract and migrates to mesenteric lymph nodes, liver, lungs, spleen, rarely eyes, and other tissues where it encysts as infective nymphal stage (so-called “C-shaped”) (Sievänen et al., 2021; Berberich et al., 2022). When ingested by a definitive host, nymphs migrate to the nasal cavity and frontal sinus where they develop into adult parasites within 6–7 months and can survive for up to 15 months (Melhorn, 2008). Humans can be aberrant final hosts after ingesting raw or poorly cooked viscera (Hajipour and Tavassoli, 2019). Reported prevalence values in dogs range from 37.45% in owned dogs in Nigeria (Oluwasina et al., 2014) to 76.5% in stray dogs in Iran (Oryan et al., 2008) and in wild canids range from 1.3% in foxes (Hodžić et al., 2016) to 67.6% in dingos (Shamsi et al., 2017). Moreover, some cases of occasional infections have been reported, sometimes as autochthonous cases (Principato et al., 1994; Paoletti et al., 2003; Bordicchia et al., 2014; Ioniță and Mitrea, 2016; Campbel and Jones, 2023) but more commonly as cases detected in dogs moved from a country to another country (Globokar Vrhovec et al., 2005; Mitchell et al., 2016; Villedieu et al., 2017; Springer et al., 2018; Thomas, 2018; Nagamori et al., 2019; Sievänen et al., 2021; Berberich et al., 2022; Macrelli and Mackintosh, 2022). This has led some authors to hypothesize that L. serrata infection might be considered an emerging parasitosis (Berberich et al., 2022). In Italy, little is known about the epidemiology of L. serrata. Data available in the literature are sporadic and limited or date back to several years ago. To the best of our knowledge, L. serrata has previously been reported in three dogs in Umbria (Principato et al., 1994), in a dog in Abruzzo (Paoletti et al., 2003), as a nymphal stage in a dog affected by nasal carcinoma (Bordicchia et al., 2014), in a dog exported from Sicily to Germany (Globokar Vrhovec et al., 2005), and more recently in a grey wolf (Raele et al., 2022). Two cases of human infections have also been reported in association with pulmonary tuberculosis (Parenzan and Chieffi, 1951) or with HIV positivity (Pampiglione et al., 2001). In order to give further insight into this topic, we report a case of L. serrata infection in a 1-year-old rescued female dog imported from Romania to Italy. We also thoroughly reviewed all previous case reports of linguatulosis in dogs and included an up-to-date overview of the relevant literature. Case DetailsThe dog was a 1-year-old intact female Borzoi that had been adopted from a Romanian shelter 3 months prior to the consultation. Upon arrival at the Italian sighthound rescue association, the dog received deworming treatment with febantel/pyrantel/praziquantel at the recommended dose (Drontal® Plus Flavour, Bayern S.p.A) and was vaccinated with core vaccines. In her recent clinical history, watery diarrhea and increased defecation frequency (5–6 times daily) were reported. The dog’s diet was biologically appropriate raw food (BARF) with mixed meats. The owner reported that the dog had multiple reverse sneezing episodes the day before the examination. At clinical examination, the dog was mildly underweight with a body condition score of 3/9 and a muscular condition score of 2 out of 3 (mild muscle loss). No nasal discharge or any other respiratory signs were reported by the owner and were observed during the clinical examination. A complete hematobiochemical profile, urinalysis, fecal examination, and abdominal ultrasound were performed. The results of blood and urine analyses were within reference ranges. The fecal examination performed by routine flotation technique on a single sample was negative. The abdominal ultrasound showed ultrasonographic changes compatible with chronic enteropathy. The dog was discharged on an intestinal diet and support therapy (vitamins and probiotics). Once returned home, upon sneezing, a worm-like parasite was expelled about 2 weeks later. The owner collected the fresh parasite in a glass jar filled with tap water and stored the specimen at room temperature. A recheck examination of the dog was quickly scheduled. The owner brought the specimen along with three fecal samples collected every other day to the Veterinary Teaching Hospital of the University of Pisa for parasitological examinations. A complete blood count showed no abnormalities, besides the presence of mild lymphocytosis with activated lymphocytes. No abnormalities in the biochemistry and urine analyses were found. The abdominal ultrasound showed again ultrasonographic changes compatible with chronic enteropathy. Upon macroscopic examination, the collected parasite was readily identified as a specimen of L. serrata on the basis of the host species and the main morphological characteristics such as the typical elongated and tongue-like shape (Fig. 1). The body of the parasite was whitish, claviform, relatively large in size (8.7 cm in length and 0.8 cm in width at the wider anterior portion), dorsally slightly convex and ventrally flattened with transversely striated cuticle on body surface, rounded anterior end and pointed posterior end. More than 90 annuli could be distinguished on the body of the parasite. Therefore, a diagnosis of linguatulosis was done. Detailed morphological examination of the specimen by stereo microscope and scanning electron microscopy (SEM) as well as molecular analysis for identification to species level were planned. The three fecal samples collected on alternate days were individually examined by a passive fecal flotation technique using a commercial sodium nitrate solution with a specific gravity of 1.2 (Coprosol; Candioli Farmaceutici). Fecal examination revealed the presence of embryonated eggs of L. serrata (Fig. 2), Ancylostomatidae eggs, and oocysts of Isospora spp. L. serrata eggs had an oval shape and smooth shell. Four eggs were measured. The mean length and width were 90 × 60 µm. A sample of mucus was also obtained by nasal lavage with sterile warmed saline, but no L. serrata eggs were detected by microscopy examination of the nasal lavage fluid. Febantel/pyrantel/praziquantel (Drontal® Plus Flavor, Bayer) and the association of emodepside with toltrazuril (Procox®, Vétoquinol Italia S.r.l.) were prescribed according to the doses recommended by the manufacturers.
Fig. 1. Female specimen of L. serrata with a ruler showing centimeters and millimeters and indicating the actual length and general body size of the parasite. After 3 weeks, the owner reported a marked improvement in watery diarrhea and defecation frequency with only occasional self-limiting episodes of diarrhea. A follow-up visit was scheduled. The coprological examination of three samples collected on three consecutive days was negative. An aero-digestive endoscopy was performed to check the possible presence of other adult or juvenile pentastomids and to grade the chronic enteropathy. The respiratory endoscopy finding was a mild-moderate activation of the nasal-associated lymphoid tissue in the nasopharynx with no other abnormalities. The digestive endoscopy found only a mild conglutination of the duodenal villi with an irregular mucosal profile. The histopathology revealed focally moderate lymphocytic gastritis and mild to moderate lymphoplasmacytic enteritis.
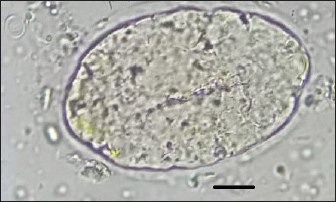
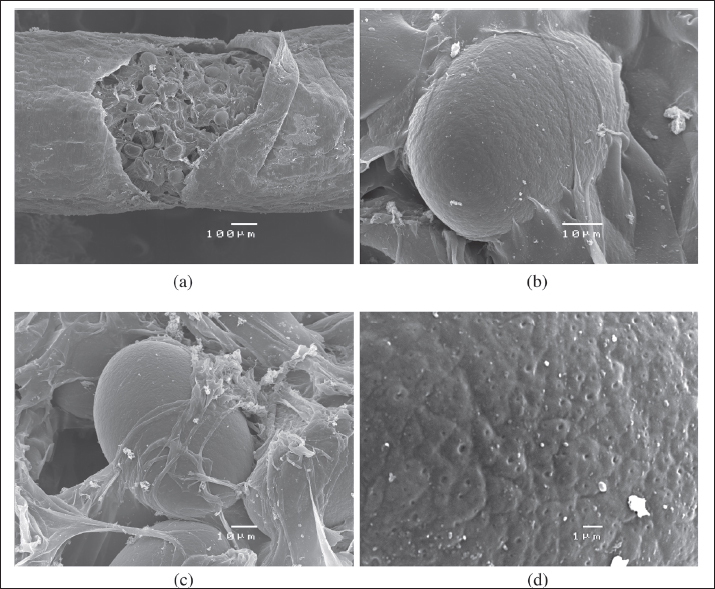
Fig. 2. Linguatula serrata egg found in the feces of a 1-year-old intact female Borzoi imported from Romania to Italy (100×, oil immersion; scale bar 10 μ). Three months after the diagnosis and treatments, the dog was re-checked. No abnormalities were identified at clinical examination, in the complete blood count, and in the biochemical profile. Morphological examination of the specimen was performed by stereoscopic microscope and SEM as follows. Under stereoscopy, the adult of L. serrata was measured again and the number of annuli was accurately counted. Length (8.7 cm) and width (0.8 cm) were confirmed and 97 annuli were counted. On the ventral view of the anterior end, a sub-terminal mouth located at the level of the first ring and four prominent hooks (two on each side of the mouth) were present. The first pair of hooks was located lateral to and closer to the mouth at the level of the first ring, while the second pair was located lateral but somewhat further away and slightly lower at the level of the second ring. The cephalic central part and caudal parts of the adult were then dissected and examined using SEM (JEOL JSM 5410SEM). To obtain SEM images, the samples were washed in 70% ethanol and dehydrated using an ethanol graduated series. The alcohol has been replaced by liquid CO2 which, through the drying procedure at the critical point, has passed from the liquid state to the gaseous state. The dried samples were mounted on the stubs using double-sided conductive tape. The samples were metalized with a layer of gold of about 10 nm using the "sputtering." Finally, the samples were observed at the SEM Jeol JSM-5410 equipped with the Jeol SemAfore system for image acquisition. SEM photographs showed that the mouth was rounded, measured 3.78 × 4.03 µm, and was surrounded by a chitinous buccal cadre, while the four hooks were slightly curved and had a sharp tip (Fig. 3A and B). Each annulus had a row of pores (chloride cell caps) located on the upper part (Fig. 4A and B). A distinct terminal cleft was present at the posterior tip (Fig. 5A and B). The uterus filled with thousands of embryonating eggs was clearly visible (Fig. 6A). SEM also highlighted some morphological characteristics of the L. serrata eggs: though the egg shell appears to be smooth (Fig. 6B and C), as seen by stereomicroscopy, the surface presents irregularly distributed small pits, which give the eggs a slightly porous heterogeneous appearance (Fig. 5).
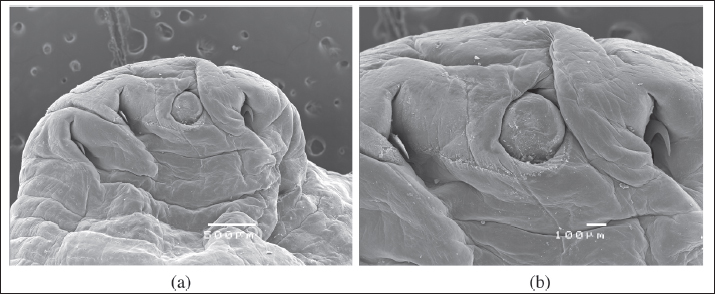
Fig. 3. SEM of the cranial ventral view of a female L. serrata specimen, showing the sub-terminal mouth, surrounded by a chitinous oral cadre, and two pairs of protractile and slightly curved hooks with a sharp tip (A: scale bar 500 μ; B: scale bar 100 μ).
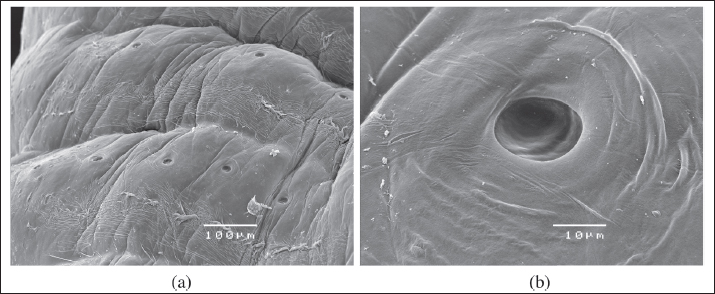
Fig. 4. SEM of the sensory pore structure of the annuli of a female L. serrata specimen (A: scale bar 100 μ; B: scale bar 10 μ). For molecular identification to the species level, two small pieces of the body of the specimen were cut for DNA extraction. Molecular analyses were performed by BMR Genomics (Padua, Italy, https://www.bmr-genomics.it/). Genomic DNA was extracted using the commercial PCRBIO Rapid Extract Lysis Kit (PCR Biosystems) following the manufacturer's instructions. A fragment (about 700 base pairs in length) of the mitochondrial gene for cytochrome C oxidase subunit 1 (COX-1) was used as a DNA barcoding system and amplified. The polymerase chain reaction (PCR) amplification was carried out in a final mixture containing 12.5 μl of AmpliTaq Gold™ 360 Master Mix (Thermo Fisher), 2 μl of gDNA extracted, 1 μl (10 μM) of LCO1490 primer (5′-GGTCAACAAATCATAAAGATATTGG-3′), 1 μl (10 μM) of HCO2198 primer (5′-TAAACTTCAGGGTGACCAAAAAATCA-3′), and deionized water, reaching a total reaction volume of 25 μl. The PCR reactions were subjected to the following conditions in a thermal cycler (Mastercycler®, Eppendorf): 95°C × 2′, then 5 cycles (95°C × 40ʺ, 45°C for 90ʺ, and 72°C for 90ʺ), followed by 35 cycles (95°C for 40ʺ, 50°C for 90ʺ minutes, and 72°C for 60ʺ minutes), and finally 72°C for 10′. The amplification products were visualized after electrophoresis on 1.5% agarose gel. PCR products were purified with ExoSAP-IT™ (Thermo Fisher), sequenced, and aligned via BLAST analysis to detect their identity by retrieving similar sequences deposited in NCBI's GenBank database (Zhang et al., 2000; Morgulis et al., 2008).
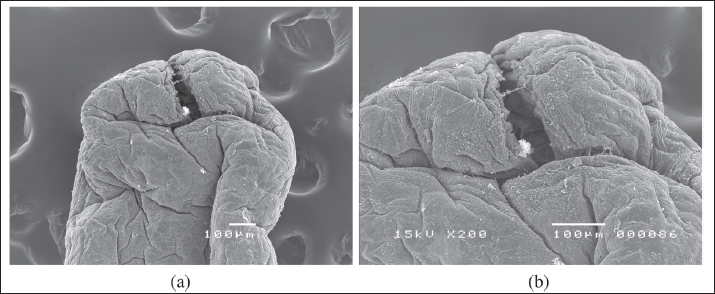
Fig. 5. SEM of the posterior end of a female L. serrata specimen showing a terminal cleft posteriorly (A, B: scale bar 100 μ).
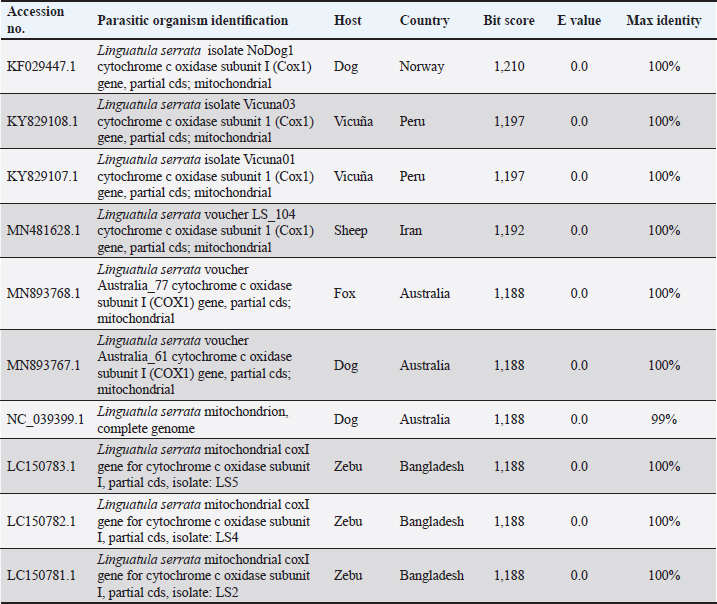
Fig. 6. SEM of the uterus of L. serrata filled with eggs (A: scale bar 10 µ), one single egg (B, C: scale bar 10 µ), and a detail of the egg shell (D: scale bar 1 µ). The BLAST search results showed that COX-1 of the adult Linguatula specimen had 99%–100% DNA homology with sequences of L. serrata available in the GenBank database. The pairwise identity between the partial nucleotide sequence of the COX1 gene from the specimen collected in this case report and other corresponding sequences from L. serrata isolates available in GenBank is shown in Table 1. DiscussionTo the best of our knowledge, this is the first report of linguatulosis in an imported dog in Italy. Previous cases of linguatulosis in Italy were sporadically reported as autochthonous cases of infection, including reports in dogs (Principato et al., 1994; Paoletti et al., 2003; Globokar Vrhovec et al., 2005; Bordicchia et al., 2014) and humans (Parenzan and Chieffi, 1951; Pampiglione et al., 2001) as well as a recent report in a grey wolf (Raele et al., 2022). This suggests that the parasite has been circulating in the country although it has been infrequently detected over the years. The dog in this case report was imported from Romania 3 months prior to presentation and had been in the same owner’s possession since its arrival in Italy. As a previous investigation has identified specimens of L. serrata in various wild canid species in Romania (Barton et al., 2022) and some cases of linguatulosis have been previously reported in dogs imported from Romania (Villedieu et al., 2017; Springer et al., 2018; Thomas, 2018; Berberich et al., 2022; Macrelli and Mckintosh, 2022), it can be assumed that L. serrata seems to be rather common in canids, including dogs, in that country. Studies showing that infections with nymphs of L. serrata are common in slaughtered domestic ruminants in Romania support this assumption (Ioniță and Mitrea, 2016). It can also be assumed that the dog had a free-roaming lifestyle for an unspecified amount of time prior to entering a shelter (Norman et al., 2020), thus was exposed to eating viscera of infected herbivores likely found dead in the field or from offal (Ioniță and Mitrea, 2016; Norman et al., 2020). Furthermore, the dog was regularly fed with BARF both during the stay in a Romanian kennel for an unspecified period and immediately after the adoption in Italy (3 months before the consultation). The risk of transmission of foodborne bacteria and parasites in dogs feeding on this diet and in humans sharing the same environment has been well documented (Strohmeyer et al., 2006; van Bree et al., 2018; Davies et al., 2019; Bottari et al., 2020), including two case reports of L. serrata in dogs regularly fed with BARF (Berberich et al., 2022; Campbel and Jones, 2023). Taking into consideration that the prepatent period of linguatulosis in dogs is about 6–7 months (Nagamori et al., 2019) and that the infection was in the patent phase 3 months after the arrival in Italy as eggs were seen in feces, an autochthonous infection taken place in Italy is really unlikely and it is very evident that the dog acquired the L. serrata infection in Romania, or during the period of free-roaming lifestyle by feeding on infected viscera, muscles, and offal from infected intermediate hosts found in the field, or during the stay in a shelter by feeding on BARF. Table 1. Accession numbers and descriptions of sequences retrieved from GenBank producing significant alignments with COX1 mitochondrial gene from an L. serrata specimen spontaneously sneezed by a 1-year-old intact female Borzoi imported from Romania to Italy.
Linguatula serrata infection in dogs can often be asymptomatic (Globokar Vrhovec et al., 2005; Nagamori et al., 2019; Sievänen et al., 2021) or manifested through mild, as in this case, to severe clinical signs, implying serious ocular involvement (Villedieu et al., 2017) and progressive worsening of cough climaxing in episodes of dyspnea (Berberich et al., 2022). Moreover, a report from Italy showed the association between the development of nasal carcinoma and infection with a nymphal stage of L. serrata in a dog, suggesting that this parasitosis may lead to more serious consequences than previously thought (Bordicchia et al., 2014). In most of the previously reported cases of linguatulosis in dogs, the infection was diagnosed following the accidental expulsion of female parasites by coughing or, much more frequently, by sneezing (Globokar Vrhovec et al., 2005; Mitchell et al., 2016; Villedieu et al., 2017; Springer et al., 2018; Thomas, 2018; Sievänen et al., 2021; Berberich et al., 2022; Macrelli and Mackintosh, 2022), as in this report. In some cases, the expulsion of the parasite occurred by self-cure and because of this phenomenon, the parasitic infection disappeared on its own after adult pentastomids were expelled (Globokar Vrhovec et al., 2005; Springer et al., 2018; Sievänen et al., 2021; Berberich et al., 2022), as in this case. In other reports, expulsion occurred several days (up to 2 weeks) after the administration of ivermectin (Ioniță and Mitrea, 2016) or febantel/praziquantel/pyrantel embonate, either alone (Globokar Vrhovec et al., 2005; Macrelli and Mackintosh, 2022) or associated with selamectin (Villedieu et al., 2017). The drugs were administered for reasons completely different from linguatulosis (Ioniță and Mitrea, 2016; Macrelli and Mackintosh, 2022) such as broad-spectrum antiparasitic treatment in imported dogs (Globokar Vrhovec et al., 2005; Villedieu et al., 2017). The administration of these antiparasitic drugs may have somehow influenced the expulsion of the pentastomids. Only in a few cases, the infection was detected by chance upon the identification of L. serrata eggs in stool samples (Principato et al., 1994; Globokar Vrhovec et al., 2005; Nagamori et al., 2019). Linguatula serrata females lay up to 5,000,000 embryonated eggs per day after mating (Melhorn, 2008). Most of the eggs are expelled through nasal secretion while only a small part of them is swallowed and then pass through the intestinal tract being expelled with the feces. Therefore, fecal flotation for the detection of Linguatula eggs cannot be considered a sensitive method for diagnosis of linguatulosis in dogs, since the eggs are intermittently expelled with the feces and a negative result does not rule out the infection. On the other hand, samples of nasal discharge are not usually analyzed to detect parasite eggs. It is worth noting that, since L. serrata eggs in feces are uncommon, they can easily be misdiagnosed as spurious parasitic eggs, mainly as arthropod eggs because the presence of appendages with hooks in the larval stage within eggs is suggestive of larvated eggs of mites (Nagamori et al., 2019). There are no commercial drugs licensed for the treatment of linguatulosis in dogs. As already mentioned, sometimes the expulsion of the parasites occurred accidentally after the administration of different antiparasitic drugs for purposes other than the treatment of the infection (Globokar Vrhovec et al., 2005; Ioniță and Mitrea, 2016; Villeneuve et al., 2017; Macrelli and Mackintosh, 2022). However, anecdotal use of various systemic antiparasitic drugs aimed specifically at the treatment of linguatulosis in dogs has been reported in some case reports, such as the use of ivermectin (Paoletti et al., 2003; Globokar Vrhovec et al., 2005), fenbendazole plus praziquantel/pyrantel (Villedieu et al., 2017), milbemycin oxime/praziquantel (Springer et al., 2018; Berberich et al., 2022; Macrelli and Mackintosh, 2022), imidacloprid/moxidectin (Thomas, 2018), and fluralaner (Nagamori et al., 2019; Sievänen et al., 2021). Unfortunately, definitive evidence of the effectiveness of each of these treatments is still lacking. In one clinically severe case, frontal sinus trephination was required for treatment (Villedieu et al., 2017). In this case report, the expulsion of an adult parasite occurred incidentally by self-cure. A treatment attempt with febantel/pyrantel/praziquantel was administered, in agreement with the therapeutic choice of other authors (Villedieu et al., 2017). This association was prescribed as a broad-spectrum antiparasitic treatment, as the product has a broad-spectrum activity against nematodes (roundworms, hookworms, whipworms) and cestodes (Echinococcus spp., Taenia spp., Dipylidium caninum). The dog also received the association of emodepside with toltrazuril for the presence of Ancylostomatidae and Isospora spp. infection. Once the administration of the drug combination concluded, there was no further evidence of egg shedding, and the purpose of the investigation was to definitively determine whether other adults or juvenile parasites were still present in the upper respiratory tract. For this purpose, endoscopy was a very useful diagnostic tool as it allowed a thorough investigation to exclude the presence of any other parasite specimens and to detect pathological changes, as previously reported by other authors (Thomas, 2018). In one clinically severe case, computed tomography was required for diagnosis (Villedieu et al., 2017). Concerning the pathological lesions associated with adult L. serrata parasites in dogs, little information is available. Investigations of histopathological changes in the upper respiratory tract of infected dogs revealed that the nasal tract, nasal turbinates, and sinuses were soft, edematous, necrotic, ulcerated, and hemorrhagic with infiltration of macrophages, lymphocytes, plasma cells, and eosinophils within the epithelium and lamina propria (Oryan et al., 2008; Hajipour et al., 2018), mainly in heavily infected dogs (Oryan et al., 2008). Severe diffuse lymphoplasmacytic rhinitis has also been reported (Villedieu et al., 2017). In addition, infected wild canids (i.e., dingoes, dingo-dog hybrids, and red foxes) showed destruction of the nasal and paranasal mucosa with the presence of multiple foci of erosion, congestion, hemorrhage, and hemosiderin-laden macrophages indicative of chronic hemorrhage (Shamsi et al., 2018). In our case report, the pathogenicity of the parasite was investigated but histopathology was not performed since the endoscopic alterations found in the upper respiratory tract (i.e., mild-moderate activation of the nasal-associated lymphoid tissue) were almost irrelevant. Since the endoscopy was performed several weeks after the expulsion of the parasite, the mucosal repair processes had probably taken place in the meantime. Linguatula serrata is a zoonotic parasite. Because eggs dispersed in the feces or nasal discharges are immediately infective to intermediate hosts, human beings can easily become exposed to the risk of infection by contact with infected definitive hosts and can act as accidental intermediate hosts when they ingest water or vegetables contaminated by embryonated eggs of the parasite (Hajipour and Tavassoli, 2019). Ingestion of infective eggs causes visceral linguatulosis with the migration and encapsulation of larvae in internal organs, including cases of liver lesions (Symmers and Valteris, 1950; Tappe and Büttner, 2009), pulmonary nodules mimicking lung carcinoma (Pampiglione et al., 2001), and mesenteric lymph node localization mimicking acute appendicitis (Mateva et al., 2013). There are also reports about rare cases of human ocular linguatulosis caused by immature stages of L. serrata (Lazo et al., 1999; Koehsler et al., 2011; Bhende et al., 2014). Owners and veterinary practitioners, who are likely to be in direct contact with canine nasal secretions and feces-containing eggs, are at risk of zoonotic infection with visceral linguatulosis. Two autochthonous human cases of visceral linguatulosis have been described in Italy. One case reported the finding of three similar specimens in the buccal cavity of a woman suffering from pulmonary tuberculosis, 1–3 months apart, one of which was identified as a nymph of L. serrata (Parenzan and Chieffi, 1951). Another case reported a coin-shaped pulmonary lesion accidentally detected in an HIV-seropositive man during a routine X-ray examination, requiring thoracotomy with surgical removal and histological analysis of a lung nodule with a diameter of 1.8 cm, as lung cancer was suspected (Pampiglione et al., 2001). Additionally, humans may act as definitive hosts when they feed on raw or undercooked viscera-containing infectious nymphs from a parasitized intermediate host, commonly sheep and goats (Hajipour and Tavassoli, 2019). The ingestion of immature stages from an infected intermediate host, usually a sheep or a goat, causes nasopharyngeal linguatulosis known as “Halzoun syndrome” or “Marrara syndrome” with irritation of the nasopharyngeal region, sneezing, coughing, and respiratory discharges (Hajipour and Tavassoli, 2019). In endemic regions, it is often linked to traditional food containing raw meat, mostly liver (Hajipour and Tavassoli, 2019). Since the consumption of raw liver and other internal organs is not usually part of the diet in Italy and other European countries, this route of infection is likely to be neglected. The morphological characters of the recovered specimen showed that the parasite was belonging to the species L. serrata, as confirmed later by molecular analysis, and presented both similarities and differences with respect to previous reports. The length (87 mm) of the specimen examined was greater than 30 and 60 mm reported by Sievänen et al. (2021) and Paoletti et al. (2003), respectively, but falls within the ranges of 65–92 and 55–93 mm previously reported by Principato et al. (1994) and Berberich et al. (2022), respectively. As regards the width, the specimen (8 mm) was in line with the range of 8–12 mm reported by Berberich et al. (2022). In previous case reports, the number of annuli was not investigated in the expulsed specimens. The number of 97 annuli counted in our specimen falls within the range of 86–109 (mean 92) annuli reported for specimens collected from wild dogs in Australia (Shamsi et al., 2020) but was greater than the range of 74–93 (mean 85) annuli found in stray dogs in Iran (Rezaei et al., 2016). Therefore, it is likely that some intraspecific morphological variations may occur within different strains of L. serrata with different geographical origins and from different hosts. Linguatula serrata was first found and described in Europe (Nicoli, 1963). In the last years, the results of phylogenetic analyses showed that two groups of L. serrata specimens with different geographic distributions can be clearly differentiated. One group includes specimens from Europe and Australia and another group includes specimens from India and Bangladesh (Sudan et al., 2018; Shamsi et al., 2020). The genetic distance between the two groups suggests that the specimens of L. serrata found in these two geographic ranges belong to two distinct species (Sudan et al., 2018; Shamsi et al., 2020). Linguatula serrata differs morphologically from Neolinguatula nuttalli (parasite of lions) and Linguatula recurvata (parasite of Baird’s tapirs) since its terminal cleft is not as marked and deep as in the other two species (Shamsi et al., 2020) as well as from Linguatula arctica (parasite of reindeers) due to the different number of abdominal annuli (Gjerde, 2013). However, morphological differentiation between Linguatula species can be challenging for non-experts. In addition to morphological features, DNA barcoding provides a reliable molecular tool for species identification of medically important parasites and vectors (Ondrejicka et al., 2014), including Linguatula species (Sievänen et al., 2021), as shown in this case report. Remarkable genetic diversity has been reported between distinct species of Linguatula (Gjerde, 2013; Shamsi et al., 2020, 2022). According to Shamsi et al. (2022), reports that do not provide adequate morphological or molecular data, justifying how the species identification was determined, must be regarded with caution. In this case, the sequence of COX-I from the Linguatula specimen showed 99%–100% homology with sequences of L. serrata deposited in the GenBank database. Moreover, additional factors such as the dog as the definitive host, the morphological characters of the specimen and eggs in nasal discharge, and the geographical area of origin (Europe) allowed us to identify the specimen as L. serrata species without any doubt. To our knowledge, in addition to this report of the COX-1 gene sequence from mitochondrial DNA of L. serrata isolated from a dog, only one previous molecular investigation of a specimen of L. serrata from a gray wolf strain has been published to date in Italy (Raele et al., 2022). BARF is a type of dog diet that is based on a mixture of raw meat, bones, fruits, and vegetables. The diet has become increasingly popular as many owners view raw food as a more natural and healthier alternative to commercial pet food, but the actual benefits remain unproven (Morelli et al., 2019). Many studies raise the question of whether BARF can be considered an appropriate type of diet from a health (Strohmeyer et al., 2006; van Bree et al., 2018; Davies et al., 2019; Bottari et al., 2020) and nutritional point of view (Mack and Kienzle, 2016). The findings of this case report and those of two previous reports (Berberich et al., 2022; Campbel and Jones, 2023) underline that there is a real risk in regularly feeding dogs with homemade BARF recipes, as these can be accidentally prepared with raw meat contaminated by L. serrata infective nymphs. This case report shows that imported dogs may carry L. serrata infection without any clinical signs and with positive coprological examination and raises awareness of the potential importation of linguatulosis due to the increased international movement of dogs. Other case reports of L. serrata infection in dogs imported into central and northern European countries have been published in recent years (Globokar Vrhovec et al., 2005; Mitchell et al., 2016; Villedieu et al., 2017; Springer et al., 2018; Thomas, 2018; Sievänen et al., 2021; Berberich et al., 2022; Macrelli and Mackintosh, 2022), suggesting a possible spread of the infection (Berberich et al., 2022). However, it is still unclear whether this case and cases reported elsewhere in Europe represent isolated imported cases or the expansion of areas endemic for linguatulosis (Villedieu et al., 2017). Given the growing number of dog adoptions from abroad (Norman et al., 2020) and of pets imported from Eastern Europe (Wright and Elsheikha, 2017), especially dogs in Romania (Norman et al., 2020), the importation of dogs could favor the spread of various pathogens and represent a risk even in non-endemic areas (Wright and Elsheikha, 2017). Consequently, pathogenic agents not commonly observed in Italy and other European countries, such as linguatulosis, should increasingly be investigated in veterinary practices in imported or traveled dogs (Wright and Elsheikha, 2017; Norman et al., 2020). Imported dogs sneezing worm-like parasites or exhibiting the above clinical signs should be examined for the infection. The zoonotic potential of L. serrata should be addressed at the time of diagnosis. Since canine linguatulosis is often subclinical, the infection easily goes undetected, and the dog owner may be exposed to visceral linguatulosis without being aware of the risk. Linguatulosis should be included in the differential diagnosis of imported dogs with upper respiratory signs. Due to the zoonotic potential of this parasite, prompt diagnosis and adequate treatment of infected dogs are essential to limit the zoonotic risk. Once the diagnosis of canine linguatulosis has been made, the veterinarian should also provide advice to owners to reduce the risk of direct contact with canine nasal secretions and feces, which may contain infected eggs. In conclusion, it is important to note that this case report emphasizes that the risk of linguatulosis transmission should be included in the canine health risks associated with BARF. Conflict of interestThe authors declare that there is no conflict of interest. Author contributionsThis work was carried out in collaboration between all authors. Performed clinical activity: VM, EG, and LV. Performed laboratory work: FM and RAP; wrote the original draft of the manuscript: RAP, VM, LV, and FM. Reviewed and edited the final version of the manuscript: RAP, FM, VM, LV, and EG. Supervision: RAP. All authors have read and agreed to the published version of the manuscript. AcknowledgmentsWe would like to thank Mr. Simone Gabrielli for the SEM photos. FundingUniversity funds of the University of Pisa. Data availabilityAll data supporting the findings of this study are available within the manuscript. Any extra data needed are available from the corresponding author upon reasonable request. ReferencesBarton, D.P., Baker, A., Porter, M., Zhu, X., Jenkins, D. and Shamsi, S. 2020. Verification of rabbits as intermediate hosts for Linguatula serrata (Pentastomida) in Australia. Parsitol. Res. 119, 1553–1562. Barton, D.P., Gherman, C.M., Zhu, X. and Shamsi, S. 2022. Characterization of tongue worms, Linguatula spp. (Pentastomida) in Romania, with the first record of an unknown adult Linguatula from roe deer (Capreolus capreolus Linnaeus). Parasitol. Res. 121, 2379–2388. Barton, D.P., Porter, M., Baker, A., Zhu, X., Jenkins, D. and Shamsi, S. 2019. First report of nymphs of the introduced pentastomid, Linguatula serrata, in red-necked wallabies (Notamacropus rufogriseus) in Australia. Aust. J. Zool. 67, 106–113. Bhende, M., Abhishek, J.B., Raman, M. and Bhende, P.S. 2014. Linguatula serrata in the anterior chamber of the eye. Ind. J. Ophthalm. 62, 1159–1161. Berberich, M., Grochow, T., Roßner, N., Schmäschke, R. and Rentería-Solís, Z. 2022. Linguatula serrata in an imported dog in Germany: single-case or emerging disease? Vet. Parasitol. Reg. Stud. Rep. 30, 100717. Bordicchia, M., Falcioni, D., Scarpona, S. and Rossi, G. 2014. Nasal carcinoma in a dog with Linguatula serrata infection. Vet. Rec. Case Rep. 2, e000015. Bottari, B., Bancalari, E., Barera, A., Ghidini, S. and Gatti., M. 2020. Evaluating the presence of human pathogens in commercially frozen, biologically appropriate raw pet food sold in Italy. Vet. Rec. 187, e50. Campbell, E. and Jones, A. 2023. Tongue worm in an untraveled dog in UK. Vet. Rec. 192, 337–338. Davies, R.H., Lawes, J.R. and Wales, A.D. 2019. Raw diets for dogs and cats: a review, with particular reference to microbiological hazards. J. Small Anim. Pract. 60, 329–339. Diakou, A., Sokos, C. and Papadopoulos, E. 2014. Endoparasites found in European brown hares (Lepus europaeus) hunted in Macedonia, Greece. Helminthologia 51, 345–351. Globokar Vrhovec, M., Pantchev, N., Norden, C. and Kilo, A. 2005. Linguatula-serrata-Befall bei vier importierten Hunden. Kleintierpraxis 50, 779–784. Gjerde, B. 2013. Phylogenetic position of Linguatula arctica and Linguatula serrata (Pentastomida) as inferred from the nuclear 18S rRNA gene and the mitochondrial cytochrome c oxidase subunit I gene. Parasitol. Res. 112, 3517–3525. Hajipour, N. and Tavassoli, M. 2019. Prevalence and associated risk factors of Linguatula serrata infection in definitive and intermediate hosts in Iran and other countries: a systematic review. Vet. Parasitol. Reg. Stud. Rep. 16, 100288. Hajipour, N., Tavassoli, M., Abbasi Eslamlo, A., Javadi S.H. 2018. Investigation of histopathological changes caused by adult stage of Linguatula serrata in dog. Comp. Clin. Pathol. 27, 717–720. Hodžić, A., Alić, A., Klebić, I., Kadrić, M., Brianti, E. and Duscher, G.G. 2016. Red fox (Vulpes vulpes) as a potential reservoir host of cardiorespiratory parasites in Bosnia and Herzegovina. Vet. Parasitol. 223, 63–70. Ioniță, M. and Mitrea, I.L. 2016. Linguatula serrata (Pentastomida: Linguatulidae) infection in dog, Romania: a case report. AgroLife Sci. J. 5, 85–89. Koehsler, M., Walochnik, J., Georgopoulos, M., Pruente, C., Boeckeler, W., Auer, H. and Barisani-Asenbauer, T. 2011. Linguatula serrata tongue worm in human eye, Austria. Emerg. Infect. Dis. 17, 870–872. Lazo, R.F., Hidalgo, E., Lazo, J.E., Bermeo, A., Llaguno, M., Murillo, J. and Teixeira, V.P. 1999. Ocular linguatuliasis in Ecuador: case report and morphometric study of the larva of Linguatula serrata. Am. J. Trop. Med. Hyg. 60, 405–409. Macrelli, M. and Mackintosh, A. 2022. Tongue worm (Linguatula serrata) infection in a dog imported into the United Kingdom from Romania. Vet. Rec. Case Rep. 10, e281. Mack J.K. and Kienzle, E. 2016. Inadequate nutrient supply in "BARF" feeding plans for a litter of Bernese Mountain Dog-puppies. A case report. Tierarztl. Prax. Ausg. K. Kleintiere Heimtiere 44, 341–347. Mateva, S.A., Nikolova, M.R., Karavainov, M.P. and Maynova, P.E. 2013. Rare case of human visceral linguatulosis in Bulgaria diagnosed on biopsy specimen. J. Biomed. Clin. Res. 6, 131–133. Melhorn, H. 2008. Encyclopedia of parasitology, 3rd edition. Berlin, Germany: Springer-Verlag, vol. 1, pp: 583, 720–721. Mitchell, S., Bell, I., Wright, R., Wall, S., Jeckel, D., Blake, P., Marshall, C., Andrews, M. and Lee, A. 2016. Walsh Tongue worm (Linguatula species) in stray dogs imported into the UK. Vet. Rec. 179, 259–260. Mohtasebi, S., Teimouri A, and Abbaszadeh Afshar MJ. 2021. Demonstration of visceral linguatulosis in steppe field mouse (Apodemus witherbyi): a neglected potential intermediate host? Clin. Microbiol. Infect. 27, 1629–1630. Morelli, G., Bastianello, S., Catellani, P. and Ricci, R. 2019. Raw meat-based diets for dogs: survey of owners’ motivations, attitudes and practices. BMC Vet. Res. 15, 74. Morgulis, A., Coulouris, G., Raytselis, Y., Madden, T.L., Agarwala, R. and Schäffer, A.A. 2008. Database indexing for production MegaBLAST searches. Bioinformatics 24, 1757–1764. Nagamori, Y., Ramachandran, A., Kuzma, C., Nafe, L. and Johnson, E.M. 2019. A zoonotic parasite, Linguatula serrata, infection in a dog imported from Ethiopia to the United States. Vet. Parasitol. Reg. Stud. Reports. 16, 100273. Nicoli, R.M. 1963. Phylogénèse et systématique: le phylum des Pentastomida. Ann. Parasitol. Hum. Comp. 38, 483–516. Norman, C., Stavisky, J. and Westgarth, C. 2020. Importing rescue dogs into the UK: reasons, methods and welfare considerations. Vet. Rec. 186, 248. Oluwasina, O.S., ThankGod, O.E., Augustine, O.O. and Gimba, F.I. 2014. Linguatula serrata (Porocephalida: Linguatulidae) infection among client-owned dogs in Jalingo, North Eastern Nigeria: prevalence and public health implications. J. Parasitol. Res. 2014, 916120. Ondrejicka, D.A., Locke, S.A., Morey, K., Borisenko, A.V. and Hanner, R.H. 2014. Status and prospects of DNA barcoding in medically important parasites and vectors. Trends Parasitol. 30, 582–591. Oryan, A., Sadjjadi, S.M., Mehrabani, D. and Rezaei, M. 2008. The status of Linguatula serrata infection of stray dogs in Shiraz, Iran. Comp. Clin. Path. 17, 55–60. Pampiglione, S., Gentile, A., Maggi, P., Scattone, A. and Sollitto, F.A. 2001. A nodular pulmonary lesion due to Linguatula serrata in an HIV-positive man. Parassitologia 43, 105–108. Paoletti, B., Peli, A., Traversa, D. and Ragaini, L. 2003. La linguatulosi nel cane. Analisi della letteratura e segnalazione di un caso in Abruzzo. Ob. Doc. Vet. 24, 37–43. Parenzan, P. and Chieffi, G. 1951. First clinical case of human infestation with Linguatula serrata in Italy. Acta Med. Ital. Mal. Infett. Parassit. 6, 67–69. Principato, M., Polidori, G.A., Dacomo, F. and Giannetto, S. 1994. Nasal pentastomiasis in dogs by Linguatula serrata Frohlich 1789: notes on its very high biological potentiality. Parassitologia 36, 119. Raele, D.A., Petrella, A., Troiano, P. and Cafiero, M.A. 2022. Linguatula serrata (Fröhlich, 1789) in Gray Wolf (Canis lupus) from Italy: a neglected zoonotic parasite. Pathogens 11, 1523. Rezaei, F., Tavassoli, M., Hashemnia, M., Sorraya, N. and Gholizadeh, M. 2016. Some morphological data of various stages of Linguatula serrata. Acta Vet. Eurasia 42, 38–46. Shamsi, S., McSpadden, K., Baker, S. and Jenkins, D.J. 2017. Occurrence of tongue worm, Linguatula cf. serrata (Pentastomida: Linguatulidae) in wild canids and livestock in south-eastern Australia. Int. J. Parasitol. Parasites Wildl. 6, 271–277. Shamsi, S., Loukopoulos, P., McSpadden, K., Baker, S. and Jenkins, D. 2018. Preliminary report of histopathology associated with infection with tongue worms in Australian dogs and cattle. Parasitol. Int. 67, 597–600. Shamsi, S., Barton, D.P., Zhu, X. and Jenkins, D.J. 2020. Characterization of the tongue worm, Linguatula serrata (Pentastomida: Linguatulidae), in Australia. Int. J. Parasitol. Parasites Wildl. 11, 149–157. Shamsi, S., Zhu, X., Halajian, A. and Barton, D.P. 2022. 28S rRNA sequences for Linguatula spp. Parasitol. Res. 121, 1799–1804. Sievänen, M., Pohjoismäki, J., Saari, S., Miro, G. and Näreaho, A. 2021. The first Linguatula serrata case in an imported dog in Finland. Vet Parasitol. Reg. Stud. Rep. 26, 100654. Symmers, W.S. and Valteris, K. 1950. Two cases of human infestation by larvae of Linguatula serrata. J. Clin. Pathol. 3, 212–219. Springer, A., Fiedler H., Raue, K. and Strube, C. 2018. Linguatula serrata-Befall bei einem Importhund aus Rumänien. Tierarztl. Prax. Ausg. K Kleintiere Heimtiere 46, 260–264. Strohmeyer, R.A., Morley, P.S., Hyatt, D.R., Dargatz, D.A, Scorza, A.V. and Lappin, M.R. 2006. Evaluation of bacterial and protozoal contamination of commercially available raw meat diets for dogs. J. Am. Vet. Med. As. 228, 537–542. Sudan, V., Shanker, D. and Jaiswal, A.K. 2018. First report of molecular characterization and sequence phylogenetic analysis of Linguatula serrata from India. Acta Parasitol. 63, 781–783. Tappe, D. and Büttner, D.W. 2009. Diagnosis of human visceral pentastomiasis. PLoS Negl. Trop. Dis. 3, e320. Tavassoli, M., Tamaddonfard, E., Mirshekar, F., Hajipour, N. and Erfanparast, A. 2018. A behavioral evaluation of the effects of ingestion of Linguatula serrata nymphs in rats. Vet Parasitol. 254, 78–81. Thomas, M. 2018. Linguatula serrata in an imported Romanian street dog. Vet. Rec. 182, 112–113. van Bree, F.P.J, Bokken, G.C.A.M, Mineur, R., Franssen, F., Opsteegh, M, van der Giessen, J.W.B., Lipman, L.J.A. and Overgaauw, P.A.M. 2018. Zoonotic bacteria and parasites found in raw meat-based diets for cats and dogs. Vet. Rec. 182, 50. Villedieu, E., Sanchez R.F, Jepson, R.E. and Ter, Haar G. 2017. Nasal infestation by Linguatula serrata in a dog in the UK: a case report. J. Small Anim. Pract. 58, 183–186. Wright, I. and Elsheikha, H. 2017. Pet parasite risks from Eastern Europe: an emerging problem. Companion Anim. 22, 564–571. Zhang, Z., Schwartz, S., Wagner, L. and Miller, W.A. 2000. Greedy algorithm for aligning DNA sequences. J. Comput. Biol. 7, 203–214. | ||
| How to Cite this Article |
| Pubmed Style Marchetti V, FM, Gori E, LV, Papini RA. Linguatula serrata (Pentastomida: Linguatulidae) infection in a paucisymptomatic greyhound imported from Romania to Italy: A case report and literature overview. Open Vet J. 2023; 13(8): 1044-1055. doi:10.5455/OVJ.2023.v13.i8.12 Web Style Marchetti V, FM, Gori E, LV, Papini RA. Linguatula serrata (Pentastomida: Linguatulidae) infection in a paucisymptomatic greyhound imported from Romania to Italy: A case report and literature overview. https://www.openveterinaryjournal.com/?mno=146376 [Access: October 06, 2024]. doi:10.5455/OVJ.2023.v13.i8.12 AMA (American Medical Association) Style Marchetti V, FM, Gori E, LV, Papini RA. Linguatula serrata (Pentastomida: Linguatulidae) infection in a paucisymptomatic greyhound imported from Romania to Italy: A case report and literature overview. Open Vet J. 2023; 13(8): 1044-1055. doi:10.5455/OVJ.2023.v13.i8.12 Vancouver/ICMJE Style Marchetti V, FM, Gori E, LV, Papini RA. Linguatula serrata (Pentastomida: Linguatulidae) infection in a paucisymptomatic greyhound imported from Romania to Italy: A case report and literature overview. Open Vet J. (2023), [cited October 06, 2024]; 13(8): 1044-1055. doi:10.5455/OVJ.2023.v13.i8.12 Harvard Style Marchetti, V., , . F. M., Gori, . E., , . L. V. & Papini, . R. A. (2023) Linguatula serrata (Pentastomida: Linguatulidae) infection in a paucisymptomatic greyhound imported from Romania to Italy: A case report and literature overview. Open Vet J, 13 (8), 1044-1055. doi:10.5455/OVJ.2023.v13.i8.12 Turabian Style Marchetti, Veronica, Fabio Macchioni, Eleonora Gori, Luigi Venco, and Roberto Amerigo Papini. 2023. Linguatula serrata (Pentastomida: Linguatulidae) infection in a paucisymptomatic greyhound imported from Romania to Italy: A case report and literature overview. Open Veterinary Journal, 13 (8), 1044-1055. doi:10.5455/OVJ.2023.v13.i8.12 Chicago Style Marchetti, Veronica, Fabio Macchioni, Eleonora Gori, Luigi Venco, and Roberto Amerigo Papini. "Linguatula serrata (Pentastomida: Linguatulidae) infection in a paucisymptomatic greyhound imported from Romania to Italy: A case report and literature overview." Open Veterinary Journal 13 (2023), 1044-1055. doi:10.5455/OVJ.2023.v13.i8.12 MLA (The Modern Language Association) Style Marchetti, Veronica, Fabio Macchioni, Eleonora Gori, Luigi Venco, and Roberto Amerigo Papini. "Linguatula serrata (Pentastomida: Linguatulidae) infection in a paucisymptomatic greyhound imported from Romania to Italy: A case report and literature overview." Open Veterinary Journal 13.8 (2023), 1044-1055. Print. doi:10.5455/OVJ.2023.v13.i8.12 APA (American Psychological Association) Style Marchetti, V., , . F. M., Gori, . E., , . L. V. & Papini, . R. A. (2023) Linguatula serrata (Pentastomida: Linguatulidae) infection in a paucisymptomatic greyhound imported from Romania to Italy: A case report and literature overview. Open Veterinary Journal, 13 (8), 1044-1055. doi:10.5455/OVJ.2023.v13.i8.12 |