| Research Article | ||
Open Vet J. 2023; 13(6): 705-714 Open Veterinary Journal, (2023), Vol. 13(6): 705–714 Original Research Experimental infection of post-weaned pigs with F18-encoding enterotoxigenic and enterotoxigenic/shigatoxigenic Escherichia coli strain isolated from the diarrheic feces in KoreaKang-Hyun Baek1, Warisraporn Tangchang3, Eun-Jin Choi1, Wan-Kyu Lee2, Kyung-Hyun Lee1, Hyun-Kyung Lee1, Jae-Won Byun1 and Hwa-Young Son3*1Animal and Plant Quarantine Agency, Gimcheon, Republic of Korea 2College of Veterinary Medicine, Chungbuk National University, Cheongju, Republic of Korea 3College of Veterinary Medicine, Chungnam National University, Daejeon, Republic of Korea *Corresponding Author: Hwa-Young Son. College of Veterinary Medicine, Chungnam National University, Daejeon, Republic of Korea. Email: hyson [at] cnu.ac.kr Submitted: 06/04/2023 Accepted: 08/05/2023 Published: 07/06/2023 © 2023 Open Veterinary Journal
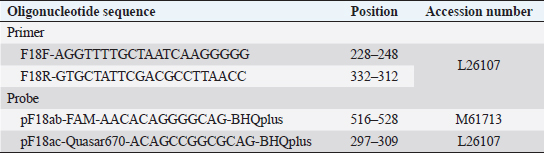
AbstractBackground: In the porcine industry, Escherichia coli (E. coli) infections have been causing post-weaning diarrhea (PWD) and edema disease (ED) for many years. It is classified into pathotypes and serotypes in animals according to virulence factors. Serotyping is performed for O, K, H, and F antigens, essential for discriminating pathogenicity and epidemiology. Furthermore, E. coli strains that produce F18 fimbriae are major sources of ED and PWD associated with Shiga-toxin producing E. coli (STEC) expressing F18ab and enterotoxigenic E. coli (ETEC) expressing F18ac, respectively. Aim: To investigate the pathogenicity potential and infection characteristics of experimental infection and confirm the pathological features of the Korean STEC/ETEC strains F18ab and F18ac in piglets. Methods: Three-week-old pigs were randomized into three experimental groups: infected G1 (F18ab), infected G2 (F18ac), and G3 (control). General health status was monitored daily, and pathological changes were evaluated. Results: Diarrhea occurred in all infected piglets. Pathological changes were only observed in the small intestine and regional lymph nodes. In G1, mucosal necrosis, inflammatory cell infiltration with hemorrhagic lesions, and apoptotic cell death in the tunica media of arterioles in the small intestine were observed. In contrast, the mucosa and epithelium appeared almost intact, with no abnormal vessel lesions in G2. Conclusion: Both strains, isolated from pigs in Korea, could be infected and did not spread from the alimentary tract to other organs. The pathological features were quite different among the F18 subtypes. The F18ab strain was more virulent than F18ac, and the virulence characteristics of the F18ac strain were more similar to ETEC than STEC. Keywords: Edema disease, Enteropathogenic Escherichia coli, F18 variants, Korean strains, Post-weaning diarrhea. IntroductionEscherichia coli (E. coli) is a Gram-negative bacterium that belongs to Enterobacteriaceae (Fairbrother and Nadeau, 2019). It is known as one of the common bacterial flora. However, some E. coli acquires virulence factors, including pili and enterotoxins encoded in plasmids, and are mediated by bacteriophages and subsequently cause disease (Pittman, 2010). E. coli is generally subdivided by pathotype and serotype, associated with certain virulence traits. Pathogenic E. coli in animals is classified as enterotoxigenic E. coli (ETEC), Shiga toxin-producing E. coli (STEC), enterohemorrhagic E. coli (EHEC); enteropathogenic E. coli (EPEC), and extraintestinal pathogenic E. coli (ExPEC) by virulence mechanism, as indicated by the presence of virulence factors (Gyles and Fairbrother, 2010). ETEC colonizes the small intestine via specific adhesion factors (fimbriae) (Nagy and Fekete, 1999) and produces heat-stable enterotoxins (STa or STb) and heat-labile enterotoxins (LT). STEC produces the Shiga toxin variant (stx2e), which causes vascular lesions in the intestine, subcutis, and brain, leading to edema and neurological symptoms (Imberechts et al., 1992). EPEC, originally isolated from humans, resembles ETEC, which causes diarrhea. Intimin, the outer membrane protein of EPEC, is involved in the intimate attachment of the bacteria to enterocytes (Lee et al., 2022). For serotypes, they are designated by O (somatic), K (capsular), H (flagellar), and F (fimbria) antigens. At least 188 O, 103 K, 56 H, and over 20 F antigens have been officially recognized to date (Fairbrother and Nadeau, 2019). E. coli infections are commonly associated with diarrhea, edema disease (ED), and septicemia in pigs (Pittman, 2010). Among these, post-weaning diarrhea (PWD) and ED are major causes of economic loss in the porcine industry worldwide (Pittman, 2010; Nagy and Fekete, 1999). According to the Korea Animal Health Integrated System (www.kahis.go.kr), 880 cases of E. coli-associated pig infections were diagnosed from 2012 to 2014. Notably, these included E. coli isolation (423), detection of pathogenic E. coli (285), colibacillosis (110), ED (52), suspected colibacillosis (4), polyserositis (2), and suspected ED (2). The data showed that ED and PWD are constant causes of major concern in Korea. PWD and ED could occur independently or simultaneously in pig farms, as it is difficult for the practitioner or farmer to distinguish between them because their symptoms overlap (Imberechts et al., 1992; Fairbrother and Nadeau, 2019). Furthermore, F18-expressing E. coli is associated with the pathogenesis of ED and PWD in post-weaned pigs (Gannon et al., 1989; Do et al., 2019). In Korea, recent studies have reported that F18-positive E. coli is the predominant strain causing diarrhea (Kim et al., 2010). F18 is subtyped into three antigenic variants: F18ab (formerly F107), F18ac (2134P and 8813), and F18 (new variant) (Byun et al., 2013). Generally, STEC-expressing F18ab fimbriae is associated with ED, whereas ETEC-expressing F18ac fimbriae is associated with PWD (Zhang et al., 2007). The cause of the disease might be identified based on the pathological findings. However, the virulence traits and pathogenesis of ETEC/STEC that produce F18ac fimbriae are obscure, and only a few reports of in vivo experimental data exist. Therefore, the present study aimed to determine the pathogenicity and pathological characteristics of the domestically isolated F18ab (STEC) and F18ac (STEC/ETEC) strains isolated in experimentally infected post-weaning piglets. Materials and MethodsEscherichia coli strainsTwo strains (O139:stx2e:F18ab:AIDA, O35:LT:STa:stx2e:F18ac:AIDA) isolated from pigs in Korea were used in this study. The strains were grown on blood agar (Asan, Korea) for 24 hours at 37°C. The bacteria were washed thrice with phosphate-buffered saline (PBS) and centrifuged at 8,000 × g. Colony forming units/ml (CFU/ml) were counted by the standard 10-fold dilution method. For animal experiments, both strains were adjusted to 1010 CFU/ml. Animals and inoculationFifteen 3-week-old crossbred (Landrace × Yorkshire × large white) pigs, with a body weight range of 3–4 kg, were purchased from a commercial pig farm and randomized into three experimental groups: group 1 (G1, n=5), group 2 (G2, n=5), and group 3 (G3, n=5). They were acclimated for 7days in animal isolation facilities (CAVAC, Korea) and fed commercial feed without antimicrobials. Before the experiment, the absence of pathogenic E. coli and other pathogenic agents, such as rotavirus, transmissible gastroenteritis virus, porcine epidemic diarrhea virus, porcine circovirus type 2, porcine reproductive, and respiratory syndrome virus, was confirmed in the pigs. Water was provided ad libitum. Sodium deoxycholate (Sigma, USA) was added at 2 g/kg of feed (except in G3) to increase the permeability of intestinal mucosa (Waddell and Gyles, 1995). After acclimation, 3 ml of bacterial suspension of O139:F18ab and O35:F18ac strains were fed to the G1 and G2 pigs, respectively. The same volume of PBS was fed to G3. Clinical signs, sample collection, and feces scoringClinical signs, such as neurologic disorders, lateral recumbency, or death, were monitored daily. Body weight and rectal temperature were measured at 0, 2, 4, 6, and 7 days post-inoculation (dpi). The fecal condition was scored (0, constipation; 1, normal; 2, mucoid; 3, watery; 4, watery and hemorrhage) and subjected to E. coli identification by real-time PCR. Discrimination of F18 subtypes in fecesTo discriminate F18 subtypes in the feces, DNA templates were extracted using the QIAampDNA Stool Kit (Qiagen, USA) and subjected to real-time PCR, as previously described (Byun et al., 2013). Briefly, it was performed using a Rotor-Gene 3000 (Corbette Research, Australia) with a reaction volume of 20 μl containing 2 μl of template, 2 × TAKARA Mastermix (TAKARA, Japan), 1 μM of each primer, and 0.5 μM of each probe (Table 1). The thermocycling profile was 95°C for 3 minutes, followed by 40 cycles at 95°C for 20 seconds and 64°C for 25 seconds. Table 1. Oligonucleotide primers and probes used in real-time PCR.
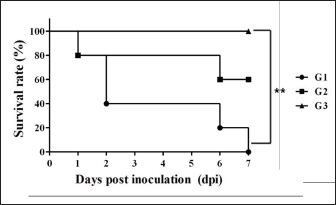
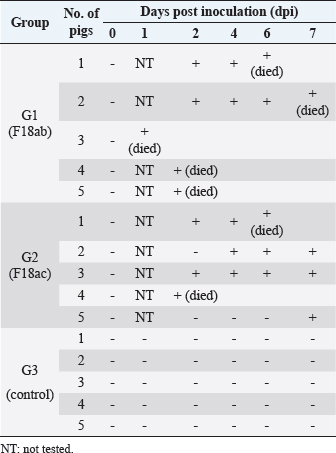
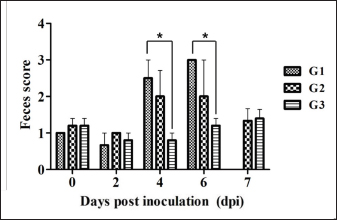
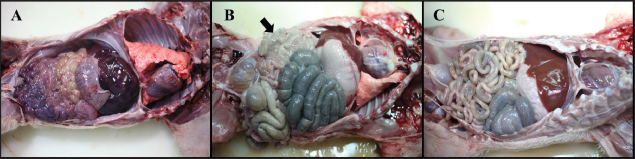
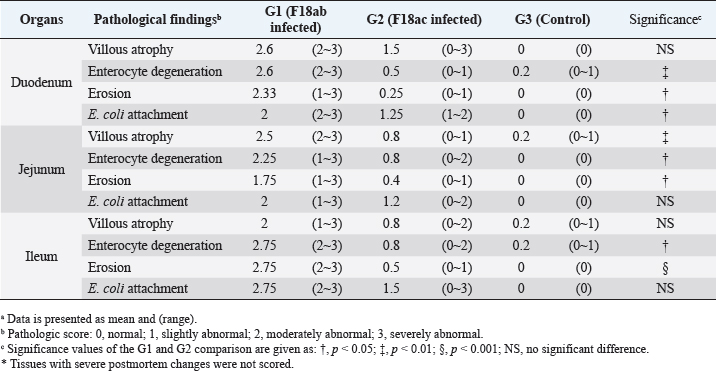
Necropsy and histopathologyThe piglets were euthanized with an overdose of barbiturate. Tissues, including the brain, spinal cords, tonsil, lung, heart, liver, kidney, stomach, duodenum, jejunum, ileum, and mesenteric lymph nodes, were collected and fixed in 10% neutral buffered formalin. They were processed, trimmed, embedded in paraffin, and sectioned at a thickness of 2.5 μm. Hematoxylin and eosin stain (H&E) was performed by an automatic staining system (Ventana Symphony, USA). Pathological findings of the mucosa (villous atrophy, enterocyte degeneration, erosion, and E. coli attachment) were evaluated in the duodenum, jejunum, and ileum, and then scored (0, normal; 1, slightly abnormal; 2, moderately abnormal; 3, severely abnormal). Ultrastructure electron microscopyIlea were fixed in 2.5% glutaraldehyde and 1% paraformaldehyde for 24 hours and post-fixed in 1% osmium tetroxide for 2 hours. The tissues were rinsed, dehydrated, and incubated in propylene oxide. Afterward, the tissues were embedded in an Epon mixture in Eponate 12 Embedding kit (Pelco, USA) and sectioned. Thin sections were stained with uranyl acetate and lead citrate and examined by a transmission electron microscope (Hitachi, Japan). TUNEL assayDNA fragmentations in myocytes of the ileal arterioles were evaluated by the terminal dUTP nick-end labeling (TUNEL) assay using ApopTag peroxidase in situ apoptosis detection kit (Millipore, USA), according to the manufacturer’s instructions. In brief, deparaffinized sections were treated with proteinase K (Takara, Japan) at room temperature (RT), approximately 20°C‒25°C, for 15 minutes, washed in deionized water, and treated with 3% hydrogen peroxide in PBS for 5 minutes. Subsequently, the sections were treated directly with equilibration buffer, and a solution of terminal deoxynucleotidyl transferase and digoxigenin-dUTP was added in a humidified chamber at 37°C for 1 hour. After incubation, the sections were dipped in STOP buffer for 15 seconds and incubated for 10 minutes at RT. After treatment with anti-digoxigenin peroxidase, digoxigenin-dUTP end-labeled DNA was visualized by peroxidase detection with 0.05% diaminobenzidine and 0.02% hydrogen peroxidase. Sections were counterstained with Gill’s hematoxylin, dehydrated, cleaned in xylene substitute, and mounted for microscopic examination. Statistical analysisStatistical analysis was performed using SPSS version 21 (IBM, Armonk, NY). The survival rate was calculated using the Log-rank test. Body conditions (body weight changes, rectal temperature changes, and feces score) are expressed as mean ± SEM and analyzed using an independent t-test for group-group comparison. Pathologic scores are expressed as mean and range and analyzed using an independent t-test for group-group comparison. p values less than 0.05 were regarded as statistically significant. Ethical approvalAll animal experiments were approved by the Institutional Animal Care and Use Committee of Chungbuk National University (Approval no. CBNUA-759-14-01). ResultsSurvival rateSurvival rates were different in each group (Fig. 1). Three of the 10 animals in infected groups died on the first day after inoculation (Table 2). As a result, the survival rates of G1 and G2 on 7 dpi were 0% and 60%, respectively, while G3 was 100%. Body conditionsNo statistically significant differences in body weight or rectal temperature were observed between groups. However, the feces scores in G1 were significantly higher than those in G3 on days 4 and 6 (Fig. 2), and the fecal condition in both groups was mucoid. Furthermore, watery and hemorrhagic feces were observed in two animals from G2 on days 4 and 6. The feces score of G1 (3.0 ± 0.00) was highest at 6 dpi; at 7 dpi, the feces score of G1 was absent, while that of G2 decreased due to the death or euthanasia of fatal pigs. Detection of F18-encoding strain in fecesMore than half of the feces were positive for each F18 positive strain at 2 dpi in G1 (4/5) and G2 (3/5). All samples from the infected groups tested positive by the end of the experiment. In G3, there were no positive results during the experiment (Table 2). Necropsy findingsAll other organs were macroscopically normal. However, a thin small intestinal wall with redness was observed in G1 (Fig. 3A) with hyperemia and enlargement of the mesenteric lymph node. In G2, parts of the intestinal wall were thin, with no hyperemic lesions (Fig. 3B). No significant abnormalities were found in G3 (Fig. 3C).
Fig. 1. Survival rate of the pigs after inoculation. Survival rate of the pigs are 0% in the G1, 60% in the G2, and 100% in the G3 at 7 dpi. Each group consists of five pigs. Groups: G1, F18ab infected; G2, F18ac infected; G3, control. ** p < 0.01. Table 2. Detection of F18ab or F18ac strains in feces.
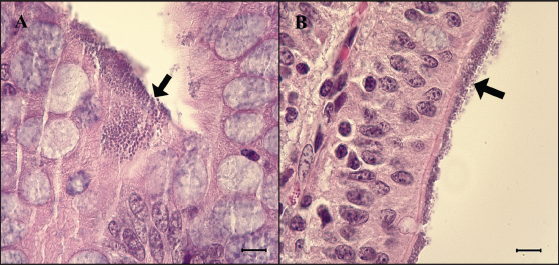
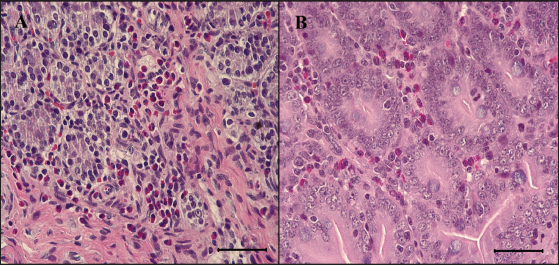
Fig. 2. Feces score of pigs after inoculation. Data are presented as means ± SEM of pigs per group. Fecal condition is scored as 4 grades (0, constipation; 1, normal; 2, mucoid; 3, watery; 4, watery and hemorrhage). Feces scores are increased markedly in the G1 and G2 than in the G3 on 4 and 6 dpi. On 7 dpi, feces score is absent or decreased in the G1 and G2 due to death or euthanasia of fatal pigs. Groups: G1, F18ab infected; G2, F18ac infected; G3, control. * p < 0.05 versus the G3. Histopathological findingsPathological changes were prominent in the small intestine (Table 3). Additionally, abnormal findings of the mucosa in all parts of the small intestine (villous atrophy, enterocyte degeneration, erosion, and E. coli attachment) of G1 were of a moderate to severe grade and had the highest score compared with G2 and G3. The scores of villous atrophy in the duodenum and ileum were higher but not statistically significant as opposed to those of the jejunum. Severe enterocyte degeneration and erosion were observed in three parts of the intestine of G1, and these were significantly higher than in G2. E. coli attachment to the villi was most prominent in the ileum of both G1 and G2 (Fig. 4). Mucosal necrosis and detached villi with hemorrhagic lesions were observed in the duodenum and jejunum of the G1 group. These lesions were more severe, with necrotic tissue and colonies of E. coli in the ileum (Fig. 5). The small intestinal mucosa and epithelium of G2 were relatively intact (Fig. 5). Inflammatory cells were also observed in the ileum. Among the inflammatory cells, markedly increased eosinophils were observed in the lamina propria of G1 and G2 (Fig. 6). In G1, mild perivascular edema and karyorrhectic cell death of myocytes in the tunica media were observed (Fig. 7). Thickened endothelial cells were also observed in affected blood vessels. These lesions were not observed in G2 or G3. Ultrastructure findingsTransmission electron microscopy of the ileum revealed numerous E. coli located on the microvilli (Fig. 8). Fimbriae were visualized between the bacteria and microvilli of G1 and G2. Vacuolation and loss of microvilli were more easily detectable in G1 than in G2. TUNEL assayTUNEL assay was performed to assess apoptotic cell death in the tunica media of blood vessels. In G1, one or two TUNEL-positive apoptotic bodies were observed in the tunica media of blood vessels (Fig. 9). In contrast, TUNEL-positive apoptotic bodies were not observed in G2 and G3. DiscussionPathogenic E. coli strains that cause diseases in pigs are generally characterized by their fimbriae type (F4, F5, F6, F41, and F18) and the presence of toxins (Pittman, 2010). Previous studies have shown that both F4 receptors and age-dependent F18 receptors are present in enterocytes, usually during the post-weaning period (Luppi, 2017; García et al., 2020), while F5, F6, and F41-positive strains affect pigs at a younger age (Luppi, 2017). F18-positive strains are known to cause diseases in the post-weaning period according to the subtype, either F18ab or F18ac (Cheng et al., 2005). Antigenic differences in F18 are related to the fedA fimbrial protein, which encodes the major fimbrial subunit of F18. In the fedA gene, F18ac has an additional proline at position 121, whereas it is either substituted or missing in F18ab (Debroy et al., 2009). We used pigs as subjects to understand the pathologic features of F18ab and F18ac E. coli isolated in Korea by inoculation of bacterial suspensions via the oral route.
Fig. 3. Gross findings. (A) Pleural and abdominal cavity of G1. Redness of the small intestines and thin intestinal wall are observed, 6 dpi. (B) Pleural and abdominal cavity of G2. Thin intestinal wall is observed in parts (arrow) of the small intestines, with no hyperemic lesion, 7 dpi. (C) Pleural and abdominal cavity of the G3. No significant findings, 7 dpi. Groups: G1, F18ab infected; G2, F18ac infected; G3, control. Table 3. Histopathological findings in the small intestine of pigs infected with E. colia.
Almost all feces (8 of 10) of the infected group were confirmed positive through real-time PCR for each subtype within 2 dpi. Mucoid diarrhea occurred at 4 dpi and subsequently became watery and hemorrhagic. Body weight loss due to diarrhea was observed in both infected groups. These results indicated that the inoculated E. coli was proliferative in intestinal organs and caused diarrhea, similar to the results in a previous F18 E. coli experimental infection study (Mclamb et al., 2013). However, clinical signs, such as edema of the eyelids and subcutaneous area, were not observed in the present study. Clinical signs are not easily detectable through ED-STEC oral; approximately 30% of challenged pigs showed these signs at 3‒10 dpi (Bertschinger and Pohlenz, 1983). Due to these rationales, some studies have attempted to administer the stx2e intravenously, and clinical signs developed 1–2 days after injection (Gannon et al., 1989; Macleod et al., 1991). As there were no clinical signs in the case of F18ab E. coli infection, adjustment of inoculum will be needed to reduce mortality and extend the experimentation period long enough to develop clinical, edematous, and other signs. It was reported that among 11 pigs that were orally inoculated with 2 × 1011 of F18ab STEC, 5 pigs died within 8 dpi (Konstantinova et al., 2008). In the present study, all pigs from the F18ab infected group died within 7 days of the experiment, despite the concentration of the inoculum being decreased to 1010. The survival rate of the F18ac infected group was 60%, suggesting that the F18ab strain was more virulent than the F18ac strain.
Fig. 4. Attachment of the E. coli in the ileum. (A) Ileum of G1. Small round-shaped E. coli are attached to the villi (arrow) and some of them penetrate into tissue, 6 dpi. H&E, Bar=10 μm. (B) Ileum of G2. E. coli is also attached to the villi (arrow). H&E, Bar=10 μm. Groups: G1, F18ab infected; G2, F18ac infected.
Fig. 5. Eosinophils in the small intestine of the infected pigs. (A) Large numbers of eosinophils are infiltrated in the lamina propria of G1, 6 dpi. H&E, Bar=50 μm. (B) Eosinophils are also infiltrated in the lamina propria of G2, 7 dpi. H&E, Bar=50 μm. Groups: G1, F18ab infected; G2, F18ac infected. Pathological findings for both infected groups showed different features since the stx2e toxin of F18ab E. coli can cause vascular damage, resulting in edema and hemorrhage (Clugston et al., 1974). Stx2e toxin circulates throughout the body by binding to Gb3 and Gb4 receptors on erythrocytes, and the key target organs affected by stx2e toxin are the brain and intestinal tract (Matise et al., 2000). Acutely affected pigs showed minimal microscopic lesions, which consisted of mild perivascular edema or karyorrhectic debris within myocytes of blood vessels (Methiyapun et al., 1984). The G1 group, infected with the F18ab E. coli, showed necrotic enteritis with hemorrhagic lesions, which had been observed in previous studies (Bertschinger and Pohlenz, 1983). The lesions were more severe in the ileum than in the proximal part of the small intestine because of the intense attachment of E. coli to the villi in the ileum. Moreover, karyorrhectic nuclei were also observed in the tunica media of blood vessels and positively identified by TUNEL labeling. TUNEL assay has been used in previous studies for the detection of apoptotic DNA fragmentation caused by stx2e toxin damage (Wada et al., 1997; Matise et al., 2000). Thus, the TUNEL assay results supported that the stx2e toxin released by STEC caused vascular damage in G1.
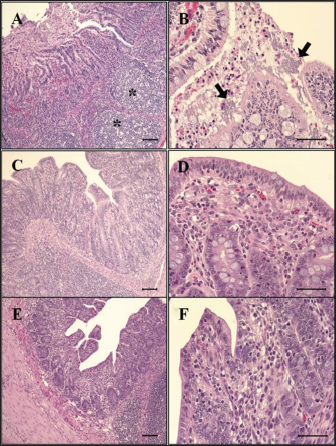
Fig. 6. Histopathological findings in the ileum. (A) Mucosal necrosis and lymphoid depletion (asterisk) are observed in G1, 2 dpi. H&E, Bar=100 μm. (B) Note the compound of necrotic enterocytes and E. coli (arrow) in G1, 6 dpi. H&E, Bar=50 μm. (C) No significant findings in mucosa of G2, 7 dpi. H&E, Bar=100 μm. (D) Few neutrophils are observed in the epithelium and lamina propria of G2, 7 dpi. H&E, Bar=50 μm. (E) No significant findings in mucosa of G3, 7 dpi. H&E, Bar=100 μm. (F) Intact epithelium and lamina propria of G3, 7 dpi. H&E, Bar=50 μm. Groups: G1, F18ab infected; G2, F18ac infected; G3, control.
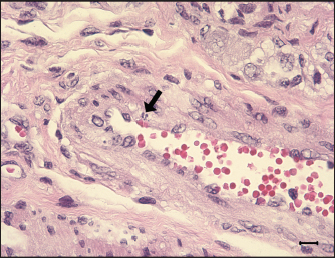
Fig. 7. Apoptosis in tunica media of G1. An ilea arteriole of G1. Karyorrhectic single cell death (arrow) and thickened endothelial cell of the blood vessel are observed. H&E, Bar=10 μm. Group: G1, F18ab infected. In contrast, G2 pigs infected with F18ac E. coli showed mild lesions in the small intestine. Moreover, E. coli colonized on the villi, mucosa, and epithelium were almost intact with infiltration of a few polymorphonuclear neutrophils in the lamina propria, and the inflammatory cells were most easily detectable in the ileum. Pyknotic or karyorrhectic nuclei were not observed in the blood vessels of G2 pigs. These findings were in accordance with lesions of PWD caused by enterotoxins. Enterotoxins (LT, STa, and STb) released by E. coli do not lead to pathological lesions or morphological alterations in the mucosa, but they do give rise to functional changes (Nagy and Fekete, 1999). LT toxin activates the adenylate-cyclase system within the cell, resulting in increased fluid and electrolyte secretion and decreased absorption (Konstantinova et al., 2008). STa toxin stimulates the guanylate-cyclase system, leading to intracellular accumulation of cGMP and reduced absorption of water and electrolytes on villi (Francis et al., 1998). The mechanism and characteristics of STb toxin are less known, but STb seems to stimulate a non-chloride anion secretion by intestinal epithelial cells (Whipp et al., 1981). The toxins stimulate the small intestine by increasing water and electrolyte secretion and/or decreasing fluid absorption, resulting in diarrhea (García et al., 2020). In cases of severe PWD, some investigators have reported that marked infiltration of neutrophils in the superficial lamina propria and necrosis of villi could occur (Faubert and Drolet, 1992). However, the strain inoculated in the G2 group was F18ac E. coli, which included both enterotoxins (LT and STa) and Shiga toxin (stx2e). Expression of stx2e was not pathologically detected in this study. Therefore, the results of this group suggest that the virulence trait of the strain was similar to that of ETEC rather than STEC. Structural study of E. coli on microvilli using electron microscopy has been conducted in previous studies (Methiyapun et al., 1984). E. coli was surrounded by an electron-lucent zone, which appeared to prevent the bacteria from making direct contact with each other and the microvilli. In the present study, an electron-lucent zone was also observed, and the E. coli were slightly apart from each other. In another study, 1–2 μm long fine flexible fimbriae that cover the bacterial surface were identified in the peribacterial zone (Hahn et al., 2000). Therefore, the electron-lucent zone in this study could be evidence of bacterial structures, such as fimbriae and flagella.
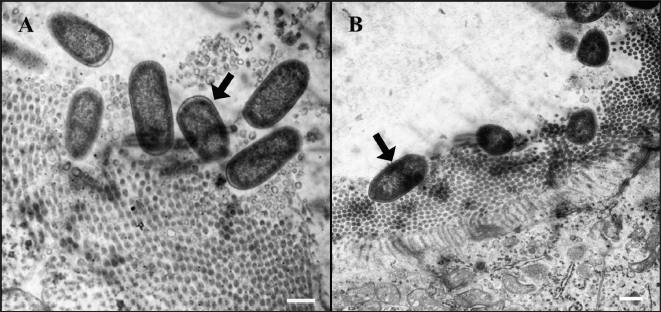
Fig. 8. Ultrastructure of E. coli attachment. (A) Ilea microvilli of G1. Numerous rod-shaped E. coli are on the microvilli of G1 (arrow). Bar=500 nm. (B) Ilea microvilli of G2. Numerous rod-shaped E. coli are also on the microvilli of G2 (arrow). Fine space between E. coli and microvilli suggests the presence of fimbriae. Bar=1,000 nm. Groups: G1, F18ab infected; G2, F18ac infected.
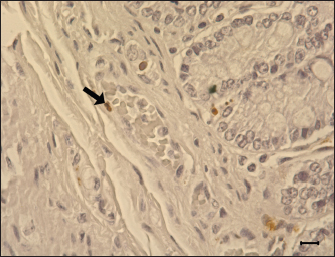
Fig. 9. Apoptosis of the blood vessel in the ileum of G1. TUNEL-positive (brown) apoptotic body (arrow) is presented in the tunica media, 6 dpi. Gill’s hematoxylin counterstain. Bar=10 μm. Group: G1, F18ab infected. In both infected groups, large numbers of eosinophils infiltrated the lamina propria. This lesion has been reported in STEC-infected pigs by previous studies (Kurtz et al., 1969), and another study suggested that eosinophil bactericidal activity against E. coli is comparable to that of neutrophils (Persson et al., 2001). Nevertheless, the specificity of eosinophil infiltration in STEC infection is currently unclear. In conclusion, the results of this study suggest that both Korean F18ab and F18ac E. coli cause diarrhea in post-weaned pigs. However, pathological lesions were distinctively observed in each subtype. The F18ab strain was more virulent than the F18ac strain, and the virulence characteristics of the F18ac strain were more similar to ETEC than STEC. AcknowledgmentsThis study was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry, and Fisheries (IPET) through the Agri-Bio Industry Technology Development Program, funded by the Ministry of Agriculture, Food, and Rural Affairs (MARFRA) (114058-03-3-CG000) and Chungnam National University. Conflict of interestAll authors have no potential conflicts of interest. Author contributionsConceptualization: Baek KH, Lee WK, Byun JW, Son HY; Formal analysis: Choi EJ, Lee KH, Lee HK; Funding acquisition: Lee WK, Son HY, Byun JW; Methodology: Lee WK; Supervision: Son HY, Byun JW; Writing - original draft: Baek KH; Writing - review & editing: Tangchang W. ReferencesBertschinger, H.U. and Pohlenz, J. 1983. Bacterial colonization and morphology of the intestine in porcine Escherichia coli enterotoxemia (edema disease). Vet. Pathol. 20(1), 99–110. Byun, J.W., Jung, B.Y., Kim, H.Y., Fairbrother, J.M., Lee, M.H. and Lee, W.K. 2013. Real-time PCR for differentiation of f18 variants among enterotoxigenic and shiga toxin-producing Escherichia coli from piglets with diarrhoea and oedema disease. Vet. J. 198(2), 538–540. Cheng, D., Sun, H., Xu, J. and Gao, S. 2005. Prevalence of fimbial colonization factors f18ab and f18ac in Escherichia coli isolates from weaned piglets with edema and/or diarrhea in China. Vet. Microbiol. 110(1–2), 35–39. Clugston, R.E., Nielsen, N.O. and Smith, D.L. 1974. Experimental edema disease of swine (E coli enterotoxemia). 3. Pathology and pathogenesis. Can. J. Comp. Med. 38(1), 34–43. Debroy, C., Roberts, E., Scheuchenzuber, W., Kariyawasam, S. and Jayarao, B.M. 2009. Comparison of genotypes of Escherichia coli strains carrying f18ab and f18ac fimbriae from pigs. J. Vet. Diagn. Invest. 21(3), 359–364. Do, K.H., Byun, J.W. and Lee, W.K. 2019. Prevalence of O-serogroups, virulence genes, and F18 antigenic variants in Escherichia coli isolated from weaned piglets with diarrhea in Korea during 2008-2016. J. Vet. Sci. 20(1), 43–50. Fairbrother, J.M. and Nadeau, É. 2019. Colibacillosis. In Diseases of swine. 11th ed. Eds., Zimmerman, J J, Locke, A, Karriker L.A, Ramirez, A, Schwartz K.J, Stevenson, GW. and Zhang J. New York, NY: John Wiley Sons, pp: 807–832. Faubert, C. and Drolet, R. 1992. Hemorrhagic gastroenteritis caused by Escherichia coli in piglets: clinical, pathological and microbiological findings. Can. Vet. J. 33(4), 251–256. Francis, D.H., Grange, P.A., Zeman, D.H., Baker, D.R., Sun, R. and Erickson, A.K. 1998. Expression of mucin-type glycoprotein k88 receptors strongly correlates with piglet susceptibility to k88(+) enterotoxigenic Escherichia coli, but adhesion of this bacterium to brush borders does not. Infect. Immun. 66(9), 4050–4055. Gannon, V.P., Gyles, C.L. and Wilcock, B.P. 1989. Effects of Escherichia coli shiga-like toxins (verotoxins) in pigs. Can. J. Vet. Res. 53(3), 306–312. García, V., Gambino, M., Pedersen, K., Haugegaard, S., Olsen, J.E. and Herrero-Fresno, A. 2020. F4- and F18-positive enterotoxigenic Escherichia coli isolates from diarrhea of postweaning pigs: genomic characterization. Appl. Environ. Microbiol. 86(23), e01913–20. Gyles, C.L. and Fairbrother, J.M. 2010. Escherichia coli. In Pathogenesis of bacterial infections in animals. 4th ed. Eds., Gyles, C.L., Prescott, J.F. and Songer, J.G. Ames, IA: Wiley-Blackwell, pp: 267–308. Hahn, E., Wild, P., Schraner, E.M., Bertschinger, H.U., Häner, M., Müller, S.A. and Aebi, U. 2000. Structural analysis of f18 fimbriae expressed by porcine toxigenic Escherichia coli. J. Struct. Biol. 132(3), 241–250. Imberechts, H., De Greve, H. and Lintermans, P. 1992. The pathogenesis of edema disease in pigs. A review. Vet. Microbiol. 31(2–3), 221–233. Kim, Y.J., Kim, J.H., Hur, J. and Lee, J.H. 2010. Isolation of Escherichia coli from piglets in South Korea with diarrhea and characteristics of the virulence genes. Can. J. Vet. Res. 74(1), 59–64. Konstantinova, L., Hamrik, J., Kulich, P., Kummer, V., Maskova, J. and Alexa, P. 2008. The effect of intramuscular administration of colistin on the development and course of experimentally induced oedema disease in weaned piglets. Vet. Microbiol. 128(1-2), 160–166. Kurtz, H.J., Bergeland, M.E. and Barnes, D.M. 1969. Pathologic changes in edema disease of swine. Am. J. Vet. Res. 30(5), 791–806. Lee, J.B., Kim, S.K. and Yoon, J.W. 2022. Pathophysiology of enteropathogenic Escherichia coli during a host infection. J. Vet. Sci. 23(2), e28. Luppi, A. 2017. Swine enteric colibacillosis: diagnosis, therapy and antimicrobial resistance. Porcine Health Manag. 3, 16. Macleod, D.L., Gyles, C.L. and Wilcock, B.P. 1991. Reproduction of edema disease of swine with purified shiga-like toxin-ii variant. Vet. Pathol. 28(1), 66–73. Matise, I., Sirinarumitr, T., Bosworth, B.T. and Moon, H.W. 2000. Vascular ultrastructure and DNA fragmentation in swine infected with shiga toxin-producing Escherichia coli. Vet. Pathol. 37(4), 318–327. Mclamb, B.L., Gibson, A.J., Overman, E.L., Stahl, C. and Moeser, A.J. 2013. Early weaning stress in pigs impairs innate mucosal immune responses to enterotoxigenic E. coli challenge and exacerbates intestinal injury and clinical disease. PLos One. 8(4), e59838. Methiyapun, S., Pohlenz, J.F. and Bertschinger, H.U. 1984. Ultrastructure of the intestinal mucosa in pigs experimentally inoculated with an edema disease-producing strain of Escherichia coli (0139: K12: H1). Vet. Pathol. 21(5), 516–520. Nagy, B. and Fekete, P.Z. 1999. Enterotoxigenic Escherichia coli (etec) in farm animals. Vet. Res. 30(2-3), 259–284. Persson, T., Andersson, P., Bodelsson, M., Laurell, M., Malm, J. and Egesten, A. 2001. Bactericidal activity of human eosinophilic granulocytes against Escherichia coli. Infect. Immun. 69(6), 3591–3596. Pittman, J.S. 2010. Enteritis in grower-finisher pigs caused by f18-positive Escherichia coli. J. Swine Health Prod. 18, 81–86. Wada, Y., Mori, K. and Iwanaga, T. 1997. Apoptosis of enterocytes induced by inoculation of a strain of attaching and effacing Escherichia coli and verotoxin. J. Vet. Med. Sci. 59(9), 815–818. Waddell, T.E. and Gyles, C.L. 1995. Sodium deoxycholate facilitates systemic absorption of verotoxin 2e from pig intestine. Infect. Immun. 63(12), 4953–4956. Whipp, S.C., Moon, H.W. and Argenzio, R.A. 1981. Comparison of enterotoxic activities of heat-stable enterotoxins from class 1 and class 2 Escherichia coli of swine origin. Infect. Immun. 31(1), 245–251. Zhang, W., Zhao, M., Ruesch, L., Omot, A. and Francis, D. 2007. Prevalence of virulence genes in Escherichia coli strains recently isolated from young pigs with diarrhea in the us. Vet. Microbiol. 123(1–3), 145–152. | ||
| How to Cite this Article |
| Pubmed Style Baek K, Tangchang W, Choi E, Lee W, Lee K, Lee H, Byun J, Son H. Experimental infection of post-weaned pigs with F18-encoding enterotoxigenic and enterotoxigenic/shigatoxigenic Escherichia coli stain isolated from the diarrheic feces in Korea. Open Vet J. 2023; 13(6): 705-714. doi:10.5455/OVJ.2023.v13.i6.5 Web Style Baek K, Tangchang W, Choi E, Lee W, Lee K, Lee H, Byun J, Son H. Experimental infection of post-weaned pigs with F18-encoding enterotoxigenic and enterotoxigenic/shigatoxigenic Escherichia coli stain isolated from the diarrheic feces in Korea. https://www.openveterinaryjournal.com/?mno=148029 [Access: September 01, 2024]. doi:10.5455/OVJ.2023.v13.i6.5 AMA (American Medical Association) Style Baek K, Tangchang W, Choi E, Lee W, Lee K, Lee H, Byun J, Son H. Experimental infection of post-weaned pigs with F18-encoding enterotoxigenic and enterotoxigenic/shigatoxigenic Escherichia coli stain isolated from the diarrheic feces in Korea. Open Vet J. 2023; 13(6): 705-714. doi:10.5455/OVJ.2023.v13.i6.5 Vancouver/ICMJE Style Baek K, Tangchang W, Choi E, Lee W, Lee K, Lee H, Byun J, Son H. Experimental infection of post-weaned pigs with F18-encoding enterotoxigenic and enterotoxigenic/shigatoxigenic Escherichia coli stain isolated from the diarrheic feces in Korea. Open Vet J. (2023), [cited September 01, 2024]; 13(6): 705-714. doi:10.5455/OVJ.2023.v13.i6.5 Harvard Style Baek, K., Tangchang, . W., Choi, . E., Lee, . W., Lee, . K., Lee, . H., Byun, . J. & Son, . H. (2023) Experimental infection of post-weaned pigs with F18-encoding enterotoxigenic and enterotoxigenic/shigatoxigenic Escherichia coli stain isolated from the diarrheic feces in Korea. Open Vet J, 13 (6), 705-714. doi:10.5455/OVJ.2023.v13.i6.5 Turabian Style Baek, Kang-Hyun, Warisraporn Tangchang, Eun-Jin Choi, Wan-Ky Lee, Kyung-Hyun Lee, Hyun-Kyung Lee, Jae-Won Byun, and Hwa-Young Son. 2023. Experimental infection of post-weaned pigs with F18-encoding enterotoxigenic and enterotoxigenic/shigatoxigenic Escherichia coli stain isolated from the diarrheic feces in Korea. Open Veterinary Journal, 13 (6), 705-714. doi:10.5455/OVJ.2023.v13.i6.5 Chicago Style Baek, Kang-Hyun, Warisraporn Tangchang, Eun-Jin Choi, Wan-Ky Lee, Kyung-Hyun Lee, Hyun-Kyung Lee, Jae-Won Byun, and Hwa-Young Son. "Experimental infection of post-weaned pigs with F18-encoding enterotoxigenic and enterotoxigenic/shigatoxigenic Escherichia coli stain isolated from the diarrheic feces in Korea." Open Veterinary Journal 13 (2023), 705-714. doi:10.5455/OVJ.2023.v13.i6.5 MLA (The Modern Language Association) Style Baek, Kang-Hyun, Warisraporn Tangchang, Eun-Jin Choi, Wan-Ky Lee, Kyung-Hyun Lee, Hyun-Kyung Lee, Jae-Won Byun, and Hwa-Young Son. "Experimental infection of post-weaned pigs with F18-encoding enterotoxigenic and enterotoxigenic/shigatoxigenic Escherichia coli stain isolated from the diarrheic feces in Korea." Open Veterinary Journal 13.6 (2023), 705-714. Print. doi:10.5455/OVJ.2023.v13.i6.5 APA (American Psychological Association) Style Baek, K., Tangchang, . W., Choi, . E., Lee, . W., Lee, . K., Lee, . H., Byun, . J. & Son, . H. (2023) Experimental infection of post-weaned pigs with F18-encoding enterotoxigenic and enterotoxigenic/shigatoxigenic Escherichia coli stain isolated from the diarrheic feces in Korea. Open Veterinary Journal, 13 (6), 705-714. doi:10.5455/OVJ.2023.v13.i6.5 |