| Research Article | ||
Open Vet J. 2023; 13(10): 1359-1365 Open Veterinary Journal, (2023), Vol. 13(10): 1359–1365 Original Research The effect of body condition on alfaxalone induction dosage requirement in dogsBartolome Rico Pérez*, Cristina Parra Martinez and Carolina Palacios JiménezClinical Science and Services, The Royal Veterinary College, Hawkshead Lane, Hatfield, UK *Corresponding Author: Bartolome Rico Pérez. Clinical Science and Services, The Royal Veterinary College, Hawkshead Lane, Hatfield, UK. Email: bricoperez20 [at] rvc.ac.uk Submitted: 09/07/2023 Accepted: 26/09/2023 Published: 31/10/2023 © 2023 Open Veterinary Journal
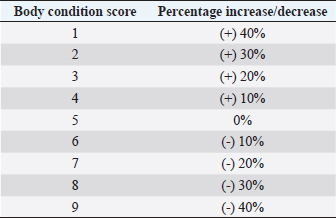
AbstractBackground: Alfaxalone is commonly used in veterinary anesthesia for the induction of general anesthesia (GA) in dogs. However, it has been associated with dose-dependent cardiovascular depression. Therefore, the administration of liposoluble, intravenous (IV)-administered injectable induction agents, such as alfaxalone, is recommended to be based on the dog’s lean body mass (LBM). Aim: To determine the influence of body condition score (BCS) on IV alfaxalone dose requirements to achieve endotracheal intubation in dogs. Methods: Prospective clinical study. A group of 34 dogs undergoing GA for diagnostic and/or surgical procedures, body weight (BW) > 4 kg, BCS > 2, age 1–14 years, American Society of Anesthesiologists (ASAs) classification I–III. Dogs were allocated to two different groups according to their BCS: non-overweight group (NOW) BCS: 3–5 and over-weight group (OW) BCS: 6–9. All dogs were premedicated IV with methadone 0.2 mg kg−1, and anesthesia was induced by a slow IV infusion of alfaxalone at 1 mg kg−1 minute−1, delivered with a syringe driver, until loss of jaw tone and no/minimal gagging reflex sufficient to allow endotracheal intubation was achieved. The total dose of alfaxalone and the occurrence of post-induction apnoea were recorded. The Shapiro–Wilk test was performed to test for normality. A Chi-square test was performed to compare the incidence of post-induction apnoea between groups, and the Mann–Whitney U test was performed to compare the induction dose of alfaxalone between groups. A p-value < 0.05 was considered statistically significant. Results: The mean dose ± standard deviation of alfaxalone in NOW was 2.18 ± 0.59 mg kg−1, and in OW, it was 1.63 ± 0.26 mg kg−1 (p=0.002). The sedation score did not differ between groups. Postinduction apnoea (PIA) occurred in 6 of 17 animals in NOW and 15 of 17 in OW (p=0.002). Conclusion: The dose of IV alfaxalone per kg of total body mass required to achieve endotracheal intubation was lower in overweight dogs, suggesting that LBM should be considered when calculating IV anesthetic doses. The incidence of post-induction apnoea was higher in overweight/obese dogs with alfaxalone administered at a rate of 1 mg kg−1 minute−1. Keywords: Alfaxalone, BCS, Induction, Obesity, Overweight. IntroductionThe term obesity refers to a medical condition characterized by the accumulation of excessive amounts of fat in the form of adipose tissue, with adverse effects on the individual’s health status (Panuganti et al., 2022). The definition of obesity in pets is controversial, but the Global Pet Obesity Initiative and the American Veterinary Medical Association (AVMA) now define it as 30% over the ideal weight. Obesity among domestic animals is an important health concern, much like it is among humans. Nevertheless, owners are often not aware of this threat. Previously published research has indicated that 60% of domestic dogs are overweight or significantly obese as a result of various factors (Ronja and Kölle, 2021). General anesthesia (GA) can be defined as a state of controlled, reversible intoxication of the central nervous system (CNS) characterized by unconsciousness, sensory deprivation to noxious stimuli, and muscle relaxation, while maintaining adequate tissue perfusion and oxygen delivery to the tissues (Brown et al., 2018). However, many injectable and inhalant anesthetic agents used to achieve these goals can have negative side effects, such as a decrease in cardiac output (CO) (Hardie et al., 1995). A limited number of studies attempted to assess the effects of extreme obesity on the pharmacology of anesthetics. Love and Cline (2015) showed how physiological and pharmacological variables can be affected by obesity in small animal patients, with possible changes in coronary, respiratory, and endocrine functions in dogs and cats. Based on these results, it may be necessary to modify the perioperative care in obese animals due to changes in body composition. These changes may include an increase in fat percentage, an increase in CO, an increase in kidney filtration, an increase in the volume of distribution of liposoluble drugs, a decrease in the volume of body water, and a change in the plasmatic protein bond (Gouju and Legeay, 2023). Alfaxalone is a synthetic neuroactive steroid that modulates muscle contractions and induces anesthesia by binding to gamma-aminobutyric acid type A receptors in the CNS (Muir et al., 2008). Alfaxalone is characterized by a rapid and smooth induction followed by rapid recovery and moderate respiratory depression related to the rate of administration, total dose, and premedication agents. The characteristics can be attributed to its short plasma half-life (Ferre et al., 2006; Muir et al., 2008; Martín Bellido and Vettorato, 2022). Alfaxalone is a highly lipid-soluble and water-insoluble molecule with an extensive volume of distribution in cats (Whittem et al., 2008). However, research conducted in dogs revealed a significantly reduced volume of distribution compared with propofol (Dehuisser et al., 2019). Alfaxalone is commonly used in dogs for induction of GA. As a short-acting IV anesthetic agent, it causes rapid loss of consciousness within 20–40 seconds of administration. In dogs and cats, however, alfaxalone produces dose-dependent depression of the cardiovascular system following IV administration (Amengual et al., 2013). Current recommendations based on previously published data (Lotia and Bellamy, 2008; Green and McLeay, 2011; Boveri et al., 2013) state that the administration of liposoluble injectable anesthetic induction agents, such as propofol and alfaxalone, should be adjusted to the animal’s lean body mass (LBM) due to the effect of the fat sink on the delay of drug elimination time. Boveri et al. (2013) demonstrated that obese dogs need a lower propofol dose per kilogram than normal body condition score (BCS) animals to enable intubation, suggesting that IV anesthesia doses should be calculated based on LBM. The aim of this study was to determine the influence of BCS on the dose of IV alfaxalone required to achieve endotracheal intubation in dogs. Our hypothesis was that induction of GA and intubation after alfaxalone administration in overweight dogs should be achieved with lower doses compared to normal-weight animals. Materials and MethodsThe study included 34 client-owned dogs who underwent diagnostic and surgical procedures under GA. To ensure that all dogs were suitable for inclusion in the study, a veterinary surgeon performed a complete history reading and physical examination. The inclusion criteria were: body weight (BW) > 4 kg, BCS [Body Condition Scoring System, 1 (emaciated) to 9 (obese) (LaFlamme 1997)] ≥ 3, age 1–14 years, American Society of Anesthesiologists (ASAs) physical status category (2011) I–II (BCS 4–5) and ASA physical status II-III (BCS ≥6). Dogs were excluded from the study when they did not allow an IV catheter to be placed without sedation due to behavioral reasons. Animals with endocrinopathies, hepatic, renal, or cardiac diseases were also excluded from the study based on clinical examination and pre-anesthetic blood tests. The BCS of the dogs was categorized out of 9. Animals were divided into two groups: Non-Over-weight Group (NOW) if BCS 3–5 and Over-weight Group (OW) if BCS ≥ 6. Each group consisted of 17 dogs. Behavior was scored using a simple descriptive scale (SDS) of 1–3 previously published (Jiménez et al., 2012), where a score of 1 represents a calm dog and a 3 is a consistently nervous one. An IV catheter was placed with an aseptic technique in a cephalic vein in every dog. An IV dose of 0.2 mg kg−1 methadone (Synthadon 10 mg ml−1, Animalcare Limited, UK) was administered to all the dogs before induction of GA. A sedation score was assigned to dogs 10 minutes after premedication. Sedation was scored using the scale published by Grint et al. (2010), where a score of 0 represents no sedation while a score of 21 represents profound sedation in the dog. Anesthesia was induced immediately after the sedation score by administering a slow IV infusion of alfaxalone (Alfaxan multidose 10 mg ml−1, Jurox Pty Limited, UK) at a rate of 1 mg kg−1 minute−1 delivered by a precision syringe driver (Syringe Pump, BeneFusion SP5, Mindray, China). This rate was determined from a literature search concluding that, following methadone premedication and low level of sedation, the velocity of administration was expected to achieve a good quality induction without a high incidence of postinduction apnoea (PIA) (Bigby et al., 2017; Martín Bellido and Vettorato, 2022). This induction rate was continued until endotracheal intubation was achieved, which was characterized by loss of palpebral reflexes and absent or minimal gagging was present, enabling laryngoscopy. Previously published scoring systems (Amengual et al., 2013) were used to categorize the anesthetic induction based on different scores, including induction score (from 0–a smooth transition to 3–marked paddling, struggling, or vocalization) and intubation score (from 0–smooth intubation to 3–swallowing, coughing, and gagging). The total dose of alfaxalone administered and the quality of induction and intubation were recorded. The ideal weight of each dog (in kg) was calculated using the actual weight and the BCS of that dog. The ideal BCS was defined as BCS 5; thus, every BCS value above or below the ideal was taken as a 10% decrease or increase, respectively, in percentage of kilograms of the actual weight to calculate the ideal weight (Table 1) (LaFlamme 2006). Once the ideal weight for each dog was obtained, the corrected dose of the induction agent, in mg kg−1, was calculated by dividing the total amount administered (mg) by the ideal weight of the dog (kg). Table 1. Percentage of change in actual body weight for calculation of ideal body weight based on BCS (LaFlamme 2006).
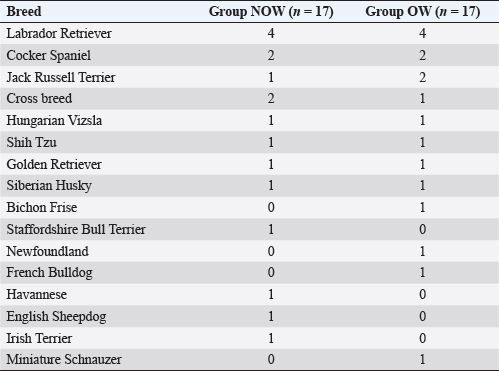
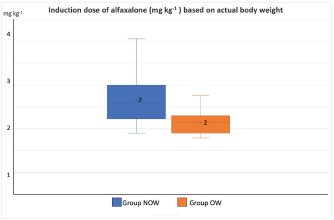
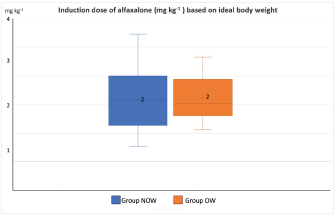
Post-induction apnoea, defined as a period after endotracheal intubation in which a patient fails to breathe spontaneously for at least 15 seconds, was also recorded (Murison 2001). As part of the procedure, anesthesia was maintained for as long as it was required. Statistical analysisStatistical analysis was performed in SPSS Statistics Editor Version 28 (IBM SPSS Statistics, International Business Machines Corporation IBM, NY, USA). A preliminary analysis of the sample size in dogs based on previous research about alfaxalone induction requirements but given “by hand,” suggested that for 80% power to detect a significant difference in the amount of alfaxalone that was required for induction (estimated at 0.5 mg kg−1), a sample size of 17 individuals per group was required, with an alpha value of 0.05. The Shapiro–Wilk normality test was used to determine the normality of the distributions of parametric data. Unpaired t-tests were used to compare demographic continuous data (ages, weights) between groups, while chi-square tests were used to compare categorical variables (sex breed, ASA status) between groups. A Mann–Whitney U test was used to compare alfaxalone induction requirements between groups. Anesthesia-related post-induction apnoea and all the reported scoring systems (induction, intubation, sedation, and behavior score) were compared among the groups using the Chi-square test. p-values <0.05 were considered statistically significant. Ethical approvalThis study protocol was approved by the Ethics Committee of the Royal Veterinary College (URN: M2020 0157). ResultsThe study recruited 34 dogs, 17 dogs per group. The NOW group included eight females (6 of which were neutered) and nine males (6 of which were neutered). The OW group included five females (4 of which were neutered) and 12 males (9 of which were neutered). Results are presented as mean (± standard deviation, SD) unless otherwise stated. No significant differences were found between the groups regarding age (NOW 7.08 ± 3.61 years; OW 7.9 ± 2.04 years), breed (Table 2), or sex distribution (p=0.84). In addition, no differences were found regarding ASA status between groups (p =0.45). The actual BW (NOW 20.9 ± 9.97 kg; OW 30.71 ± 15.59 kg) was different between groups (p=0.02), but this difference became non-significant (p=0.32) when corrected to the ideal BW (NOW 22.01 ± 10.01 kg; OW 23.97 ± 13.94 kg). In group NOW, 2 were categorized as BCS 3, 5 as BCS 4, and 10 as BCS 5. In group OW, five dogs were classed as BSC 6, 5 as BCS 7, 6 as BCS 8, and 1 as BCS 9. The sedation score and intubation score were 1 and 0 for all the dogs included in the study, respectively. The median (range) behavior score was 2 (1–3) for both groups, with no differences found between the groups (p=0.81). The induction median (range) score was 1 (0–2) for both groups, and the differences in induction scores were not significant between groups (p=0.77). The mean alfaxalone dose required for anesthetic induction based on the total BW was statistically significantly different between groups (p=0.002). Dogs in group NOW required 2.15 ± 0.59 mg kg−1, compared with dogs in group OW, which required 1.63 ± 0.26 mg kg−1 (Fig. 1). The mean values for the dose based on ideal BW (mg kg−1) were 2.08 ± 0.53 for the NOW and 2.12 ± 0.38 for the overweight group, and no statistical differences were seen between the groups (p=0.30) (Fig. 2). Post-induction apnoea of more than 15 seconds occurred in 5 of 17 dogs in group NOW and in 15 of 17 dogs in group OW (p=0.002). DiscussionAccording to the findings of this veterinary study, overweight dogs required lower doses per unit of BW of alfaxalone to achieve endotracheal intubation compared to normal-weight dogs. As in the veterinary literature about other liposoluble anesthetics (Boveri et al., 2013), the induction dose of alfaxalone was statistically significantly lower in overweight dogs. In obese human patients, it is recommended to induce GA using LBM-based doses because of the increased risk of side effects associated with relative overdose (Ingrande et al., 2010). Considering the direct correlation between LBM and CO, LBM would be an appropriate scalar of IV drug dosing (Collis et al., 2001; Ingrande et al., 2011). While it is true that LBM can be calculated as the difference between the total BW and the body fat mass, it is not always possible to determine an accurate measurement of body fat mass unless expensive techniques, including deuterium oxide dilution and DEXA scanning, are used (German, 2006). Table 2. Distribution of dog breed among study groups, expressed in a total number of dogs. NOW: non-overweight group. OW: over-weight group.
Fig. 1. Induction dose of alfaxalone in mg kg−1 administered at a dose rate of 1 mg kg−1 hour−1 to dogs based on actual body weight. NOW: non-over-weight group (body condition score [BCS] 3–5). OW: over-weight group (BCS 6–9).
Fig. 2. Induction dose of alfaxalone in mg kg−1 administered at a dose rate of 1 mg kg−1 hour−1 to dogs based on ideal body weight. NOW: non-over-weight group (body condition score [BCS] 3–5). OW: over-weight group (BCS 6–9). Body condition scoring, which utilizes visual assessment and palpation, is widely accepted as the most reliable method of quantifying and evaluating body composition and fat mass in clinical settings (Burkholder and Toll, 2000). Typically, palpation is used to evaluate the amount of fat surrounding the ribs, the presence of muscle mass in limbs, the appearance of spine prominences, and loose skin in the neck region. The scoring system that uses nine integers has become the most widely accepted (Laflamme, 1997). However, based on DEXA results (LaFlamme, 1997), no empirical data have been provided regarding whether BCS can be validated against gold standard methods of determining fat mass. BCS ratings of 1–2 out of 9 are considered to be in very poor condition, as the animals are extremely thin, and all their bone features are clearly visible, indicating poor health. A BCS of 8–9 out of 9 is an indicator that an animal is on the other extreme end of the scale of obesity, whereas a BCS of 6–7 out of 9 is associated with a moderately overweight animal. We used the nine-point BCS scale as a subjective measure of body fat in the present study, i.e., BCS scores were used as conversion factors for estimating body fat percentages from BCS scores. We found that there were differences in the alfaxalone induction requirements between overweight dogs (BCS 6–7, n=11) and obese dogs (BCS 8–9, n=6). However, there were not enough obese dogs for meaningful tests to be conducted, and therefore, further research is needed. Based on our data and the comparison of animals of similar BW (approximately 24 kg) but different BCS, 36 mg of alfaxalone was required to induce GA in a dog with a BSC of 7/9, while 63 mg was required in a dog with a BSC of 4/9. There would have been almost no difference between the induction requirements based on ideal BW (36/19.2=1.9 mg kg−1 and 63/26.5=2.3 mg kg−1). Moreover, no significant differences were observed in the induction dose requirements of alfaxalone calculated based on ideal BW between groups. Behavior score was recorded as different behaviors or levels of anxiety could have affected induction dose requirements. No differences in behavior scores between groups were noted before premedication; therefore, this is unlikely to have influenced overall induction dose requirements. However, as the behavior score was not repeated after premedication, this cannot be completely excluded as a confounding factor. As an additional potential confounding factor, the age of the patient might have affected our results since it has been reported that anesthetic induction requirements decrease as the patient ages (Robinson et al., 1985). It is important to note, however, that in this study, there were no statistically significant differences between the ages of both groups, so it is unlikely that the age of the dogs influenced the outcome of the study. Both groups had similar distributions of breeds between them. Labrador Retrievers, Cocker Spaniels, Cavalier King Charles Spaniels, and Scottish Terriers have been found to have a significantly higher predisposition to obesity than other breeds (Edney and Smith, 1986). Furthermore, neutering can also have a significant impact on the BCS (Edney and Smith, 1986; McGreevy et al., 2005; Lund et al., 2006). As the breeds and genders were equally distributed among the groups in our study, these factors did not appear to influence the results. It should be recognized, however, that this study was not intended to determine the impact of individual breeds or sexes on BCS and, therefore, the required dose of alfaxalone. It was statistically significant that overweight dogs were more likely to develop post-induction apnoea in our study, despite the same alfaxalone dose requirements between groups when scaled according to ideal BW. Obesity reduces the total lung volume and functional residual capacities and may increase the risk of hypoventilation during anesthesia. Another possible factor influencing the incidence of apnoea is the differences in body fat content and pharmacokinetic differences between non-overweight and obese patients. These differences cause a relatively higher alfaxalone concentration to reach the brain in individuals with an excessive body fat percentage and could have influenced the incidence of apnoea. According to Amengual et al. (2013), alfaxalone has been reported to induce transient respiratory depression when given by IV injection following rapid administration. Some redistribution into the fat mass may have been possible after the anesthesia induction process due to the relatively slow infusion of alfaxalone rather than a rapid bolus. It is important to consider that the fat mass available for such redistribution is relatively greater in overweight and obese dogs compared to normal-weight dogs. Theoretically, an increase in fat percentage should equate to an increase in CO, kidney filtration, and an increase in the volume of distribution of liposoluble drugs. However, the results of this study are in contradiction with this concept, which may raise some controversy about the correlation between alfaxalone plasma concentration and its effects at the site of action. However, the study design was limited to the clinical endpoint, and no pharmacokinetic data was collected. Further pharmacokinetic studies are needed to evaluate the correlation between alfaxalone plasmatic concentrations and these effects in overweight dogs. There are several limitations in our study. Our study was conducted in relatively healthy patients categorized as ASA physical status I–III as it was appropriate first to quantify adverse effects in a relatively healthy population of dogs. Furthermore, the inclusion of underweight and ideal BW dogs in the same group could have affected the interpretation of the results. One limitation is the lack of a repeated behavior score after methadone was administered IV to every dog. However, because of the low sedation scores recorded during the study, the authors believe that a repeated behavior score would have been similar and unlikely to affect the results of the study. Another limitation is the subjectivity of the nine-point BCS scale. However, to standardize this bias, all the dogs were assessed and assigned a BCS by the same assessor. To improve the accuracy of this subjectivity, further studies with the median of multiple assessments by different assessors could be considered. A possible limitation is that the dosage of methadone was calculated based on the total BW, which could have influenced the results. However, there were no significant differences between groups in the degree of sedation after the administration of methadone; hence, it seems unlikely any bias was added by the dosage of methadone. The endpoint of the anesthetic administration, defined as loss of palpebral reflexes and no/minimal gagging sufficient to allow endotracheal intubation, was assessed by the same operator in all the dogs and who was not blinded to the group allocation, which could have introduced bias in the results. Thus, this is a limitation of our study. Nevertheless, the same assessor intubated all patients, and alfaxalone was delivered by syringe driver to minimize variation in this subjective endpoint. All dogs were anesthetized with a slow IV infusion of alfaxalone at a rate of 1 mg kg−1 minute−1 delivered with a precision syringe driver. In healthy dogs, alfaxalone has been studied in both clinical and supraclinical dosages (Muir et al., 2008), but very little information has been gathered regarding the effect of injection speed. The induction doses of alfaxalone required to achieve intubation increases when these drugs are administered at faster rates (Bigby et al., 2017), so the rate chosen in the present study may have affected the total amount of alfaxalone administered. Based on the findings of Bigby et al., 2017, the rate at which alfaxalone is administered has a significant impact on the amount of alfaxalone needed to induce GA in healthy dogs. In our study, the rate of administration was lower than the faster rate described by Bigby et al. (2 mg kg−1 minute−1). However, the average induction dosage of alfaxalone required based on ideal BW is similar. Infusion rates used to achieve the same dosage of alfaxalone varied between studies, possibly because of differences in premedication protocols. In the study previously mentioned, dexmedetomidine and methadone were administered IM, whereas in this study, only methadone was administered. Thus, it is likely that there was a difference in sedation and CO in dogs receiving dexmedetomidine that caused a difference in the alfaxalone dose required. Nevertheless, all the dogs included in this study received the same infusion rate, so it is unlikely to have influenced the differences between the groups. No cardiovascular parameters were evaluated in the study, so further studies are indicated to elaborate if the differences found between groups are accompanied by any differences in the cardiovascular system. ConclusionIn conclusion, the dose of IV alfaxalone per kg of total body mass required to achieve endotracheal intubation was lower in overweight dogs, suggesting that LBM should be considered when calculating IV anesthetic induction doses. The incidence of post-induction apnoea was higher in overweight/obese dogs when alfaxalone was administered at a rate of 1 mg kg−1 minute−1. AcknowledgmentsThis work and the research behind it would not have been possible without the exceptional support of the Anaesthesia and Analgesia Team (Royal Veterinary College, University of London). Conflict of interestThe authors declare that there is no conflict of interest. Authors contributionsBRP: study idea and design, execution of the study, data collection, data management, interpretation and statistical analysis, preparation of the manuscript. CPM: preparation and revision of the manuscript. CP: study design, supervision of the study, revision of the manuscript. All authors have read and approved the manuscript. FundingThere was no funding source for this study, and it was all a contribution from the authors. Data availabilityAll data supporting the findings of this study are available within the manuscript, and no additional data sources are required. ReferencesAmengual, M., Flaherty, D., Auckburally, A., Bell, A.M, Scott, E.M., Pawson, P. (2013) An evaluation of anaesthetic induction in healthy dog using rapid intravenous injection of propofol or alfaxalone. Vet. Anaesth. Analg. 40, 115–123. American Society of Anesthesiologists Physical Status Classification System. 2011. http://www.asahq.org/For-Members/Clinical-Information/ASA-Physical-Status-Classification-System.aspx. [Accessed 05/05/2023]. Bigby, S.E., Beths, T., Bauquier, S., Carter, J.E. 2017. Effect of rate of administration of propofol or alfaxalone on induction dose requirements and occurrence of apnea in dogs. Vet. Anaesth. Analg. 44(6), 1267–1275. Boveri, S., Brearley, J.C. and Dugdale, A.H. 2013. The effect of body condition on propofol requirement in dogs. Vet. Anaesth. Analg. 40(5), 449–454. Brown, E.N., Pavone, K.J. and Naranjo, M. 2018. Multimodal general anesthesia: theory and practice. Anesth. Analg. 127(5), 1246–1258. Burkholder, W.J. and Toll, P.W. 2000. Obesity. In: Small animal clinical nutrition. 4th ed. Hand, M.S., Thatcher, C.D., Remillard, R.L. Topeka, Kan: Mark Morris Institute, pp: 401–430. Collis, T., Devereux, R.B., Roman, M.J., de Simone, G, Yeh, J., Howard, B.V., Fabsitz, R.R., Welty, T.K. 2001. Relations of stroke volume and cardiac output to body composition: the strong heart study. Circulation 103, 820–825. Dehuisser, V., Bosmans, T., Devreese, M., Gehring, R., Croubels, S., Duchateau, L., Polis, I. 2019. Alfaxalone total intravenous anaesthesia in dogs: pharmacokinetics, cardiovascular data and recovery characteristics. Vet. Anaesth. Analg. 46(5), 605–612. Edney, A.T.B. and Smith, P.M. 1986. Study of obesity in dogs visiting veterinary practices in the United Kingdom. Vet. Rec. 118, 391–396. Ferré, P.J., Pasloske, K., Whittem, T., Ranasinghe, M.G., Li, Q., Lefebvre, H.P. 2006. Plasma pharmacokinetics of alfaxalone in dogs after an intravenous bolus of Alfaxan-CD RTU. Vet. Anaesth. Analg. 33(4), 229–236. German, A.J. 2006. The growing problem of obesity in dogs and cats. J. Nutr. 136, 1940–1946. Gouju, J. and Legeay, S. 2023. Pharmacokinetics of obese adults: not only and increase in weight. Biomed. Pharmacother. 11(166), 115281. Green, B. and McLeay, S.C. 2011. Anesthetizing the obese. Anesth. Analg. 113, 1–3. Grint, N.J., Alderson, B. and Dugdale, A.H. 2010. A comparison of acepromazine-buprenorphine and medetomidine-buprenorphine for preanesthetic medication of dogs. J. Am. Vet. Med. Assoc. 237(12), 1431–1437. Ingrande, J., Brodsky, J.B. and Lemmens, H.J. 2011. Lean body weight scalar for the anesthetic induction dose of propofol in morbidly obese subjects. Anesth. Analg. 113(1), 57–62. Ingrande, J. and Lemmens, H.J.M. 2010. Dose adjustment of anaesthetics in the morbidly obese. Br. J. Anaesth. 105(1), 16–23. Hardie, E.M., Jayawickrama, J., Duff, L.C. and Becker, K.M. 1995. Prognostic indicators of survival in high-risk canine surgery patients. J. Vet. Emerg. Crit. Care 5, 42–49. Jiménez, C.P., Mathis, A., Mora, S.S., Brodbelt, D. and Alibhai, H. 2012. Evaluation of the quality of the recovery after administration of propofol or alfaxalone for induction of anaesthesia in dogs anaesthetized for magnetic resonance imaging. Vet. Anaesth. Analg. 39(2), 151–159. LaFlamme, D.P. 1997. Development and validation of a body condition score system for dogs. Canine Pract. 22, 10–15. LaFlamme, D.P. 2006. Understanding and managing obesity in dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 36(6), 1283–1295. Lotia, S. and Bellamy, M.C. 2008. Anaesthesia and morbid obesity. Contin. Educ. Anaesth. Crit. Care Pain 8, 151–156. Love, L. and Cline, M.G. 2015. Perioperative physiology, and pharmacology in the obese small animal patient. Vet. Anaesth. Analg. 42(2), 119–132. Lund, E.M., Armstrong, P.J., Kirk, C.A.,Klausner J.S. 2006. Prevalence and risk factors for obesity in adult dogs from private US veterinary practices. Intern. J. Appl. Res. Vet. Med. 4, 177–186. Martín Bellido, V. and Vettorato, E. 2022. Clinical review of the pharmacological and anaesthetic effects of alfaxalone in dogs. J. Small Anim. Prac. 63, 341–361. McGreevy, P.D., Thomson, P.C., Pride, C., Fawcett, A., Grassi, T., Jones, B. 2005. Prevalence of obesity in dogs examined by Australian veterinary practices and the risk factors involved. Vet. Rec. 156(22), 695–702. Muir, W., Lerche, P., Wiese, A., Nelson, L., Pasloske, K., Whittem, T. 2008. Cardiorespiratory and anesthetic effects of clinical and supraclinical doses of alfaxalone in dogs. Vet. Anaesth. Analg. 35(6), 451–462. Murison, P.J. 2001. Effect of propofol at two injection rates or thiopentone on post-intubation apnoea in the dog. J. Small Anim. Pract. 42(2), 71–74. Panuganti, K.K., Nguyen, M., Kshirsagar, R.K., Doerr C. 2022. Obesity (Nursing) [Updated 2022 Aug 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls. Robinson, F.P., Dundee, J.W. and Halliday, N.J. 1985. Age affects the induction dose of propofol (‘Diprivan’). Postgrad. Med. J. 61(3), 157–159. Ronja, N. and Kölle, P. 2021. Adipositas beim Hund—ein Überblick zu den Ursach [Obesity in dogs—a review of underlying reasons]. Tierarztl. Prax. Ausg. K. Kleintiere. Heimtiere. 49(4), 284–293. Whittem, T., Pasloske, K.S., Heit, M.C. and Ranasinghe, M.G. 2008. The pharmacokinetics and pharmacodynamics of alfaxalone in cats after single and multiple intravenous administration of Alfaxan at clinical and supraclinical doses. J. Vet. Pharmacol. Ther. 31(6), 571–579. | ||
| How to Cite this Article |
| Pubmed Style Perez BLR, Martinez CP, Jimenez CP. The effect of body condition on alfaxalone induction dosage requirement in dogs. Open Vet J. 2023; 13(10): 1359-1365. doi:10.5455/OVJ.2023.v13.i10.16 Web Style Perez BLR, Martinez CP, Jimenez CP. The effect of body condition on alfaxalone induction dosage requirement in dogs. https://www.openveterinaryjournal.com/?mno=152125 [Access: July 27, 2024]. doi:10.5455/OVJ.2023.v13.i10.16 AMA (American Medical Association) Style Perez BLR, Martinez CP, Jimenez CP. The effect of body condition on alfaxalone induction dosage requirement in dogs. Open Vet J. 2023; 13(10): 1359-1365. doi:10.5455/OVJ.2023.v13.i10.16 Vancouver/ICMJE Style Perez BLR, Martinez CP, Jimenez CP. The effect of body condition on alfaxalone induction dosage requirement in dogs. Open Vet J. (2023), [cited July 27, 2024]; 13(10): 1359-1365. doi:10.5455/OVJ.2023.v13.i10.16 Harvard Style Perez, B. L. R., Martinez, . C. P. & Jimenez, . C. P. (2023) The effect of body condition on alfaxalone induction dosage requirement in dogs. Open Vet J, 13 (10), 1359-1365. doi:10.5455/OVJ.2023.v13.i10.16 Turabian Style Perez, Bartolome Luis Rico, Cristina Parra Martinez, and Carolina Palacios Jimenez. 2023. The effect of body condition on alfaxalone induction dosage requirement in dogs. Open Veterinary Journal, 13 (10), 1359-1365. doi:10.5455/OVJ.2023.v13.i10.16 Chicago Style Perez, Bartolome Luis Rico, Cristina Parra Martinez, and Carolina Palacios Jimenez. "The effect of body condition on alfaxalone induction dosage requirement in dogs." Open Veterinary Journal 13 (2023), 1359-1365. doi:10.5455/OVJ.2023.v13.i10.16 MLA (The Modern Language Association) Style Perez, Bartolome Luis Rico, Cristina Parra Martinez, and Carolina Palacios Jimenez. "The effect of body condition on alfaxalone induction dosage requirement in dogs." Open Veterinary Journal 13.10 (2023), 1359-1365. Print. doi:10.5455/OVJ.2023.v13.i10.16 APA (American Psychological Association) Style Perez, B. L. R., Martinez, . C. P. & Jimenez, . C. P. (2023) The effect of body condition on alfaxalone induction dosage requirement in dogs. Open Veterinary Journal, 13 (10), 1359-1365. doi:10.5455/OVJ.2023.v13.i10.16 |