| Research Article | ||
Open Vet J. 2023; 13(8): 977-982 Open Veterinary Journal, (2023), Vol. 13(8): 977-982 Original Research Intraoperative management of axial posterior capsular opacities during cataract surgery in six dogsFrédéric Goulle*Centre Hospitalier Vétérinaire AniCura Aquivet, Eysines, France *Corresponding Author: Frédéric Goulle. Centre Hospitalier Vétérinaire AniCura Aquivet, Eysines, France. Email: frederic.goulle [at] anicura.fr Submitted: 05/06/2023 Accepted: 22/07/2023 Published: 31/08/2023 © 2023 Open Veterinary Journal
AbstractBackground: Posterior capsular opacification (PCO) is a cause of decreased vision after canine cataract surgery. PCO can appear in the months following surgery but can also be present at the time of surgery. Aims: To describe the intraoperative management of marked axial PCO during canine cataract surgery through a retrospective case series study of six cases. Methods: Six dogs (six eyes) with cataracts were included in this study. A complete ophthalmologic examination including electroretinography and ocular ultrasound was performed. After conventional phacoemulsification, a marked PCO obstructing the visual axis was found in five cases, previously diagnosed by ultrasound in the sixth. An axial posterior capsulorhexis (APC) was performed in all cases, before or after implantation of the intraocular lens (IOL). For the four cases implanted before APC, the posterior capsule (PC) was visualized either by positioning the IOL laterally in the bag with viscoelastic or through the optic of the implant. After perforation of the PC with a 30-Gauge needle, APC was initiated with Vannas curved scissors, then finalized with Utrata forceps to obtain a circular axial opening (3 mm diameter). In each case, a moderate anterior vitrectomy was performed through the APC (under the IOL when initially placed), then the IOL centered and the viscoelastic was removed. Results: Six dogs (Beagle, German Shepherd, Cavalier King Carles, French pointing dog, American bully, Beagle Harrier) aged 11 to 94 months (mean 51.8) were included. The mean follow-up period was 15.5 months (range 10–22). Visual function with capsular axial transparency and well-centered IOL, without complications during the follow-up period, was preserved for each eye. Conclusion: APC combined with moderate anterior vitrectomy appears to be effective in the treatment of marked axial PCO obstructing the visual axis during canine cataract surgery. Keywords: Posterior capsular opacification, Cataract, Dog. IntroductionCataract, defined as an opacity of the lens or lens capsule, is the most common cause of visual impairment in dogs and humans (Davidson and Nelms, 1999; Chandler et al., 2007). Phacoemulsification cataract extraction with intraocular lens (IOL) implantation is the standard surgical procedure in veterinary and human medicine. The most common long-term vision-threatening complication following cataract surgery in both species is posterior capsular opacification (PCO) (Khalifa, 1992; Sigle and Nasisse, 2005) (Fig. 1). PCO, which is therefore a cause of reduced visual acuity after canine cataract surgery, can appear in the months following surgery [100% of dogs that undergo phacoemulsification cataract surgery develop PCO within 1 year postoperatively (Davidson and Nelms, 1999; Bras et al., 2006)] but can also be present at the time of surgery. In the latter case, PCO is discovered either intraoperatively after cataract removal or at preoperative ultrasonography assessment. The purpose of this short case series study is to describe in detail a surgical technique available for treating axial PCO intraoperatively during cataract surgery in dogs, the objective being to restore axial capsular transparency. Materials and MethodsThis retrospective study includes six dogs (six eyes) that underwent cataract surgery by conventional phacoemulsification with prosthetic IOL implantation. A thorough ophthalmologic examination was performed in each case, including tonometry (Tonovet®, Icare, Vantaa, Finland), slit-lamp examination (Hawk Eye®, Dioptrix, Toulouse, France), electroretinography (Visiosystem®, Siem Biomedicale, Nîmes, France), and ocular ultrasonography (Vu Max HD®, Sonomed Escalon, Lake Success, NY, USA). Obstacle-run test, cotton-ball test, menace response, palpebral reflex, dazzle reflex, and pupillary light reflexes were evaluated. Preoperative mydriasis was obtained with one drop of phenylephrine 10% (Neosynephrine Faure 10%; Europhta, Monaco) followed by tropicamide eye drops (Mydriaticum 2 mg/ 0.4 ml, Thea Pharma, Clermont Ferrand, France) (1 drop every 5 minutes for 20 minutes).
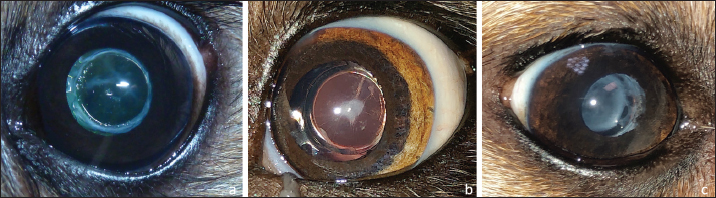
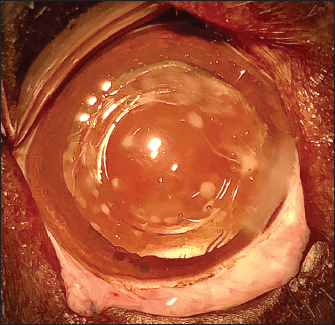
Fig. 1. Three different stages of long-term postoperative PCO: a-discrete (PCO blocked by square edges of IOL optic), b-moderate, and c-marked.
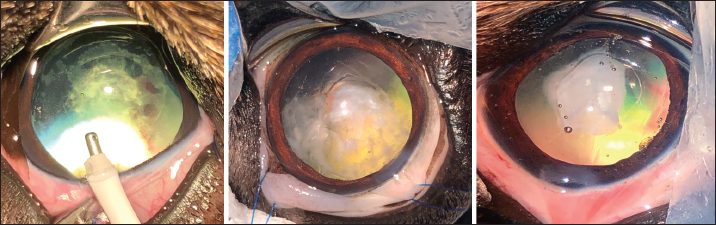
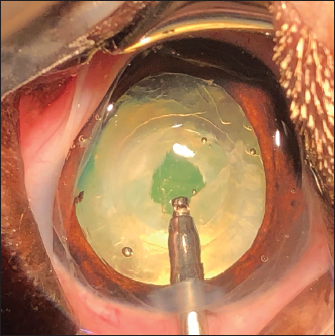
Fig. 2. Three cases of marked PCO (increasing intensity from left to right) obstructing the visual axis (intraoperative view). After 3.2-mm clear corneal incision using an angled microsurgical knife (Unique Technologies, Inc, Mohnton, USA) and inflation of the anterior chamber with 1.6% sodium hyaluronate viscoelastic substance (Ophteis Bio 1.6, Rayner, Worthing, UK), anterior capsulorhexis was performed. Then conventional phacoemulsification (Infiniti Vision System, Alcon Laboratories, Inc. Fort Worth, USA) using a one-handed technique was completed, followed by meticulous irrigation-aspiration of the residual cortical material and polishing of the posterior capsule (PC). At this stage, a marked PCO obstructing the visual axis was found in five cases (Fig. 2), previously diagnosed by ultrasound in the sixth. In order to restore the transparency of the visual axis, an axial posterior capsulorhexis (APC) was performed in all cases, before or after implantation of an IOL (Intraocular implant PFI, Medicontur, Zsambék, Hungary). The IOL implantation inside the capsular bag was performed in a conventional manner using an injection cartridge through the limbal keratotomy. For the four cases implanted before APC, the approach of the PC was to inject viscoelastic gel into the capsular bag to mobilize the IOL (inside the bag) to access the PC, using two variations. The first method was to push and position the IOL laterally and obliquely toward the equator of the bag by gently injecting the viscoelastic gel, to reach the central area of the PC for the surgical approach (Fig. 3). A second method was to leave the IOL centered in the bag and inject the viscoelastic gel between the IOL and the PC, allowing visualization of the PC through IOL optic for its surgical approach under it. In all cases, the amount of gel injected was visually controlled by the degree of concave swelling of the PC to avoid overinflating it. The APC was initiated by very gently making small perforations of the PC with a 30-gauge needle. Then, long curved Vannas scissors were inserted through this narrow posterior capsulotomy to cut PC to the right, removed from the eye, and then reintroduced for a cut to the left (Fig. 4). APC was then performed continuously with Utrata forceps, from one flap to the other to obtain a circular axial opening of about 3 mm diameter (Fig. 4). A slight anterior vitrectomy (Anterior Vitrectomy Probe w/21 GA Infusion Cannula, Alcon Laboratories, Inc. Fort Worth, USA) was then performed through the PC opening (under the IOL when initially placed) to prevent a possible vitreous presentation (Fig. 5). Using the irrigation/aspiration handpiece, IOL was the centered in the bag and viscoelastic removed. As mentioned above and depending on the case, the instruments (needle, Vannas scissors, Utrata forceps, vitreotome) were manipulated either with direct access to the PC thanks to the lateral positioning of IOL inside the capsular bag or in the space between the IOL optic and PC. The corneal wound was sutured by separate Polyglactin 910 absorbable stitches (Vicryl 9-0, Ethicon, Puerto Rico, USA). Administration of intracameral 25 mcg of tissue plasminogen activator (Actilyse, Boehringer, France) and 0.5 ml of 0.01% carbachol (Carbachol Intraocular Solution USP, Freedom Ophthalmic Pvt Inc, NY, USA) were achieved immediately after corneal incision closure in all cases. Methylprednisolone acetate (Depomedrol, Zoetis, Malakoff, France) was injected subconjunctivally at a dose of 5 mg in all cases, with no dog being diabetic.
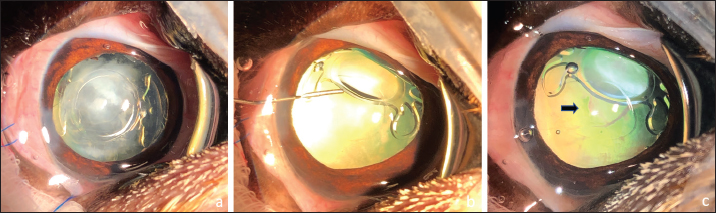
Fig. 3. Posterior capsulotomy after IOL lateral displacement toward the equator of the capsular bag: IOL in the bag, note the marked PCO (a). Injection of viscoelastic gel to move laterally the IOL inside the bag (b). Posterior capsulotomy (black arrow) (c).
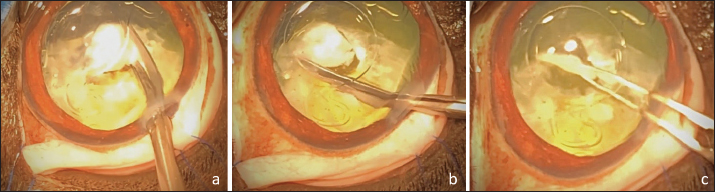
Fig. 4. APC performed in the space between the IOL optic and the PC. Cutting of the PC to the right (a), then to the left (b). Removal of the central capsular opacity with the Utrata forceps (c). Dogs were discharged from the hospital the day after surgery with the following prescription: meloxicam (0.1 mg/kg, PO, q 24 hours for 5 days), amoxicillin–clavulanic acid (12.5 mg/kg, PO, q 12 hours for 10 days), dexamethasone–neomycin sulfate–polymixin B sulfate ophthalmic solution (Maxidrol, Novartis Pharma S.A.S., Rueil-Malmaison, France), 1 drop, q 12 hours for a minimum of 6 months and dorzolamide chlorhydrate-timolol maleate drops (Cosopt, Santen OY, Tampere, Finland), 1 drop, q 8 hours for 1 month. Follow-up visits were performed at 1, 2, 4, 8, 10–12 weeks, then 4, 6, 9, 12 months postoperatively. Some cases had a longer follow-up (see below). At every visit, a complete ophthalmologic examination was performed including cotton-ball test, menace response, dazzle reflex and pupillary light reflexes evaluation, tonometry, and slit lamp examination. IOL centration and visual axis transparency through PC opening were assessed by both slit lamp and direct ophthalmoscopy examination. The final visual outcome of each operated eye was assessed by evaluating menace response and unilateral cotton ball test (performed while masking the fellow eye).
Fig. 5. Anterior vitrectomy under the IOL centered in the capsular bag. Ethical approvalNo ethical approval was needed for this study. ResultsSix dogs were included in the study, from July 2021 to May 2023, represented by six breeds: Beagle, German Shepherd, Cavalier King Charles Spaniel, American Bully, Beagle Harrier, and French pointing dog. The average age was 51.8 months (ranging from 11 to 94). Case 1 was a 6-year-old male Beagle presented for inherited partial bilateral cataract, important in the left eye (oculus sinister (OS)), moderate in the right eye (oculus dexter (OD)), which had been evolving for 6 months. Case 2 was a 7.5-year-old spayed female Cavalier King Charles Spaniel presented for inherited bilateral cataract, total OS for 2 years, and partial OD, this latter evolving for 3 months. Case 3 was an 11-month-old male German Shepherd presented for an important inherited partial bilateral cataract rapidly evolving for 2 months. Case 4 was an 11-month-old female French pointing dog presented for important inherited partial bilateral cataract rapidly evolving for 4 months. Case 5 was a 4-year-old male American Bully presented for inherited bilateral cataract, total OD, and tiny posterior subcapsular axial OS, that had been evolving for 1 year. Case 6 was a 6-year-old male Beagle Harrier presented for inherited bilateral cataract, moderate OD, and tiny posterior subcapsular axial OS, that had been evolving for 2 months. Electroretinography revealed for all cases a normal retinal function. Ocular ultrasound revealed no specific anatomic abnormalities (cases 1, 2, 3, 6), bilateral microphakia with posterior lenticone and PC thickening (case 4), and a slightly flattened right lens (case 5). Both eyes were operated in the same anesthetic time for case 3. Only unilateral cataract surgery was performed for the five other cases, OD (cases 4, 5, 6), OS (cases 1, 2), either by our decision (cases 5, 6), or at the request of the owner (cases 1, 2, 4). Phacoemulsification was performed for all eyes in a conventional manner (one-handed technique). After cataract removal, a marked PCO obstructing the visual axis was observed intraoperatively at the end of the procedure for each case, which was suspected only for case 4, given the ultrasound findings. Because of the intraoperative discovery of axial PCO in all cases except case 4, APC was not planned for these five cases and was decided immediately after phacoemulsification, while APC was planned for case 4. APC was performed after IOL implantation (cases 1, 2, 3, 4) and before implantation (cases 5, 6). To carry out APC after implantation, the method of pushing and positioning IOL laterally and obliquely toward the equator of the bag was used in cases 1 and 2, while the second surgical approach by visualization of the PC through IOL optic was used in cases 3 and 4. The mean follow-up period was 15.5 months (range 10–22). From the first postoperative day, a functional vision with axial capsular transparency through APC was preserved for each operated eye. Furthermore, IOL was well-centered in all cases and no complications were observed during the follow-up period. DiscussionThe most common long-term vision-threatening complication following cataract surgery in dogs is PCO (Khalifa, 1992; Sigle and Nasisse, 2005). The PC itself does not opacify (Khalifa, 1992; Glover and Constantinescu, 1997). There is a combination of three different coexisting mechanisms in which capsular opacification occurs: (1) fibrous metaplasia of lens epithelial cells which are secretory, producing basement membrane and collagen in aberrant attempts to form new lens fibers (Hiles and Johnson, 1980; Glover and Constantinescu, 1997); (2) myofibroblastic differentiation of these cells with the ability to migrate and contract, forming capsular folds resulting in optical distortion (McDonnell et al., 1984; Glover and Constantinescu, 1997); and (3) Elschnig pearl formation (McDonnell et al., 1983; Glover and Constantinescu, 1997). Postoperative PCO occurs in the months following canine cataract surgery and thus represents a cause of decreased visual acuity and potential blindness (Cobo et al., 1984; Dziezyc, 1990; Davidson and Nelms, 1999; Bras et al., 2006). PCO can also be present at the time of surgery and be discovered either intraoperatively after cataract removal (Cobo et al., 1984; Dziezyc, 1990) or at preoperative ultrasonography assessment. In the latter case, the abnormality encountered may be marked axial PC hyperechogenicity, in which case preoperative planned APC may reasonably be considered and performed if confirmed intraoperatively. In our experience, however, there does not seem to be a clear correlation between PC hyperechogenicity and the eventual presence of PCO. On the other hand, in the specific case of posterior lenticonus, especially if associated with a persistent hyperplastic tunica vasculosa lentis/persistent hyperplastic primary vitreous, the surgeon may have to perform a planned APC (Gemensky-Metzler and Wilkie, 2004).
Fig. 6. Simple scattered spots on the PC that do not need to be removed. The intensity and location of PCO vary from case to case. Simple scattered spots of variable location are frequently observed and often do not interfere with visual acuity and, therefore, do not need to be removed (Fig. 6). If these spots are located in the central visual axis, the surgeon will decide whether or not to perform an APC depending on their number and intensity and therefore on whether he considers that the animal's vision will be sufficiently impaired. However, when large areas of PCO that significantly obstruct the visual axis are observed, it seems important and necessary to perform an APC to restore transparency to the visual axis and allow vision to be restored. Another important point to consider is the age of the animal. In young human patients, there is indeed a higher risk to observe a PCO following phacoemulsification surgery, causing a decreased visual acuity (Hiles and Wallar, 1974; McDonnell et al., 1983). Similarly, the tendency for the degree of secondary capsular opacification in dogs is inversely related to age. Therefore, in very young dogs undergoing cataract surgery, long-term visual axis clarity may be improved by a primary posterior capsulotomy (Parks, 1983). Hence, the intraoperative finding of even moderate axial PCO in a young puppy may be considered by some surgeons as an indication for APC, in order to prevent possible accentuation of PCO in the long term. APC in dogs can be performed either prior to (Gemensky-Metzler and Wilkie, 2004) or after IOL insertion, according to the surgeon's preference. In human patients, some surgeons are reluctant to implant IOL after posterior capsulectomy and suggest performing this latter after placing IOL within the bag, fearing enlargement or tearing of posterior capsulectomy (BenEzra and Cohen, 1997). One study demonstrates that posterior capsular tears in humans extend to the equator in 100% of cases when subjected to forces (e.g., IOL implantation) (Castaneda et al., 1992). Furthermore, a retrospective and noncomparative case series describes a technique used in the intraoperative management of PC tears during human cataract surgery. This technique (posterior continuous curvilinear capsulorhexis) is performed to avoid an anticipated extension of the inadvertent tear during such maneuvers as IOL placement (Gimbel et al., 2001). Although APC can be performed before or after IOL implantation, our preference is for post-implantation APC with the goal of preserving as much of the potentially fragile PC as possible, especially in juvenile cases. A difference can be made between opaque fibrotic plaque eventually on wrinkled capsules and a slightly opaque juvenile PC in dogs, the latter being much more fragile than the former. Thus, it seems to us that post-implantation APC is more likely to preserve an inherently fragile PC, particularly in juvenile cases. Moreover, in case 4, microphakia would have made implantation after APC difficult because of the thin and fragile PC and the tension exerted by the IOL on the small bag. Surgeons performing continuous tear anterior capsulorhexis for cataract removal in dogs observe that some capsules tear in a smooth and controlled fashion while others are brittle or tough and tearing is more difficult to control (Bernays and Peiffer, 2000). We believe by experiment that the same is true for PC and the ease with which PC can be torn varies according to cases. In this short case series, we systematically used long and curved Vannas capsulotomy scissors to start and facilitate the capsulectomy, which was finished using Utrata forceps. Using Vannas capsulotomy scissors was useful, especially in cases of severe fibrosis. In one juvenile case, we encountered some difficulties before grasping the flap, as sometimes described in humans, due to the thinness of the capsule (Castaneda et al., 1992). In both juvenile cases (cases 3, 4), we refined the shape of the APC by using the vitrectomy probe to round the fenestration. We performed a slight anterior vitrectomy in all cases to prevent possible vitreous presentation because the anterior hyaloid membrane was ruptured after posterior capsulectomy despite a viscoelastic injection to move the vitreous away from the PC. To perform APC after implantation, we used two variations: the first was to push and position IOL laterally and obliquely toward the equator of the bag, while the second surgical approach was by PC visualization through IOL optic. The first technique allows direct access to the PC and if the surgeon is bothered by a possible IOL return to the center, it requires additional use of viscoelastic to reposition it laterally. In the second technique, viscoelastic is injected under the IOL while leaving it well centered in the bag to visualize the PC, without being hindered by IOL, but involving sliding the instruments between it and PC. It is important to take care not to overinflate the bag in both techniques. Functional vision with axial capsular transparency was preserved without complications during the follow-up period for each eye and the IOL was well-centered in all cases. ConclusionDuring cataract surgery in dogs, opacification of the PC that blocks the visual axis can be operated with an APC combined with moderate anterior vitrectomy, as described in the literature, whether planned or decided intraoperatively. To the author's knowledge, this is the first case series about the intraoperative management of posterior capsular opacities during cataract surgery in dogs. Despite the retrospective nature and small size of this series, the outcome described in this short case series seems to show that this technique is effective and safe, inasmuch axial posterior capsular transparency was restored in all cases without complications. Further additional studies on a larger number of cases are needed to confirm this conclusion. AcknowledgmentsNot applicable. Conflict of interestThe author declares no conflict of interest. FundingThis research received no specific grant or funding. Data availabilityThe data supporting the findings of this study are available within the manuscript. Any other data are available from the corresponding author upon reasonable request. ReferencesBenEzra, D. and Cohen, E. 1997. Posterior capsulectomy in pediatric cataract surgery: the necessity of a choice. Ophthalmology 104(1), 2168–2174. Bernays, M.E. and Peiffer, R.L. 2000. Morphologic alterations in the anterior lens capsule of canine eyes with cataracts. Am. J. Vet. Res. 61, 1517–1519. Bras, I.D., Colitz, C.M., Saville, W.J., Gemensky-Metzler, A.J. and Wilkie, D.A. 2006. Posterior capsular opacification in diabetic and nondiabetic canine patients following cataract surgery. Vet. Ophthalmol. 9, 317–327. Castaneda, V.E., Legler, U.F., Tsai, J.C. and Hoggatt, J.P. 1992. Posterior continuous curvilinear capsulorhexis: an experimental study with clinical applications. Ophthalmology 99, 45–50. Chandler, H.L., Barden, C.A., Lu, P., Kusewitt, D.F. and Colitz, C.M. 2007. Prevention of posterior capsular opacification through cyclooxygenase-2 inhibition. Mol. Vis. 13, 677–691. Cobo, L.M., Ohsawa, E., Chandler, D., Arguello, R. and George, G. 1984. Pathogenesis of capsular opacification after extracapsular cataract extraction: an animal model. Ophthalmology 91, 857–863. Davidson, M.G. and Nelms, S.R. 1999. Diseases of the lens and cataract formation, 3rd ed. Philadelphia, PA: Lippincott/Williams & Wilkins, pp: 797–825. Dziezyc, J. 1990. Cataract surgery. Current approaches. Vet. Clin. North. Am. Small. Anim. Pract. 20(3), 737–754. Gemensky-Metzler, A.J. and Wilkie, D.A. 2004. Surgical management and histologic and immunohistochemical features of a cataract and retrolental plaque secondary to persistent hyperplastic tunica vasculosa lentis/persistent hyperplastic primary vitreous (PHTVL/PHPV) in a Bloodhound puppy. Vet. Ophthalmol. 7, 369–375. Gimbel, H.V., Sun, R., Ferensowicz, M., Penno, E.A. and Kamal, A. 2001. Intraoperative management of posterior capsule tears in phacoemulsification and intraocular lens implantation. Ophthalmology 108, 2186–2189. Glover, T.D. and Constantinescu, G.M. 1997. Surgery for cataracts. Vet. Clin. North. Am. Small. Anim. Pract. 27, 1143–1173. Hiles, D.A. and Johnson, B.L. 1980. The role of the crystalline lens epithelium in postpseudophakos membrane formation. J. Am. Intraocul. Implant. Soc. 6(2), 141–147. Hiles, D.A. and Wallar. P.H. 1974. Phacoemulsification versus aspiration in infantile cataract surgery. Ophthalmic. Surg. 5, 13–16. Khalifa, M.A. 1992. Polishing the posterior capsule after extracapsular extraction of senile cataract. J. Cataract. Refract. Surg. 18, 170–173. McDonnell, P.J., Stark, W.J. and Green, W.R. 1984. Posterior capsule opacification: a specular microscopic study. Ophthalmology 91, 853–856. McDonnell, P.J., Zarbin, M.A. and Green, W.R. 1983. Posterior capsule opacification in pseudophakic eyes. Ophthalmology 90(12), 1548–1553. Parks, M.M. 1983. Posterior lens capsulectomy during primary cataract surgery in children. Ophthalmology 90(4), 344–345. Sigle, K.J. and Nasisse, M.P. 2005. Long-term complications after phacoemulsification for cataract removal in dogs: 172 cases (1995–2002). J. Am. Vet. Med. Assoc. 228, 74–79. | ||
| How to Cite this Article |
| Pubmed Style Frédéric Goulle. Intraoperative management of axial posterior capsular opacities during cataract surgery in six dogs. Open Vet J. 2023; 13(8): 977-982. doi:10.5455/OVJ.2023.v13.i8.3 Web Style Frédéric Goulle. Intraoperative management of axial posterior capsular opacities during cataract surgery in six dogs. https://www.openveterinaryjournal.com/?mno=156266 [Access: July 27, 2024]. doi:10.5455/OVJ.2023.v13.i8.3 AMA (American Medical Association) Style Frédéric Goulle. Intraoperative management of axial posterior capsular opacities during cataract surgery in six dogs. Open Vet J. 2023; 13(8): 977-982. doi:10.5455/OVJ.2023.v13.i8.3 Vancouver/ICMJE Style Frédéric Goulle. Intraoperative management of axial posterior capsular opacities during cataract surgery in six dogs. Open Vet J. (2023), [cited July 27, 2024]; 13(8): 977-982. doi:10.5455/OVJ.2023.v13.i8.3 Harvard Style Frédéric Goulle (2023) Intraoperative management of axial posterior capsular opacities during cataract surgery in six dogs. Open Vet J, 13 (8), 977-982. doi:10.5455/OVJ.2023.v13.i8.3 Turabian Style Frédéric Goulle. 2023. Intraoperative management of axial posterior capsular opacities during cataract surgery in six dogs. Open Veterinary Journal, 13 (8), 977-982. doi:10.5455/OVJ.2023.v13.i8.3 Chicago Style Frédéric Goulle. "Intraoperative management of axial posterior capsular opacities during cataract surgery in six dogs." Open Veterinary Journal 13 (2023), 977-982. doi:10.5455/OVJ.2023.v13.i8.3 MLA (The Modern Language Association) Style Frédéric Goulle. "Intraoperative management of axial posterior capsular opacities during cataract surgery in six dogs." Open Veterinary Journal 13.8 (2023), 977-982. Print. doi:10.5455/OVJ.2023.v13.i8.3 APA (American Psychological Association) Style Frédéric Goulle (2023) Intraoperative management of axial posterior capsular opacities during cataract surgery in six dogs. Open Veterinary Journal, 13 (8), 977-982. doi:10.5455/OVJ.2023.v13.i8.3 |