| Research Article | ||
Open Vet J. 2023; 13(8): 1003-1011 Open Veterinary Journal, (2023), Vol. 13(8): 1003-1011 Original Research Evaluation of renal disturbance in animal models of polycystic ovary syndromeYudit Oktanella*, Handayu Untari, Dyah Kinasih Wuragil, Hana Ismiawati, Nur Afidatul Hasanah, Galuh Chandra Agustina and Dyah Ayu Oktavianie PratamaFaculty of Veterinary Medicine, Brawijaya University, Malang, Indonesia *Corresponding Author: Yudit Oktanella. Faculty of Veterinary Medicine, Brawijaya University, Malang, Indonesia. Email: yudito [at] ub.ac.id Submitted: 03/06/2023 Accepted: 25/07/2023 Published: 31/08/2023 © 2023 Open Veterinary Journal
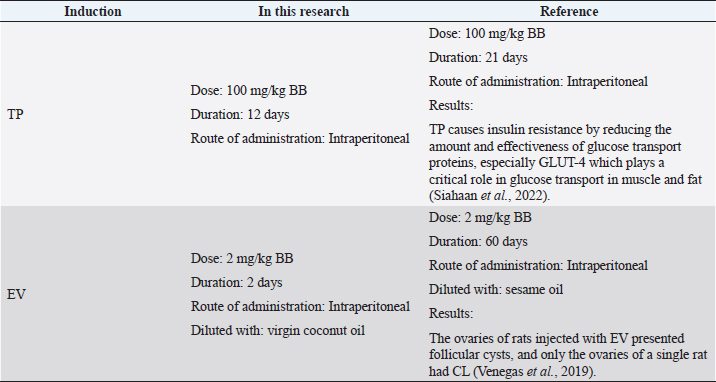
AbstractBackground: Polycystic ovary syndrome (PCOS) is an endocrine disease characterized by hyperandrogenism and hyperinsulinemia, followed by luteinizing hormone and follicle-stimulating hormone deficiency. PCOS conditions cause metabolic disorders that increase uric acid levels and malondialdehyde (MDA) levels. Animal models of PCOS have been used extensively in research to study the pathogenesis, clinical characteristics, and treatment of PCOS. Aim: This study aimed to identify the pathological mechanisms underlying renal dysfunction in PCOS by observing several parameters, including blood urea nitrogen (BUN), creatinine, uric acid, and renal MDA levels. Methods: This research was an experimentally designed study using a Wistar rat (Rattus norvegicus) as an animal model of PCOS which were divided into three groups: negative control group (n=6), Testosterone propionate (TP) induction group (n=6), and estradiol valerate (EV) induction group (n=6). Results: According to statistical analysis it indicated that induction of TP and EV can increase blood uric acid levels in PCOS model rats (p < 0.05), TP induction can increase kidney BUN and MDA levels significantly (p < 0.05), However, the observation of creatinine levels did not show significant differences in all treatment groups (p > 0.05). Conclusion: Based on the results of this study, it can be concluded that the induction of animal models with TP can trigger significant renal damage compared to EV. Keywords: PCOS, Animal model, Renal disturbance, Testosterone propionate, Estradiol valerate. IntroductionPolycystic ovary syndrome (PCOS) affects 1 in 10 women of reproductive age worldwide. The World Health Organization states that 116 million women, or 3.4% of women worldwide, suffer from PCOS (Jabeen et al., 2022). In Indonesia, 44.8% of PCOS patients have both ovulatory disorders and polycystic ovarian phenotype, and the incidence of PCOS patients is 20–30 years, for example, 45.7% (Pangastuti and Sumapradja, 2011). The symptoms of women with PCOS are variable. In general, women with PCOS have menstrual irregularity, androgen excess (such as hirsutism and acne). 40% of women with PCOS have infertility due to anovulation (Sirmans and Pate, 2013). PCOS is also present in animals, especially cows. Cystic ovarian disease is a very common condition with a reported prevalence of 5%–10%. Cystic ovarian disease is a common cause of infertility in cattle, and ovarian cysts resemble atretic follicles (Jeengar et al., 2014). The effects of cysts on the ovaries can cause infertility in cows, repeat breeders, poor egg quality, and infertility (Gümüşay et al., 2018). In addition, the decrease in livestock production has caused economic losses for farmers. Animal models of PCOS have been used extensively in research to study the pathogenesis, clinical characteristics, and treatment of PCOS (Walters et al., 2018). In addition, using PCOS animal models allows us to understand the impact of PCOS on various organs, including the kidney. By observing renal organ parameters in PCOS animal models, we can gain insight into the physiological and pathological changes in the kidney due to PCOS. The stimulating hormones used in this study are testosterone propionate (TP) as an androgen and estradiol valerate (EV) as an estrogen. TP induction can cause hyperandrogenism, abnormal reproductive morphology, hyperinsulinemia, promote atretic follicular changes, and produce luteinizing theca cells (Corrie et al., 2021). Stimulation of EV causes a decrease in follicle-stimulating hormone hormones and their transformation into large follicles in ovarian morphology, such as cysts with atresia. While estradiol is the main female sex hormone controlling female reproduction during estrus and menstruation, high estrogen levels in the body can cause PCOS (Oyebanji et al., 2018). Cardiovascular risk factors, obesity, glucose intolerance, dyslipidemia, and increased inflammation are disorders that are often seen in PCOS patients (Can et al., 2020). Increased production of excess androgens in the ovaries is a cause of PCOS which is called hyperandrogenism. Hyperandrogenism causes disturbances in the cardiovascular system such as hypertension through the activation of the androgen vasoconstrictor, namely Endothelin-1 (Kataoka et al., 2022). Vasoconstriction of blood vessels results in oxidative stress (Dalmasso et al., 2016) which initiates the membrane lipid peroxidase process and triggers the increase in free radicals. Lipid peroxide is unstable, so it breaks down easily into complex compounds, one of which is malondialdehyde (MDA). Increased MDA production catalyzed by nicotinamide adenine dinucleotide phosphate (NADPH) oxidation, insulin, and xanthine oxidase (Anita, 2015). Increased uric acid is strongly associated with metabolic disorders in PCOS, including insulin resistance, obesity, and metabolic and dyslipidemia. Dyslipidemia can increase androgen levels, which can worsen PCOS (Anisya and Graharti, 2019). In addition, dyslipidemia is one of the conditions that affect the increase in the level of uric acid in the body. Uric acid is the end product of purine (adenine and guanine) metabolism. In terms of PCOS, gout is considered a different metabolic disease and is one of the risk factors for PCOS exacerbation (Toda et al., 2018). The evaluation of uric acid levels can be used as a simple diagnostic marker to determine the risk of metabolic syndrome in PCOS patients. Studies using animal models of PCOS are used to examine the etiology, long-term health, pathophysiology, physiology, and treatment of PCOS (Koçak, 2021). An animal model of PCOS can be established by inducing androgens, estrogens, and aromatase inhibitors (Walters et al., 2018). It is crucial to understand the urgency of observing renal organ parameters in animal models of PCOS as this provides insight into the impact of PCOS on renal health. This study may help identify pathological mechanisms underlying renal dysfunction in PCOS and provide a better understanding of the relationship between PCOS and renal disease. In addition, research in animal models of PCOS may provide a foundation for developing effective prevention and treatment strategies to protect and restore kidney health in individuals with PCOS. Observing renal organ parameters in PCOS animal models involves various analytical techniques and methods. Frequently observed parameters include renal function, histological structure, molecular expression, and enzyme activity associated with renal function. Focusing on these parameters, we can identify changes in the kidney due to PCOS manifestations, including inflammation, fibrosis, and renal dysfunction. Materials and MethodsThis research is an experimental laboratory study using white rats (Rattus norvegicus) as an animal model experiment weighing between 130–150 g aged 6–8 months. The animals were then acclimatized for 14 days and maintained at the Animal Laboratory of the Faculty of Veterinary Medicine, Universitas Brawijaya. Eighteen female rats were divided into three groups (Table 1): the negative control (CN) group, the TP induction group, and the EV induction group. Animal preparationThere were 18 female Wistar strain rats (R. norvegicus) with body weights between 130–180 g and 6–8 months old. Before treatment, acclimatization was carried out for approximately 7 days, sot the experimental animals adapted to the new environment. During the study, rats (R. norvegicus) were given feed and drinking water ad-libitum. Rats were fed twice a day in the morning and evening. Animals were housed based on the treatment group. Administration of TP and EVTP was given at 100 mg/kg BW with a volume of 0.13 ml/tail for 12 days. EV was given with 2 mg/kg BW with a volume of 0.13 ml/head for 2 days. EV was dissolved with virgin coconut oil and induced intraperitoneally. The CN group was given physiological NaCl orally with a gastric sonde. TP and EV were given as liquid preparations. EV was dissolved in virgin coconut oil and administered by intraperitoneal injection. TP was administered at 100 mg/kg BW in group P1 for 12 days. EV was administered at 2 mg/kg BW in group P2 for 2 days. EV is used as an induction of PCOS model animals by modifying the technique of Venegas et al. (2019), which uses EV of 2 mg diluted with sesame oil for 60 days. TP used as PCOS induction is also a modification of Siahaan et al. (2022), which uses 100 mg/kg BW TP for 21 days via the intraperitoneal route. Table 1. Table of group distribution and treatment in the study.
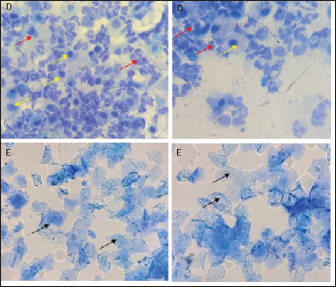
Vaginal swabThe examination of the estrous cycle using the vaginal swab method was carried out twice a day. Vaginal swab cytological sampling uses a cotton swab moistened with NaCl 0.9%. Then insert into the vulva up to the part of the vagina adjacent to the cervix, then make a circular motion to get epithelial cells. Then the cotton swab was applied to the glass object. After drying, the slides were stained with methylene blue dye and observed under a microscope at 400×—–1,000× magnification to identify the various stages of the estrous cycle. Euthanasia methodEuthanasia and kidney organ collection in experimental animals were divided into days 4 and 14. The euthanasia method was carried out by injecting ketamine-xylazine anesthetic drugs with ten times the dose of anesthesia intramuscularly, followed by cervical dislocation. Sample collectionSerum samples can be used to identify changes in protein levels associated with PCOS pathomechanisms (Rheza et al., 2023). Before the necropsy, blood samples are taken first through the intracardiac as much as 3–5 ml. Then, the necropsy of the experimental animals was started by making an incision on the abdomen to the thorax so that all organs could be seen clearly. After that, one of the kidneys was collected and washed with 0.9% physiological NaCl. Then the kidneys were wrapped in aluminum foil, put into a plastic zip-lock, and stored at −20ºC to examine MDA levels. Examination of uric acid levelsThe procedure for checking uric acid levels using the point of care testing stick method with three and one blood tests (Nesco Multicheck, India) using blood samples. Rat blood samples were taken through the intracardiac as much as 0.3 ml. Examination of uric acid levels is carried out by inserting the chip and strip into the device with the same code. The device is ready for use if “UA” and a picture of blood drops appear. The blood sample is inserted through the strip gap until the end of the strip and beeps; wait 10 seconds, then the tool reads a few seconds, and the results will appear on the screen. Examination of blood urea nitrogen (BUN) and creatinine levels in blood serumBUN and creatinine levels in blood serum were examined by biochemical blood tests using a spectrophotometer (RIELE Photometer 5010 Version 5). Examination of kidney MDA levelIn the procedure for examining kidney MDA levels using the thiobarbituric acid method, the kidney organs were cut, then weighed to 0.1 g, crushed with a cooled mortar (mortar placed on ice gel), and 0.9% physiological NaCl was added. The kidney homogenate was transferred into a microtube and centrifuged at 8,000 rpm for 20 minutes. Afterward, 100 µl of supernatant was transferred to a new microtube, and 550 µl of distilled water, 100 µl of 10% TCA, 250 µl of 1 N HCl, and 100 µ of 1% Na-Thio were added and homogenized with a vortex. The solution was centrifuged at 500 rpm for 10 minutes. The resulting supernatant was heated on a water bath/water bath at 100ºCfor 20 minutes, and the absorbance was measured with a spectrophotometer at λ=532 nm. The resulting absorbance value was entered into the equation to determine the MDA level. Data analysisThe parameters used in this study, namely uric acid levels, kidney organ MDA levels, and serum creatinine BUN levels, were analyzed quantitatively using One-way Analysis of Variance data analysis with a confidence level of 95% (α=0.05) to determine differences between treatment groups and continued with post hoc Test using the least real difference to determine which treatment gave the best results. Software used for quantitative data analysis is Statistical Program for Social Science 016 Version 2.9 for Windows. Ethical approvalThe Research Ethics Committee (Animal Care and Use Committee) of Brawijaya University approved the research with an ethical certificate number No. 090-KEP-UB-2022. ResultsThe method used to ensure animal models have been induced PCOS is by examining vaginal swabs and checking testosterone levels by the ELISA method. The vaginal swab determines changes in cells found in the vaginal mucosa during one estrous cycle (Foeh et al., 2020). Animals with PCOS conditions will show a dominant reproductive cycle in the estrus phase through vaginal swab examination results (Haslan et al., 2021). In this study, both TP and EV induction groups experienced irregularity of the estrous cycle characterized by the presence of persistent estrus and or persistent diestrus phases followed by abnormal estrous cycles (Fig. 1). The results of measuring testosterone levels by ELISA method showed that TP-induced rats experienced an increase from normal levels of 423.08 ng/l. In contrast, the testosterone levels of EV-induced rats were 482.32 ng/l. Fernanda et al. (2019) stated that the limit of normal levels of testosterone levels in rats is 200–400 ng/l and so these conditions indicate an increase in testosterone levels, indicating a biochemical hyperandrogenism condition which is one of the diagnoses of PCOS.
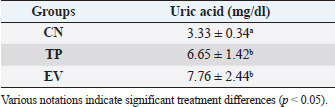
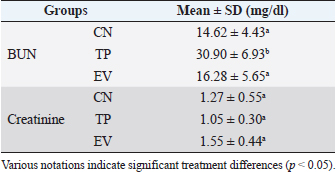
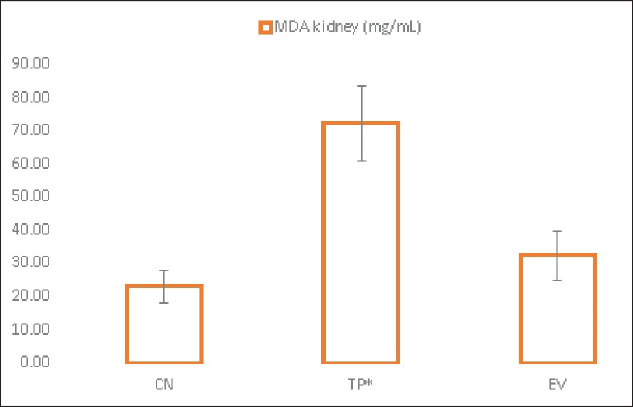
Fig. 1. Histological features of vaginal swabs in TP and EV groups indicated estrous dan diestrus phase. (D): Diestrus; (E): Estrous; (Yellow arrows): nucleated epithelial cells; (Red arrows): leukocytes cells; (Black arrows): cornified epithelial cells. Uric acid measurementUric acid is the end product of exogenous and endogenous purine metabolism (Chaudhary et al., 2013). Data from the calculation of uric acid levels in the blood of rats in the CN group, the TP induction group at a dose of 100 mg/kg BW, and the EV induction group at a dose of 2 mg/kg BW are presented in Table 3. The results of uric acid measurements in PCOS animal models show that acid levels in the TP induction group at a dose of 100 mg/kg BW and the EV induction group at a dose of 2 mg/kg BW are significantly different (p < 0.05) from the CN. The CN group (K−) has an average uric acid level of 3.33 mg/dl which is still considered normal. This follows Umboh et al. (2019), the standard level of blood uric acid in rats is 1.2–5.0 mg/dl. These measurements indicate that induction of TP and EV can increase blood uric acid levels in PCOS model rats. BUN and creatinine levelsBUN is a marker parameter for acute kidney damage (Fitria et al., 2019). This is because the urea in the blood is the main element produced from the process of decomposing proteins and other chemical compounds containing nitrogen which will typically be excreted through the kidneys, so an increase in blood serum levels can indicate a failure of kidney function (Suryawan, 2016). The results of the measurement of BUN and creatinine levels are presented in Table 4. According to the data in Table 2, it can be stated that the TP group obtained the highest BUN levels and were significantly different (p < 0.05) compared to the CN group and EV. This indicates that the induction of TP in PCOS animal models caused a significant increase in BUN levels. In contrast, the induction of PCOS model animals with EV did not increase BUN levels. Statistical results of serum creatinine levels in all treatment groups showed no significant differences (p > 0.05), so in this study, it can be concluded that induction of TP and EV did not trigger an increase in creatinine levels. Kidney MDAMDA level was measured to determine oxidative damage to the kidney organs, which is thought to be caused by increased free radicals in PCOS animal models. The statistical analysis results of the calculation of kidney MDA levels can be seen in Figure 2. Examination of MDA levels is used as an indicator of oxidative stress (Judiono et al., 2014), considering that MDA is the end product of excess lipid peroxidation due to oxidative stress (Morales and Munne, 2019). Statistically, TP induction can increase kidney MDA levels significantly different from the other two groups (p < 0.05). Table 2. The difference between the induction method in this research and the reference research.
Table 3. Uric acid level values of various treatment groups.
Table 4. BUN and creatinine levels in various treatment groups.
DiscussionUnder normal circumstances, uric acid, as an end product of purine metabolism, describes the body's metabolic state and can maintain the level of oxidation in the body's physiological conditions (Chaudhary et al., 2013). High levels of testosterone in the blood are associated with increased uric acid levels and the prevalence of hyperinsulinemia (Hu et al., 2021). The testosterone hormone can induce the hepatic metabolism of purine nucleotides. High concentrations of uric acid levels affect lipid synthesis and distortion of lipid oxidation. Abnormal lipid metabolism in PCOS results in lipotoxicity that affects cells produces large amounts of reactive oxygen species (ROS), and induces damage to intracellular organelles, leading to cell death (Lima et al., 2015). The increased testosterone level in PCOS can contribute to uric acid levels. This is in line with the study's results, which found that induction of TP and EV can increase blood uric acid levels in PCOS model rats. According to Liu et al. (2021), hyperandrogenism, insulin resistance, abnormal lipid metabolism, and complications in PCOS can increase uric acid. In cases of PCOS in humans, it also shows an increase in uric acid due to androgens and insulin encouraging reabsorption of uric acid in the proximal tubules of the kidney and reducing uric acid secretion, thereby accelerating the occurrence of hyperuricemia (Nasrul and Sofitri, 2012). The mechanism of this condition involves the role of testosterone, which can increase the activation of the xanthine oxidase enzyme, which plays a role in converting xanthine into uric acid. In addition, testosterone causes insulin resistance which can interfere with uric acid excretion in the kidneys. Increased blood uric acid levels in patients with PCOS are also associated with hyperinsulinemia, resulting in low uric acid excretion. In conditions of insulin resistance, the kidneys cannot excrete uric acid effectively, resulting in increased blood uric acid levels (Battelli et al., 2018; Paleva, 2019). In addition, according to Gill (2013), elevated uric acid levels in PCOS are associated with increased insulin resistance. EV induction increases purine biosynthesis due to the activity of the hexose monophosphate pathway, which is characterized by hyperinsulinemia. Increased glucose-6-phosphate flux caused by hexose monophosphate is characterized by glycolytic damage and increased lipogenesis. Uric acid is formed from purines via hypoxanthine. Uric acid is excreted from the body through urine as a reflection of purine catabolism in the body. Excessive accumulation of uric acid can cause metabolic disorders in the body (Mu et al., 2018). PCOS cases in humans also show increased uric acid because androgens and insulin encourage uric acid reabsorption in the proximal tubules of the kidneys and reduce uric acid secretion, thus accelerating hyperuricemia (Nasrul and Sofitri, 2012). BUN is the end product of protein metabolism and amino acid catabolism produced by the liver and distributed throughout intracellular and extracellular fluids and excreted through the urine. Elevated BUN associated with kidney disease or renal failure, obstruction of the urinary tract by kidney stones, congestive heart failure, dehydration, fever, shock, bleeding in the gastrointestinal tract, and rapid muscle damage can also cause a transient increase in serum urea concentration (Gowda et al., 2010). TP induction (TP group) can cause BUN levels to increase significantly; the following research by Fitria et al. (2019) indicates acute renal impairment in PCOS cases. However, this increase did not occur in the EV induction group (EV group). This is thought to be because, in this study, EV induction only lasted for 2 days, while based on the literature, EV is most effective in causing PCOS at a level of 4.5 mg/kg BW for 60 days resulting in effects on changes in rat BUN levels (Ramadoss et al., 2019). Therefore, EV at 2 mg/kg BW for 2 days still cannot significantly affect BUN levels. In this study, the value of the CN group (K−) is the standard value of rat MDA levels (R. norvegicus) in normal conditions, where rats are not given TP and EV induction. This is under the statement of Valko et al. (2006) that in normal rats, it is known that MDA remains due to biochemical processes in the body that produce free radicals. Free radicals are beneficial for biological functions such as phagocytosis. Endogenous antioxidants in the body can neutralize free radicals in low amounts; if the amount of free radical compounds exceeds the number of antioxidants in the body, free radicals will damage lipid components resulting in oxidative stress (Srivastava and Kumar, 2015). Hyperandrogenism in PCOS has been shown to cause decreased kidney function (Dalmasso et al., 2016). The kidneys play a role in various homeostatic functions of the body, namely the excretion of metabolic waste and foreign chemicals, both produced by the body and entering the body (Guyton and Hall, 2016). According to Zhang et al. (2018), TP induction can escalate MDA levels by inducing lipid peroxidation and reducing antioxidant activity. A significant elevation in MDA levels occurred in the P1 group due to oxidative stress caused by exposure to TP. The results of this study are also in line with Serrano et al. (2018), which state that TP induction at a dose of 100 mg/kg BW causes glucose intolerance, dyslipidemia, and increased levels of oxidative stress which potentially causes lipid peroxidation, ROS toxicity and decreased antioxidant function. In addition, the half-life of TP is about 33 hours and can stay in the body for 5 days. This is because the extended structure of the ester group in TP correlates with a long half-life so that TP will remain in the bloodstream for a long time (Turza et al., 2022).
Fig. 2. Kidney MDA levels in treatment groups. (CN): Negative control; (TP): Testosterone propionate; (EV): Estradiol valerate; (*): indicate significant differences (p < 0.05). EV is used to create animal models of PCOS by inducing hormonal changes from normal conditions. Exposure to EV induction in rats causes reproductive cycle irregularities, anovulation, and polycystic ovaries showing increased atresia follicles. The PCOS condition in mice due to EV induction is similar to human PCOS cases (Walters et al., 2018). It is also evidenced that EV induction has a reproductive cycle with a consistent, persistent estrus phase and higher testosterone, uric acid, and renal MDA levels as metabolic disorders in PCOS. EV also has a half-life of about 11–14 hours and provides a faster effect on the appearance of PCOS conditions (Ndefo and Mosely, 2010). The role of oxidative stress and renal tissue damage response in the pathogenesis of PCOS shows the relationship of renal MDA levels as a result of lipid peroxidation in metabolic syndrome conditions that trigger increased apoptosis and decreased mitochondrial function (Olatunji et al., 2021)—the formation of ROS in the mitochondrial respiration chain as second messengers for NF-κβ activation. Increased MDA production is catalyzed through NADPH oxidation, insulin, and xanthine oxidase (Anita, 2015). Several studies have shown a correlation between high levels of androgen hormones and the development of kidney damage (Gozukara et al., 2015; Valdivielso et al., 2019; Lau et al., 2022). However, the exact pathomechanisms associated with this still need to be fully understood. Androgens are known to influence the renin-angiotensin-aldosterone system (RAAS) profoundly. Hyperandrogenism due to PCOS syndrome may affect RAAS regulation by increasing the production of angiotensinogen and angiotensin II. This can lead to vasoconstriction of the renal arteries and increased reabsorption of salt and water, which can trigger blood pressure disorders and kidney damage. In addition, hyperandrogenism may also contribute to inflammation and oxidative stress (Dalmasso et al., 2016), which are factors also associated with kidney damage. Androgen hormones can influence immune system activity and stimulate the production of inflammatory mediators such as pro-inflammatory cytokines (Traish et al., 2018). This can trigger an inflammatory response and oxidative stress in the kidney, which can cause structural damage to kidney function. ConclusionBased on the results of this study, it can be concluded that the induction of animal models with TP can trigger more significant renal damage compared to EV. Although both compounds can induce PCOS conditions in animal models with similar clinical symptoms, researchers still need to pay attention to the side effects caused, especially those related to kidney function. This is because there are many variations in the duration of induction of both drugs that can potentially trigger other disorders/complications in PCOS animal models. AcknowledgmentsNone. Conflict of interestThe Authors declare that there is no conflict of interest. FundingThis research was supported through internal research funding from the Faculty of Veterinary Medicine, Brawijaya University, Malang, East Java, Indonesia. Data availabilityThe data used to support the findings of this study are included within the article and no additional data sources are required. Authors contributionsY, Oktanella: devised the project, drafted the manuscript, the main conceptual ideas, and the proof outline. H, Untari, DK, and Wuragil were involved in planning and supervising the work. H, Ismiawati and NA, Hasanah processed the experimental data and performed the analysis. GC, Agustina and DAO, Pratama were performed in interpreting the results and worked on the revised manuscript. ReferencesAnisya, V. and Graharti, R. 2019. Policystic ovary syndrom: infertility risks that can be prevented through weight loss in obese women. Medula 9(2), 257–265. Anita, D.C. 2015. Blood glucose and kidney malondialdehyde levels of physically exercised diabetic rats. Indones. J. Nurs. Pract. 1(2), 109–116. Battelli, M.G., Bortolotti, M., Polito, L. and Bolognesi, A. 2018. The role of xanthine oxidoreductase and uric acid in metabolic syndrome. Biochim. Biophys. Acta (BBA) - Mol. Basis. Dis. 1864(8), 2557–2565. Can, M., Duran, C., Guney, I., Elmas, H., Ayhan, M. and Erdem, S.S. 2020. The relationship between glomerular filtration rate and metabolic and inflammatory parameters in obese and non-obese patients with polycystic ovary syndrome. Clín. Investig. Arterioscler. (English Edition). 32(6), 256–262. Chaudhary, K., Malhotra, K., Sowers, J. and Aroor, A. 2013. Uric acid—key ingredient in the recipe for cardiorenal metabolic syndrome. Card. Med. 3(3), 208–220. Corrie, L., Gulati, M., Singh, S.K., Kapoor, B., Khursheed, R., Awasthi, A., Vishwas, S., Chellappan, D.K., Gupta, G., Jha, N.K., Anand, K. and Dua, K. 2021. Recent updates on animal models for understanding the etiopathogenesis of polycystic ovarian syndrome. Life. Sci. 280, 119753. Dalmasso, C., Maranon, R., Patil, C., Bui, E., Moulana, M., Zhang, H., Smith, A., Yanes Cardozo, L.L. and Reckelhoff, J.F. 2016. Cardiometabolic effects of chronic hyperandrogenemia in a new model of postmenopausal polycystic ovary syndrome. Endocrinology 157(7), 2920–2927. Fernanda, M.A., Sa’adi, A. and Sudjarwo. 2019. Lineage verification of testosterone standard curve using ELISA method (Enyme-linked immunosorbent assay). J. Res. Technol. 5(1), 2460–5972. Fitria, L., Lukitowati, F. and Kristiawati, D. 2019. Reference values for evaluation of liver and kidney functions in rats ( Rattus norvegicus Berkenhout, 1769) wistar strain. J. Pend. Mat. Dan IPA. 10(2), 81. Foeh, N., Datta, F.U., Detha, A.I.R., Ndaong, N.A. and Moi, M. 2020. Distribution of vagina smear results of local bean goats (Capra aegagrus) in Kupang City. J. Vet. Stud. 7(2), 128–133. Gill, A. 2013. Correlation of the serum insulin and the serum uric acid levels with the glycated haemoglobin levels in the patients of type 2 diabetes mellitus. J. Clin. Diagnost. Res. 7(7), 1295–1297. Gowda, S., Desai, P.B., Kulkarni, S.S., Hull, V.V., Math, A.A. and Vernekar, S.N. 2010. Markers of renal function tests. North. Am. J. Med. Sci. 2(4), 170. Gozukara, I.O., Gozukara, I., Gozukara, K., Kucur, S. and Karakılıc, E. 2015. Association of glomerular filtration rate with inflammation in polycystic ovary syndrome. Int. J. Fertil. Steril. 9(2), 176–182. Gümüşay, B.K., Gökçek, S. and Yavaş, L. 2018. Investigation of immunological infertility in repeat breeder cows. Harran. Üniv. Veteriner. Fak. Derg. 7(1), 89–92. Guyton, A.C. and Hall, J.E. 2016. Guyton and hall textbook of medical physiology, 14th ed. Amsterdam, The Netherlands Elsevier, pp: 303–442. Haslan, M.A., Samsulrizal, N., Hashim, N., Zin, N.S.N.M., Shirazi, F.H. and Goh, Y.M. 2021. Ficus deltoidea ameliorates biochemical, hormonal, and histomorphometric changes in letrozole-induced polycystic ovarian syndrome rats. BMC. Complement. Med. Ther. 21(1), 291. Hu, J., Xu, W., Yang, H. and Mu, L. 2021. Uric acid participating in female reproductive disorders: a review. Reprod. Biol. Endocrinol. 19(1), 65. Jabeen, A., Yamini, V., Amberina, A.R. and Eshwar, M.D. 2022. Polycystic ovarian syndrome: prevalence, predisposing factors, and awareness among adolescent and young girls of South India. Cureus 14(8), e27943. Jeengar, K., Chaudhary, V., Kumar, A., Raiya, S., Gaur, M. and Purohit, G.N. 2014. Ovarian cysts in dairy cows: old and new concepts for definition, diagnosis and therapy. Anim. Reprod. 11(2), 63–73. Judiono, Djokomoeljanto, and dan Hadisaputro, S. 2014. Effects of oral clear kefir probiotics on glycemic status, lipid peroxidation, antioxidative properties of streptozotocin induced hyperglycemia wistar rats. Gizi. Indonesia. 34(1), 1–6. Kataoka, T., Fukamoto, A., Hotta, Y., Sanagawa, A., Maeda, Y., Furukawa-Hibi, Y. and Kimura, K. 2022. Effect of high testosterone levels on endothelial function in aorta and erectile function in rats. Sex. Med. 10(5), 100550. Koçak, S. 2021. PCOS animal models: an approach induced by dehydroepiandrosterone. Gaziantep. Islam. Sci. Technol. Univ. 2(1), 136–145. Lau, L.H.Y., Nano, J., Prehn, C., Cecil, A., Rathmann, W., Zeller, T., Lechner, A., Adamski, J., Peters, A. and Thorand, B. 2022. Associations of endogenous androgens and sex hormone-binding globulin with kidney function and chronic kidney disease. Front. Endocrinol. 13, 1000650. Lima, W.G., Martins-Santos, M.E.S. and Chaves, V.E. 2015. Uric acid as a modulator of glucose and lipid metabolism. Biochimie 116, 17–23. Liu, Y.N., Luo, H., Che, X., Peng, H., Li, M. and Liu, K.X. 2021. Uric acid metabolism in polycystic ovary syndrome. Clin. Chim. Acta. 517, 74–80. Morales, M. and Munne, S. 2019. Malondialdehyde: facts and artifacts. Plant. Physiol. 180(3), 1246–1250. Mu, L., Pan, J., Yang, L., Chen, Q., Chen, Y., Teng, Y., Wang, P., Tang, R., Huang, X., Chen, X. and Yang, H. 2018. Association between the prevalence of hyperuricemia and reproductive hormones in polycystic ovary syndrome. Reprod. Biol. Endocrinol. 16(1), 104. Nasrul, E. and Sofitri, S. 2012. Hyperuricaemia in pre diabetes. J. Kesehatan. Andalas. 1(2), 86–91. Ndefo, U.A. and Mosely, N. 2010. Estradiol valerate and estradiol valerate/dienogest (Natazia) tablets. Pharm. Therap. 35(11), 614. Olatunji, L.A., Areola, E.D., Usman, T.O., Badmus, O.O. and Olaniyi, K.S. 2021. Treatment with acetate during late pregnancy protects dams against testosterone-induced renal dysfunction. Heliyon 7(1), e05920. Oyebanji, O.G., Asaolu, M.F. and Amonimo, E.O. 2018. Hormonal imbalance in polycystic ovarian syndrome (PCOS) in teaching hospitals in Ekiti State, Nigeria. Open. J. Obstet. Gynecol. 08(13), 1456–1464. Paleva, R. 2019. Mechanisms of obesity-related insulin resistance. J. Ilmiah. Kesehatan. Sandi. Husada. 10(2), 354–358. Pangastuti, N.P. and Sumapradja, K. 2011. Profile of policystic ovarian syndrome patients in Dr. Cipto Mangunkusumo General Hospital Jakarta March 2009—March 2010. Indones. J. Obstet. Gynecol. 35(1), 6. Ramadoss, M., Vijayaraman, M. and Jessica, A. 2019. Estradiol valerate dose determination for PCOS induction. Int. J. Pharm. Biol. Sci. 9(2), 1131–1136. Rheza, A., Santoso, B. and Widjiati, W. 2023. Correlation of serum kisspeptin levels, ovarian kisspeptin expression, and ovarian BMP15 expression in rat model of polycystic ovarian syndrome. Open. Vet. J. 13(3), 288–296. Serrano, L., Bridi, A., Della Mea, R., Braga Rissi, V., dos Santos Guarda, N., Moresco, R.N., Premaor, M.O., Antoniazzi, A.Q., Gonçalves, P.B.D. and Comim, F.V. 2018. Oxidative stress and metabolic markers in pre-and postnatal polycystic ovary syndrome rat protocols. J. Inflamm. Res. 11(2), 193–202. Siahaan, S.C.P.T., Santoso, B. and Widjiati. 2022. Effectiveness of Moringa oleifera leaves on TNF-α expression, insulin levels, glucose levels and follicle count in Rattus norvegicus PCOS model. Diabetes. Metab. Syndr. Obes. Targets. Ther. 15, 3255–3270. Sirmans, S.M. and Pate, K.A. 2013. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin. Epidemiol. 6, 1–13. Srivastava, K.K. and Kumar, R. 2015. Stress, oxidative injury and disease. Indian J. Clin. Biochem. 30(1), 3–10. Suryawan, D.G.A. 2016. Overview of serum urea and creatinine levels in patients with chronic renal failure undergoing haemodialysis therapy at Sanjiwani Gianyar Hospital. J. Med. Lab. 4(2), 145–153. Toda, E.S.M., Natalia, L. and Astuti, A.T. 2018. The relationship between obesity and the incidence of hyperuricaemia at Puskesmas Depok III, Sleman, Yogyakarta. J. Ilmu. Gizi. Indonesia. 1(2), 113–119. Traish, A., Bolanos, J., Nair, S., Saad, F. and Morgentaler, A. 2018. Do androgens modulate the pathophysiological pathways of inflammation? Appraising the contemporary evidence. J. Clin. Med. 7(12), 549. Turza, A., Popescu, V., Mare, L. and Borodi, G. 2022. Structural aspects and intermolecular energy for some short testosterone esters. Materials (Basel, Switzerland) 15(20), 7245. Umboh, D.Y., De Queljoe, E. and Yamlean, P.V.Y. 2019. Antihyperuricemia activity test of green gedi leaf ethanol extract (Abelmoschus manihot (L.) Medik) in wistar strain male white rats (Rattus norvegicus). Pharmacon 8(4), 878. Valdivielso, J.M., Jacobs-Cachá, C. and Soler, M.J. 2019. Sex hormones and their influence on chronic kidney disease. Curr. Opin. Nephrol. Hyperten. 28(1), 1–9. Valko, M., Rhodes, C.J., Moncol, J., Izakovic, M. and Mazur, M. 2006. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 160(1), 1–40. Venegas, B., De León Gordillo, L.Y., Rosas, G., Espinoza, J.A., Morán, C., Domínguez, R. and Morales-Ledesma, L. 2019. In rats with estradiol valerate-induced polycystic ovary syndrome, the acute blockade of ovarian β-adrenoreceptors improve ovulation. Reprod. Biol. Endocrinol. 17(1), 95. Walters, K.A., Bertoldo, M.J. and Handelsman, D.J. 2018. Evidence from animal models on the pathogenesis of PCOS. Best practice and research. Clin. Endocrinol. Metab. 32(3), 271–281. Zhang, G., Kang, Y., Zhou, C., Cui, R., Jia, M., Hu, S., Ji, X., Yuan, J., Cui, H. and Shi, G. 2018. Amelioratory effects of testosterone propionate on age-related renal fibrosis via suppression of TGF-β1/Smad signaling and activation of Nrf2-ARE signaling. Sci. Rep. 8(1), 10726. | ||
| How to Cite this Article |
| Pubmed Style Oktanella Y, Untari H, Wuragil DK, Agustina GC, Pratama DAO. Evaluation of renal disturbance in animal models of polycystic ovary syndrome. Open Vet J. 2023; 13(8): 1003-1011. doi:10.5455/OVJ.2023.v13.i8.6 Web Style Oktanella Y, Untari H, Wuragil DK, Agustina GC, Pratama DAO. Evaluation of renal disturbance in animal models of polycystic ovary syndrome. https://www.openveterinaryjournal.com/?mno=158814 [Access: July 27, 2024]. doi:10.5455/OVJ.2023.v13.i8.6 AMA (American Medical Association) Style Oktanella Y, Untari H, Wuragil DK, Agustina GC, Pratama DAO. Evaluation of renal disturbance in animal models of polycystic ovary syndrome. Open Vet J. 2023; 13(8): 1003-1011. doi:10.5455/OVJ.2023.v13.i8.6 Vancouver/ICMJE Style Oktanella Y, Untari H, Wuragil DK, Agustina GC, Pratama DAO. Evaluation of renal disturbance in animal models of polycystic ovary syndrome. Open Vet J. (2023), [cited July 27, 2024]; 13(8): 1003-1011. doi:10.5455/OVJ.2023.v13.i8.6 Harvard Style Oktanella, Y., Untari, . H., Wuragil, . D. K., Agustina, . G. C. & Pratama, . D. A. O. (2023) Evaluation of renal disturbance in animal models of polycystic ovary syndrome. Open Vet J, 13 (8), 1003-1011. doi:10.5455/OVJ.2023.v13.i8.6 Turabian Style Oktanella, Yudit, Handayu Untari, Dyah Kinasih Wuragil, Galuh Chandra Agustina, and Dyah Ayu Oktavianie Pratama. 2023. Evaluation of renal disturbance in animal models of polycystic ovary syndrome. Open Veterinary Journal, 13 (8), 1003-1011. doi:10.5455/OVJ.2023.v13.i8.6 Chicago Style Oktanella, Yudit, Handayu Untari, Dyah Kinasih Wuragil, Galuh Chandra Agustina, and Dyah Ayu Oktavianie Pratama. "Evaluation of renal disturbance in animal models of polycystic ovary syndrome." Open Veterinary Journal 13 (2023), 1003-1011. doi:10.5455/OVJ.2023.v13.i8.6 MLA (The Modern Language Association) Style Oktanella, Yudit, Handayu Untari, Dyah Kinasih Wuragil, Galuh Chandra Agustina, and Dyah Ayu Oktavianie Pratama. "Evaluation of renal disturbance in animal models of polycystic ovary syndrome." Open Veterinary Journal 13.8 (2023), 1003-1011. Print. doi:10.5455/OVJ.2023.v13.i8.6 APA (American Psychological Association) Style Oktanella, Y., Untari, . H., Wuragil, . D. K., Agustina, . G. C. & Pratama, . D. A. O. (2023) Evaluation of renal disturbance in animal models of polycystic ovary syndrome. Open Veterinary Journal, 13 (8), 1003-1011. doi:10.5455/OVJ.2023.v13.i8.6 |