| Research Article | ||
Open Vet J. 2023; 13(10): 1299-1307 Open Veterinary Journal, (2023), Vol. 13(10): 1299–1307 Original Research Molecular identification and cross-immunogenic study on two field isolates of Mycoplasma synoviae isolated from broilers in five districts of Khyber PakhtunkhwaAtta Ur Rehman1, Abdul Haleem Shah2, Sajjad Ur Rahman3, Saifur Rehman1,4, Muhammad Kamal shah1, Widya Paramita Lokapirnasari5*, Imdadullah Khan1, Muhammad Inamullah Malik1 and Andreas Berny Yulianto61Faculty of Veterinary and Animal Sciences, Gomal University, Dera Ismail Khan, Pakistan 2Institute of Biological Sciences, Gomal University, Dera Ismail Khan, Pakistan 3Institute of Microbiology, Faculty of Veterinary Science, University of Agriculture, Faisalabad, Pakistan 4Division of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 5Division of Animal Husbandry, Faculty of Veterinary Medicine, Universitas Airlangga, Indonesia 6Faculty of Veterinary Medicine, University of Wijaya Kusma, Surabaya, Indonesia *Corresponding Author: Widya Paramita Lokapirnasari. Division of Animal Husbandry, Faculty of Veterinary Medicine, Universitas Airlangga, Indonesia. Email: widya-p-l [at] fkh.unair.ac.id Submitted: 14/07/2023 Accepted: 18/09/2023 Published: 31/10/2023 © 2023 Open Veterinary Journal
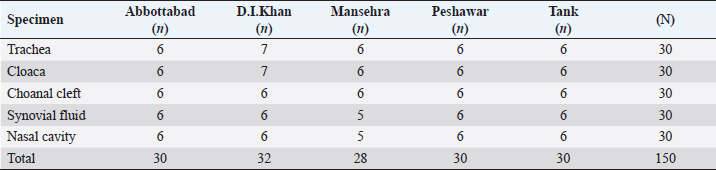
AbstractBackground: Mycoplasma synoviae (MS) is an important poultry pathogen causing heavy economic losses Worldwide. Subclinical persistence of this pathogen is the major issue to control its prevalence. Aim: This study aimed to determine the molecular and cross-immunogenicity of MS among broilers in five Districts of Khyber Pakhtunkhwa (KP). Methods: This study was conducted by collecting 434 specimen samples from 40 broiler farms and desi poultry in five districts of KP. Specimen samples from the broiler birds (n=150), broiler farm environment (n=264), and desi poultry birds (n=20) were aseptically collected and serially passaged in Modified Frey’s broth. The homologous and heterologous antibody reactions were studied in rabbits. Before inoculation into rabbits, the MS isolates were inactivated by formalin and adjuvanted with Montanide. Results: The overall turbidity prevalence in Frey’s broth was observed as 109/434 (25.11%) samples, and these turbidity-positive samples were shifted on Frey’s agar. After the appearance of classic fried egg colonies, the Biochemical confirmation was supported by the production of catalase and phosphatase, reduction of tetrazolium, film and spot assay, and fermentation of glucose for species differentiation in avian mycoplasma. The MS prevalence percentage was recorded as 2% (9/434) through biochemical tests. The PCR results showed 0.5% MS prevalence with two field isolates (named MS-1 and MS-2). Both MS-1 and MS-2 field isolates showed similar values (42.2) of homologous geometric mean titer (GMT). While the heterologous GMT for MS-1 serum against MS-2 isolate was lower (27.9) as compared to MS-2 serum against MS1 isolate (38.9). No titer was detected in the control group (Group-III). Conclusion: In conclusion, the results indicated the existence of MS in broiler birds and high homologous titers recorded between field isolates, which is a perpetual menace to poultry. Keywords: Mycoplasma synoviae, PCR characterization, Homologous and heterologous antigenic responses. IntroductionGenus Mycoplasma contains a lot of vital poultry pathogens, which are responsible for heavy economic losses all over the World (Dufour-Gesbert et al., 2006). Among these avian Mycoplasma spp: Mycoplasma synoviae (MS) and Mycoplasma gallisepticum are known as the worst of all the avian species (Buim et al., 2009). MS is a wall-less bacteria having a high number of sterols in the cell membrane (Kleven, 2008). MS has the smallest genome with 23%–40% G+C contents (Nicholas and Ayling, 2003). The main target of MS is the nose, throat, pharynx, trachea, and bronchi, resulting in subclinical upper respiratory disease. When MS is accompanied by MG, it causes air sacculitis (Silva et al., 2008). Horizontal as well as vertical modes of transmission are found in MS. From infected birds, MS can easily be transferred through aerosols generated by diseased birds. Direct contact as well as contaminated fomites can also be a source of infection (Marois et al., 2005). Immunocompromised birds are more affected by MS (Dhondt et al., 2007). Infectious synovitis, respiratory sickness, lowered egg yield, miss shaped eggs, and slow weight gain are the main clinical infections caused by MS. Death rates are very low due to these infections, but their economic impact is long-lasting in the poultry birds all over the Globe (Kursa et al., 2019). Less effectivity of many antibiotics against various Mycoplasma spp: has increased the interest in the prevention of the diseases caused by these bacteria. However, very few effective vaccines against mycoplasma are available to provide long-lasting immunity (Nicholas and Ayling, 2003). The production of more efficient vaccines is mandatory to control the diseases caused by avian mycoplasma. The variations in the antigenicity were witnessed in many species of mycoplasma (Wise, 1993) as documented by 2 (Lin and Kleven, 1982) in MG strains. Virulence of these MG isolates is also associated with antigenicity (Lin and Kleven, 1982). Homologous and heterologous titers were studied by Vardaman and Yoder (1969) using HI antigens (M. gallisepticum and MS). The researchers found higher homologous HI titers than the heterologous titers. Rehman et al. (2022) also found high homologous GMT values among M. gallisepticum field isolates in KP (Rehman et al., 2022). Quick and timely diagnosis of MS is imperative to avert MS spread to minimize the economic losses in commercial broilers. There are three approaches to diagnose mycoplasma infection: identification and isolation of the bacterium by culture recognition of its DNA. Serological methods like ELISA, serum plate agglutination test, and Haemagglutination inhibition tests (Dufour-Gesbert et al., 2006; Abbas et al., 2018) are used to detect the specific antibodies. VlhA gene was found more satisfactory in MS strain differentiation (Beylefeld, 2018). Hemagglutinin and pro-lipoprotein are present on VlhA gene (Dijkman et al., 2014). The antigenic and phase differentiation are expressed by hemagglutinin (Matucci et al., 2020). The precise and fast characterization of MS is mostly based on the amplification (Diene et al., 2016) of the conserved region of the vlhA gene using the most familiar conventional PCR-based technique. Due to a lack of applicable written data about MS isolation, PCR conformation, and antigenic cross immunity in various districts of Khyber Pakhtunkhwa (KP), the current study was planned to characterize MS isolates through various tools, including PCR. Materials and MethodsA total of 434 specimens as well as environmental samples, were collected for an unknown population with a 95% confidence interval from the forty commercial poultry (broiler) farmhouses of various localities (districts) of KP. Eight (n=8) closed sheds were selected from Abbottabad and Mansehra 4 from each district. Thirty-two (n=32) open broiler sheds were nominated from D.I. Khan, Peshawar, and Tank as 12, 10, and 10, respectively. Specimen samples were collected aseptically from the birds with clinical symptoms of respiratory distress. Only one specific specimen sample was collected from individual birds using a separate, properly disinfected cotton bud. The detail of specimen samples (n=150) collected from broiler birds is mentioned below in Table 1. Two hundred and sixty-four (264) samples from housing premises including: droppings (n=51), drinkers (n=54), feeder (n=57), feathers (n=51), and dust (n=51) were taken from the forty (n=40) selected broiler farmhouses in various localities of KP. Only 20 tissue samples from desi (local) poultry were also collected from various desi poultry processing points in the District Dera Ismail Khan (n=10) and Tank (n=10). For further processing, every sample was aseptically put into a sealed test tube filled with Modified Frey’s broth (Kleven, 1998) in an iced container and transported to the University of Agriculture Faisalabad. The Biochemical characterization was completed by performing some specific tests such as film and spot assay, catalase test, tetrazolium reduction test, phosphatase test, and glucose test to segregate different species of Genus Mycoplasma. DNA extraction of MSAfter biochemical conformation, the molecular characterization was done by extracting the DNA from the recovered Mycoplasma cells as initially characterized on the basis of biochemical tests. The DNA extraction kit (TIANGEN, China) was used to extract MS DNA, succeeding the procedure as instructed by Cheng et al. (2014). Table 1. District wise and organ-based distribution of specimen samples from 40 commercial broiler farms.
Table 2. PCR protocols to amplify MS DNA.
MS DNA amplificationAmplification of MS vlhA gene was performed using the below primer sequence;
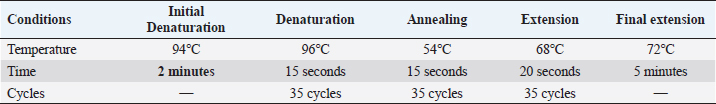
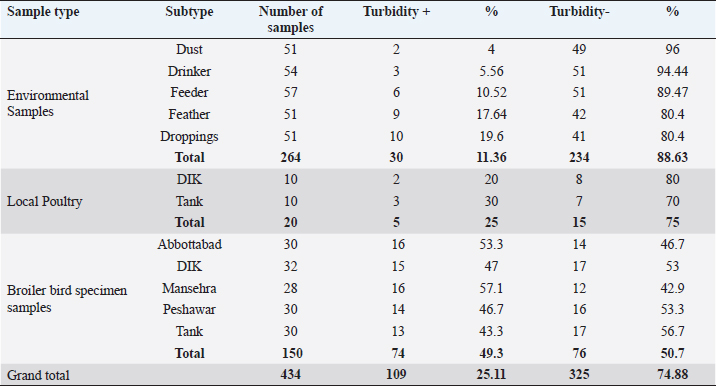
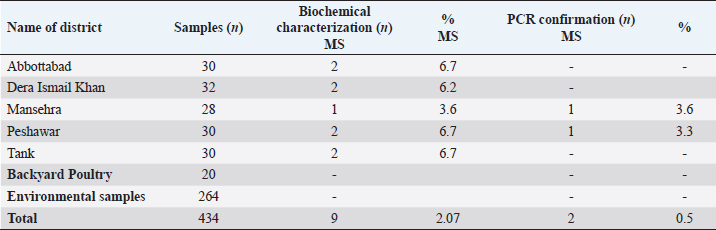
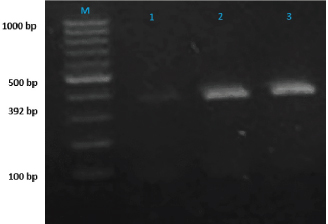
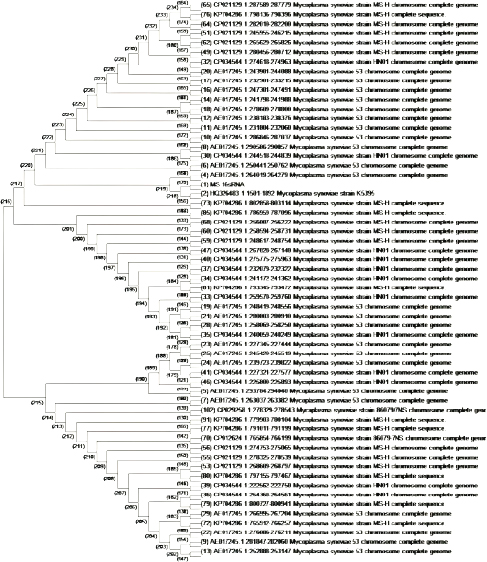
A 25 µL of master mix and 3 µL of MS DNA template were put into a PCR tube along with 1 µL of forward and reverse primers, 1 µL Taq DNA polymerase was mixed with 20 µL of nuclease-free water. Following the instructions as documented by Jeffery et al. (2007), the extracted DNA of MS was intensified in a thermal cycler, as illustrated in Table 2. After thermal cycling, the amplified product was processed for visualization. The process of electrophoresis was completed by making one percent agarose solutions, and Ethidium bromide was applied to stain the product of PCR. The stained product was finally pictured under an ultraviolet source (Sajid et al., 2020). MS antigen preparationTo study the immunogenic cross reactivity between the MS isolates recovered in this research, the Haemagglutination antigen was prepared as used by Ibrahim et al. (2018). The recovered isolates were dipped into Frey’s broth (Douma et al., 1989) along with phosphate buffer saline to maintain pH at 7.2. The suspension was then centrifuged three times, and the final dilution was prepared with a protein concentration of 1 mg per ml and kept in a freezer (-20°C) for future use. A 0.3 percent solution of formaldehyde and antigen suspension were assorted with each other for 15 minutes and placed in the refrigerator for six hours. After formaldehyde treatment, the inactivated MS antigen was then mixed with Montanide (SEPPIC, France) adjuvant and kept in a refrigerator for future use (Lind et al., 1984). The safety and sterility tests were completed before being administered to rabbits. Relative study on cross antigenic titersA total of fifteen rabbits were distributed in 3 groups (Group I, Group II, and Group III). Five rabbits were allotted to every group. All the rabbits of Group I and Group II were injected ½ ml of inactivated adjuvanted MS-1 and MS-2 antigen using subcutaneous route, respectively, as an initial dose and booster dose (0.5 ml) was given after seven days of the initial dose. The Group III was left un-inoculated (control). This practice of rising antiserum against whole MS cells in rabbits was also documented by Poveda et al. (1990). Fourteen days after the second (booster) dose, the sera samples were obtained from the rabbits of Group I, Group II, and Group III. Fresh poultry blood was taken in a sterilized test tube with EDTA as an anticoagulant to prepare a 1% solution of three times washed RBCs for the HI test. The highest dilution of the serum completely preventing Haemagglutination, was taken as the HI titer (Allan and Gough, 1974). ResultsIn environmental samples, the overall percentage of turbidity positive is 11.36% (30/264). The droppings were found with the highest prevalence (19.6%), then feathers (17.64%), feeders (10.52%), drinkers (5.56%), and dust (4%), as indicated in Table 3. Out of 20 tissue samples collected from Desi (Local) poultry in Tank, the turbidity positive prevalence was high (30%) as compared to DIK (20%), as shown in Table 3. Broiler specimen samples (n=150), 74 samples (49.3%) showed turbidity out of the total samples. The highest percentage of positive samples was recorded in Mansehra (57.3%), then Abbottabad (53.3%), DIKhan (47%), Peshawar (46.7%) and Tank (43.3%) as shown in Table 3. On the basis of biochemical characterization, the MS positivity percentage was documented as 6.7% in Peshawar, Tank, and Abbottabad, as indicated in Table 4. The overall MS prevalence percentage based on biochemical tests was recorded as 2.07%. PCR confirmed two MS isolates from the specimen samples with 0.5% as the overall percentage. These MS isolates were, named MS-1 and MS-2, that were only detected positive for MS by PCR. The PCR amplicon of 392 bp was recorded as a positive sample in Lane 2 and Lane 3, as indicated in Figure 1. Lane 1 showed a negative sample. After amplification, the product amplified product was processed for visualization. For the purpose of electrophoresis, one percent agarose solutions were prepared, and the molecular product was pictured under an ultraviolet source after staining with Ethidium bromide (Sajid et al., 2020), as shown in Figure 1. The recovered MS sequence was submitted to National Centre for Biotechnology Information (NCBI) under accession number MW397013 and on the basis of vlhA gene HQ326483.1, was found to be similar to MS isolates as shown in Figure 2. The molecular product was sent to Advanced Bioscience Internationals, Singapore, for sequencing through BLAST. The Phylogenetic analysis was performed using Mega X software, as indicated in Figure 2. Table 3. Distribution and Turbidity based results of specimen samples taken from Broiler Farm environment, local poultry and broiler birds of various broiler farms in five major districts of Khyber Pakhtunkhwa.
Table 4. Biochemical and molecular confirmation of specimen samples collected from various localities (Districts) of KP.
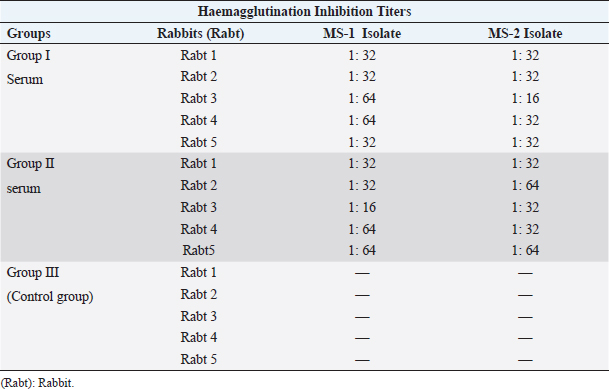
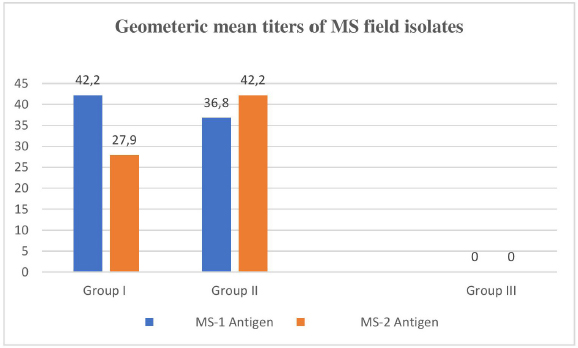
Immunogenic cross reactivityThe minimum homologous antibody titer of the serum of Group I MS (rabt) against MS-1 was recorded as1:32 and the maximum titer was 1:64. The maximum heterologous HI titers of Group I MS rabbit’s serum against MS-2 isolate antigen ranged as (1:16 and 1:32) as shown in Table 5. Whereas the homologous and heterologous HI titers of Group II MS rabbit’s serum against MS-2 isolate antigen were recorded as 1:16–1:64 and 1:32–1:64, respectively, as indicated in Table 5. Null titer was recorded in Group III as it was denoted as the control group. In both groups of experimental rabbits, the homologous GMT value (42.2) of MS-1 and MS-2 isolates was similar and recorded as (100%). However, the heterologous GMT value in the serum of MS I against MS 2 isolate was lower (27.9% or 66%), as shown in Figure 3. Likewise, the heterologous GMT for MS-2 serum against MS-1 isolate was recorded as (38.9% or 92.2%) GMT. DiscussionThe molecular confirmatory study of MS was performed using PCR technique as documented by Jeffery et al. (2007). The present study was conducted on commercial poultry (broilers) to study the occurrence of MS, and it was found to be 0.5% with an amplicon of 392 base pairs, while (Gharibi et al., 2018) found 0% MS occurrence in broilers. In Iranian broilers, 38% MS prevalence was confirmed (Ghaniei, 2016) through vlhA gene amplification. Amer et al. (2019) documented a high prevalence rate (100%) in Egyptian broiler breeders using vlhA gene primers. An amplicon of 396 bp was generated from all 12 joint samples. Rajkumar et al. (2018) carried out MS PCR through the amplification of vlhA gene, and a product of 392 bp was generated in most of the field isolates with 33.0% MS prevalence, which is higher than the current study. In contrast to our study, Tomar et al. (2017) also documented a higher (2.1%) MS prevalence in broiler birds in Haryana state of India. The differentiation study of Genus Mycoplasma, MG, and MS using distinct primers for each was carried out by Malekhoseini et al. (2017). The MG was identified by amplifying its 16S rRNA gene with an amplicon of (185 bp), and MS was recognized using vlhA gene (392 bp) with precise primers similar to the procedure applied in the current study.
Fig. 1. Lane M shows PCR marker (1,000 base pairs) while Lane1-3 symbolize samples.
Fig. 2. MS with extreme resemblance by Phylogenetic study using Mega XI software (neighbor joining). Table 5. The Haemagglutination inhibition titers documented among the MS field strains in rabbits.
Fig. 3. Geometric mean titers of MS field isolates. Using the molecular method of MS characterization (Multiplex PCR) through the amplification of vlhA gene of MS,found a 25.3% prevalence of MS in Egyptian uninhabited birds. Rasool et al. (2018) also amplified and sequenced the 16 S r RNA (213 bp) and vlhA gene (375 bp) to differentiate MS from various field strains. Out of 40 Mycoplasma isolates, 20 (50%) were identified as MS through 16 S r RNA, and five (12.5%) were characterized as MS through fractional vlhA gene analysis. Both MS field isolates (MS-1 and MS-2) showed the same homologous immunogenic titer of (42.2 GMT) as a result of the Haemagglutination Inhibition test. There were some variations in heterologous values. The MS-2 isolate showed a lower (66%) heterologous immune response with MS-1 serum as compared to MS-1 isolate (87%) against MS-2 serum, as indicated in Figure 3. Similar findings were recorded by Weinack and Snoeyenbos (1976), who also elaborated on the immunogenic alterations amongst three stains of MS. The differential study using the haemagglutination inhibition test amongst the field isolates of MS and standard WVU 1853 MS strain was carried out by Vardaman and Drott (1980). Vardaman and Yoder also found increased levels of HI titers in homologous MG and MS antigens than the homologous HI levels (1969). A high level of homologous antigenic cross reactivity as compared to heterologous was also studied in different M. gallisepticum field strains isolated from commercial poultry (broiler) farmhouses in KP (Pakistan) by Rehman et al. (2022). A variety of serological tests were used to explore the existence of antigenic cross reactivity in different isolates of M. pneumoniae and M. genitallium. The study was carried out in the rabbits that were previously immunized with the different isolates of M. pneumoniae and M. genitallium, and antiserum was raised, as documented by Lind et al. (1984). Fakruddin et al. performed various serological techniques on food samples to study the cross reactions in different bacterial species and recorded robust immunological cross reactivity between Enterobacteriaceae and virulent bacteria present in various types of food stuff (2015). Current advancements in the invention of apparatuses and procedures to divine (Agrawal, 2019) cross immunity against various types of microorganisms will be helpful in the production of vaccines. ConclusionThe presence of MS in broiler birds was confirmed by cultural, biochemical, and molecular tests in five districts of KP. The results of the immunological cross-reactivity showed that homologous GMT titers were high in comparison to heterologous GMT titers. Routine monitoring of the pathogen will be helpful to overcome the disease. The most authentic, easy, and reliable technique was PCR assay among all the techniques performed in this study. AcknowledgmentsThe authors would like to express their sincere gratitude to Professor Sajjad Ur Rahman for his invaluable assistance and guidance during the duration of the study. Conflict of interestAll authors declare that there is no conflict of interest. Authors contributionsConceptualization: Sajjad Ur Rahman and Abdul Haleem Shah. Data curation: Atta Ur Rehman Formal analysis Saifur Rehman, Kamal Shah, and Inamullah Malik, Funding acquisition: Widya Paramita Lokapirnasari and Atta Ur Rehman. Investigation: Sajjad Ur Rahman and Atta Ur Rehman. Methodology: Atta Ur Rehman, Sajjad Ur Rahman, and Abdul Haleem Shah. Project administration: Atta Ur Rehman; Imdadullah Khan, and Inamullah Malik Resources: Widya Paramita Lokapirnasari; Software: Atta Ur Rehman and Saifur Rehman Supervision: Sajjad Ur Rahman and Abdul Haleem Shah Validation: Atta Ur Rehman, Abdul Haleem Shah, Sajjad Ur Rahman; Saifur Rehman Muhammad Kamal shah, Widya Paramita Lokapirnasari, Imdadullah Khan, Inamullah Malik and Andreas Berny Yulianto, Writing - original draft: Attaur rehman; Saifur rehman; Writing - review and editing: All authors: FundingThe Faculty of Veterinary Medicine (Fakultas Kedokteran Hewan), Universitas Airlangga, as indicated by document number (769/UN3.14/PT/2020), supported the APC. Data availabilityAdditional data may be obtained from the primary author and corresponding author upon a reasonable request. ReferencesAbbas, N., Muhammad, S., Naveed, M., Rahman, S., Ahmad, K.N., Tariq, N.A., Khalid, A. and Faheem, J. 2018. Seropositivity, involvement in suspected cases of chronic respiratory diseases and comparative efficacy of various sero-diagnostic tests of Mycoplasma gallisepticum. J. Appl. Environ. Biol. Sci. 8(3), 137–141. Agrawal, B. 2019. Heterologous immunity: role in natural and vaccine-induced resistance to infections. Front. Immunol. 10, 2631. Allan, W.H. and Gough, R.E. 1974. A standard haemagglutination inhibition test for newcastle disease. (1). A comparison of macro and micro methods. Vet. Rec. 95(6), 120–123. Amer, M.M., Mekky, H.M., & Fedawy, H.S. 2019. Molecular identification of Mycoplasma synoviae from breeder chicken flock showing arthritis in Egypt. Vet. World 12(4), 535–541. Beylefeld, A. 2018. Genomic comparison of mycoplasma species isolated from commercial chickens in South Africa (Doctoral dissertation, University of Pretoria). Buim, M.R., Mettifogo, E., Timenetsky, J., Kleven, S. and Ferreira, A.J.P. 2009. Epidemiological survey on Mycoplasma gallisepticum and M. synoviae by multiplex PCR in commercial poultry. Brazil J. Vet. Res. 29, 552–556. Cheng, X., He, W., Huang, F., Huang, M. and Zhou, G., 2014. Multiplex real-time PCR for the identification and quantification of DNA from duck, pig and chicken in Chinese blood curds. Food Res. Int. 60, 30–37. Dhondt, A.A., Dhondt, K.V., Hawley, D.M. and Jennelle, C.S. 2007. Experimental evidence for transmission of Mycoplasma gallisepticum in house finches by fomites. Avian Pathol. 36(3), 205–208. Diene, S.M., Bertelli, C., Pillonel, T., Jacquier, N., Croxatto, A., Jaton, K. and Greub, G. 2016. Comparative genomics of Neisseria meningitidis strains: new targets for molecular diagnostics. Clin. Microbiol. Infect. 22(6), 568.e1–7. Dijkman, R., Feberwee, A. and Landman, W.J. 2014. Variable lipoprotein haemagglutinin (vlhA) gene sequence typing of mainly Dutch Mycoplasma synoviae isolates: comparison with vlhA sequences from Genbank and with amplified fragment length polymorphism analysis. Avian pathol. 43(5), 465–472. Douma, R., Dular, R., Brodeur, B.R. and Kasatiya, S.S. 1989. Development and application of monoclonal antibodies for the detection of cell infecting mycoplasmas. J. Immunol. Methods 124(2), 197–203. Dufour-Gesbert, F., Dheilly, A., Marois, C. and Kempf, I. 2006. Epidemiological study on Mycoplasma synoviae infection in layers. Vet. Microbiol. 114(1–2), 148–154. Fakruddin, M., Rahaman, M.M., Ahmed, M.M. and Hoque, M.M. 2015. Occurrence of enterobacteriaceae with serological cross reactivity towards Salmonella spp., Shigella spp. and Vibrio cholerae in food. Br. Microbiol. Res. J. 5(1), 44. Gharibi, D., Ghadimipour, R. and Mayahi, M. 2018. Detection of Mycoplasma gallisepticum and Mycoplasma synoviae among commercial poultry in Khouzestan Province Iran. Arch. Razi. Inst. 73(2), 139–146. Ghaniei A. 2016. Molecular characterization of Mycoplasma synoviae isolated from broiler chickens of West Azarbaijan province by PCR of vlhA gene. Vet. Res. Forum 7(3), 197–202. Ibrahim, F.F., Abd El-Ghany, W.A., El Rawy, E.M., Shaker, M.M. and El-Jakee, J. 2018. The protective efficacy of locally prepared combined inactivated Mycoplasma gallisepticum and Pasteurella multocida vaccine in chickens. Bio. Res. 15(2), 702–707. Jeffery, N., Gasser, R.B., Steer, P.A. and Noormohammadi, A.H. 2007. Classification of Mycoplasma synoviae strains using single-strand conformation polymorphism and high-resolution melting-curve analysis of the vlhA gene single-copy region. Microbiol. Russ. Acad. Sci. 153(8), 2679–2688. Kleven, S. 1998. Mycoplasmas in the etiology of multifactorial respiratory disease. Poult. Sci. 77(8), 1146–1149. Kleven, S. 2008. Control of avian mycoplasma infections in commercial poultry. Avian Dis. 52(3), 367–374. Kursa, O., Tomczyk, G. and Sawicka, A. 2019. Prevalence and Phylogenetic analysis of Mycoplasma synoviae strains isolated from polish chicken layer flocks. J. Vet. Res. 63(1), 41–49. Lin, M. and Kleven, S. 1982. Cross-immunity and antigenic relationships among five strains of Mycoplasma gallisepticum in young Leghorn chickens. Avian Dis. 26(3), 496–507. Lind, K., Lindhardt, B.O., Schütten, H.J., Blom, J. and Christiansen, C. 1984. Serological cross-reactions between Mycoplasma genitalium and Mycoplasma pneumoniae. J. Clin. Microbiol. 20(6), 1036–1043. Malekhoseini, G., Pourbakhsh, S., Homayounimehr, A., Zolfeghari, M., Ashtari, A. and Abtin, A. 2017. Simultaneous identification of Mycoplasma gallisepticum and Mycoplasma synoviae by duplex PCR assay. J. Immunol. Case Rep. 1(1), 12–16. Marois, C., Picault, J.P., Kobisch, M. and Kempf, I. 2005. Experimental evidence of indirect transmission of Mycoplasma synoviae. Vet. Res. 36(5–6), 759–769. Matucci, A., Stefani, E., Gastaldelli, M., Rossi, I., De Grandi, G., Gyuranecz, M. and Catania, S. 2020. Molecular differentiation of Mycoplasma gallisepticum Outbreaks: a last decade study on Italian farms using GTS and MLST. Vaccines 8(4), 665. Nicholas, R.A. and Ayling, R.D. 2003. Mycoplasma bovis: disease, diagnosis, and control. Res. Vet. Sci. 74(2), 105–112. Poveda, J.B., Carranza, J., Miranda, A., Garrido, A., Hermoso, M., Fernandez, A. and Domenech, J. 1990. An epizootiological study of avian mycoplasmas in southern Spain. Avian Pathol. 19(4), 627–633. Rasool, A., Anjum, A.A., Rabbani, M., Lateef, M., Akhter, F., Afroz, H., Muhammad, J. and Nawaz, M. 2018. Molecular characterization and phylogenetic analysis of Mycoplasma synoviae isolated from chicken. J. Anim. Plant Sci. 28(2), 491–497. Sajid, S., ur Rahman, S., Nayab, S., Khan, I.U. and Javeed, A., 2020. 71. Infectious bursal disease virus cloning and structural protein (VP2) expression in Escherichia coli. Pure Appl. Biol. 9(1), 743–749. Silva, R., do Nascimento, E.R., de Almeida Pereira, V.L., Barreto, M.L. and Do Nascimento, M. 2008. Mycoplasma synoviae infection on Newcastle disease vaccination of chickens. Brazil. J. Microbiol. 39(2), 384–389. Tomar, P., Singh, Y., Mahajan, N., Jindal, N. and Singh, M. 2017. Molecular detection of avian mycoplasmas in poultry affected with respiratory infections in Haryana (India). Int. J. Curr. Microbiol. App. Sci. 6(6), 2155–2162. Rehman, A., Shah, A.H., Ur Rahman, S., Sajid, S., Khan, I.U., Ullah, Q., Farid, A. and Khan, M.H. 2022. Molecular Confirmation and Immunological Cross Reactivity among Mycoplasma gallisepticum Isolates Recovered from Broiler Chicken in Khyber Pakhtunkhwa, Pakistan. Pak. Vet. J. 42(4), 1–6. Vardaman, T.H. and Drott, J.H. 1980. Comparison of Mycoplasma synoviae hemagglutinating antigens by the hemagglutination-inhibition test. Avian Dis. 24(3), 637–640. Vardaman, T.H. and Yoder Jr.H.W. 1969. Preparation of Mycoplasma synoviae hemagglutinating antigen and its use in the hemagglutination-inhibition test. Avian Dis. 13(3), 654–661. Weinack, O.M. and Snoeyenbos, G.H. 1976. Serologic studies with Mycoplasma synoviae in experimentally inoculated chickens. Avian Dis. 20(2), 253–259. Wise K.S. 1993. Adaptive surface variation in mycoplasmas. Trend. Microbiol. 1(2), 59–63. | ||
| How to Cite this Article |
| Pubmed Style Rehman AU, Shah AH, Rahman SU, Rehman S, Shah MK, Lokapirnasari WP, Khan I, Malik I, Yulianto AB. Molecular identification and cross immunogenic study on two field isolates of Mycoplasma synoviae isolated from broilers in five districts of Khyber Pakhtunkhwa. Open Vet J. 2023; 13(10): 1299-1307. doi:10.5455/OVJ.2023.v13.i10.9 Web Style Rehman AU, Shah AH, Rahman SU, Rehman S, Shah MK, Lokapirnasari WP, Khan I, Malik I, Yulianto AB. Molecular identification and cross immunogenic study on two field isolates of Mycoplasma synoviae isolated from broilers in five districts of Khyber Pakhtunkhwa. https://www.openveterinaryjournal.com/?mno=161053 [Access: July 27, 2024]. doi:10.5455/OVJ.2023.v13.i10.9 AMA (American Medical Association) Style Rehman AU, Shah AH, Rahman SU, Rehman S, Shah MK, Lokapirnasari WP, Khan I, Malik I, Yulianto AB. Molecular identification and cross immunogenic study on two field isolates of Mycoplasma synoviae isolated from broilers in five districts of Khyber Pakhtunkhwa. Open Vet J. 2023; 13(10): 1299-1307. doi:10.5455/OVJ.2023.v13.i10.9 Vancouver/ICMJE Style Rehman AU, Shah AH, Rahman SU, Rehman S, Shah MK, Lokapirnasari WP, Khan I, Malik I, Yulianto AB. Molecular identification and cross immunogenic study on two field isolates of Mycoplasma synoviae isolated from broilers in five districts of Khyber Pakhtunkhwa. Open Vet J. (2023), [cited July 27, 2024]; 13(10): 1299-1307. doi:10.5455/OVJ.2023.v13.i10.9 Harvard Style Rehman, A. U., Shah, . A. H., Rahman, . S. U., Rehman, . S., Shah, . M. K., Lokapirnasari, . W. P., Khan, . I., Malik, . I. & Yulianto, . A. B. (2023) Molecular identification and cross immunogenic study on two field isolates of Mycoplasma synoviae isolated from broilers in five districts of Khyber Pakhtunkhwa. Open Vet J, 13 (10), 1299-1307. doi:10.5455/OVJ.2023.v13.i10.9 Turabian Style Rehman, Atta Ur, Abdul Haleem Shah, Sajjad Ur Rahman, Saifur Rehman, Muhammad Kamal Shah, Widya Paramita Lokapirnasari, Imdadullah Khan, Inamullah Malik, and Andreas Berny Yulianto. 2023. Molecular identification and cross immunogenic study on two field isolates of Mycoplasma synoviae isolated from broilers in five districts of Khyber Pakhtunkhwa. Open Veterinary Journal, 13 (10), 1299-1307. doi:10.5455/OVJ.2023.v13.i10.9 Chicago Style Rehman, Atta Ur, Abdul Haleem Shah, Sajjad Ur Rahman, Saifur Rehman, Muhammad Kamal Shah, Widya Paramita Lokapirnasari, Imdadullah Khan, Inamullah Malik, and Andreas Berny Yulianto. "Molecular identification and cross immunogenic study on two field isolates of Mycoplasma synoviae isolated from broilers in five districts of Khyber Pakhtunkhwa." Open Veterinary Journal 13 (2023), 1299-1307. doi:10.5455/OVJ.2023.v13.i10.9 MLA (The Modern Language Association) Style Rehman, Atta Ur, Abdul Haleem Shah, Sajjad Ur Rahman, Saifur Rehman, Muhammad Kamal Shah, Widya Paramita Lokapirnasari, Imdadullah Khan, Inamullah Malik, and Andreas Berny Yulianto. "Molecular identification and cross immunogenic study on two field isolates of Mycoplasma synoviae isolated from broilers in five districts of Khyber Pakhtunkhwa." Open Veterinary Journal 13.10 (2023), 1299-1307. Print. doi:10.5455/OVJ.2023.v13.i10.9 APA (American Psychological Association) Style Rehman, A. U., Shah, . A. H., Rahman, . S. U., Rehman, . S., Shah, . M. K., Lokapirnasari, . W. P., Khan, . I., Malik, . I. & Yulianto, . A. B. (2023) Molecular identification and cross immunogenic study on two field isolates of Mycoplasma synoviae isolated from broilers in five districts of Khyber Pakhtunkhwa. Open Veterinary Journal, 13 (10), 1299-1307. doi:10.5455/OVJ.2023.v13.i10.9 |