| Review Article | ||
Open Vet J. 2023; 13(12): 1504-1516 Open Veterinary Journal, (2023), Vol. 13(12): 1504–1516 Review Article Chitosan hydrogel nanoparticle enhance therapeutic effect of bovine umbilical mesenchymal stem cell conditioned medium on canine cognitive dysfunction or canine Alzheimer’s like mediated by inhibition of neuronal apoptoticHevi Wihadmadyatami1*, Muhammad Ali Zulfikar2, Herawati Herawati3, Dyah Ayu O.A. Pratama4, Golda Rani Saragih1, Ulayatul Kustiati5 and Nurrahmi Handayani21Department of Anatomy, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia 2Department of Chemistry, Bandung Institute of Technology, Bandung, Indonesia 3Laboratory of Veterinary Public Health, Faculty of Veterinary Medicine, Brawijaya University, Malang, Indonesia 4Laboratory of Pathology, Faculty of Veterinary Medicine, Brawijaya University, Malang, Indonesia 5Laboratory of Pharmacology, Faculty of Veterinary Medicine, Brawijaya University, Malang, Indonesia *Corresponding Author: Hevi Wihadmadyatami. Department of Anatomy, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia. Email: heviwihadmadyatami [at] ugm.ac.id Submitted: 26/07/2023 Accepted: 18/12/2023 Published: 31/12/2023 © 2023 Open Veterinary Journal
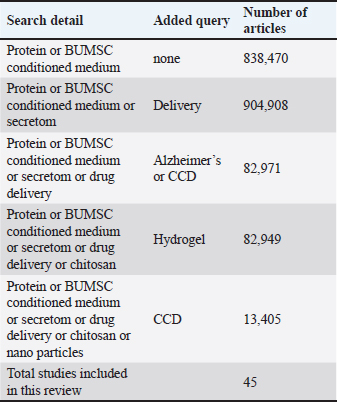
ABSTRACTIn treating brain diseases, such as canine cognitive dysfunction (CCD), most currently available potent drugs have weak therapeutic efficacy. One of the causes is the inability of the substance to reach the brain in therapeutic quantities. These pharmaceuticals lacked targeted mechanisms for drug delivery, coming about in an elevated drug concentration in imperative organs, which drove to drug harmfulness. In recent years, cell-free treatment (conditioned medium) determined from animal and human stem cells has provided new promise for treating brain diseases, as CM can stimulate the regeneration of neurons and prevent the inflammation and apoptotic of neurons caused by pathology or aging. On the other hand, it is well known that chitosan-hydrogel (CH) is a polymer derived from natural sources. It has been authorized for use in biomedical use because of its uncommon biodegradability, biocompatibility, and mucoadhesive properties. CH modification has been utilized to generate nanoparticles (NPs) for intranasal and intravenous brain targeting. NPs shown upgraded drug take-up to the brain with decreased side impacts due to their drawn out contact time with the nasal mucosa, surface charge, nanosize, and capacity to extend the tight intersections inside the mucosa. Due to the aforementioned distinctive characteristics, developing Chitosan Hydrogel Nanoparticles load with bovine umbilical mesenchymal stem cell conditioned medium is crucial as a new therapeutic strategy for CCD. Keywords: Bovine umbilical mesenchymal stem cell, Canine cognitive dysfunction, Cell-free therapy, Chitosan hydrogel nanoparticle, Secretome. IntroductionNeurodegenerative diseases are becoming a worldwide well-being concern. One of the foremost extreme neurodegenerative diseases is Alzheimer’s disease (AD) and its prevalence is increasing exponentially around the world. About 50 million individuals are right now beset by the illness, and its predominance is anticipated to triple by 2050. As same as like AD, it is estimated that canine cognitive dysfunction (CCD) affects over 15 and 30 million dogs in the Europe and United States, respectively. Recently in the United States, the prevalence of CCD ranges from 14% to 60% (Dewey et al., 2019). Age is the foremost noteworthy risk figure for the advancement of CCD, which influences up to 60% of elderly canines, fundamentally those with the age of more than 11 years. In addition, research of neurodegenerative diseases in animals has uncovered striking likenesses among cognitive dysfunction in canines as well as the majority of other primates and AD in humans (Braidy et al., 2015; Schütt et al., 2016; Urfer et al., 2021). CCD and AD share numerous similarities. Alzheimer’s and CCD patients exhibit comparable neuropathological alterations influencing cerebral angiopathy, cerebral gyri (cerebral atrophy), sulcal widening, and ventricular broadening. As the illness advances, the dogs slowly lose the capacity to communicate and display decreased cognitive capacities. Clinically, CCD is characterized by a few behavioral changes, counting confusion, diminished social interplay, changes in sleep schedule, diminished motion, expanded uneasiness, and shortfalls in learning and memory. CCD is analyzed by recognizing behavioral indications and administering other restorative conditions that will imitate CCD or bewilder its determination. CCD, alluded to as CCD syndrome, canine Alzheimer’s, or canine dementia, influences up to sixty percent of senior canines, essentially those older than eleven years (Fast et al., 2013). There are no breed-specific contrasts within the predominance of CCD (Mihevc and Majdič, 2019), nor are there any breed-specific contrasts within the pathology or clinical signs of the illness. Since bigger breeds of canines have a briefer life hope than smaller breeds (MacQuiddy et al., 2022), clinical signs of CCD are watched and detailed more as frequently as possible in smaller canines (Schmidt et al., 2015). CCD is difficult to treat because there are few available medications, and systemically administered pharmaceuticals cannot enter the brain because of decreased blood-brain barrier (BBB) permeability. Recently only one medication “Selegiline” used to treat the CCD. In the consequence of the predominance of age as a factor of dementia in canines and humans, finding an effective treatment for these debilitating diseases is crucial. Furthermore, to its closeness to AD, CCD is additionally amazingly attractive as a human infection model of AD. CCD is additionally a great issue for the wellbeing of older dogs. It is, hence, moreover of intrigued for creating novel demonstrative strategies or markers and solutions for medicating this illness in dogs. In recent years, the application of stem cells and secretome therapy (conditioned medium) derived from animals and humans have become extremely popular and offers new hope for the treatment of incurable diseases. This article examines the chitosan hydrogel nanoparticles (CHNPs) loaded bovine umbilical mesenchymal stem cell (BUMSC) conditioned medium as a modern medical approach for CCD in greater detail. MethodsThis review undertook a protocol-driven search of the literature in PubMed using the keywords bovine umbilical stem cell, conditioned medium, protein, drug, delivery, CCD disorder, nanoparticle (NP), and chitosan to identify recent studies. In order to complement and provide a comprehensive review and qualitative analysis of the literature search results, snowballing techniques such as reference chasing and citation searching in relevant articles and journals were employed. Search ResultsTable 1 presents a comprehensive information search of details, queries, and research in this review. The keywords were utilized to locate previous research that employed chitosan hydrogel (CH) as a drug-delivering vehicle in the context of stem cell and cell-free therapy (conditioned medium/secretome). Although there is great potential and numerous studies on cell and cell-free therapy research in the field of neurodegeneration, particularly in CCD studies, the utilization of CHNPs as an adjuvant or drug delivery system for BUMSC conditioned medium has not been thoroughly investigated. There is potential to enhance the system in order to create a new method for enhancing and establishing consistent use of CHNPs, particularly for mesenchymal stem cell (MSC) conditioned medium in cases of CCD (Fig. 1). Table 1. Comprehensive information search of details, queries, and research in this review.
Canine cognitive disfunction disorder (canine Alzheimer’s Like)CCD and or canine Alzheimer’s Like is a medical illness that impairs the cognitive abilities of aging canines. Like humans, our furry companions are susceptible to alterations in memory, problem-solving abilities, and overall mental function. The most prevalent symptom of CCD is confusion or disorientation, getting disoriented in familiar environments, or having trouble returning home during walks. The dogs may also display altered sleep patterns, such as restlessness or excessive daytime dozing. Another clinical sign to watch out for is altered social behavior. Dogs with CCD may become withdrawn and less interested in interacting with their human family members or other pets. They may also display increased anxiety, pacing back and forth or exhibiting repetitive behaviors. Importantly, the prevalence of CCD increases with age. As both canines and people live longer, neurodegenerative diseases are getting to be more predominant. In 2016, there were 77 million possessed canines within the United States, of which 15% (just over 11.5 million) were 11 years old. The predominance of CCD is assessed to run from 14% to over 60%, expanding as the dog ages. According to a few studies, the predominance of CCD in canines aged 11–12 years was 28%, and 68% in 15–16 years old (Madari et al., 2015; Katina et al., 2016; MacQuiddy et al., 2022).
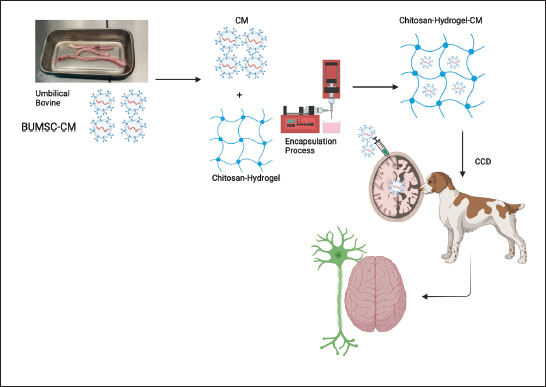
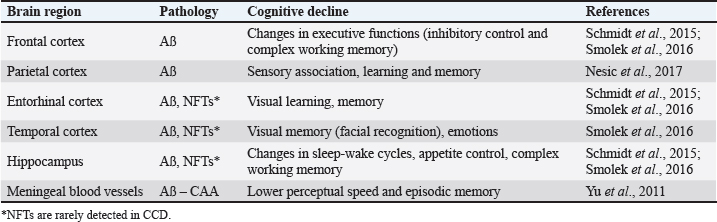
Fig. 1. Use of CHNPs, particularly for MSC conditioned medium in cases of CCD. The etiology of CCD could be caused by genetics, alterations in brain chemistry, oxidative stress, and brain inflammation. CCD and AD in humans share striking similarities. Both diseases are characterized by the collection of unusual proteins (amyloid plaques) in brain tissue, which disturb ordinary neural work (Fig. 2). An extracellular plaques accumulation in the brain parenchyma and abnormally phosphorylated protein TAU accumulation in neuro fibrile tangles (NFT) also prominent neuropathological signs of AD and CCD (Abey et al., 2020). It is believed that these pathological characteristics disrupt cognitive and behavioral shifts. The amino acid sequence of APP and the enzymes that regulate the execution of APP are extremely similar in humans and canines. APP-770, APP-751, APP-695, and APP-714 are the major APP isoforms in canines. The canine APP-770 shares 98.3% resemblance with the amyloid-beta precursor protein in humans and 96.9% amino acid identity, rendering them virtually identical. Amyloid was found as insoluble plaques within the cerebral cortex of people as well as canines, and cognitive disability in old canines was unequivocally connected to the hoarding of amyloid within the brain (Barnes et al., 2016). On the canine, the arrangement and development of amyloid stores were seen by immunostaining all over the cortical gray matter layers, which is additionally characteristic of human AD. The accumulation of amyloid protein relates to the seriousness of cognitive disability in the dog and varies with age and size (Schmidt et al., 2015). The amyloid plaque was more frequent in little and medium-sized canines due to the longer life span of small-breed dogs and, subsequently, the longer time required to construct up amyloid stores. Most research on CCD was conducted on different breeds of old dogs; subsequently, the pathology of the brain is essentially depicted with no advance elaboration on the characteristics of individual dogs. Normal disease onset occurs in the canine prefrontal cortex, which then extends to the parietal, occipital cortices, entorhinal, and hippocampus (Table 2) (Schmidt et al., 2015; Ozawa et al., 2016; Schütt et al., 2016; Smolek et al., 2016; Borghys et al., 2017). Moreover, pE3AA, an extremely neurotoxic subspecies of pyroglutamyl A, was also detected in the hippocampus of demented canines. The prevalence of these plaques was greatest in small and medium-sized canines (Schmidt et al., 2015). In addition, research proposes that amyloid oligomers and protofibrils may be neurotoxic within the canine brain. The extent of amyloid-soluble oligomers within the cerebrospinal fluid (CSF) was conversely proportional to the amount of amyloid in the brains of CCD-affected beagles. Precipitates in CSF of a senile Samoyed dog with CCD were more neurotoxic than synthetic oligomeric and fibrillary forms of A (Rusbridge et al., 2018). In canines with CCD, besides the presence of amyloid plaque, activation of microglial and astrocytes along with hypertrophy of astrocytes have been observed (Smolek et al., 2016). Additionally, the levels of pathogenic amyloid within the prefrontal cortex of the canine were related favorably with age but not with the seriousness of cognitive shortages or neuronal cell loss (Gómez-Pinedo et al., 2016). Neprilysin (NEP) mRNA, which encodes a significant amyloid-degrading protein, was drained within the prefrontal cortex of matured canines with CCD (Canudas et al., 2014), This can be compared to AD, where regions with higher amyloid accumulation express lower levels of NEP.
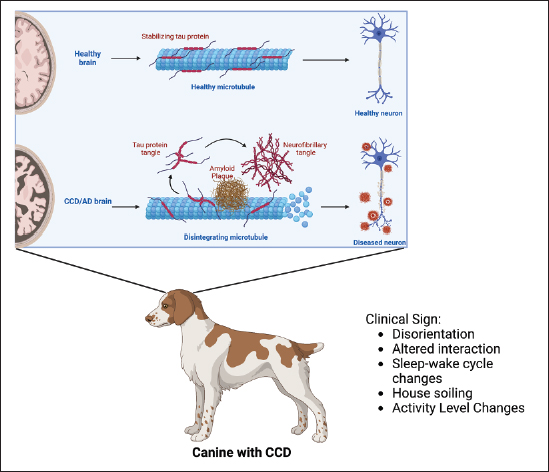
Fig. 2. CCD occurs due to the accumulation of aberrant proteins (amyloid plaques) in brain tissue and extracellular plaques in the brain parenchyma and abnormally phosphorylated protein TAU accumulation in NFT, which disrupts normal neural function so that it has an impact on symptoms that appear in dogs with CCD such as disorientation, altered interaction between the dog and its environment, sleep-wake cycle changes, house soiling, and activity level changes. Images is creating by Biorender. Table 2. Affected brain region on the dog with CCD.
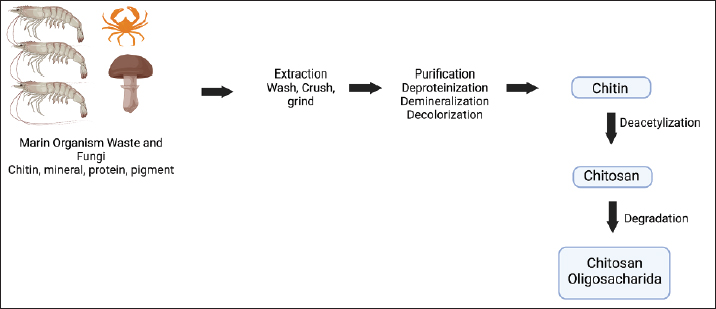
Degeneration of noradrenergic neurons connects with expanded amyloid deposition within the prefrontal cortex of geriatric canines with cognitive disability. Similarly, loss of cortical noradrenergic neurons and locus coeruleus degeneration occur early on in AD in humans (Kelly et al., 2017). Age also decreases the action of the canine cholinergic system. In aged cognitively impaired dogs, the total of basal forebrain cholinergic neurons was notably lower than in matured cognitively unimpaired and youthful canines. However, not at all like for noradrenergic neurons, this decrease did not connect with the degree of amyloid cortical stack. In AD, cholinergic neurons on the basal forebrain region, counting the neurons comprising the nucleus basalis of Meynert, are typically absent, as they are in CCD. Cholinergic neurons are the most susceptible to amyloid pathology in human patients, taken after by glutamatergic and GABAergic neurons. The loss of synapses correlates with the progression of the disease, and the destruction of cholinergic neurons in AD triggers memory and attention deficits. These neurotransmitter system alterations, accompanied by weakened synaptic and neuronal function, have also been noticed in canines, resulting in CCD symptoms. Like AD in humans, there is currently no exact treatment for CCD. Selegiline, or L-deprenyl, is the only drug licensed for CCD in certain countries, e.g., the United States. This drug may have a symptomatic effect on canines by slowing their cognitive decline. To increase the drug’s effectiveness, antioxidants, L-carnitine, or omega-3 fatty acids may be used as supplementation. Mental stimulation, such as training, playing, and sustaining daily routines, may contribute to maintaining a high standard of living. Of note, giving the selegiline may affect some side effect such as anorexia, diarrhea, and vomiting/salivation. Lastly, there needs to be a concern, although Selegiline prescription is able to maintain CCD, this drug was developed to maintain Parkinson’s Disease. This condition makes it important to have a new choice of medication for CCD. BUMSCsStem cells are non-specialized cells with the power to transform into any type of cell and possess the capability of self-regeneration (Zakrzewski et al., 2019). Somatic or mature stem cells are unspecialized and located among specialized cells throughout the entire body post-development. The purpose of these cells is to facilitate the repair, expansion, and substitution of cells that are lost on a daily basis. These cells have a limited selection of differentiation possibilities. Among numerous varieties, there are MSCs. MSC can be isolated from several tissues. In bone marrow, these cells primarily differentiate into bone, cartilage, and adipose cells. As stem cells, they are unique because they possess pluripotent capabilities and can specialize in cells from any embryonic layer (Debbarma et al., 2020). Research has demonstrated the capacity of MSC to change into different categories of skin cells, clusters of islet-like cells, and renal epithelial cells (Brasile et al., 2019; Jo et al., 2021; Jayasinghe et al., 2022). These three studies are merely a few illustrations of the substantial amount of information that has been gathered in the past ten years, which supports the potency of MSC to transdifferentiate when transplanted in vivo. Taking these findings into account, it has been conclusively established that MSCs can practically differentiate into three germ layers. In case MSCs display pluripotent markers and have the capacity to distinguish in vitro and transdifferentiate in vivo into three germ layers, it is possible that there is imprecision in the terminology used in some papers when referring to them as multipotent (Debbarma et al., 2020). Domestic bovines (Bos taurus) share resemblances with humans in terms of numerous anatomical and physiological traits, such as an extended lifespan. As a result, stem cells obtained from cattle could be a substitute cellular model in regenerative medicine. Furthermore, delving into the biology of stem cells in domestic animals possesses distinct attributes, especially when it comes to veterinary regenerative therapy (Hill et al., 2019). The initial investigation that isolated bovine umbilical cord blood MSC noted that the cells proliferated into single-layer cells and could be amplified into advanced generations. Furthermore, the cells displayed CD73 and OCT4 and had the capability to transform into bone-forming, cartilage-forming, and fat-forming lineages (Hill et al., 2019). In a different investigation, separate cells were sub-cultured until reaching passage 32 and exhibited the presence of CD29, CD73, CD44, CD166, and CD90. CD29 (integrin beta-1) is a member of the integrin families, a protein that acts as a beta subunit. It can combine with extracellular and surface proteins like CD51 and CD49a-f in MSCs to facilitate interactivity between cells in the extracellular matrix. CD44 is a glycoprotein found on the cell surface, and it functions as an adhesion molecule. It facilitates interactions between cells and the extracellular matrix, and it can attach to type I collagen and fibronectin. Additionally, it provides sites for MSCs to anchor and grow. Furthermore, these cells had the capacity to transform into bone-forming cells, fat-storing cells, liver cells, pancreatic cells, and nerve cells, suggesting their potential for use in research and medical purposes for cattle (Xiong et al., 2014). BUMSCs conditioned medium (BUMSC-CM)Despite suggestions that the transplantation of different MSCs is a reliable method for the regeneration of functional tissue, there are still numerous critical obstacles that need to be overcome before it can be implemented in a clinical setting (Uhlén et al., 2019). In the last few years, the secretory capabilities of MSCs have been extensively studied, particularly in relation to the bioactive molecules they release into their surrounding environment, known as the conditioned media (El Moshy et al., 2020). A crucial group of proteins are those that are effectively transported inside the secretory pathway for destinations outside the cytoplasm and nucleus of the cell. The assembly of effectively emitted proteins, hereinafter mentioned as “secretome” or conditioned medium (Uhlén et al., 2019). It is believed that Secretome or conditioned media has several advantages over stem cells and has the same benefits as stem cells, such as being rich in various growth factors, cytokines, chemokines, and enzymes, without tumorigenicity or immunogenicity. CM can be stored in the freezer, is readily packaged and transported, and does not need to be compatible with the donor. Research has indicated comparable or improved healing impact of secretome in different models of disease in contrast to the transplantation of stem cells (Muhammad, 2019). These elements are released into the outer cellular environment, where they control the growth, specialization, movement, and programmed cell death of various immune cells (Barrachina et al., 2016). Conditioned media can be easily extracted and have been shown to have impressive effects on the regeneration of mesenchymal tissue (Pawitan, 2014). Conditioned media has demonstrated numerous chemotactic proteins and growth factors (e.g., EGF, VEGF, FGF, HGF, TGF, TNF, IL-6, IL-8, and IGF). They function as a transduction signal, enabling intercellular communication to stimulate angiogenesis and the forming of new blood vessels, reduce inflammation, boost new neuron growth, modulate the immune system, and prevent cell death (Muhammad, 2019). It also includes molecules that promote adhesion, receptors, and substances that break down proteins, which play a role in the migration of MSCs (e.g., matrix metalloproteinases [MMPs], CCL-2, CXCL-12, CCR4, CCL-3, CXCR4, ICAM, VCAM, ADAM-12, and PECAM) (Kusindarta and Wihadmadyatami, 2021). The results by Kusindarta and Wihadmadyatami (2021) utilizing the metabolomic technique (LC-MS) uncovered that BUMSCs comprise numerous amino acids such as isoleucine, leucine, valin, ethanol, etc. These amino acids additionally encompass α-glucose and β-glucose, nicotinamide, nutrients, as well as the final outcome of the oxidation of amino acid and choline. Currently, there are not many studies using BUMSC-CM. Research conducted by Larasati et al. (2022) and Saragih et al. (2023) inspected the capacity of BUMSC-CM to diminish apoptosis and inflammation in in vitro AD models (SH-SY5Y and PC12 cell lines). The findings revealed that BUVEC-CM was effective in substantially down-regulate the IL-1β and regulating caspase-9 and caspase-3 in a downward manner (Fig. 3). CH as drug delivery vehicleChitosan, derived from the exoskeletons of crustaceans like shrimp, crabs, and other sources like mushrooms and yeast. Chitosan is a natural polymer that has been gaining attention in the subject of drug delivery. Chitosan can also be obtained from other sources like mushrooms and yeast. The extraction process involves deproteinization, demineralization, decolorization, and deacetylation to obtain pure chitosan (Fig. 4). This remarkable substance possesses unique characteristics that make it an ideal candidate for various applications. One key characteristic of chitosan is its biocompatibility. When used in drug delivery systems, chitosan exhibits minimal toxicity and immunogenicity, making it safe for use in humans. This opens up exciting possibilities for targeted drug delivery without causing harmful side effects. Chitosan possesses mucoadhesive properties that enable it to adhere to mucosal surfaces; this property enables protracted contact between the drug-loaded hydrogel and the target tissue, thereby enhancing drug absorption and bioavailability. This polysaccharide’s mucoadhesive properties and capability to unbind tight junctions in respiratory and olfactory epithelia make it an incredible molecule for enhancing brain bioavailability. Moreover, chitosan can be readily chemically modified to improve its functionality. By incorporating specific functional groups onto the polymer backbone, the physical properties of chitosan could be modified to meet specific needs, such as increasing the solubility and degradation rate. Owing to its positive charge, which increases cell absorption and makes it fit for loading with negatively charged therapeutics, chitosan has also demonstrated promise in brain delivery (Karlsson et al., 2018), the high cerebral uptake of antibody-modified PEG-chitosan NPs was credited to the collaboration between the positive chitosan charge and antibody (Monsalve et al., 2015). In addition, investigations of combination NPs coated with low molecular weight chitosan via polydopamine layer (PLGA-pD-LMWC) revealed that PLGA-pD-LMWC NPs and the cell membrane of the cancer cell line (SK-OV-3) made interplay after 1 hour of contact. In addition, the NPs penetrated the cells following a 3-hour incubation period. On the contrary, NPs lacking a chitosan coating were not taken up by cells (Fig. 5) (Del Prado-Audelo et al., 2020). Additionally, chitosan has been shown to possess antimicrobial properties. This makes it particularly useful in preventing infections at the site of administration when used as a drug carrier. These unique characteristics make chitosan an attractive material for developing innovative drug delivery systems with improved therapeutic outcomes. With ongoing research and advancements in technology, we can expect even more exciting applications for this remarkable substance in the future. Chitosan’s ability to form hydrogels represents a further advantage. Hydrogels are three-dimensional networks capable of retaining significant quantities of water or biological fluids without compromising their structural integrity. Hydrogels derived from chitosan have demonstrated outstanding swelling properties and controlled release capabilities, making them highly versatile drug carriers. Hydrogels, meanwhile, are three-dimensional networks capable of absorbing and retaining vast quantities of water or other biological fluids. Due to their distinctive characteristics and diverse applications, hydrogels have acquired immense popularity in drug delivery. These three-dimensional networks comprise hydrophilic polymers that absorb vast water or biological fluids without losing structural integrity. This hydrogels ability makes them an ideal matrix for controlled drug release and delivery. The ability of hydrogels to pack away a wide variety of medications, including small molecules, proteins, peptides, and even genetic materials such as DNA or RNA, is one of their chief advantages.
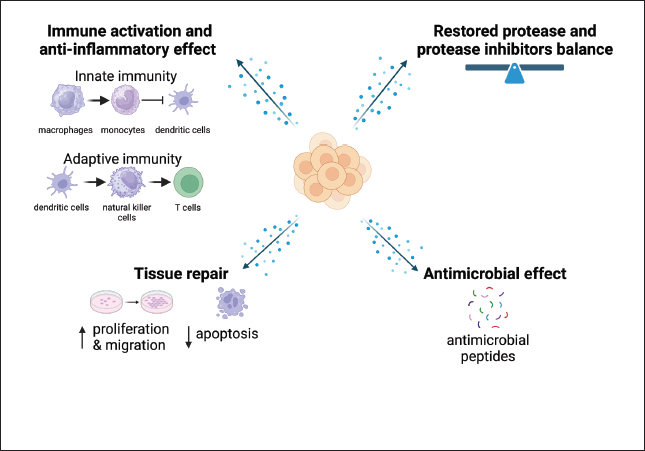
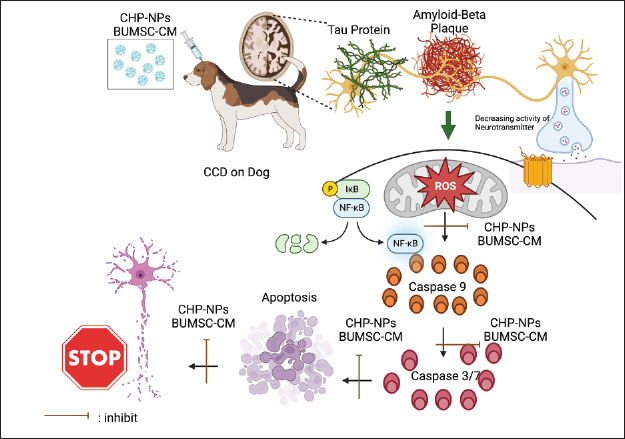
Fig. 3. Effects of BUMSC-CM (secretom) on neurodegeneration disease (CCD/AD) treatment. BUMSC-CMa inflammation by inducing macrophage polarization from a pro-inflammatory (M1) to an anti-inflammatory (M2) phenotype, inhibiting monocyte differentiation into dendritic cells, inhibiting NK cell cytotoxicity and proliferation, suppressing T cell proliferation, and modulating the inflammatory profile of helper T cells. The growth factors contained in BUMSC-CM regenerate neuron by increasing neuronal cell proliferation and decreasing apoptosis. Antimicrobial peptides present in the secretome exert an antimicrobial effect, whereas protease inhibitors reduce the excess of protease activity in the brain, restoring the protease/anti-protease equilibrium. Imaging creating by Biorender.
Fig. 4. Outline of chitosan’s production. The extraction process involves deproteinization, demineralization, decolorization, and deacetylation to obtain pure chitosan. Images is creating by Biorender.
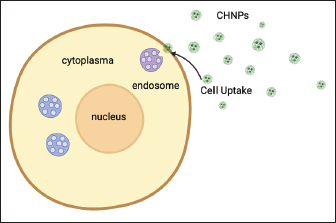
Fig. 5. CHNPs are able to increase NP absorption by cells. Imaging is creating by Biorender. By adjusting the composition and crosslinking density of the hydrogel, researchers can modulate drug release kinetics to achieve sustained or pulsatile drug delivery profiles. In addition to acting as carriers for therapeutic agents, hydrogels can also be designed to respond to specific stimuli in the body. For example, temperature-sensitive hydrogels undergo phase transition upon exposure to body heat, facilitating on-demand release at desired sites. Similarly, pH-responsive hydrogels can exploit variations in acidity levels within different tissues or cellular compartments. Moreover, researchers have explored incorporating various functional groups into hydrogel structures to enhance their biocompatibility and promote cell adhesion and growth. These modifications enable localized drug delivery directly at the site of injury or disease while minimizing adverse effects on healthy tissues. The versatility offered by hydrogels extends beyond traditional pharmaceuticals; they also hold promise as scaffolds for tissue engineering applications. Their high water content mimics the natural extracellular environment necessary for cellular proliferation and differentiation. Hydrogel-based systems allow cells seeded within them to organize into complex tissue structures with improved functionality. Due to its unique properties and advantages, combination CH has become a promising drug delivery system. Moreover, CH-based drug delivery systems offer targeted delivery to specific sites within the body. By modifying the formulation parameters such as particle size, crosslinking density, and surface charge of the hydrogel particles, the drug, with the help of NPs, can achieve site-specific targeting and improve bioavailability. Furthermore, CHs have been explored in various fields, including ocular drug delivery for treating eye diseases like glaucoma or dry eye syndrome; oral drug delivery for improving gastrointestinal absorption; transdermal drug delivery for enhanced skin permeation; and even in tissue engineering applications for regenerative medicine. CH holds tremendous potential as a versatile platform for delivering drugs effectively and safely. Its unique characteristics and those offered by hydrogels make it an ideal candidate for developing advanced drug delivery systems tailored to specific therapeutic needs mainly brain disease. Drug release of CHNPsThe therapeutic effect is greatly affected by the release of drugs from CHNPs. The drug release in NPs is affected by several factors, including their water absorption capacity, degradation rate, chemical composition, molecular weight, solubility, and crystallinity. Interactions between pharmaceuticals and polymer drugs have a substantial impact on the release of drugs from the delivery mechanism. CHNPs control the release, augment the bioavailability of degraded compounds, and improve the absorption of hydrophilic medications at the desired location. The release of drugs from polymer CHNPs can be controlled through one of the following mechanisms: (a) Surface erosion of the polymer matrix, (b) cleavage of polymer bonds on the surface or inside the bulk of the matrix, or (c) diffusion of the loaded drug. In contemporary technology, these three methods can be used to administer the drug. The release of drugs from CHNPs was also affected by the pH of the environment, which can be attributed to the solubility of CS. CS derivatives can formulate drug release based on the anticipated pharmacokinetic characteristics of the drug. The process by which CHNPs achieve continuous drug release can be described as follows: (a) The drugs that are absorbed or trapped on the surface layer of the particles are released. (b) The drugs diffuse through the swelling rubber matrix. (c) The release of drugs is caused by the erosion, fracture, hydrolysis, or degradation of the polymer, resulting in long-term drug release. The release of drugs from CHNPs exhibited a characteristic biphasic pattern, consisting of an initial rapid release followed by a subsequent slower and regulated release. Incorporating hydrogel will enhance the visibility and precision of the biphasic release mechanism (Samy et al., 2020; Herdiana et al., 2021). CHNPs loaded BUMSC-CM as therapeutic for the CCDBecause of the lower permeability of the BBB, most systemically administered medications are inaccessible to the brain, posing a significant challenge in treating brain diseases. A continuous layer of brain micro-vessel epithelial cells with intricate tight junctions constitutes the BBB. Brain microvessel endothelial cells (BMECs) with typical luminal (apical) and abluminal (basolateral) membrane sections maintain the BBB’s integrity. BMECs, along with neurons, glial cells, pericytes, and vascular smooth muscle cells of the vessel wall, are pivotal components of the neurovascular unit. The appearance of metabolizing enzymes, limited pinocytosis, and multiple efflux transport systems further inhibit the entrance of materials into the brain. The neurovascular unit preserves the chemical contexture of brain interstitial fluid and sets brain blood flow and permeability of the BBB, which is crucial for holding functional neuronal circuits (Daneman and Prat, 2015). The breach of the BBB permits plasma components and neurotoxins to enter the brain. The BBB prevents 98% of small molecular substances and nearly 100% of large molecular substances from entering the brain from circulation (Fig. 6). Numerous techniques, such as temporary modulation of BBB, ultrasound-based BBB opening, endogenous transporters, prodrugs usage, and chemical drug delivery systems and carriers, such as NPs, and liposomes, have been learned to get over the BBB-related issues and load of drugs into the brain. The development of nanomedicine, a branch of nanotechnology, particularly NPs and liposomes, is altering the methods of drug delivery used to treat fatal diseases, such as neurodegenerative diseases. NPs are dense colloidal particles that are manufactured with a variety of polymers. Even though the particle measure of NPs varied from 1 to 1000 nm, particles under 200 nm are typically utilized for drug targeting. NPs have been examined in detail for their ability to bring drugs to the brain. NPs have numerous benefits, including high drug loading, constancy in biological fluids, improved bioavailability, extended blood circulation time, cationic nature, and site-specific drug delivery. A handful of NPs (Doxil®, Abraxane®, Caelyx®, OnivydeTM, MyocetTM, and Vyxeos®) have been recognized for the therapy of cancer (Wilson, 2020). The medication of CCD with CHNPs laden with BUMSC-conditioned medium (BUMSC-CM) is intriguing and promising. But how do these minute particles function? Chitosan, which is derived from chitin, a natural polysaccharide found in crustacean shells, has unique properties that make it a great choice candidate for drug delivery systems. Its biocompatibility, biodegradability, and ability to form hydrogels are crucial to its effective use. Due to their immunomodulatory and regenerative capabilities, MSCs have shown enormous therapeutic potential. When administered as same as the stem cell, MSC-conditioned medium contains a potent cocktail of growth factors, cytokines, and other bioactive molecules that promote tissue repair and regeneration. The focus of this review was BUMSC-CM as a part of cell free therapy from the stem cells.
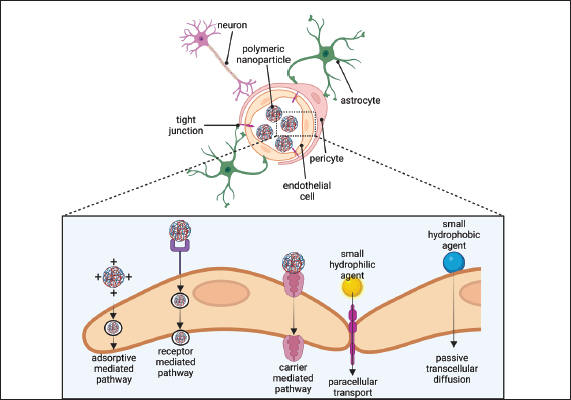
Fig. 6. The neurovascular unit. A schematic cross-section of a brain capillary illustrating endothelial cells connected by tight junctions. On the brain side of the endothelial cells, the basement membrane encases pericytes that span multiple endothelial cells and surrounds the endothelial cells. Endothelial cells are in contact with astrocyte. Neurons are present in the brain’s tissue. The endothelium carrier transports polymeric NPs via carrier-mediated, receptor-mediated, and adsorptive-mediated pathways. Imaging creating by Biorender. CHNPs serve as carriers for BUMSC-CM, allowing for the controlled dispersion of the therapeutic cargo at specific body locations. The CH matrix provides both stability and prolonged release kinetics. These NPs, when administered locally or via systemic routes such as intranasal, intramuscular and or intravenous injection, progressively release BUMSC-CM components directly into surrounding tissues or the bloodstream, where they exert their beneficial effects on damaged neuronal tissues. CHNPs containing BUMSC-CM combines the unique properties of chitosan as a carrier system with the regenerative goods of medium obtained from MSC. This novel new strategy holds great promise for treating CCD by neuroprotection, promoting neuro-regeneration, synaptogenesis, reducing inflammation, and stimulating regeneration via the controlled delivery of potent bioactive molecules directly to the central nervous system, where they are most required. This finding also appears in numerous studies. Aggregation of activated microglia may cause the reduction of neurons by discharging cytotoxic and proinflammatory factors. MSC-CM importantly cut the quantity of activated microglia. This led to neuroprotection in the CNS, improving cell survival by inhibiting neuronal apoptosis, promoting cell proliferation, promoting cell regeneration, and preventing b amyloid deposition. (Kuroda et al., 2020; Saha et al., 2020; Kuo et al., 2021; Park et al., 2021; Alidous et al., 2023) (Fig. 7). The therapeutic potential of CHNPs loaded with BUMSC conditioned medium for treating CCD is promising. This novel combination enhanced cognitive function in CCD dogs and reduced oxidative stress and inflammation. Chitosan’s mode of action is an intriguing characteristic. It has been employed widely in drug delivery systems as a biocompatible and biodegradable material that can encapsulate and protect therapeutic agents. In this investigation, CH was an efficient delivery system for BUMSC-CM, enhancing its therapeutic effects. In addition, using secretome acquired from BUMSCs adds a layer of intrigue. These cells have demonstrated promising regenerative properties in numerous conditions, including neurological disorders. The BUMSC-CM generated contains numerous growth factors and anti-inflammatory substances that may promote neuronal repair and reduce neuroinflammation in canines with CCD. While the results are promising, additional research is required to optimize the formulation and dosage regimen for clinical use. It would also be beneficial to investigate this treatment’s long-term and potential adverse effects. This review emphasizes the potential therapeutic benefits of using CHNP-loaded BUMSC conditioned medium to treat CCD. Future PerspectiveA complex interaction exists between maturing, hereditary risk variables, and natural impacts in developing age-related CCD. CCD in canines resembles AD regarding amyloid plaque deposition, APP processing, and cognitive impairment. A peptide gathers in the extracellular space of the canine brain as soluble oligomers, fibrils, and A plaques as the dog ages. There are no effective therapies for CCD disorders; the vast majority of treatments as of now accessible are symptomatic. This is often in part due to a dearth of knowledge regarding the pathogenesis of these illnesses and the absence of suitable animal models. In dogs with CDD, secretase or cholinesterase immunotherapy or inhibitor therapy has been undertaken, with comparable comes about to Alzheimer’s trials. The neuroprotective impacts of cognitive improvement, in conjunction with high-antioxidant diet, are beneficial for managing illness progression and seriousness in people and dogs. Comparable studies with different drugs recommend that dogs are a valuable model for future research into both the pathogenesis and the development of novel treatments for AD. It has been reported that chitosan-based carriers are potent for the in vivo transmission of medications for the medication of certain brain diseases, such as CCD. The charge on chitosan-based drug transport systems has been observed to result in targeted drug delivery, which increases drug concentration at the brain site and improves therapeutic outcomes. The model of chitosan-based drug delivery systems affects brain tissue drug absorption. Chitosan NPs coated on their surface with BUMSC-CM were able to get across the BBB and also provided neuroprotection. The reported dimension of the NPs was either below or above 200 nm. Additional studies on the consistency of the particle size variety of NPs that are efficacious for brain absorption are in demand. The conjugation of CM to the surface of the NPs and the coating of the NPs surface may be a viable strategy for developing a formula with added brain uptake and decreased toxicity. Nevertheless, additional in vivo investigations on NP formula conjugated with BUMSC-CM are urgently required. It is also vital to conduct additional in vivo research on the long-term toxicity of these NPs. As stated by to the achieved data, several angles of the use of BUMSC-CM to the treatment of CCD remain obscure. In addition, the next suggestions are able to be considered future study goals to identify the principal therapeutic effect mechanism. Evaluation of the presence of an immune response to conditioned medium. Comparison of the effect of various CM varieties on the prognosis of CCD. It is still necessary to investigate the effect of CMs on diverse populations and ages at various stages of CCD.
Fig. 7. BUMSC-CM contains a potent cocktail of growth factors, cytokines, and other bioactive molecules that promote tissue repair and regeneration CHNPs serve as carriers for BUMSC-CM, allowing for the controlled dispersion of the therapeutic cargo at specific body locations. The CH matrix provides both stability and prolonged release kinetics. This novel new strategy holds great promise for treating CCD by inhibiting of b amyloid plaque deposition thus promoting neuro-regeneration, reducing inflammation, and stimulating regeneration via the controlled delivery of potent bioactive molecules directly to the central nervous system, where they are most required. Imaging creating by Biorender. ConclusionDue to chitosan biocompatibility, ability to bind hydrogels with controlled release capabilities, mucoadhesive properties, and ease of chemical modification, CHNP possesses tremendous potential as a versatile material in drug delivery systems. With advances in veterinary medicine, there is optimism that effective treatments for conditions such as CCD Disorder can be discovered. Combining cutting-edge technologies such as CHNPs with regenerative cell-based therapies such as BUMSC conditioned medium may enhance the lives of our cherished canine companions suffering from cognitive decline. It should be noted, however, that additional research is required to comprehend this therapy’s efficacy and safety profile thoroughly. Before widespread implementation, researchers will need to undertake rigorous clinical trials and gather more evidence. AcknowledgmentThe authors wish to thank Universitas Gadjah Mada, Bandung Institute of Technology, and Brawijaya University, for the Indonesian Collaboration Research 2023 (RKI 2023), with the grant number 2635/UN1.P.II/Dit-Lit/PT.01.03/2023. ReferencesAbey, A., Davies, D., Goldsbury, C., Buckland, M., Valenzuela, M. and Duncan, T. 2020. Distribution of tau hyperphosphorylation in canine dementia resembles early Alzheimer’s disease and other tauopathies. Brain Pathol. 31(1),144–162. Alidoust, E., Akhoondian, M., Atefi, A.H., Keivanlou, M.H., Ch, M.H. and Jafari, A. 2023. Stem cell-conditioned medium is a promising treatment for Alzheimer’s disease. Behav. Brain Res. 452, 114543. Barnes, J., Cotton, P., Robinson, S. and Jacobsen, M. 2016. Spontaneous pathology and routine clinical pathology parameters in aging beagle dogs: a comparison with adolescent and young adults. Vet. Pathol. 53(2), 447–455. Barrachina, L., Remacha, A.R., Romero, A., Vázquez, F.J., Albareda, J., Prades, M., Ranera, B., Zaragoza, P., Martín-Burriel, I. and Rodellar, C. 2016. Effect of inflammatory environment on equine bone marrow derived mesenchymal stem cells immunogenicity and immunomodulatory properties. Vet. Immunol. Immunopathol. 171, 57–65. Borghys, H., Van Broeck, B., Dhuyvetter, D., Jacobs, T., de Waepenaert, K., Erkens, T., Brooks, M., Thevarkunnel, S. and Araujo, J.A. 2017. Young to middle-aged dogs with high amyloid-β levels in cerebrospinal fluid are impaired on learning in standard cognition tests. J. Alzheimers Dis. 56(2), 763–774. Braidy, N., Poljak, A., Jayasena, T., Mansour, H., Inestrosa, N.C. and Sachdev, P.S. 2015. Accelerating Alzheimer’s research through ‘natural’ animal models. Curr. Opin. Psychiatry 28(2), 155–164. Brasile, L., Henry, N., Orlando, G. and Stubenitsky, B. 2019. Potentiating renal regeneration using mesenchymal stem cells. Transplantation 103(2), 307. Canudas, J., Insua, D., Sarasa, L., Gonzalez-Martinez, A., Suárez, M.L., Santamarina, G., Pesini, P. and Sarasa, M., 2014. Neprilysin is poorly expressed in the prefrontal cortex of aged dogs with cognitive dysfunction syndrome. Int. J. Alzheimer’s Dis. 2014, 483281. Daneman, R. and Prat, A. 2015. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 7(1), a020412. Debbarma, P., Mondal, T., Manna, C., Kumar, K., Mukherjee, J., Das, B.C., Bag, S. and Das, K. 2020. Post-calving umbilical cord tissue offcut: a potential source for the isolation of bovine mesenchymal stem cells. Vet. World 13(12), 2772–2779. Del Prado-Audelo, M.L., Caballero-Florán, I.H., Sharifi-Rad, J., Mendoza-Muñoz, N., González-Torres, M., Urbán-Morlán, Z., Florán, B., Cortes, H. and Leyva-Gómez, G. 2020. Chitosan-decorated nanoparticles for drug delivery. J. Drug Deliv. Sci. Technol. 59, 101896. Dewey, C.W., Davies, E.S., Xie, H. and Wakshlag, J.J. 2019. Canine cognitive dysfunction: pathophysiology, diagnosis, and treatment. Vet. Clin. North Am. Small. Anim. Pract. 49, 477–499. El Moshy, S., Radwan, I.A., Rady, D., Abbass, M.M., El-Rashidy, A.A., Sadek, K.M., Dörfer, C.E. and El-Sayed, K.M.F., 2020. Dental stem cell-derived secretome/conditioned medium: the future for regenerative therapeutic applications. Stem Cells Int. 2020, 7593402. Fast, R., Schütt, T., Toft, N., Møller, A. and Berendt, M. 2013. An observational study with long-term follow-up of canine cognitive dysfunction: clinical characteristics, survival, and risk factors. J. Vet. Intern. Med. 27(4), 822–829. Gómez-Pinedo, U., Villar-Quiles, R.N., Galán, L., Matías-Guiu, J.A., Benito-Martin, M.S., Guerrero-Sola, A., Moreno-Ramos, T. and Matías-Guiu, J. 2016. Immununochemical markers of the amyloid cascade in the hippocampus in motor neuron diseases. Front. Neurol. 7, 195. Herdiana, Y., Wathoni, N., Shamsuddin, S. and Muchtaridi, M. 2021. Drug release study of the chitosan-based nanoparticles. Heliyon 8(1), 1–16. Hill, A.B.T., Bressan, F.F., Murphy, B.D. and Garcia, J.M. 2019. Applications of mesenchymal stem cell technology in bovine species. Stem Cell Res. Ther. 10(1), 1–13. Jayasinghe, M., Prathiraja, O., Perera, P.B., Jena, R., Silva, M.S., Weerawarna, P.S.H., Singhal, M., Kayani, A.M.A., Karnakoti, S. and Jain, S. 2022. The role of mesenchymal stem cells in the treatment of type 1 diabetes. Cureus 14(7), e27337. Jo, H., Brito, S., Kwak, B.M., Park, S., Lee, M.G. and Bin, B.H. 2021. Applications of mesenchymal stem cells in skin regeneration and rejuvenation. Int. J. Mol. Sci. 22(5), 2410. Karlsson, J., Vaughan, H.J. and Green, J.J. 2018. Biodegradable polymeric nanoparticles for therapeutic cancer treatments. Annu. Rev. Chem. Biomol. Eng. 9, 105–127. Katina, S., Farbakova, J., Madari, A., Novak, M. and Zilka, N. 2016. Risk factors for canine cognitive dysfunction syndrome in Slovakia. Acta Vet. Scand. 58, 17. Kelly, S.C., He, B., Perez, S.E., Ginsberg, S.D., Mufson, E.J. and Counts, S.E., 2017. Locus coeruleus cellular and molecular pathology during the progression of Alzheimer’s disease. Acta Neuropathol. Commun. 5(1), 1–14. Kuo, S.C., Chio, C.C., Yeh, C.H., Ma, J.T., Liu, W.P., Lin, M.T., Lin, K.C. and Chang, C.P. 2021. Mesenchymal stem cell-conditioned medium attenuates the retinal pathology in amyloid-β-induced rat model of Alzheimer’s disease: underlying mechanisms. Aging Cell 20(5), p.e13340. Kuroda, E., Nishimura, K., Kawanishi, S., Sueyoshi, M., Ueno, F., Toji, Y., Abo, N., Konishi, T., Harada, K., Satake, S. and Shima, C. 2020. Mouse bone marrow-derived microglia-like cells secrete transforming growth factor-β1 and promote microglial Aβ phagocytosis and reduction of brain Aβ. Neuroscience 438, 217–228. Kusindarta, D.L. and Wihadmadyatami, H. 2021. Conditioned medium derived from bovine umbilical mesenchymal stem cells as an alternative source of cell-free therapy. Vet. World 14(10), 2588. Larasati, V.A., Lembang, G.V., Tjahjono, Y., Winarsih, S., Ana, I.D., Wihadmadyatami, H. and Kusindarta, D.L. 2022. In vitro neuroprotective effect of the bovine umbilical vein endothelial cell conditioned medium mediated by downregulation of IL-1β, caspase-3, and caspase-9 expression. Vet. Sci. 9(2), 48. MacQuiddy, B., Moreno, J.A., Kusick, B. and McGrath, S. 2022. Assessment of risk factors in dogs with presumptive advanced canine cognitive dysfunction. Front. Vet. Sci. 9, 958488. Madari, A., Farbakova, J., Katina, S., Smolek, T., Novak, P., Weissova, T., Novak, M. and Zilka, N. 2015. Assessment of severity and progression of canine cognitive dysfunction syndrome using the CAnine DEmentia Scale (CADES). Appl. Anim. Behav. Sci. 171, 138–145. Mihevc, P.S. and Majdič, G. 2019. Canine cognitive dysfunction and alzheimer’s disease—two facets of the same disease? Front Neurosci. 12(13), 604. Monsalve, Y., Tosi, G., Ruozi, B., Belletti, D., Vilella, A., Zoli, M., Vandelli, M.A., Forni, F., López, B.L. and Sierra, L. 2015. PEG-g-chitosan nanoparticles functionalized with the monoclonal antibody OX26 for brain drug targeting. Nanomedicine (Lond.) 10(11), 1735–1750. Muhammad, S.A. 2019. Mesenchymal stromal cell secretome as a therapeutic strategy for traumatic brain injury. BioFactors 45(6), 880–891. Nesic, S., Kukolj, V., Marinković, D., Vučićević, I. and Jovanović, M. 2017. Histological and immunohistochemical characteristics of cerebral amyloid angiopathy in elderly dogs. Vet. Q. 37(1), 1–7. Ozawa, M., Chambers, J.K., Uchida, K. and Nakayama, H. 2016. The relation between canine cognitive dysfunction and age-related brain lesions. J. Vet. Med. Sci. 78(6), 997–1006. Park, H.S., Pang, Q.Q., Kim, Y.S., Kim, J.H. and Cho, E.J. 2021. Neuroprotective effect of membrane-free stem cell extract against amyloid beta 25–35-induced neurotoxicity in SH-SY5Y cells. Appl. Sci. 11(5), 2219. Pawitan, J.A. 2014. Prospect of stem cell conditioned medium in regenerative medicine. BioMed. Res. Int. 2014, 965849. Rusbridge, C., Salguero, F.J., David, M.A., Faller, K.M.E., Bras, J.T., Guerreiro, R.J., Richard-Londt, A.C., Grainger, D., Head, E., Brandner, S.G.P., Summers, B., Hardy, J. and Tayebi, M. 2018. An aged canid with behavioral deficits exhibits blood and cerebrospinal fluid amyloid beta oligomers. Front. Aging Neurosci. 10(7), 1–8. Saha, P., Sarkar, S., Paidi, R.K. and Biswas, S.C. 2020. TIMP-1: a key cytokine released from activated astrocytes protects neurons and ameliorates cognitive behaviours in a rodent model of Alzheimer’s disease. Brain. Behav. Immun. 87, 804–819. Samy, M., Abd El-Alim, S.H., Rabia, A.E.G., Amin, A. and Ayoub, M.M.A. 2020. Formulation, characterization and in vitro release study of 5-fluorouracil loaded chitosan nanoparticles. Int. J. Biol. Macromol. 156, 783–791. Saragih, G.R., Winarsih, S., Wihadmadyatami, H. and Kusindarta, D.L. 2023. Bovine umbilical vein endothelial cells conditioned medium prevents TMT-induced neurotoxicity mediated by the upregulation of brain-derived neurotropic factors on the human neuroblastoma SH-SY5Y cells. F1000 Res [In press]. Schmidt, F., Boltze, J., Jäger, C., Hofmann, S., Willems, N., Seeger, J., Härtig, W. and Stolzing, A. 2015. Detection and quantification of β-amyloid, pyroglutamyl Aβ, and tau in aged canines. J. Neuropathol. Exp. Neurol. 74(9), 912–923. Schütt, T., Helboe, L., Pedersen, L.Ø., Waldemar, G., Berendt, M. and Pedersen, J.T. 2016. Dogs with cognitive dysfunction as a spontaneous model for early Alzheimer’s disease: a translational study of neuropathological and inflammatory markers. J. Alzheimers Dis. 52, 433–449. Smolek, T., Madari, A., Farbakova, J., Kandrac, O., Jadhav, S., Cente, M., Brezovakova, V., Novak, M. and Zilka, N. 2016. Tau hyperphosphorylation in synaptosomes and neuroinflammation are associated with canine cognitive impairment. J. Comp. Neurol. 524(4), 874–895. Uhlén, M., Karlsson, M.J., Hober, A., Svensson, A.S., Scheffel, J., Kotol, D., Zhong, W., Tebani, A., Strandberg, L., Edfors, F. and Sjöstedt, E., 2019. The human secretome. Sci. Signal. 12(609), 0274. Urfer, S.R., Darvas, M., Czeibert, K., Sándor, S., Promislow, D.E., Creevy, K.E., Kubinyi, E. and Kaeberlein, M. 2021. Canine cognitive dysfunction (CCD) scores correlate with amyloid beta 42 levels in dog brain tissue. GeroScience 43(5), 2379–2386. Wilson, C. 2020. An evolution in cancer treatment. New Scientist 245(3272), 44–47. Xiong, H., Bai, C., Wu, S., Gao, Y., Lu, T., Hu, Q., Guan, W. and Ma, Y. 2014. Biological characterization of mesenchymal stem cells from bovine umbilical cord. Anim. Cells Syst. 18(1), 59–67. Yu, C.H., Song, G.S., Yhee, J.Y., Kim, J.H., Im, K.S., Nho, W.G., Lee, J.H. and Sur, J.H. 2011. Histopathological and immunohistochemical comparison of the brain of human patients with Alzheimer's disease and the brain of aged dogs with cognitive dysfunction. J. Comp. Pathol. 145(1), 45–58. Zakrzewski, W., Dobrzyński, M., Szymonowicz, M. and Rybak, Z. 2019. Stem cells: past, present, and future. Stem Cell Res. Ther. 10(1), 1–22. | ||
| How to Cite this Article |
| Pubmed Style Wihadmadyatami H, Zulfikar MA, Herawati H, Pratama DAO, GRS, UK, Handayani N. Chitosan hydrogel nanoparticle enhance therapeutic effect of bovine umbilical mesenchymal stem cell conditioned medium on canine cognitive dysfunction or canine alzheimer’s like mediated by inhibition of neuronal apoptotic. Open Vet J. 2023; 13(12): 1504-1516. doi:10.5455/OVJ.2023.v13.i12.1 Web Style Wihadmadyatami H, Zulfikar MA, Herawati H, Pratama DAO, GRS, UK, Handayani N. Chitosan hydrogel nanoparticle enhance therapeutic effect of bovine umbilical mesenchymal stem cell conditioned medium on canine cognitive dysfunction or canine alzheimer’s like mediated by inhibition of neuronal apoptotic. https://www.openveterinaryjournal.com/?mno=162450 [Access: May 13, 2024]. doi:10.5455/OVJ.2023.v13.i12.1 AMA (American Medical Association) Style Wihadmadyatami H, Zulfikar MA, Herawati H, Pratama DAO, GRS, UK, Handayani N. Chitosan hydrogel nanoparticle enhance therapeutic effect of bovine umbilical mesenchymal stem cell conditioned medium on canine cognitive dysfunction or canine alzheimer’s like mediated by inhibition of neuronal apoptotic. Open Vet J. 2023; 13(12): 1504-1516. doi:10.5455/OVJ.2023.v13.i12.1 Vancouver/ICMJE Style Wihadmadyatami H, Zulfikar MA, Herawati H, Pratama DAO, GRS, UK, Handayani N. Chitosan hydrogel nanoparticle enhance therapeutic effect of bovine umbilical mesenchymal stem cell conditioned medium on canine cognitive dysfunction or canine alzheimer’s like mediated by inhibition of neuronal apoptotic. Open Vet J. (2023), [cited May 13, 2024]; 13(12): 1504-1516. doi:10.5455/OVJ.2023.v13.i12.1 Harvard Style Wihadmadyatami, H., Zulfikar, . M. A., Herawati, . H., Pratama, . D. A. O., , . G. R. S., , . U. K. & Handayani, . N. (2023) Chitosan hydrogel nanoparticle enhance therapeutic effect of bovine umbilical mesenchymal stem cell conditioned medium on canine cognitive dysfunction or canine alzheimer’s like mediated by inhibition of neuronal apoptotic. Open Vet J, 13 (12), 1504-1516. doi:10.5455/OVJ.2023.v13.i12.1 Turabian Style Wihadmadyatami, Hevi, Muhammad Ali Zulfikar, Herawati Herawati, Dyah Ayu O.A Pratama, Golda Rani Saragih, Ulayatul Kustiati, and Nurrahmi Handayani. 2023. Chitosan hydrogel nanoparticle enhance therapeutic effect of bovine umbilical mesenchymal stem cell conditioned medium on canine cognitive dysfunction or canine alzheimer’s like mediated by inhibition of neuronal apoptotic. Open Veterinary Journal, 13 (12), 1504-1516. doi:10.5455/OVJ.2023.v13.i12.1 Chicago Style Wihadmadyatami, Hevi, Muhammad Ali Zulfikar, Herawati Herawati, Dyah Ayu O.A Pratama, Golda Rani Saragih, Ulayatul Kustiati, and Nurrahmi Handayani. "Chitosan hydrogel nanoparticle enhance therapeutic effect of bovine umbilical mesenchymal stem cell conditioned medium on canine cognitive dysfunction or canine alzheimer’s like mediated by inhibition of neuronal apoptotic." Open Veterinary Journal 13 (2023), 1504-1516. doi:10.5455/OVJ.2023.v13.i12.1 MLA (The Modern Language Association) Style Wihadmadyatami, Hevi, Muhammad Ali Zulfikar, Herawati Herawati, Dyah Ayu O.A Pratama, Golda Rani Saragih, Ulayatul Kustiati, and Nurrahmi Handayani. "Chitosan hydrogel nanoparticle enhance therapeutic effect of bovine umbilical mesenchymal stem cell conditioned medium on canine cognitive dysfunction or canine alzheimer’s like mediated by inhibition of neuronal apoptotic." Open Veterinary Journal 13.12 (2023), 1504-1516. Print. doi:10.5455/OVJ.2023.v13.i12.1 APA (American Psychological Association) Style Wihadmadyatami, H., Zulfikar, . M. A., Herawati, . H., Pratama, . D. A. O., , . G. R. S., , . U. K. & Handayani, . N. (2023) Chitosan hydrogel nanoparticle enhance therapeutic effect of bovine umbilical mesenchymal stem cell conditioned medium on canine cognitive dysfunction or canine alzheimer’s like mediated by inhibition of neuronal apoptotic. Open Veterinary Journal, 13 (12), 1504-1516. doi:10.5455/OVJ.2023.v13.i12.1 |