| Research Article | ||
Open Vet J. 2023; 13(12): 1708-1717 Open Veterinary Journal, (2023), Vol. 13(12): 1708–1717 Original Research Mechanism of long-term high-dose prednisolone administration producing myocardial fibrosis in beagle dogsSachiyo Tanaka1*, Shuji Suzuki1, Satoshi Soeta2, Takeharu Kaneda3 and Yasushi Hara11Division of Veterinary Surgery, Department of Veterinary Science, Faculty of Veterinary Medicine, Nippon Veterinary and Life Science University, Tokyo, Japan 2Division of Veterinary Anatomy, Department of Veterinary Science, Faculty of Veterinary Medicine, Nippon Veterinary and Life Science University, Tokyo, Japan 3Division of Veterinary Pharmacology, Department of Veterinary Science, Faculty of Veterinary Medicine, Nippon Veterinary and Life Science University, Tokyo, Japan *Corresponding Author: Sachiyo Tanaka. Division of Veterinary Surgery, Department of Veterinary Science, Faculty of Veterinary Medicine, Nippon Veterinary and Life Science University, Tokyo, Japan. Email: sachiyoct [at] gmail.com Submitted: 29/07/2023 Accepted: 06/11/2023 Published: 31/12/2023 © 2023 Open Veterinary Journal
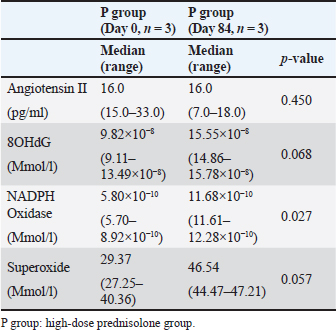
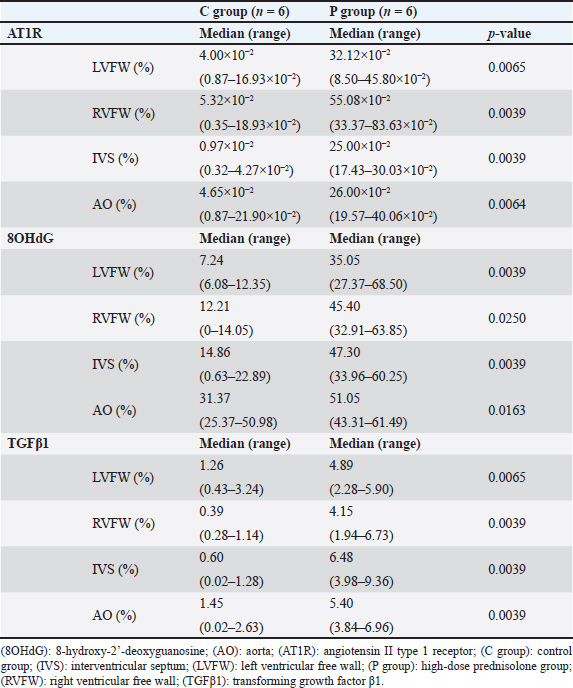
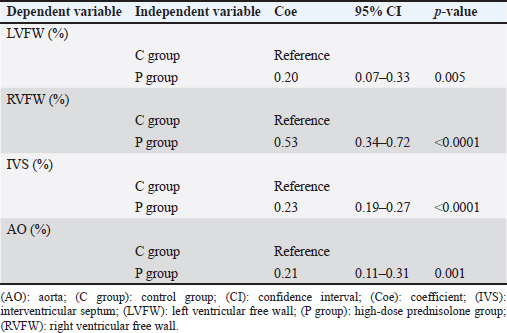
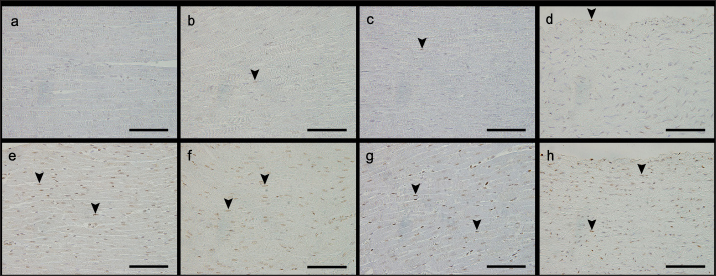
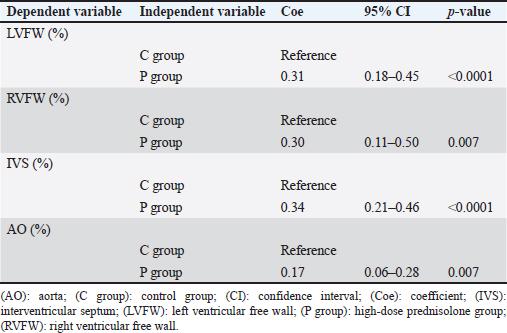
AbstractBackground: We previously reported that myocardial fibrosis may be one of the causes of left ventricular hypertrophy and cardiac dysfunction in dogs with hyperglucocorticism (HGC). The detailed mechanism by which myocardial fibrosis of the left ventricle occurs in dogs with HGC remains unclear. Aim: Th is study investigated the mechanism by which HGC causes fibrosis of the left ventricle. Methods: The impa cts of HGC on the heart by comparing samples obtained from high-dose glucocorticoid (GC)-treated (P) and untreated (C) dogs. The P group included healthy Beagle dogs (n=6) treated with prednisolone (2 mg/kg, bid, po) for 84 days, and the C group included healthy Beagle dogs (n=6) euthanized for unrelated reasons. In three of the P group dogs, serum was collected before the start of administration (Day 0) and on Day 84 to measure angiotensin II concentrations and oxidative stress markers (8-hydroxy-2′-deoxyguanosine (8OHdG), NADPH oxidase, and superoxide levels). Samples of the left ventricular free wall (LVFW), right ventricular free wall (RVFW), interventricular septum (IVS), and aortic root were harvested from both groups (n=6 for each group). Using these tissue samples, angiotensin II type 1 receptor (AT1R), 8OHdG, and transforming growth factor β1 (TGFβ1) immunohistochemical stains were performed. Results: The blood N ADPH oxidase concentration was significantly higher (p =0.027) in the P group 84 days after initiation of the medication compared to that before prednisolone treatment. By contrast, there was no significant difference in serum angiotensin II (p =0.450), 8OHdG (p =0.068), and superoxide (p =0.057) concentrations. The positive staining rates of AT1R, 8OHdG, and TGFβ1 in the heart (LVFW, RVFW, IVS, and aortic root) were significantly higher in the P group than those in the C group. Conclusion: Angiotensin II and oxidative stress in HGC may cause left ventricular fibrosis in dogs. Keywords: Canine, Cushing syndrome, Hyperglucocorticism, Left ventricular, Fibrosis. IntroductionThe incidence of canine Cushing syndrome (CS) has been estimated to be 0.2%, rendering it the most common endocrine disease (Carotenuto et al., 2019; de Bruin et al., 2009). Hypercortisolism (HAC) is one of the causes of CS (Boscaro et al., 2001; Behrend et al., 2013; Nieman, 2015; Nivy et al., 2018). Cardiovascular complications in human patients with HAC are associated with mortality rates four times higher than those in the healthy population, indicating that the systemic changes associated with hyperglucocorticism (HGC) increase cardiovascular risk (Boscaro et al., 2001; Nieman, 2015; van der Pas et al., 2013). Specific cardiovascular changes in human HAC patients include morphological and functional changes in the heart that result in myocardial hypertrophy and cardiac diastolic and systolic dysfunction (Sugihara et al., 1992; Isidori et al., 2015). Notably, hypertrophy of cardiomyocytes and an increase in the cardiac fiber count were reported as histopathological changes(Sugihara et al., 1992). In addition, left ventricular hypertrophy and cardiac dysfunction in human and canine CS cases are influenced by disease durability (Fallo et al., 1994; Muiesan et al., 2003). Recently, left ventricular hypertrophy and cardiac dysfunction were reported in dogs with HAC and dogs treated experimentally treated with high-dose prednisolone (Chen et al., 2014; Oui et al., 2015; Takano et al., 2015; Tanaka et al., 2021). In a histopathological study in dogs, 2 mg/kg prednisolone every 12 hours for 28 days produced no apparent changes in the myocardium (Oui et al., 2015). However, our recent study revealed that the same dose of prednisolone treated for 84 days caused fibrosis in the left ventricular myocardium and ventricular septum. In addition, there was a decrease in glucocorticoid receptor (GCR) levels and an increase in mineralocorticoid receptor (MCR) at these regions (Tanaka et al., 2021). It has been suggested that elevated levels of reactive oxygen species activate the glucocorticoid (GC)-MCR complex in the heart and upregulate the expression of transforming growth factor β1 (TGFβ1), resulting in fibrosis (Funder, 2017). However, the details of the mechanism by which canine HGC causes fibrosis of the left ventricle have not been elucidated. Therefore, in this study, as a follow-up to Tanaka and colleagues (Tanaka et al., 2021) we investigated the mechanism by which HGC causes fibrosis of the left ventricle. Materials and MethodsDogsTwelve healthy male Beagles were included in this study. Six dogs included in the high-dose prednisolone (P) group (weight: 9.5–11.2 kg; median weight: 10.8 kg; age: 15–17 months; median age: 16 months) received 2 mg/kg synthetic corticosteroid (5 mg prednisolone tablet YD; Yoshindo Company Ltd., Toyama, Japan) every 12 hours for 84 days by orally administered. The clinical parameters of the P group included physical examination findings, complete blood count, blood biochemistry, electrocardiography, noninvasive blood pressure measurement, and cardiac echocardiography findings at baseline (Day 0) and on Days 7, 28, 56, and 84 after initiation of the synthetic corticosteroid administration. On Day 0, all six dogs in the P group were carefully evaluated to ensure that 1) they were healthy, 2) their test results were not outside the normal range, 3) did not show any evidence of systemic disease, and 4) did not show any signs of cardiac disease or congenital abnormalities of the heart. At each examination time point after the start of medication, the dog's general condition was confirmed to be well enough to continue the experiment, and the changes over time were evaluated. In addition, in the P group, tetracosactide (0.25 mg Cortrosyn injection; Daiichi Sankyo Company Ltd., Tokyo, Japan) was administered intramuscularly on Day 0 and Day 84 (125 μg for dogs weighing less than 5 kg and 250 μg for dogs weighing more than 5 kg), and blood samples were collected at baseline and 1 hour after injection for adrenocorticotropic hormone (ACTH) stimulation tests. Serum cortisol concentrations were measured using a chemiluminescence enzyme immunoassay (Fuji Film, Monolith, Tokyo, Japan). After the administration period, the dogs were euthanized with pentobarbital (90 mg/kg, iv; Kyoritsu Seiyaku, Tokyo, Japan) for histopathological examination. The control (C) group consisted of six dogs (body weight: 8.5–10.5 kg, median body weight: 9.6 kg, age: 8–19 months, median age: 17.5 months), which were euthanized using the same procedure but for other purposes (anatomy and surgical training of veterinary students). The dogs in C group underwent a preliminary physical examination, electrocardiography, and noninvasive blood pressure measurement to ensure that there were no abnormalities or values outside of the reference range. The dogs in both groups were housed in cages (0.7 m wide × 1.2 m deep × 1.5 m high). The temperature was 23°C, humidity was 50%–60%, and light and dark hours were from 6:00 am to 8:00 pm and from 8:00 pm to 6:00 am, respectively. The dogs were kept in cages for the duration of the experiment but could roam freely indoors for 1 hour twice a day (morning and evening). In addition, the dogs in the P group were evaluated every 12 hours during the prednisolone administration period to ensure that they had no energy- or appetite-related problems, were drinking adequate water, and had no injuries or gastrointestinal symptoms. Serum concentrations of angiotensin II and oxidative stress markers in the P groupIn three of the P group dogs, the blood samples collected before tetracosactide administration on Day 0 and Day 84 were centrifuged at 1000 × g, 4°C for 15 minutes. The serum was then rapidly frozen in liquid nitrogen and stored frozen at −80°C until the various assays were performed. Angiotensin II concentrations were measured using a double-antibody radioimmunoassay (Fuji Film, Monolith, Tokyo, Japan). The levels of the oxidative stress markers 8-hydroxy-2′-deoxyguanosine (8OHdG), NADPH oxidase, and superoxide were measured using enzyme-linked immunosorbent assays (ELISAs). Notably, 8OHdG was determined based on a competitive ELISA technique using a commercial kit (8-hydroxy-2′-deoxyguanosine ELISA Kit, BioVision, Inc., CA, USA). NADPH oxidase was determined based on the double antibody sandwich ELISA technique (Canine NADPH Oxidase ELISA Kit, MyBioSource, Inc., San Diego, CA, USA). Superoxide was determined using a colorimetric method (Superoxide Anion Microplate Assay Kit, Bioworld Technology, Inc., MN, USA). All samples were measured in triplicate. Histological treatmentAfter euthanasia, the heart and aortic root were promptly sampled from the animals of both study groups. The heart was then cut using a sharp disposable blade (Futaba surgical blades No. 23, Futaba, Inc., Tokyo, Japan) so that the midpoint of the incision line from the coronary sulcus to the apex of the heart was parallel to the coronary sulcus. Subsequently, the left ventricular free wall (LVFW), right ventricular free wall (RVFW), interventricular septum (IVS), and aortic root were promptly harvested and immersed in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). This procedure was completed within 30 minutes after euthanasia. The tissues were then immersed in paraformaldehyde for 24 hours, and the tissue specimens were embedded in paraffin and thinly sliced to a thickness of 3 μm according to standard methods. ImmunohistochemistryAfter deparaffinization, tissue sections were immersed in 1% H2O2 for 30 minutes to block endogenous peroxidase activity and washed with 0.01 M phosphate-buffered saline (PBS). To activate the target antigens, sections for angiotensin II type 1 receptor (AT1R) and 8OHdG immunohistochemical stains were immersed in citrate buffer (0.01 M, pH 6.0) and subjected to microwave treatment at 500 W for 15 minutes. Likewise, sections for TGFβ1 immunohistochemical stains were immersed in citrate buffer (0.01 M, pH 6.0) and incubated at 60°C for 60 minutes. Sections for AT1R and 8OHdG immunohistochemistry were then incubated with 10% normal goat serum (Nichirei Bioscience, Tokyo, Japan) for 30 minutes at room temperature, and those for TGFβ1 immunohistochemistry with 5% normal goat serum (Nichirei Bioscience, Tokyo, Japan). Subsequently, sections for AT1R immunohistochemical stains were then treated with anti-rabbit AT1R polyclonal antibody (1:100; ab18801, Abcam, Cambridge, England), those for 8OHdG immunohistochemical stains with anti-mouse 8OHdG monoclonal antibody (1:50; MOG-020P, Nikken Zyle, Shizuoka, Japan), and those for TGFβ1 immunohistochemical stains with anti-mouse TGFβ1 monoclonal antibody (1:50; 3C11, Santa Cruz Biotechnology, California, USA) as primary antibodies and incubated overnight at room temperature. The next day, sections were washed with PBS and incubated with anti-rabbit immunoglobulin peroxidase-conjugated antibody [Simple Stain Rat MAX-PO (MULTI, Nichirei Bioscience, Tokyo, Japan)] as the secondary antibody for 1 hour at room temperature. The sections were then washed again with PBS, chromogenized with DAB chromogen solution (Histofine DAB Substrate Kit, Nichirei Bioscience, Tokyo, Japan), and counterstained with hematoxylin. Histological evaluationThe various immunohistochemical markers in tissue sections were evaluated by authors ST and SS. Three randomly selected fields per tissue section were photographed at 400× magnification using a digital microscope camera (DP71; Olympus, Tokyo, Japan). For AT1R and TGFβ1 immunohistochemical stains, the percentage of positively stained areas in one field of view was calculated using image analysis software (ImageJ; version 1.43u, National Institutes of Health, Bethesda, MA, USA). For the 8OHdG immunohistochemical stain, the percentage of nuclei with positive staining in one field of view was calculated. For all stains, three randomly selected fields of view were assessed, and the mean of these measurements was calculated. The assessors were blinded to the group classification at the time of imaging and evaluation of the tissue sections. Statistical analysisWeight (kg), age (months), and other continuous variables, such as serum concentrations of 8OHdG, NADPH oxidase, superoxide, and angiotensin II, percentages of positively stained area (%) in AT1R and TGFβ1 immunohistochemical stain, the percentage of nuclei with positive staining in 8OHdG immunohistochemical stain were examined. First, the Shapiro–Wilk test was performed to classify normally and non-normally distributed variables. Non-normally distributed variables were expressed as medians (range) and compared using the Kruskal–Wallis test. Univariable linear regression analysis was performed using the following dependent variables: the percentage of positively stained areas in one field of view in AT1R and TGFβ1 immunohistochemical stains (%) and the percentage of positively stained nuclei in one field of view in 8OHdG immunohistochemical stains (%). The independent variables were as follows: group (C and P), age (months), and weight (kg). Additional univariable linear regression was performed with the following dependent variable: the percentage of positively stained nuclei in one field of view in 8OHdG immunohistochemical stains (%). The independent variable was the percentage of positively stained areas in one field of view in AT1R immunohistochemical stains (%). An additional model was created in which the dependent variable was the percentage of positively stained areas in one field of view in TGFβ1 immunohistochemical stains (%). The independent variable was the percentage of positively stained areas in one field of view in AT1R stains and the percentage of positively stained nuclei in one field of view in 8OHdG immunohistochemical stains (%). Groups (C and P) were included in the model as dummy variables. The results are presented as coefficients (Coe), standard errors (SE), p-values (P), and 95% confidence intervals (CIs). Model diagnostics were performed by assessing the normality of the residuals, and data that were not normally distributed were log-transformed. Stata/IC 14.0 (StataCorp, College Station, TX, USA), a commercial statistical software package, was used for the abovementioned analyses. Statistical estimation and inference were performed using two-tailed hypotheses and tests at the 5% significance level. Ethical approvalThis study was conducted after approval by the relevant Laboratory Animal Committee (approval number: 2019S-72), and all dogs were handled in accordance with relevant institutional and national guidelines and regulations for the care and use of laboratory animals. ResultsConcentrations of angiotensin II and oxidative stress markers in the serum of dogs from the P groupIn the P group, the blood NADPH oxidase concentration (p =0.027) was significantly higher 84 days after initiation of the medication compared to that before prednisolone treatment. In addition, serum 8OHdG (p =0.068) and superoxide (p =0.057) concentrations tended to be higher 84 days after starting the medication than those before the treatment, although the increase was not statistically significant. By contrast, we observed no significant differences or trends in the changes in blood angiotensin II levels over time (p =0.450; Table 1). Immunohistochemical stainsThe descriptive statistics of immunohistochemical data are listed in Table 2. In AT1R immunohistochemical stains, we confirmed the presence of regions with brownish granular positive staining in the cytoplasm of cardiomyocytes, and the aortic tunica media, mainly in the P group (Fig. 1). Univariable linear regression showed that the percentage of positively stained areas in one field of view was significantly increased in the P group compared to that in the C group in the left ventricle (p =0.005), right ventricle (p < 0.0001), ventricular septum ( p < 0.0001), and aorta (p =0.001; Table 3). Similarly, 8OHdG immunohistochemistry showed brownish granular staining of cardiomyocytes and aortic tunica media nuclei, mainly in the P group (Fig. 2). According to univariable linear regression, the percentage of positively stained nuclei in one field of view was significantly higher in the P group than that in the C group in the left ventricle (p < 0.001), right ventricle (p =0.007), ventricular septum (p < 0.001), and aorta (p =0.007; Table 4). Table 1. Concentrations of angiotensin II and oxidative stress markers in the serum of dogs from the high-dose prednisolone (P) group.
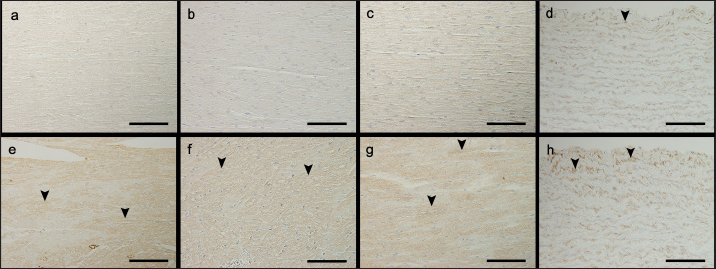
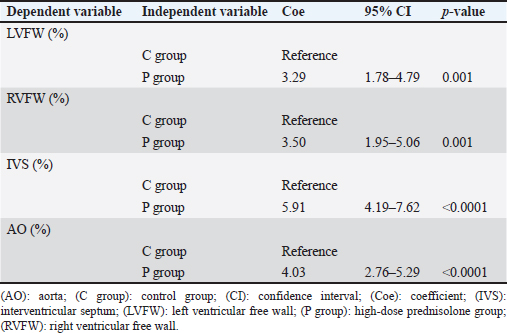
TGFβ1 immunohistochemical stains showed brownish granular positively stained areas in the cytoplasm of cardiomyocytes, and the aortic tunica media, mainly in the P group (Fig. 3). Univariable linear regression demonstrated that the percentage of positively stained areas in one field of view in the P group was significantly increased compared to that in the C group in the left ventricle (p =0.001), right ventricle (p =0.001), ventricular septum ( p < 0.0001), and aorta (p < 0.0001; Table 5). There was no relationship between body weight and age in the immunohistochemical stain parameters. However, in the ventricular septum, 8OHdG immunohistochemical stains showed a positive association with AT1R immunohistochemical stains (Coe=1.27%, 95% CI=0.57–1.98, p =0.002). Regarding TGFβ1 immunohistochemical stains, we observed positive associations with 8OHdG immunohistochemical stains in the left ventricle (Coe=8.55%, 95% CI=4.10–12.99, p =0.002), AT1R (Coe=4.92%, 95% CI=1.43–8.41, p =0.011) and 8OHdG (Coe=5.82%, 95% CI=0.09–11.54, p =0.047) stains in the right ventricle, AT1R (Coe=22.98%, 95% CI=12.91–33.05, p < 0.0001) and 8OHdG (Coe=8.83%, 95% CI=5.15–12.52, p < 0.0001) stains in the ventricular septum, and AT1R stains (Coe=12.16%, 95% CI=3.66–20.66, p =0.010) in the aorta. DiscussionThe results of this study showed a significant increase in the AT1R-, 8OHdG-, and TGFβ1-positive staining rates in the myocardium of the P group compared to that in the C group. These results may support the hypothesis that in the left ventricular myocardium of dogs with HGC, increased myocardial oxidative stress activates the GC-MCR complex, which increases the expression of TGFβ1, resulting in fibrosis (Funder, 2017). TGFβ1 is a potent inducer of connective tissue growth factor, stimulates collagen and fibronectin production, and inhibits collagen degradation by suppressing matrix metalloproteinase activity (Border and Noble, 1994; Leask and Abraham, 2004). The results of AT1R and 8OHdG immunohistochemical stains showed that the percentage of positively stained areas in P group compared to that in C group was significantly increased in all examined regions, i.e., left ventricle, right ventricle, ventricular septum, and aorta. Administration of the GC dexamethasone to rats increased AT1R gene expression and the number of AT1Rs in vascular smooth muscle cells and enhanced their responsiveness to angiotensin II (Sato et al., 1994). In addition, increased angiotensin II stimulation increases oxidative stress (Brito et al., 2015). In the present study, the increase in AT1R expression in myocardial and aortic tissues of the P group increased their sensitivity to angiotensin II stimulation, which may have led to increased levels of 8OHdG, a marker of oxidative stress. Compared to control rats, continuous administration of corticosterone, another type of GC, causes fibrosis of the myocardium with increased superoxide production and NADPH oxide activity (Hattori et al., 2013). These findings may support the result of this study that fibrosis of the left ventricular myocardium was associated with increased oxidative stress. Table 2. Percentages of positively stained area (%) in AT1R and TGFβ1 immunohistochemical stain, and the percentage of nuclei with positive staining in 8OHdG immunohistochemical stain of the left and RVFW, IVS, and aorta.
Angiotensin II stimulation has been reported to induce TGFβ1 mRNA and protein expression in cardiomyocytes and cardiac fibroblasts, and treatment with an AT1R blocker markedly reduced TGFβ1 expression in the myocardium (Gray et al., 1998). This suggests that TGFβ1 induction in the myocardium is at least partially mediated by angiotensin II stimulation. Furthermore, TGFβ1 levels in myocardial tissue increased significantly in a rat model of pressure overload-induced myocardial hypertrophy (Li and Brooks, 1997). The dogs of the P group used in the present study showed a significant increase in blood pressure 84 days after the start of prednisolone medication compared to baseline (Tanaka et al., 2021). These findings suggest that the increase in TGFβ1 expression in myocardial and aortic tissues observed in the current study is not only caused by the activation of the GC-MCR complex in myocardial tissues with elevated oxidative stress but the increased efficacy of angiotensin II stimulation due to increased AT1R expression may be involved in the elevation of blood pressure and induction of TGFβ expression in myocardial tissue.
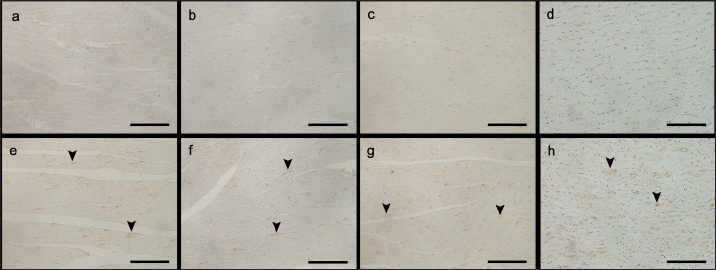
Fig. 1. Images showing AT1R immunohistochemistry of the left and RVFW, ventricular septum, and aorta in the control and prednisolone groups. Arrowheads: area positively stained for AT1R. (a) LVFW in the control group. (b) RVFW in the control group. (c) Ventricular septum in the control group. (d) Aorta in the control group. (e) LVFW in the high-dose prednisolone group. (f) RVFW in the high-dose prednisolone group. (g) Ventricular septum in the high-dose prednisolone group. (h) Aorta in the highdose prednisolone group. Table 3. Results of the univariable linear regression for the percentage of area positively stained for AT1R in the left and RVFW, ventricular septum, and aorta.
Although the mechanism of GC-induced hypertension is not fully understood, it has been reported that increased levels of H2O2, a reactive oxygen species, cause vasoconstriction due to the inactivation of nitric oxide (NO), which plays an important role in the development of hypertension in rats with HGC (Safaeian et al., 2016; Wilcox, 2002). Thus, in the P group in the current study, the increase in blood oxidative stress markers may have led to an increase in blood pressure and subsequently to an upregulation of TGFβ expression in myocardial and aortic tissue in response to the increase in blood pressure. The present study has some limitations. First, the number of dogs used in this study was small because we used the minimum number of animals required considering ethical standards. Thus, the presented findings may be affected by outliers. Notably, the measurement of serum angiotensin II and oxidative stress marker concentrations was performed only on samples from three animals in the P group, which cannot be considered statistically reliable. Second, considering the pharmacological difference between cortisol and prednisolone, with prednisolone exhibiting a four times stronger GC activity than cortisol, the effect of GCs on myocardial tissue may have been more potent than in patients with CS with a similar disease progression (Feldman and Nelson., 2003). In this regard, it has been reported that left ventricular hypertrophy and cardiac dysfunction in dogs and humans with CS are influenced by disease persistence (Fallo et al., 1994; Muiesan et al., 2003). Third, although HGC persists in spontaneous HAC, the dogs in P group were administered prednisolone twice daily, which may have caused an intermittent increase in blood counts, and the duration of systemic organ exposure to GCs may have been different from that in clinical cases of CS.
Fig. 2. Images showing 8OHdG immunohistochemistry of the left and RVFW, ventricular septum, and aorta in the control and prednisolone groups. arrowheads: 8OHdG-positive nuclei. (a) LVFW in the control group. (b) RVFW in the control group. (c) Ventricular septum in the control group. (d) Aorta in the control group. (e) LVFW in the high-dose prednisolone group. (f) RVFW in the high-dose prednisolone group. (g) Ventricular septum in the high-dose prednisolone group. (h) Aorta in the high-dose prednisolone group. Table 4. Results of the univariable linear regression for the percentage of nuclei with positive staining for 8OHdG in the left and RVFW, ventricular septum, and aorta.
Fig. 3. Images showing transforming growth factor β1 immunohistochemistry of the left and RVFW, ventricular septum, and aorta in the control and prednisolone groups. Arrowheads show areas positively stained for transforming growth factor β1. (a) LVFW in the control group. (b) RVFW in the control group. (c) Ventricular septum in the control group. (d) Aorta in the control group. (e) LVFW in the high-dose prednisolone group. (f) RVFW in the high-dose prednisolone group. (g) Ventricular septum in the high-dose prednisolone group. (h) Aorta in the high-dose prednisolone group. Table 5. Results of the univariable linear regression for the percentage of area positively stained for transforming growth factor β1 in the left and RVFW, ventricular septum, and aorta.
In conclusion, the fibrosis observed in the left ventricular myocardium of dogs with HGC may involve increased angiotensin II efficacy and oxidative stress associated with HGC. The results on myocardial fibrosis in dogs with HGC obtained in this study may contribute to the elucidation of the cardiovascular effects of HGC. AcknowledgmentsWe thank Editage (www.editage.jp) for editing this manuscript. Conflict of interestThe authors declare that they have no conflicts of interest. FundingNot applicable. Authors’ contributionSachiyo Tanaka: conceptualization, experiment design, data collection, writing the first draft, and manuscript editing. Shuji Suzuki: experiment design, data collection, and manuscript editing. Satoshi Soeta: experiment design, data collection, writing the first draft, and manuscript editing. Takeharu Kaneda: experiment design, data collection, writing the first draft, and manuscript editing. Yasushi Hara: conceptualization, experiment design, writing the first draft, and manuscript editing. All authors read and approved the final version of the paper. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesBehrend, E.N., Kooistra, H.S., Nelson, R., Reusch, C.E. and Scott-Moncrieff, J.C. 2013. Diagnosis of spontaneous canine hyperadrenocorticism: 2012 ACVIM consensus statement (small animal). J. Vet. Intern. Med. 27, 1292–1304. Border, W.A. and Noble, N.A. 1994. Transforming growth factor beta in tissue fibrosis. N. Engl. J. Med. 331, 1286–1292. Boscaro, M., Barzon, L., Fallo, F. and Sonino, N. 2001. Cushing's syndrome. Lancet. 357, 783–791. Brito, R., Castillo, G. and González, J. 2015. Oxidative stress in hypertension: mechanisms and therapeutic opportunities. Exp. Clin. Endocrinol. Diabetes. 123, 325–335. Carotenuto, G., Malerba, E., Dolfini, C., Brugnoli, F., Giannuzzi, P., Semprini, G., Tosolini, P. and Fracassi, F. 2019. Cushing's syndrome-an epidemiological study based on a canine population of 21,281 dogs. Open Vet. J. 9, 27–32. Chen, H.Y., Lien, Y.H. and Huang, H.P. 2014. Assessment of left ventricular function by two-dimensional speckle-tracking echocardiography in small breed dogs with hyperadrenocorticism. Acta Vet. Scand. 56, 88. de Bruin, C., Meij, B.P., Kooistra, H.S., Hanson, J.M., Lamberts, S.W. and Hofland, L.J. 2009. Cushing's disease in dogs and humans. Horm. Res. 71(Suppl. 1), 140–143. Fallo, F., Budano, S., Sonino, N., Muiesan, M.L., Agabiti-Rosei, E. and Boscaro, M. 1994. Left ventricular structural characteristics in Cushing's syndrome. J. Hum. Hypertens. 8, 509–513. Feldman, E.C. and Nelson, R.W. 2003. Canine and feline endocrinology and reproduction, 3rd edition. St. Louis, Mo: Saunders. Funder, J.W. 2017. Aldosterone and mineralocorticoid receptors-physiology and pathophysiology. Int. J. Mol. Sci. 18, 1032. Gray, M.O., Long, C.S., Kalinyak, J.E. Li, H.T. and Karliner, J.S. 1998. Angiotensin II stimulates cardiac myocyte hypertrophy via paracrine release of TGF-beta 1 and endothelin-1 from fibroblasts. Cardiovasc Res. 40, 352–363. Hattori, T., Murase, T., Iwase, E., Takahashi, K., Ohtake, M., Tsuboi, K., Ohtake, M., Miyauchi, M., Murohara, T. and Nagata, K. 2013. Glucocorticoid-induced hypertension and cardiac injury: effects of mineralocorticoid and glucocorticoid receptor antagonism. Nagoya. J. Med. Sci. 75, 81–92. Isidori, A.M., Graziadio, C., Paragliola, R.M., Cozzolino, A., Ambrogio, A.G., Colao, A., Corsello, S.M., Pivonello, R. and ABC Study Group 2015. The hypertension of Cushing's syndrome: controversies in the pathophysiology and focus on cardiovascular complications. J. Hypertens. 33, 44–60. Leask, A. and Abraham, D.J. 2004. TGF-beta signaling and the fibrotic response. FASEB J. 18, 816–827. Li, J.M. and Brooks, G. 1997. Differential protein expression and subcellular distribution of TGFbeta1, beta2 and beta3 in cardiomyocytes during pressure overload-induced hypertrophy. J. Mol. Cell Cardiol. 29, 2213–2224. Muiesan, M.L., Lupia, M., Salvetti, M., Grigoletto, C., Sonino, N., Boscaro, M., Rosei, E.A., Mantero, F. and Fallo, F. 2003. Left ventricular structural and functional characteristics in Cushing's syndrome. J. Am. Coll. Cardiol. 41, 2275–2279. Nieman, L.K. 2015. Cushing's syndrome: update on signs, symptoms and biochemical screening. Eur. J. Endocrinol. 173, M33–M38. Nivy, R., Refsal, K.R., Ariel, E., Kuzi, S., Yas-Natan, E. and Mazaki-Tovi, M. 2018. The interpretive contribution of the baseline serum cortisol concentration of the ACTH stimulation test in the diagnosis of pituitary dependent hyperadrenocorticism in dogs. J. Vet. Intern. Med. 32, 1897–1902. Oui, H., Jeon, S., Lee, G., Park, S., Cho, K.O. and Choi, J. 2015. Tissue Doppler and strain imaging of left ventricle in Beagle dogs with iatrogenic hypercortisolism. J. Vet. Sci. 16, 357–365. Safaeian, L., Hajhashemi, V. and Haghjoo Javanmard, S. 2016. The effect of protocatechuic acid on blood pressure and oxidative stress in glucocorticoid-induced hypertension in rat. Iran. J. Pharm. Res. 15, 83–91. Sato, A., Suzuki, H., Nakazato, Y., Shibata, H., Inagami, T. and Saruta, T. 1994. Increased expression of vascular angiotensin II type 1A receptor gene in glucocorticoid-induced hypertension. J. Hypertens. 12, 511–516. Sugihara, N., Shimizu, M., Kita, Y., Shimizu, K., Ino, H., Miyamori, I., Nakabayashi, H. and Takeda, R. 1992. Cardiac characteristics and postoperative courses in Cushing's syndrome. Am. J. Cardiol. 69, 1475–1480. Takano, H., Kokubu, A., Sugimoto, K., Sunahara, H., Aoki, T. and Fijii, Y. 2015. Left ventricular structural and functional abnormalities in dogs with hyperadrenocorticism. J. Vet. Cardiol. 17, 173–181. Tanaka, S., Shibuya, H., Suzuki, S., Kanno, N., Harada, Y., Sato, A., Soeta, S. and Hara, Y. 2021. Long-term administration of prednisolone: effects on the myocardial tissue of healthy beagle dogs. J. Vet. Med. Sci. 83, 84–93. van der Pas, R., Leebeek, F.W.G., Hofland, L.J., De Herder, W.W. and Feelders, R.A. 2013. Hypercoagulability in Cushing's syndrome: prevalence, pathogenesis and treatment. Clin. Endocrinol. (Oxf). 78, 481–488. Wilcox, C.S. 2002. Reactive oxygen species: roles in blood pressure and kidney function. Curr. Hypertens. Rep. 4, 160–166. | ||
| How to Cite this Article |
| Pubmed Style Tanaka S, Suzuki S, Soeta S, Kaneda T, Hara Y. Mechanism of long-term high-dose prednisolone administration producing myocardial fibrosis in beagle dogs. Open Vet J. 2023; 13(12): 1708-1717. doi:10.5455/OVJ.2023.v13.i12.19 Web Style Tanaka S, Suzuki S, Soeta S, Kaneda T, Hara Y. Mechanism of long-term high-dose prednisolone administration producing myocardial fibrosis in beagle dogs. https://www.openveterinaryjournal.com/?mno=162525 [Access: May 13, 2024]. doi:10.5455/OVJ.2023.v13.i12.19 AMA (American Medical Association) Style Tanaka S, Suzuki S, Soeta S, Kaneda T, Hara Y. Mechanism of long-term high-dose prednisolone administration producing myocardial fibrosis in beagle dogs. Open Vet J. 2023; 13(12): 1708-1717. doi:10.5455/OVJ.2023.v13.i12.19 Vancouver/ICMJE Style Tanaka S, Suzuki S, Soeta S, Kaneda T, Hara Y. Mechanism of long-term high-dose prednisolone administration producing myocardial fibrosis in beagle dogs. Open Vet J. (2023), [cited May 13, 2024]; 13(12): 1708-1717. doi:10.5455/OVJ.2023.v13.i12.19 Harvard Style Tanaka, S., Suzuki, . S., Soeta, . S., Kaneda, . T. & Hara, . Y. (2023) Mechanism of long-term high-dose prednisolone administration producing myocardial fibrosis in beagle dogs. Open Vet J, 13 (12), 1708-1717. doi:10.5455/OVJ.2023.v13.i12.19 Turabian Style Tanaka, Sachiyo, Shuji Suzuki, Satoshi Soeta, Takeharu Kaneda, and Yasushi Hara. 2023. Mechanism of long-term high-dose prednisolone administration producing myocardial fibrosis in beagle dogs. Open Veterinary Journal, 13 (12), 1708-1717. doi:10.5455/OVJ.2023.v13.i12.19 Chicago Style Tanaka, Sachiyo, Shuji Suzuki, Satoshi Soeta, Takeharu Kaneda, and Yasushi Hara. "Mechanism of long-term high-dose prednisolone administration producing myocardial fibrosis in beagle dogs." Open Veterinary Journal 13 (2023), 1708-1717. doi:10.5455/OVJ.2023.v13.i12.19 MLA (The Modern Language Association) Style Tanaka, Sachiyo, Shuji Suzuki, Satoshi Soeta, Takeharu Kaneda, and Yasushi Hara. "Mechanism of long-term high-dose prednisolone administration producing myocardial fibrosis in beagle dogs." Open Veterinary Journal 13.12 (2023), 1708-1717. Print. doi:10.5455/OVJ.2023.v13.i12.19 APA (American Psychological Association) Style Tanaka, S., Suzuki, . S., Soeta, . S., Kaneda, . T. & Hara, . Y. (2023) Mechanism of long-term high-dose prednisolone administration producing myocardial fibrosis in beagle dogs. Open Veterinary Journal, 13 (12), 1708-1717. doi:10.5455/OVJ.2023.v13.i12.19 |