| Research Article | ||
Open Vet J. 2023; 13(10): 1326-1333 Open Veterinary Journal, (2023), Vol. 13(10): 1326–1333 Original Research Asiatic acid increased locomotor and head width by inducing brain-derived neurotrophic factor in intrauterine hypoxia-exposed zebrafishAriani Ariani1,2*, Husnul Khotimah3, Nurdiana Nurdiana3, and Masruroh Rahayu41Doctoral Program of Medical Science, Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia 2Department of Pediatrics, Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia 3Department of Pharmacology, Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia 4Department of Neurology, Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia *Corresponding Author: Ariani Ariani. Doctoral Program of Medical Science, Faculty of Medicine, Universitas Brawijaya, Malang, Indonesia. Email: arianidr [at] ub.ac.id Submitted: 06/08/2023 Accepted: 21/09/2023 Published: 31/10/2023 © 2023 Open Veterinary Journal
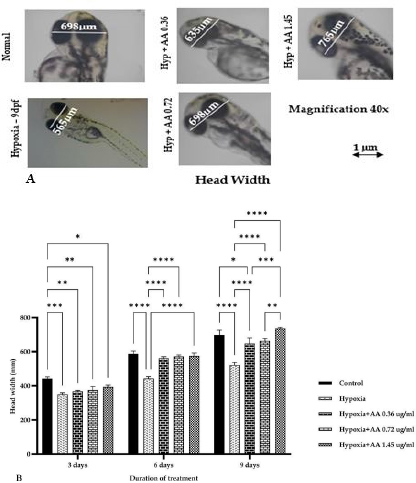
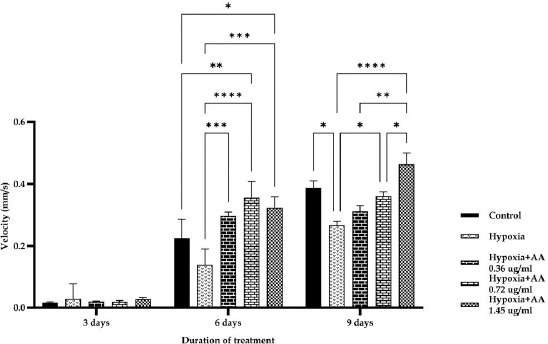
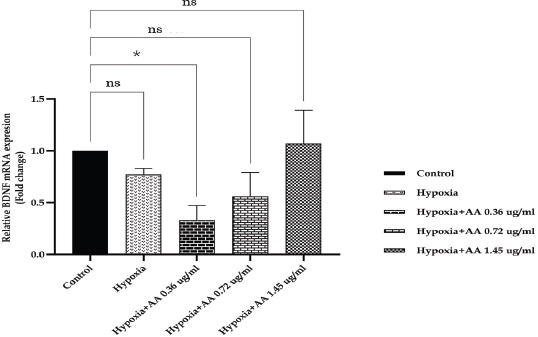
AbstractBackground: Hypoxia ischemia leads to abnormal behavior and growth. Prenatal hypoxia also decreases brain adaptive potential, which can cause fatal effects such as cell death. Asiatic acid (AA) in Centella asiatica is a neuroprotector through antioxidant and anti-inflammatory activities. Aim: This study aimed to analyze the effect of AA as a neuroprotector against hypoxia during intrauterine development on locomotor activity, head width, and brain-derived neurotrophic factor (BDNF) expression. Methods: The true experimental laboratory research used a posttest control-only design. Zebrafish embryos (Danio rerio) aged 0–2 dpf (days postfertilization) were exposed to hypoxia with oxygen levels reaching 1.5 mg/l. Then, AA was administered at successive concentrations, namely, 0.36, 0.72, and 1.45 μg/ml, at 2 hpf (hours postfertilization), 3, 6, and 9 dpf. Head width, velocity activity, and BDNF expression were observed. Results: Intrauterine hypoxia significantly decreased head width, velocity rate, and BDNF expression (<0.001). Administration of AA at all concentrations and age 9 dpf to zebrafish larvae with intrauterine hypoxia exposure increased head width ( p < 0.0001), velocity (p < 0.05), and relative mRNA expression of BDNF (p < 0.05). Conclusion: AA is potentially neuroprotective to the brain in zebrafish larvae exposed to hypoxia during intrauterine development Keywords: Asiatic acid, BDNF, Growth, Hypoxia, Neuron. IntroductionPerinatal hypoxic ischemia (HI) occurs in 1.5–3 per 1,000 live births. It is often the most common cause of neurodevelopmental disorders in infants in later life that can manifest as disturbances in adulthood, such as decreased learning, memory, and attention (Piešová and Mach, 2020). The incidence of hypoxic-ischaemic encephalopathy in live births ranges between 3/1,000 and 6/1,000, of which 15%–20% of affected infants die in the neonatal period, and 25%–30% of survivors may experience some long-term sequelae (Guan et al., 2017). Hypoxia is usually presumed to have a pathological effect but is also involved in maintaining normal physiological functions (Chen et al., 2020). HI in the brain can occur prenatally, naturally, and postnatally, but the neurodevelopmental sequelae will be more severe if they appear in the prenatal and natal periods. Several studies have proven that oxygen deficiency conditions in the brain during the prenatal period will cause damage to brain neurons, metabolic failure, and cell death (Gorgij et al., 2021). HI leads to abnormal behavior and reduced growth. Motor disabilities, such as locomotor disruption, are related to developmental hypoxic injury (Son et al., 2022). Brain-derived neurotrophic factor (BDNF) is a neuroprotective agent that functions in the growth and development of the nervous system and reduces neuronal death and the severity of brain damage after HI injury (Gorgij et al., 2021). Clinically, oxygen therapy is given to patients rationally because hyperoxia often causes lung injury (Honda et al., 2019). One of the traditional plants, Centella asiatica, is a plant that is easily found in subtropical and tropical areas such as Indonesia. This plant has a neuroprotective effect through antioxidant and anti-inflammatory mechanisms. Asiatic acid (AA) in C. asiatica suppresses oxidative stress and apoptosis in Alzheimer’s disease (Rather et al., 2018), protecting against mitochondrial damage in rat models of focal cerebral ischemia and protecting the brain against neurotoxins (Chiroma et al., 2019). A breakthrough is needed to prevent the negative impact of hypoxic conditions on the developing baby’s brain. There has been no research on the effect of AA as a neuromodulator in brain neurons. This study aimed to analyze the effect of AA as a neuroprotector in hypoxic conditions during intrauterine development on locomotor activity rate, head width, and BDNF. Material and MethodsMaterialsThis research used commercial products of AAs (2α,23-dihydroxyursolic acid, and Dammarolic acids) from C. asiatica (NSC Product Number: A2612, CAS Number: 464-92-6, Sigma Aldrich, USA). The concentrations in this experiment were based on previous studies, namely, 0.36, 0.72, and 1.45 μg/ml (Khotimah et al., 2015). Animal model (Zebrafish embryo)Wild-type zebrafish embryos (Danio rerio) from fertilizing male and female zebra types were identified and obtained from the Hydrology Laboratory, Faculty of Fisheries and Marine Sciences, Universitas Brawijaya, Malang, Indonesia. Experimental designThe true experimental laboratory research used a posttest control-only design. Zebrafish embryos (Danio rerio) aged 0–2 dpf were exposed to hypoxia. Then, AA was administered at 2 hpf, 3 dpf, 6 dpf, and 9 dpf. The zebrafish larvae at ages 3 dpf, 6 dpf, and 9 dpf were evaluated for head width and locomotor (velocity) rate. The relative mRNA expression of BDNF was observed at 9 dpf. Zebrafish embryos aged 0–2 hpf were allocated and then randomly divided into five treatment groups: (a) normal zebrafish larvae (control); (b) hypoxia zebrafish larvae; (c) hypoxia zebrafish larvae + 0.36 μg/ml AA; (d) hypoxia zebrafish larvae + 0.72 μg/ml AA; and (e) hypoxia zebrafish larvae + 1.45 μg/ml AA. The hypoxic modelThe hypoxia model in this study involves the supply of nitrogen (N2) gas to the chamber that has been provided until it reaches hypoxic conditions (PO2 ~ 5 kPa, 1,5 mg O2/l). Zebrafish larvae treated with hypoxia at age 2 hpf to 3 dpf, returned to a normoxic state (PO2 ~ 20 kPa, 8 mg O2/l) at age 4 dpf (Borowiec et al., 2015). The dissolved oxygen (DO) was measured using a DO meter. If the saturation of the solute oxygen is too high, nitrogen gas is added until the DO reaches 1.5 mg O2/l to create hypoxia (Borowiec et al., 2015). If the DO concentration is too low, it is adjusted by adding oxygen to 8 mg O2/l (Ariani et al., 2023). Measurement of head width of zebrafish larvaeThe head width of zebrafish larvae in all groups was observed and measured at 3, 6, and 9 dpf. Measurements were taken from the left to right side (Son et al., 2022) using raster image software 3.0 in millimeter (mm) units. Observation of zebrafish larvae velocityThe behavioral activity was monitored in zebrafish larvae aged 3, 6, and 9 dpf placed in 96-well round bottom plates. After acclimatization for 30 minutes, spontaneous behavior (velocity/swimming) as basal swimming activity (Davis et al., 2021) was measured for 10 minutes in 10 zebrafish of each group. The mean of velocity (mm s−) was measured. The velocity of zebrafish larvae in each group was recorded using EthoVision XT tracking software tools and DanioScope (Leesburg, VA, USA) (Sireeni et al., 2020). Measurement of the relative mRNA expression of BDNF The mRNA expression of BDNF was measured using quantitative PCR (qPCR) with a Fast RNA Tissue/Insect Kit (Zymo Research, Irvine, CA). Total RNA was extracted from 12 zebrafish larvae at 9 dpf (Köblitz et al., 2015) by following the manufacturer’s instructions. A Thermo Fisher Scientific Nanodrop 2000 Spectrophotometer (Waltham, MA) was used to confirm the quantity and purity of RNA. Each sample’s 100–200 ng of RNA was used to create cDNA using a LunaScript® RT Master Mix Kit (New England Biolabs, Ipswich, MA). The 20 µl real-time polymerase chain reaction mixtures contained 10 µl of master, eight µl of DNase/RNase-free water, one µl of 50 nM primers, and one µl of cDNA template. The reactions were carried out using Chai Open qPCR. Statistical analysisThe data are presented as each group’s mean ± SEM. The data were analyzed with GraphPad Prism 7 (La Jolla, California, USA). Statistical significance was determined through one-way analysis of variance. p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), and 0.0001 (****) were considered to be significant, highly significant, and extremely significant differences, respectively (Wang et al., 2021). Ethical approval This research was approved by the Health Research Ethics Committee of Universitas Brawijaya with reference number 179/EC/KEPK/07/2022. ResultsMeasurement of the head width of zebrafish larvaeThe hypoxic zebrafish larvae at 3, 6, and 9 dpf showed head width (in mm) increases of 350 ± 22, 443 ± 28, and 522 ± 37, respectively. The older the zebrafish larvae were, the wider the head width. The zebrafish larvae that received hypoxia and AA concentrations of 0.36 and 0.72 μg/ml at 3 and 6 dpf showed a mean head width (in mm) smaller consecutively, namely, (367 ± 15 and 561 ± 22) and (375 ± 52 and 571 ± 24) compared to the control group (442 ± 27 and 587 ± 40). Nevertheless, concentrations of AA of 1.45 μg/ml and at 9 dpf (736 ± 19) increased the head width in zebrafish larvae exposed to hypoxia compared to the control group (697 ± 74) and hypoxia control (522 ± 37) (Fig. 1). Observation of zebrafish larvae velocityAdministration of AA to zebrafish larvae exposed to hypoxia showed no significant difference in velocity rate at 3 dpf. The zebrafish larvae at 3 dpf showed a higher mean velocity rate (mm s−) in the hypoxic group (0.028 ± 0.012) than in the control group (0.028 ± 0.012). The velocity rate in zebrafish larvae increased at high concentrations and larvae age. The administration of AA at concentrations 0.72 and 1.45 µg/ml increased the velocity rate (mm/smm s−) in zebrafish larvae exposed to hypoxia at 6 (0.296 ± 0.031, 0.355 ± 0.130, and 0.323 ± 0.086) and 9 dpf 0.311 ± 0.045, 0.360 ± 0.034, and 0.463 ± 0.089 compared to the hypoxic group that was not given AA (0.138 ± 0.127 and 0.266 ± 0.031, respectively). The velocity rate in the control group (0.386 ± 0.058) was lower than that at the AA concentration of 1.45 µg/ml in zebrafish larvae exposed to hypoxia (0.463 ± 0.089) at 9 dpf (Fig. 2). The relative mRNA expression of BDNFThe control group showed a higher mean relative mRNA expression of BDNF than the hypoxic group. AA administration at concentrations of 0.36 and 0.72 µg/ml at 3 and 6 dpf did not increase BDNF expression successively compared to the hypoxic group (unpublished data). The higher the concentration of AA given to zebrafish larvae exposed to hypoxia, the higher the expression of BDNF at 9 dpf. There was an increase in BDNF expression due to the administration of AA at concentrations of 0.36, 0.72l, and 1.45 µg/ml to zebrafish larvae exposed to hypoxia (0.311 ± 0.045, 0.360 ± 0.034, and 0.463 ± 0.089, respectively) compared with hypoxic controls (0.266 ± 0.089). Administration of AA at concentrations of 0.72 and 1.45 µg/ml to zebrafish larvae exposed to hypoxia increased BDNF expression compared with that in the control and hypoxia groups (Fig. 3).
Fig. 1. A). Photomicrograph of head width of zebrafish larvae at 9 dpf in all groups with a 40× objective magnificent. Measurements are taken on the head from the tip of the right eye to the left. B). The size of the head width of zebrafish larvae after exposure to hypoxia to different concentrations of AA. Acetic acid at various concentrations increased the growth of AA administration at concentrations of 0.36, 0.72, and 1.45 μg/ml. It increased the head width of zebrafish larvae exposed to hypoxia compared to the control. The data are presented as the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 were considered significant, highly significant and extremely significant differences, respectively. The concentration of 1.45 µg/ml AA significantly increased the head width of zebrafish larvae at 9 dpf compared with normal.
Fig. 2. Velocity rate at 3, 6, and 9 dpf zebrafish larvae exposed hypoxia in all groups. The data are presented as the mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 were significant, highly significant, and extremely significant differences, respectively. The control group showed a significantly higher velocity rate than the hypoxia group at 6 and 9 dpf. Observations at 9 dpf showed that a 1.45 µg/ml concentration of AA could increase the velocity rate compared with the control group. DiscussionHead width of zebrafish larvaeZebrafish are tolerant to low concentrations of O2 and can quickly adapt by increasing the gills’ surface area and increasing myoglobin’s expression as an oxygen carrier (De Medeiros Borges et al., 2023). Fetal hypoxia during pregnancy creates an intrauterine environment that does not provide enough oxygen and nutrients to the fetus (Peebles, 2004), inhibiting fetal growth and even causing death (Iqbal and Ciriello, 2013). Head width measurement is one of the easiest methods to measure brain growth (Harris, 2015). The circle of the zebrafish larva head is measured on larvae at 3, 6, and 9 dpf according to the age analogy of children 0, 2, and 8 years in humans (Sorribes et al., 2013). The results showed that zebrafish exposed to hypoxia showed a decrease in head width compared to the control group. Hypoxia during the first 4 weeks of life leads to a significant growth delay in the body (Farahani et al., 2008). Hypoxia inhibits organogenesis and intrauterine growth in human fetuses (Kamei and Duan, 2018) by altering several vital metabolic processes, including glucose absorption and oxidative metabolism, thereby inhibiting growth (Chen et al., 2020). The barrier to fetal development is the failure of the fetus to reach its maximum growth potential. The leading causes of fetal growth obstacles are structural or genetic defects and placental insufficiency. Placental insufficiency includes the fetal inability to obtain sufficient nutrients and oxygen (Swanson and David, 2015). Hypoxia may reduce the mother’s supply of nutrients to the fetus by inhibiting placental rapamycin complex 1, which is responsible for cell growth, proliferation, and metabolism (Piešová and Mach, 2020). The brain needs oxygen and high nutrition because it undergoes aerobic metabolism (Piešová and Mach, 2020). Prenatal hypoxia in the period of brain formation causes significant changes in the development of cognitive functions at various stages of postnatal life that correlate with morphological changes in brain structures involved in learning and memory and shape individual life experiences and character (Desplats, 2014; Babenko et al., 2015). Prenatal hypoxia also leads to decreased brain adaptive potential and plasticity due to disruption in forming new contact between cells and the spread of nerve stimuli, especially in the cortex and hippocampus (Nalivaeva et al., 2018). Hypoxia significantly affects the morphology and function of various types of neurons. It disrupts the development of nerve processes and connections, involving subplate neurons and neuron synapses, which regulate brain development and play an essential role in building cortical relationships with other brain regions (McClendon et al., 2017).
Fig. 3. The relative mRNA expression of BDNF among the treatment groups showed no significant difference. AA at a 1.45 µg/ml concentration resulted in the highest BDNF expression. Data are presented as the mean ± SEM. The control group showed higher BDNF expression than the hypoxic control group. The higher the concentration of AA given, the higher the expression of BDNF in zebrafish exposed to hypoxia. AA administration at a 1.45 g/ml concentration in zebrafish exposed to hypoxia resulted in high BDNF expression compared with the other groups. AA at all concentrations increased the head width in zebrafish larvae exposed to hypoxia at 3, 6, and 9 dpf. The longer the time and concentration of AA, the greater the average head width of zebrafish larvae exposed to hypoxia. AA could modulate the PI3K/AKT/mTOR signaling pathway, affecting cell proliferation, growth, translation, migration, and survival (Hao et al., 2018). This study’s results follow the author’s previous research, which showed that administering C. asiatica extract containing AA could increase the growth of zebrafish larvae exposed to intermittent hypoxia (Ariani et al., 2023). Administration of AA at a concentration of 0.72 μg/ml on the 9th day resulted in a head width in zebrafish larvae exposed to hypoxia that was higher than that in the hypoxia group but similar to that in the normal group. This result is different from the effect of the administration of AA at a concentration of 1.45 μg/ml against zebrafish larvae exposed to hypoxia, which could decrease both normal and hypoxia control levels because concentrations that are too high and for a long time may cause an excessive increase in biological responses for morphological and locomotor improvements as well as BDNF expression in zebrafish. An exaggerated biological response may lead to more severe tissue damage if not adequately controlled. Zebrafish larval velocityVelocity rate measurements showed that zebrafish larvae aged 3 days under hypoxic conditions were higher than those under normal conditions. Administration of AA to zebrafish larvae aged 3 and 6 days also showed no significant difference because it is possible that at that age, the zebrafish larvae are still adapting to the environment so that the exposure that appears can be overly responsive without proper regulatory control. This research followed Sireeni et al. (2020). It showed that early development of zebrafish, age 5, showed a relatively small increase in cortisol levels or even nothing against mild stress, such as circles. The administration of AA to zebrafish larvae exposed to hypoxia at 9 days showed different significance, with an optimal concentration of 0.72 µg/ml. This is consistent with a study by Khotimah et al. (2015), which showed that administering C. asiatica extracts to adult zebrafish with a Parkinson’s model given rotenone improved locomotive activity. Hypoxia reduces the ability of zebrafish larvae to swim. Based on Davis et al. (2021), zebrafish larvae at an early stage of development (5–8 dpf) may show different behavioral responses depending on the concentration of the inducer administered. Hypoxia causes mitochondrial dysfunction and also causes cell damage (Kumar et al., 2015) and cell death (apoptosis) in dopaminergic neurons in the substantia nigra (Khotimah et al., 2015). A decrease in dopamine neurotransmitter synthesis leads to a decline in the modulation of neuroendocrine functions, cognition, attention, and behaviors due to the disturbance of tyrosine hydroxylase activity (Baek et al., 2014). Triterpenes in CA function to increase the levels of antioxidant enzymes, such as glutathione, catalase, and superoxide dismutase (Sun et al., 2020), and also increase the levels of the neurotransmitters serotonin, dopamine, and norepinephrine in the rat brain (Chen et al., 2005). At the age of 9 days, the velocity rate of zebrafish larvae exposed to hypoxia and AA was higher than that under normal conditions because high concentrations cause locomotor hyperactivity. This study differs from Licitra et al. (2021), which showed that administration of C. sativa at a concentration of 0.3 to 1.2 mg/l increased locomotion compared to the control. The relative mRNA expression of BDNFIn this study, hypoxia caused a decrease in BDNF expression. The control group showed a higher average expression of BDNF than the hypoxic group. Low BDNF expression results in the decompensation of neurotrophic processes, and cortical neurons are not protected, so they are insufficient to maintain nerve regeneration. The BDNF has a neuroprotective function through accelerated myelination, reorganization, and regulation of cerebral tissue neurons in hypoxia-ischemia-induced injury. BDNF can reduce cell death and infarct size and improve sensorimotor recovery after HI injury (Gorgij et al., 2021). There was an increase in BDNF expression due to the administration of AA to zebrafish larvae exposed to hypoxia compared with hypoxic controls. The administration of AA at concentrations of 0.72 and 1.45 µg/ml to zebrafish larvae exposed to hypoxia increased BDNF expression compared with both the control and hypoxia groups because BDNF plays a role in neuroinflammation in the injured central nervous system (CNS) to modulate inflammatory homeostasis (Lai et al., 2018). AA is neuroprotective by suppressing oxidative stress and apoptosis in HI-induced brain injury (Ding et al., 2018). Xu et al. (2013) found that madecassoside in C. asiatica can improve cognitive performance by increasing the expression of BDNF so that neuronal cells become more resilient (Sbrini et al., 2020). Moreover, AA inhibits transforming growth factor β–activated kinase 1 activation, suppresses p-JNK (c-Jun N-terminal kinase) expression, and targets proapoptotic factors in the brain cortex. AA significantly suppressed HI-mediated upregulation of caspase-3, p53, and p-c-Jun expression in the rat brain cortex (Wang et al., 2019). Research by Yang et al. (2016) showed that madecassic acid in C. asiatica significantly reduced hypoxia-induced oxidative stress, increased cell viability, decreased TUNEL and reactive oxygen species ratios, and lowered malondialdehyde, caspase-3 and caspase-9 activity, as well as the Bax/Bcl-2 ratios. BDNF is a potent regulatory mediator in the CNS, such as neurogenesis, growth, and physiological function of neurons to prevent cell death during hypoxic conditions (Lai et al., 2018). The existence of high BDNF expression can be followed by growth and high locomotor in hypoxic conditions. Administration of AA concentration of 0.72 μg/ml and at 9 dpf could have neuroprotective effects on the brain in zebrafish larvae exposed to hypoxia because of the increased head width, velocity rate, and relative expression of BDNF. ConclusionHypoxia during intrauterine development decreases zebrafish larvae on head width and velocity rate. An AA concentration of 0.72 µg/ml and exposure at 9 dpf was neuroprotective to the brain in zebrafish larvae exposed to hypoxia. AcknowledgmentsThe authors would like to thank the Department of Pharmacology, Medical Faculty, Universitas Brawijaya, for the support. Conflicts of interestThe authors declare that there is no conflict of interest. FundingThis research was supported by the Ministry of Research and Technology of the Republic of Indonesia with grant number 033/E5/PG.02.00/2022. Author contributionsAR, HK, NU, MA: concept and design of the research; AR, HK, NU, MA: acquisition of data, analysis, and interpretation; critical revision and final approval. Data availabilityAll data of this manuscript are provided within the manuscript. Any extra data needed can be requested from the corresponding author. ReferencesAriani, A., Ghofar, I., Khotimah, H., Nurdiana, N. and Rahayu, M. 2023. Asiatic acid in Centella asiatica extract towards morphological development in an intermittent hypoxia intrauterine embryo model and molecular prediction pathway of insulin-like growth factor-1 receptor signalling. Open. Vet. J. 13, 629–637. Babenko, O., Kovalchuk, I. and Metz, G.A.S. 2015. Stress-induced perinatal and transgenerational epigenetic programming of brain development and mental health. Neurosci. Biobehav. Rev. 48, 70–91. Baek, D., Lee, C. and Baek, S. 2014. Effect of treadmill exercise on social interaction and tyrosine hydroxylase expression in the attention-deficit/hyperactivity disorder rats. J. Exerc. Rehabil. 10, 252–257. Borowiec, B.G., Darcy, K.L., Gillette, D.M. and Scott, G.R. 2015. Distinct physiological strategies are used to cope with constant hypoxia and intermittent hypoxia in killifish (Fundulus heteroclitus). J. Exp. Biol. 218, 1198–1211. Chen, P., Chiu, W., Hsu, P., Lin, S., Peng, I., Wang, C. and Tsai, S. 2020. Pathophysiological implications of hypoxia in human diseases. J. Biomed. Sci. 27, 1–19. Chen, Y., Han, T., Rui, Y., Yin, M., Qin, L. and Zheng, H. 2005. Effects of total triterpenes of Centella asiatica on the corticosterone levels in serum and contents of monoamine in depression rat brain. J. Chinese. Med. Mater. 28, 492–496. Chiroma, S., Baharuldin, M.T., Mat Taib, C., Amom, Z., Jagadeesan, S., Adenan, M. and Moklas, M.A. 2019. Protective effect of Centella asiatica against D-galactose and aluminium chloride induced rats: behavioral and ultrastructural approaches. Biomed. Pharmacother. 109, 853–864. Davis, R., Luchtenburg, F., Richardson, M., Schaaf, M., Tudorache, C. and Slabbekoorn, H. 2021. The importance of individual variation for the interpretation of behavioural studies: ethanol effects vary with basal activity level in zebrafish larvae. Psychopharmacology (Berl) 238, 3155–3166. De Medeiros Borges, H., Dagostin, C., Córneo, E., Dondossola, E., Bernardo, H., De Poeri Pickler, K., Da Costa Pereira, B., De Oliveira, M., Scussel, R., Michels, M., Machado-deÁvila, R., Dal-Pizzol, F. and Rico, E. 2023. Zebrafish as a potential model for stroke: a comparative study with standardized models. Life. Sci. 312, 121200. Desplats, P. 2014. Perinatal programming of neurodevelopment: epigenetic mechanisms and the prenatal shaping of the brain. Adv. Neurobiol. 10, 335–361. Ding, H., Xiong, Y., Sun, J., Chen, C., Gao, J. and Xu, H. 2018. Asiatic acid prevents oxidative stress and apoptosis by inhibiting the translocation of α-synuclein into mitochondria. Front. Neurosci. 12, 1–10. Farahani, R., Kanaan, A., Gavrialov, O., Brunnert, S., Douglas, R.M. and Morcillo, P., Haddad, G.G. 2008. Differential effects of chronic intermittent and chronic constant hypoxia on postnatal growth and development. Pediatr. Pulmonol. 43, 20–28. Gorgij, E., Fanaei, H., Yaghmaei, P., Shahraki, M. and Mirahmadi, H. 2021. Treadmill exercise during pregnancy decreased vulnerability to neonatal hypoxia-ischemia through reducing inflammation and increasing antiapoptotic gene expressions and antioxidant capacity in rats. Stroke. Res. Treat. 14, 5512745. Guan, B., Dai, C., Zhang, Y., Zhu, L., He, X., Wang, N. and Liu, H. 2017. Early diagnosis and outcome prediction of neonatal hypoxic-ischemic encephalopathy with color Doppler ultrasound. Diagn. Interv. Imaging. 98, 469–475. Hao, Y., Huang, J., Ma, Y., Chen, W., Fan, Q., Sun, X., Shao, M. and Cai, H. 2018. Asiatic acid inhibits proliferation, migration and induces apoptosis by regulating Pdcd4 via the PI3K/Akt/mTOR/p70S6K signaling pathway in human colon carcinoma cells. Oncol. Lett. 15, 8223–8230. Harris, S. 2015. Measuring head circumference: update on infant microcephaly. Can. Fam. Physician. 61, 680–684. Honda, T., Hirakawa, Y. and Nangaku, M. 2019. The role of oxidative stress and hypoxia in renal disease. Kidney. Res. Clin. Pract. 38, 414–426. Iqbal, W. and Ciriello, J. 2013. Effect of maternal chronic intermittent hypoxia during gestation on offspring growth in the rat. Am. J. Obstet. Gynecol. 209, 564.e1–e9. Kamei, H. and Duan, C. 2018. Hypoxic treatment of zebrafish embryos and larvae. Methods. Mol. Biol. 1742, 195–203. Khotimah, H., Sumitro, S., Ali, M. and Widodo, M. 2015. Standardized Centella asiatica increased brain-derived neurotrophic factor and decreased apoptosis of dopaminergic neuron in rotenone-induced zebrafish. GSTF. J. Psychol. 2, 22–27. Köblitz, L., Fiechtner, B., Baus, K., Lussnig, R. and Pelster, B. 2015. Developmental expression and hypoxic induction of hypoxia inducible transcription factors in the Zebrafish. PLoS One 10(6), e0128938. Kumar, V., Babu, V., Nagarajan, K., Machawal, L. and Bajaj, U. 2015. Protective effects of Centella asiatica against isoproterenol-induced myocardial infarction in rats: biochemical, mitochondrial and histological findings. J. Phytopharm. 4, 80–86. Lai, S., Chen, J., Lin, H., Liu, Y., Tsai, C., Chang, P., Lu, D. and Lin, C. 2018. Regulatory effects of neuroinflammatory responses through brain-derived neurotrophic factor signaling in microglial cells. Mol. Neurobiol. 55, 7487–7499. Licitra, R., Martinelli, M., Jasinski, L., Marchese, M., Kiferle, C. and Fronte, B. 2021. In vivo evaluation of Cannabis sativa full extract on zebrafish larvae development, locomotion behavior and gene expression. Pharmaceuticals (Basel) 14(12), 1224. McClendon, E., Shaver, D.C., Degener-O’Brien, K., Gong, X., Nguyen, T., HoerderSuabedissen, A., Molnár, Z., Mohr, C., Richardson, B.D., Rossi, D.J. and Back, S.A. 2017. Transient hypoxemia chronically disrupts maturation of preterm fetal ovine subplate neuron arborization and activity. J. Neurosci. 37, 11912–11929. Nalivaeva, N.N., Turner, A.J. and Zhuravin, I.A. 2018. Role of prenatal hypoxia in brain development, cognitive functions, and neurodegeneration. Front. Neurosci. 12, 1–21. Peebles, D.M. 2004. Fetal consequences of chronic substrate deprivation. Semin. Fetal. Neonatal. Med. 9, 379–386. Piešová, M. and Mach, M. 2020. Impact of perinatal hypoxia on the developing brain. Physiol. Res. 69, 199–213. Rather, M., Thenmozhi, A., Manivasagam, T., Bharathi, M., Essa, M. and Guillemin, G. 2018. Neuroprotective role of asiatic acid in aluminium chloride induced rat model of Alzheimer’s disease. Front. Biosci. Sch. 10, 262–275. Sbrini, G., Brivio, P., Fumagalli, M., Giavarini, F., Caruso, D., Racagni, G., Dell’Agli, M., Sangiovanni, E. and Calabrese, F. 2020. Centella asiatica L. Phytosome improves cognitive performance by promoting Bdnf expression in rat prefrontal cortex. Nutrients 12(2), 355. Sireeni, J., Bakker, N., Jaikumar, G., Obdam, D., Slabbekoorn, H., Tudorache, C. and Schaaf, M. 2020. Profound effects of glucocorticoid resistance on anxiety-related behavior in zebrafish adults but not in larvae. Gen. Comp. Endocrinol. 292, 113461. Son, J., Gerenza, A.K., Bingener, G.M. and Bonkowsky, J.L. 2022. Hypoplasia of dopaminergic neurons by hypoxia-induced neurotoxicity is associated with disrupted swimming development of larval zebrafish. Front. Cell. Neurosci. 16, 1–16. Sorribes, A., Porsteinsson, H., Arnardóttir, H., Jóhannesdóttir, I.P., Sigurgeirsson, B., De Polavieja, G.G. and Karlsson, K. 2013. The ontogeny of sleep-wake cycles in zebrafish: a comparison to humans. Front. Neural. Circuits. 7, 1–13. Sun, B., Wu, L., Wu, Y., Zhang, C., Qin, L., Hayashi, M., Kudo, M., Gao, M. and Liu, T. 2020. Therapeutic potential of Centella asiatica and its triterpenes: a review. Front. Pharmacol. 11, 1–24. Swanson, A.M. and David, A.L. 2015. Animal models of fetal growth restriction: considerations for translational medicine. Placenta 36, 623–630. Wang, B., Liu, L., Li, Y., Zou, J., Li, D., Zhao, D., Li, W. and Sun, W. 2021. Ustilaginoidin D induces hepatotoxicity and behaviour aberrations in zebrafish larvae. Toxicology 456, 152786. Wang, H., Meng, Z., Zhou, L., Cao, Z., Liao, X., Ye, R. and Lu, H., 2019. Effects of acetochlor on neurogenesis and behaviour in zebrafish at early developmental stages. Chemosphere 220, 954–964. Xu, C.L., Qu, R., Zhang, J., Li, L.F., Ma, S.P. 2013. Neuroprotective effects of madecassoside in early stage of Parkinson’s disease induced by MPTP in rats. Fitoterapia 90, 112–118. Yang, B., Xu, Y., Hu, Y., Luo, Y., Lu, X., Tsui, C., Lu, L. and Liang, X. 2016. Madecassic acid protects against hypoxia-induced oxidative stress in retinal microvascular endothelial cells via ROS-mediated endoplasmic reticulum stress. Biomed. Pharmacother. 84, 845–852. | ||
| How to Cite this Article |
| Pubmed Style Ariani A, Husnul K, Nurdiana N, Masruroh R. Asiatic acid increased locomotor and head width through inducing brain-derived neurotrophic factor in intrauterine hypoxia-exposed zebrafish. Open Vet J. 2023; 13(10): 1326-1333. doi:10.5455/OVJ.2023.v13.i10.12 Web Style Ariani A, Husnul K, Nurdiana N, Masruroh R. Asiatic acid increased locomotor and head width through inducing brain-derived neurotrophic factor in intrauterine hypoxia-exposed zebrafish. https://www.openveterinaryjournal.com/?mno=164040 [Access: July 27, 2024]. doi:10.5455/OVJ.2023.v13.i10.12 AMA (American Medical Association) Style Ariani A, Husnul K, Nurdiana N, Masruroh R. Asiatic acid increased locomotor and head width through inducing brain-derived neurotrophic factor in intrauterine hypoxia-exposed zebrafish. Open Vet J. 2023; 13(10): 1326-1333. doi:10.5455/OVJ.2023.v13.i10.12 Vancouver/ICMJE Style Ariani A, Husnul K, Nurdiana N, Masruroh R. Asiatic acid increased locomotor and head width through inducing brain-derived neurotrophic factor in intrauterine hypoxia-exposed zebrafish. Open Vet J. (2023), [cited July 27, 2024]; 13(10): 1326-1333. doi:10.5455/OVJ.2023.v13.i10.12 Harvard Style Ariani, A., Husnul, . K., Nurdiana, . N. & Masruroh, . R. (2023) Asiatic acid increased locomotor and head width through inducing brain-derived neurotrophic factor in intrauterine hypoxia-exposed zebrafish. Open Vet J, 13 (10), 1326-1333. doi:10.5455/OVJ.2023.v13.i10.12 Turabian Style Ariani, Ariani, Khotimah Husnul, Nurdiana Nurdiana, and Rahayu Masruroh. 2023. Asiatic acid increased locomotor and head width through inducing brain-derived neurotrophic factor in intrauterine hypoxia-exposed zebrafish. Open Veterinary Journal, 13 (10), 1326-1333. doi:10.5455/OVJ.2023.v13.i10.12 Chicago Style Ariani, Ariani, Khotimah Husnul, Nurdiana Nurdiana, and Rahayu Masruroh. "Asiatic acid increased locomotor and head width through inducing brain-derived neurotrophic factor in intrauterine hypoxia-exposed zebrafish." Open Veterinary Journal 13 (2023), 1326-1333. doi:10.5455/OVJ.2023.v13.i10.12 MLA (The Modern Language Association) Style Ariani, Ariani, Khotimah Husnul, Nurdiana Nurdiana, and Rahayu Masruroh. "Asiatic acid increased locomotor and head width through inducing brain-derived neurotrophic factor in intrauterine hypoxia-exposed zebrafish." Open Veterinary Journal 13.10 (2023), 1326-1333. Print. doi:10.5455/OVJ.2023.v13.i10.12 APA (American Psychological Association) Style Ariani, A., Husnul, . K., Nurdiana, . N. & Masruroh, . R. (2023) Asiatic acid increased locomotor and head width through inducing brain-derived neurotrophic factor in intrauterine hypoxia-exposed zebrafish. Open Veterinary Journal, 13 (10), 1326-1333. doi:10.5455/OVJ.2023.v13.i10.12 |