| Case Report | ||
Open Vet J. 2023; 13(11): 1478-1484 Open Veterinary Journal, (2023), Vol. 13(11): 1478-1484 Case Report Transjugular occlusion of large patent ductus arteriosus with an Amplatzer™ muscular ventricular septal defect occluder in a 3-month-old dogLuigi Venco1*, Valentina Valenti1, Stefania Franceschini1 and Christine Castellitto21Ospedale Veterinario Città di Pavia, Pavia, Italy 2Clinica Veterinaria Poggio Piccolo, Castel Guelfo di Bologna, Italy *Corresponding Author: Luigi Venco. Ospedale Veterinario Città di Pavia, Pavia, Italy. Email: luigivenco [at] libero.it Submitted: 16/08/2023 Accepted: 13/10/2023 Published: 30/11/2023 © 2023 Open Veterinary Journal
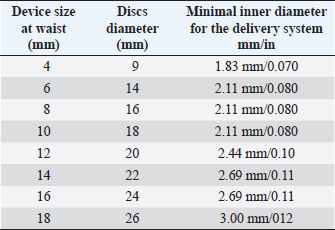
AbstractBackground: Cardiologists close most patent ductus arteriosus (PDA) defects in dogs using the Amplatz® canine duct occluder via a transarterial approach. However, this approach can be problematic in small dogs due to the small femoral artery diameter. In such cases, cardiologists have opted to use coils or vascular plugs deployed from a transvenous approach. However, in small dogs with large PDA, the risk of device protrusion into the pulmonary artery, incomplete closure, or device embolization, often leads to surgical PDA closure via thoracotomy. Case Description: The present report describes a 3-month-old male 6 kg Border collie with a large, PDA which was successfully occluded using the Amplatzer™ muscular ventricular septal defect (mVSD) device from a transvenous approach after closure attempts with an Amplatzer™ vascular plug II failed. Conclusion: This is the first case report in veterinary medicine of PDA closure with an Amplatzer™ mVSD occluder device. This approach, described in PDA closure in people, could be considered in minimally invasive PDA closure in small dogs with challenging anatomy. Keywords: PDA, Amplatzer™ muscular ventricular septal defect device, Case report, Dog. IntroductionPatent duct arteriosus (PDA), is considered the most common congenital heart disease in dogs (Detweiler et al., 1961; Hunt et al., 1995; McDonald, 2006). Certain breeds have been described as being predisposed, but the disease can occur in any breed and is more often seen in females than in males (Buchanan, 1994; Brambilla et al., 2020). Left-to-right shunting PDA can lead to pathological pulmonary overcirculation and left-sided volume overload, potentially resulting in congestive heart failure. Dogs with large PDAs that remain unclosed often die within 1 year (Eyster et al., 1976). A wide range of PDA sizes and morphologies has been described by multidimensional imaging (Doocy et al., 2018). Such variations in PDA morphology, PDA size, and arterial size used for vascular access all influence closure recommendations and device selection when considering minimally invasive transcatheter closure (Doocy et al., 2018). One medical device company has developed a transcatheter closure device specifically for use in dogs with PDA—the Amplatzer™ canine duct occluder (ACDO) (Infiniti Medical, Huddersfield, UK)—which cardiologists currently consider the treatment of choice for dogs with left-to-right shunting PDA (Nguyenba and Tobias, 2007; Gordon et al., 2010). This device has a minimally invasive controlled catheter-based delivery system, easy deployment, low complication rate, and a broad range of sizes that allow closure of a wide range of PDAs. However, the ACDO delivery system size and the need to use this device via a transarterial approach limits its use in very small dogs, especially when presenting with large-sized PDAs (Singh et al., 2012; Morgan et al., 2022). In addition, the need for femoral artery ligation or a long period of vessel compression can lead to serious local complications (Bagardi et al., 2022). Consequently, investigators have examined Amplatzer™ vascular plugs II (AVPII) (AGA Medical Corporation, Plymouth, MN), or coils for interventional closure of PDA through a transvenous approach, in dogs where routine ACDO closure is not possible (Blossom et al., 2010; Bagardi et al., 2022; Belachsen et al., 2022; Hildebrandt et al., 2022; Morgan et al., 2022). The Amplatzer™ muscular ventricular septal defect (mVSD) occluder (AGA Medical Corporation, Plymouth, MN) has been approved by the Food and Drug Association for transcatheter VSD closure, and has two concentric discs and a wide waist to accommodate the thicker portion of the ventricular septum (Table 1). The mVSD occluders have a high waist-to-disc ratio ranging from 0.44 to 0.69, similar to the ACDO devices, compared to the cylindrical shape of the AVPII. In cases in which the PDA diameter is large and its length is short, the PDA may be considered to be similar to an aorticopulmonary window for closure purposes (Salam et al., 2022). In such instances, a VSD closure device might be more appropriate than AVPII. Furthermore, double discs reduce the risk of embolization. In humans, Trehan et al. (2008) reported the successful use of mVSD occluder in three patients with aorticopulmonary windows, while Demkow et al. (2001) used a mVSD occluder to close a 16 mm hypertensive PDA. Other investigators have reported successful closure of large PDA with VSD occluders where other devices failed (Onorato et al., 2004; Zhou et al., 2006; Atiq et al., 2007; Eicken et al., 2007; Hokanson et al., 2008; Fernando et al., 2013; Cubeddu et al., 2014; García-Montes et al., 2015; Salam et al., 2022). Currently, no reports exist detailing the use of mVSD occluders in PDA closure in dogs. Our case report details the use of an mVSD occluder in a small dog with a large PDA that was not closeable by ACDO or AVPII occluders. Case DetailsA 3-month-old, 6 kg male intact Border collie with a subclinical PDA, diagnosed via transthoracic echocardiography 3 days prior, was referred for minimally invasive closure. On presentation, the dog was alert and the physical examination revealed pink, moist mucous membranes, capillary refill time of less than 2 seconds, palpable left basilar continuous murmur, and bounding femoral pulses. Thoracic radiographs showed left-sided cardiomegaly and pulmonary over circulation (Fig. 1). A transthoracic echocardiogram revealed severe left atrial and left ventricular dilation: left atrium/aorta (LA:Ao): 2.04, reference value: <1.6, normalized left ventricular internal diastolic diameter (LVIDDN): 3.02, reference value: ≤1.7) and large PDA, with an approximate minimal ostium and ampulla diameters of 5.2 and 10.3 mm (Fig. 2). Doppler echocardiography confirmed left-to-right flow across the PDA with a pressure gradient of 102 mmHg in systole and 41 mmHg in diastole. Mild mitral regurgitation was also noted. Table 1. Amplatzer™ mVSD occluder device specifications and delivery system required dimensions (length of connecting waist 7 mm for all of them).
We considered that the small diameter of the femoral artery at the access site (1.6 mm in systole on 2D ultrasound) would be too small to safely introduce an 8, 9, or 10 mm ACDO (ACDO sizes ranging from 150%–200% of the minimal ostium diameter, as recommended by the manufacturer). These ACDO, respectively, require a minimal inner diameter of the delivery sheath of 0.073, 0.086, and 0.099 inches, corresponding to 6, 7, or 8 French outer diameter introducers. Therefore, we attempted occlusion using a 16 mm (AVPII as previously described (Bagardi et al., 2022) with an expected ratio of 1.6 between device diameter and ampulla diameter, somewhat higher than other investigators have recommended (1.2–1.5) (Belachsen et al., 2022; Hildebrandt et al., 2022). We decided to use a larger up-sized device because of the minimally tapering ductus morphology and the low ratio of the maximal ampulla width to the minimal PDA diameter.
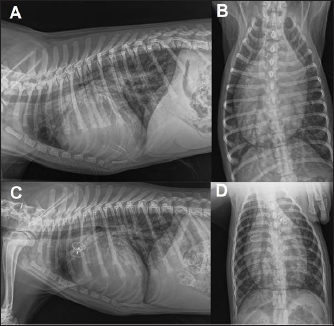
Fig. 1. Thoracic radiographs, right lateral and dorso-ventral views, (A and B) before the surgery and (C and D) 24 hours later. Post-operative films show a reduction of the cardiac silhouette and of the pulmonary over-circulation, and correct device position.
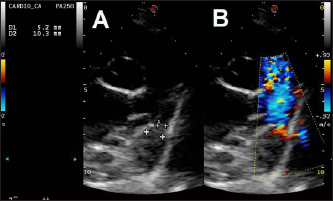
Fig. 2. Transthoracic echocardiographic (right parasternal short axis view, optimized for duct visualization). (A) PDA visualization and its measurement and (B) left to right flow on CFM Doppler (dual mode). The owner was informed and gave their written consent to use other off-label devices if needed. Anesthesia was induced and maintained as previously described (Bagardi et al., 2022) and perioperative intravenous amoxicillin (22 mg/kg) was administered. The dog was placed in left lateral recumbency on the fluoroscopy table, the right jugular vein was surgically isolated by a cut-down technique, and an 11 Fr, 10 cm long introducer vascular sheath (Pinnacle Peripheral Introducer Sheath, Terumo Medical Corporation, Somerset, NJ) was inserted into the jugular vein. A 4 Fr, 65 cm long diagnostic catheter (Berenstein Hockey stick tip catheter, Infiniti Medical, Huddersfield, UK) was then inserted into the jugular vein along with a preplaced straight tip hydrophilic 0.035″, 150 cm long guidewire (Terumo Radiofocus® Glidewire, Terumo Medical Corporation, Somerset, NJ). The guidewire and catheter were passed, under fluoroscopic guidance, through the right ventricle into the pulmonary artery and through the PDA in a retrograde manner until the tip of the catheter reached the descending aorta. After removing the guidewire, an angiographic study using 3 ml of iohexol (240 mg/ml), manually injected, was performed, showing the morphology of the PDA (length: 9 mm, maximal ampulla width:10.4 mm, minimal PDA diameter at the ostium: 5.4 mm). Following angiography, a standard stiffness 0.035″, 175 cm long J-tip guide wire (Medtronic Angiographic PTFE Rosen Wire, Medtronic Vascular, Danvers, MA) was advanced into the descending aorta and the diagnostic catheter was removed. A 7 Fr × 40 cm guiding sheath with a 2.54 mm inner diameter (Flexor Balkin KCFW-7.0-38-40-RB-BLKN-HC with Check-Flo Valve, Cook Medical Inc., Bloomington, IN) was advanced over the guidewire into the descending aorta to serve as a delivery sheath, and the guidewire was removed together with the dilator. A 16 mm AVPII was positioned via the delivery sheath into the PDA ampulla as previously described (Bagardi et al., 2022); however, an immediate “pull-through” into the pulmonary artery occurred before deployment (Fig. 3). The device was retracted and removed. Given the PDA morphology and the failure to secure the AVPII, we chose a 12 mm Amplatzer™ mVSD occluder device with a disc diameter of both discs of 20 mm, device waist size of 12 mm, and a length of connecting waist of 7 mm. This choice was based on the fact that the larger AVPII device is 18 mm wide and, given the ease and immediacy of pulling through, we thought that a device less than 2 mm wider would not have guaranteed against a post-operative embolization, even if successfully implanted. Furthermore, the next-sized AVPII (18 mm) is twice as long as the mVSD (14 vs. 7 mm). We considered that larger AVPII devices could have obstructed the aorta or pulmonary artery, as has been described in children (Salam et al., 2022). The device, previously screwed clockwise onto the delivery cable and introduced into its loader (SJM Amplaterz™ TorqVue™ Delivery 45° component 7 Fr. Loader and Delivery cable, AGA Medical Corporation, Plymouth, MN) was inserted into the delivery sheath and advanced carefully until the distal disc was expanded in the descending aorta near the PDA. The partially deployed mVSDO, attached delivery cable, and delivery sheath were then gently pulled back simultaneously until the distal disc engaged the ostium of the PDA and lodged in the ductal ampulla. The guiding sheath then was retracted further allowing expansion of the central component of the device within the PDA ampulla. The entire system was then retracted and subsequently, the proximal disk was expanded within the main pulmonary artery. The stability of the device was then tested with the push-pull “Minnesota wiggle” maneuver, the cessation of transductive flow and the correct positioning of the mVSD pulmonary disc were verified with transthoracic echocardiography, and the device was then deployed (Fig. 4). During the placement of the device all the parameters (saturation, invasive blood pressure, end-tidal CO2) remained within physiological ranges. Blood pressure increased (from 96/40/58 to 120/50/73 mmHg) while heart rate decreased (from 100 to 65 bpm) after occlusion of the PDA.
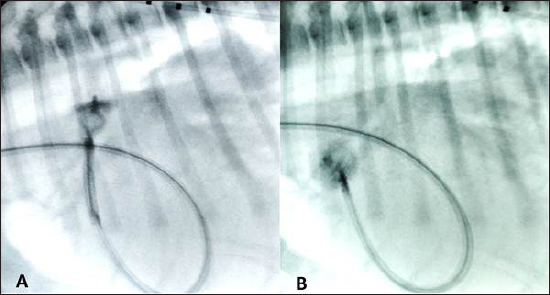
Fig. 3. (A) Fluoroscopic sequence after the AVP II first disc has been released engaging the aortic side of the PDA and the central part left unsheated and (B) subsequently the other second component has been deployed an immediate “pull-through” into the pulmonary artery occurred immediately after to deployment of the whole device An angiogram was then performed through the delivery catheter, which, in dextrophase, showed no obstruction of the pulmonary artery and, in levophase, confirmed the complete closure of the PDA. The delivery sheath and the introducer sheath were then removed, and the jugular vein was repaired with a 4–0 monofilament polydioxanone absorbable suture (PDS* II Ethicon, Raritan, NJ). Recovery from anesthesia was uneventful and the dog was able to walk and eat 3 hours later.
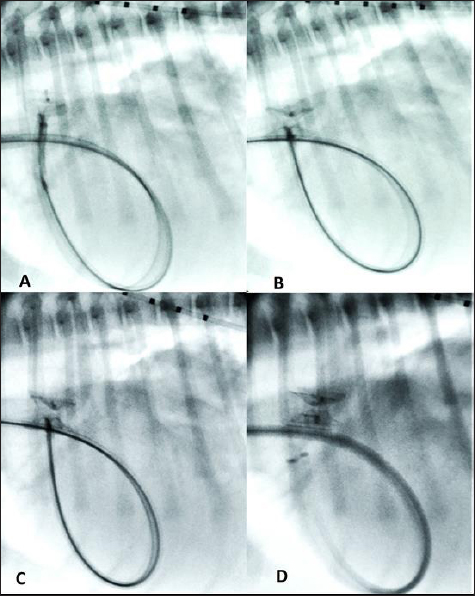
Fig. 4. Fluoroscopic sequence. (A) The first disc of the mVSD has been deployesd engaging the aortic side of the PDA and (B) then the waist and second disc fully extruded with the waist occluding the ostium and the second disc located into the pulmonary artery. (C) The stability of the device is tested with the push-pull “Minnesota wiggle” maneuver. (D) The device has been released
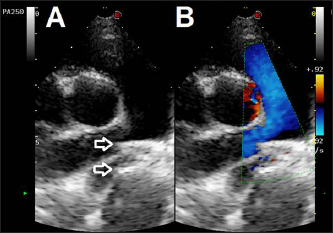
Fig. 5. Dual mode transthoracic echocardiography (right parasternal short axis view, optimized for duct visualization) 24 hours after surgery. The device discs (arrows) are correctly positioned and there is no evidence of residual shunt or pulmonary arterial obstruction given the laminar flow in the right pulmonary artery on CFM Doppler. Echocardiographic checks at 3 and 24 hours showed complete PDA closure, correct positioning of the mVSDO (Fig. 5), and a clear reduction in left heart volume overload (LA: Ao decreased from 1.72 to 1.3, LVIDDN decreased from 2.49 to 2.43). The dog was then discharged and a check-up was scheduled at 3 months. The 3-month echocardiographic examination confirmed complete occlusion of the PDA with the absence of residual shunting, correct positioning of the device, disappearance of mild mitral regurgitation, and reverse remodeling of the cardiac chambers (kg 11.9, BSA 0.54, LA:Ao 1.14, LVIDDN: 1.94). Ethical approvalEthical review and approval were waived for this study because this is not an experimental study, but the clinical report of a spontaneously occurring congenital disease and detailed informed consent were obtained from the owner for the publication of the images and the clinical case. DiscussionOur case report details the first use of an Amplatzer™ mVSD occluder device for occluding a large PDA in a small, young dog. Clinicians have reported successful closure of PDA in humans with various morphologies using the mVSD, especially large, tubular or short PDA (Onorato et al., 2004; Zhou et al., 2006; Atiq et al., 2007; Eicken et al., 2007; Hokanson et al., 2008; Fernando et al., 2013; Cubeddu et al., 2014; García-Montes et al., 2015; Salam et al., 2022). The dog in our case report mirrored the criteria used for selecting this device in humans: device waist of the same size or slightly (1–2 mm) larger than the ampulla diameter (Salam et al., 2022), and we successfully deployed the device and occluded the PDA after attempts using the AVPII failed. Our success, albeit in a single dog, demonstrates how, in small dogs with wide PDA, where stable closure of the PDA with coils, ACDO, or AVPII is unfeasible or likely to fail, the mVSD can be considered as a valid, minimally invasive alternative. In such cases, the double disc conformation of the mVSD and a waist similar to the ACDO should confer greater stability, especially when the ratio between ampulla diameter and minimal ductal diameter is low. Furthermore, our vascular approach (through the jugular vein) allowed deployment of the mVSD, whereas the transarterial approach required to deploy the ACDO would likely have failed. The mVSD has a waist-to-disc ratio ranging from 0.44 for the smallest to 0.69 for the largest one (0.6 in our case, close to 0.55, 0.56, 0.62 for the 8, 9, and 10 mm ACDO devices). In cases with large, tubular, or short PDA, a wide-waisted mVSD closure device might be more appropriate than an AVPII, because it provides a better apposition to the PDA orifice. In our case, we opted for a 12 mm mVSD (slightly larger than the maximum measured diameter) for having friction on the device’s waist with the PDA surface and a waist approximately twice the minimum ductal diameter. Furthermore, the double discs (similar to the ACDO) reduce the risk of device embolization and the two discs have clearly larger dimensions than the waist and superior rigidity due to tightly woven nitinol wire (Salam et al., 2022), compared to the AVPII which has a cylindrical shape and a softer consistency. The mVSD differs slightly from the ACDO device as it has a slightly longer connecting waist and symmetrical discs which allow both transvenous and transarterial approaches. This latter feature confers an advantage in small breed dogs with large PDA, where the size of the femoral artery either prevents or challenges the transarterial approach. Although veterinary investigators occluded a challenging PDA in a dog using an aortic closed stent device (Patata et al., 2020), and an ACDO device by a transvenous route in a cat (Kharbush and Trafny, 2021), our approach and device selection might be more familiar to cardiologists in dealing with difficult cases. Closure devices used in humans usually cost substantially more than those used in veterinary medicine (such as ACDO). However, by using short-dated devices, or devices that have passed beyond their legal shelf life, clinicians can reduce the costs of such devices for their clients. Our report provides cardiologists with a novel method of transvascular PDA occlusion in small dogs with large PDA in which ACDO, AVPII, or coil devices cannot be placed securely or safely. AcknowledgmentsThe authors sincerely thank Mark Rishniw for their valuable advice in drafting, reviewing, and editing the text. Conflict of interestThe authors declare that there is no conflict of interest. Author contributionsVenco L, Valenti V., Franceschini S.: Evaluation, anesthesia, conceptualization, and surgical management. Castellitto C.: Evaluation and follow up. Venco L.: Manuscript supervision. All the authors’ manuscript drafting and approval of the final manuscript. FundingThis research received no specific grant. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAtiq, M., Aslam, N. and Kazmi, K.A. 2007. Transcatheter closure of small-to-large patent ductus arteriosus with different devices: queries and challenges. J. Invasive Cardiol. 19(7), 295–298. Bagardi, M., Domenech, O., Vezzosi, T., Marchesotti, F., Bini, M., Patata, V., Croce, M., Valenti, V. and Venco, L. 2022. Transjugular patent ductus arteriosus occlusion in seven dogs using the Amplatzer vascular plug II. Vet. Sci. 9(8), 431. Belachsen, O., Sargent, J., Koffas, H., Schneider, M. and Wagner, T. 2022. The use of Amplatzer vascular plug II in 32 consecutive dogs for transvenous occlusion of patent ductus arteriosus. J. Vet. Cardiol. 41, 88–98. Blossom, J.E., Bright, J.M. and Griffiths, L.G. 2010. Transvenous occlusion of patent ductus arteriosus in 56 consecutive dogs. J. Vet. Cardiol. 12(2), 75–84. Brambilla, P.G., Polli, M., Pradelli, D., Papa, M., Rizzi, R., Bagardi, M. and Bussadori, C. 2020. Epidemiological study of congenital heart diseases in dogs: prevalence, popularity, and volatility throughout twenty years of clinical practice. PLoS One 15(7), e0230160. Buchanan, J.W. 1994. Patent ductus arteriosus. Semin. Vet. Med. Surg. 9, 168–176. Cubeddu, R.J., Babin, I. and Inglessis, I. 2014. The off-label use of the Amplatzer muscular VSD occluder for large patent ductus arteriosus: a case report and review. Cardiovasc. Interv. Ther. 29(3), 256–260. Demkow, M., Ruzyllo, W., Siudalska, H. and Kepka, C. 2001. Transcatheter closure of a 16 mm hypertensive patent ductus arteriosus with the Amplatzer muscular VSD occluder. Catheter Cardiovasc. Interv. 52(3), 359–362. Detweiler, D.K., Patterson, D.F., Hubben, K. and Botts, R.P. 1961. The prevalence of spontaneously occurring cardiovascular disease in dogs. Am. J. Public Health Nations Health. 51, 229–241. Doocy, K.R., Saunders, A.B., Gordon, S.G. and Jeffery, N. 2018. Comparative, multidimensional imaging of patent ductus arteriosus and a proposed update to the morphology classification system for dogs. J. Vet. Intern. Med. 32(2), 648–657. Eicken, A., Balling, G., Gildein, H.P., Genz, T., Kaemmerer, H. and Hess, J. 2007. Transcatheter closure of a non-restrictive patent ductus arteriosus with an Amplatzer muscular ventricular septal defect occluder. Int. J. Cardiol. 117(1), e40–e42. Eyster, G.E., Eyster, J.T., Cords, G.B. and Johnston, J. 1976. Patent ductus arteriosus in the dog: characteristics of occurrence and results of surgery in one hundred consecutive cases. J. Am. Vet. Med. Assoc. 168(5), 435–438. Fernando, R., Koranne, K., Loyalka, P., Kar, B. and Gregoric, I. 2013. Patent ductus arteriosus closure using an Amplatzer™ ventricular septal defect closure device. Exp. Clin. Cardiol. 18(1), e50–e54. García-Montes, J.A., Camacho-Castro, A., Sandoval-Jones, J.P., Buendía-Hernández, A., Calderón-Colmenero, J., Patiño-Bahena, E. and Zabal, C. 2015. Closure of large patent ductus arteriosus using the Amplatzer septal occluder. Cardiol. Young 25(3), 491–495. Gordon, S.G., Saunders, A.B., Achen, S.E., Roland, R.M., Drourr, L.T., Hariu, C. and Miller, M.W. 2010. Transarterial ductal occlusion using the Amplatz canine duct occluder in 40 dogs. J. Vet. Cardiol. 12(2), 85–92. Hildebrandt, N., Stosic, A., Henrich, E., Wiedemann, N., Wurtinger, G. and Schneider, M. 2022. Transvenous embolization of moderate to large patent ductus arteriosus in dogs using the Amplatzer vascular plug II. J. Vet. Intern. Med. 36(1), 20–28. Hokanson, J.S., Gimelli, G. and Bass, J.L. 2008. Percutaneous closure of a large PDA in a 35-year-old man with elevated pulmonary vascular resistance. Congenit. Heart Dis. 3(2), 149–154. Kharbush, R.J. and Trafny, D.J. 2021. Transvenous patent ductus arteriosus occlusion via canine duct occluder in a cat. J. Vet. Cardiol. 33, 6–12. Hunt, G.B., Church, D.B., Malik, R. and Bellenger, C.R. 1995. A retrospective analysis of congenital cardiac anomalies (1977–1989). Aust. Vet. Pract. 20, 70–75. McDonald, K.A. 2006. Congenital heart diseases of puppies and kittens. Vet. Clin. North Am. Small Anim. Pract. 36(3), 503–531. Morgan, K.R.S., Stauthammer, C.D., Barncord, K., Pinkos, A., Fundingsland, S. and Rishniw, M. 2022. Transvenous detachable coiling of patent ductus arteriosus in small dogs. J. Vet. Cardiol. 42, 65–73. Nguyenba, T.P. and Tobias, A.H. 2007. The Amplatz canine duct occluder: a novel device for patent ductus arteriosus occlusion. J. Vet. Cardiol. 9, 109–117. Onorato, E., Mbala-Mukendi, M., Casilli, F., Girardi, P., Canali, G., Lanzoni, L., Guilarte, N. and Barbieri, E. 2004. Amplatzer muscular VSD occluder for catheter closure of a 20 mm hypertensive patent ductus arteriosus. A case report and literature review. Minerva Cardioangiol. 52(3), 219–223. Patata, V., Scalise, F., Sorropago, G., Marchesotti, F., Nicoli, S., Auriemma, E., Rondelli, V., Pesaresi, M., Glaus, T.M., Baron Toaldo, M., Vezzosi, T. and Domenech, O. 2020. Closure of an unusual morphology patent ductus arteriosus with a covered stent in a dog. J. Vet. Cardiol. 32, 7–15. Salam, A., Bautista-Rodriguez, C., Karsenty, C., Bouvaist, H., Piccinelli, E. and Fraisse, A. 2022. Transcatheter closure of tubular patent ductus arteriosus using muscular ventricular septal defect devices in infants and small children with congestive heart failure. Arch. Cardiovasc. Dis. 115(3), 134–141. Singh, M.K., Kittleson, M.D., Kass, P.H. and Griffiths, L.G. 2012. Occlusion devices and approaches in canine patent ductus arteriosus: comparison of outcomes. J. Vet. Intern. Med., 26, 85–92. Trehan, V., Nigam, A. and Tyagi, S. 2008. Percutaneous closure of nonrestrictive aortopulmonary window in three infants. Catheter Cardiovasc. Interv. 71, 405–411. Zhou, T., Shen, X.Q., Zhou, S.H., Qi, S.S., Fang, Z.F. and Lü, X.L. 2006. Percutaneous closure of huge patent ductus arterious associated with anomalous inferior vein cava drainage and dextrocardia with muscular ventricular septal defect occluder. Chin. Med. J. (Engl). 119(1), 69–72. | ||
| How to Cite this Article |
| Pubmed Style Venco L, Valenti V, Franceschini S, Castellitto C. Transjugular occlusion of large patent ductus arteriosus with an Amplatzer™ muscular Ventricular Septal Defect occluder in a 3-month-old dog. Open Vet J. 2023; 13(11): 1478-1484. doi:10.5455/OVJ.2023.v13.i11.12 Web Style Venco L, Valenti V, Franceschini S, Castellitto C. Transjugular occlusion of large patent ductus arteriosus with an Amplatzer™ muscular Ventricular Septal Defect occluder in a 3-month-old dog. https://www.openveterinaryjournal.com/?mno=165396 [Access: May 12, 2024]. doi:10.5455/OVJ.2023.v13.i11.12 AMA (American Medical Association) Style Venco L, Valenti V, Franceschini S, Castellitto C. Transjugular occlusion of large patent ductus arteriosus with an Amplatzer™ muscular Ventricular Septal Defect occluder in a 3-month-old dog. Open Vet J. 2023; 13(11): 1478-1484. doi:10.5455/OVJ.2023.v13.i11.12 Vancouver/ICMJE Style Venco L, Valenti V, Franceschini S, Castellitto C. Transjugular occlusion of large patent ductus arteriosus with an Amplatzer™ muscular Ventricular Septal Defect occluder in a 3-month-old dog. Open Vet J. (2023), [cited May 12, 2024]; 13(11): 1478-1484. doi:10.5455/OVJ.2023.v13.i11.12 Harvard Style Venco, L., Valenti, . V., Franceschini, . S. & Castellitto, . C. (2023) Transjugular occlusion of large patent ductus arteriosus with an Amplatzer™ muscular Ventricular Septal Defect occluder in a 3-month-old dog. Open Vet J, 13 (11), 1478-1484. doi:10.5455/OVJ.2023.v13.i11.12 Turabian Style Venco, Luigi, Valentina Valenti, Stefania Franceschini, and Christine Castellitto. 2023. Transjugular occlusion of large patent ductus arteriosus with an Amplatzer™ muscular Ventricular Septal Defect occluder in a 3-month-old dog. Open Veterinary Journal, 13 (11), 1478-1484. doi:10.5455/OVJ.2023.v13.i11.12 Chicago Style Venco, Luigi, Valentina Valenti, Stefania Franceschini, and Christine Castellitto. "Transjugular occlusion of large patent ductus arteriosus with an Amplatzer™ muscular Ventricular Septal Defect occluder in a 3-month-old dog." Open Veterinary Journal 13 (2023), 1478-1484. doi:10.5455/OVJ.2023.v13.i11.12 MLA (The Modern Language Association) Style Venco, Luigi, Valentina Valenti, Stefania Franceschini, and Christine Castellitto. "Transjugular occlusion of large patent ductus arteriosus with an Amplatzer™ muscular Ventricular Septal Defect occluder in a 3-month-old dog." Open Veterinary Journal 13.11 (2023), 1478-1484. Print. doi:10.5455/OVJ.2023.v13.i11.12 APA (American Psychological Association) Style Venco, L., Valenti, . V., Franceschini, . S. & Castellitto, . C. (2023) Transjugular occlusion of large patent ductus arteriosus with an Amplatzer™ muscular Ventricular Septal Defect occluder in a 3-month-old dog. Open Veterinary Journal, 13 (11), 1478-1484. doi:10.5455/OVJ.2023.v13.i11.12 |