| Research Article | ||
Open Vet J. 2023; 13(12): 1583-1596 Open Veterinary Journal, (2023), Vol. 13(12): 1583–1596 Original Research Seroprevalence and spatial distribution of livestock brucellosis using three serological tests in Kajiado County, KenyaMahacla O. Odongo1*, Lilly C. Bebora1, James K. Gathumbi1, Gabriel O. Aboge2, Lillian W. Waiboci3 and Joseph Erume41Department of Veterinary Pathology, Microbiology and Parasitology, Faculty of Veterinary Medicine, University of Nairobi, Nairobi, Kenya 2Department of Public Health, Pharmacology and Toxicology, Faculty of Veterinary Medicine, University of Nairobi, Nairobi, Kenya 3Department of Biochemistry, Faculty of Science and Technology, University of Nairobi, Nairobi, Kenya 4Department of Biomolecular Resources and Biolab Sciences, College of Animal Resources and Biosecurity, Makerere University, Kampala, Uganda *Corresponding Author: Mahacla Omungala Odongo. Department of Veterinary Pathology, Microbiology and Parasitology, Faculty of Veterinary Medicine, University of Nairobi, Nairobi, Kenya. Email: mahacla [at] uonbi.ac.ke Submitted: 20/08/2023 Accepted: 15/11/2023 Published: 31/12/2023 © 2023 Open Veterinary Journal
AbstractBackground: Brucellosis is a serious zoonotic infection with a global socioeconomic impact on both the livestock industry and human health. In Kenya, brucellosis is endemic but there is limited information on the true burden of the disease due to weak or peace-meal surveillance. The true burden and spread of animal brucellosis in Kajiado County is not known. Aim: The aim of the study was to determine the current seroprevalence and spatial distribution of livestock brucellosis in Kajiado County and also to compare the three serological tests, namely; Rose Bengal plate test (RBPT), indirect enzyme-linked immunosorbent assay (i-ELISA), and competitive ELISA (c-ELISA) in the detection of seropositive animals. Methods: A cross-sectional study was undertaken in 5 sub-counties and 13 wards, where a total of 782 serum samples from unvaccinated bovine (n=278; 34 herds), ovine (n=256; 25 flocks), and caprine (n=248; 28 flocks), were screened for anti-Brucella antibodies using RBPT, i-ELISA, and c-ELISA tests, in parallel. Results: Overall animal seroprevalence was 6.91% (54/782); while that for bovine, ovine, and caprine was 18.35% (51/278), 0.78% (2/256), and 0.4% (1/248), respectively. Bovine seroprevalence was 2.2% (6/278), 14.4% (40/278), and 4.7% (13/278) in RBPT, i-ELISA, and c-ELISA tests, respectively; while ovine 0.78% (2/256) and caprine 0.4% (1/248) were positive only in c-ELISA. Bovine herd seropositivity was 67.65% (23/34), whereas ovine and caprine flock seropositivities were 8% (2/25) and 3.6% (1/28), respectively. Conclusion: The findings indicate a moderate seroprevalence of brucellosis in bovine, while that of ovine and caprine was low in Kajiado County. Indirect ELISA was found superior to both c-ELISA and RBPT in detecting bovine seropositive animals, while c-ELISA was superior to both RBPT and i-ELISA in detecting seropositive ovines and caprines. These results will contribute to baseline data for further study of Brucella infection and a starting point for the formulation of a strategy for the control of brucellosis in Kajiado County. Keywords: Brucellosis, Livestock, Seroprevalence, Kajiado County. IntroductionBrucellosis is a serious disease of both economic and public health importance as it affects the health and productivity of different animals including cattle, sheep, goats, pigs camels, and also humans. It is endemic in many developing countries in Africa, including Kenya (Djangwani et al., 2021). The disease is caused by small Gram-negative, nonmotile coccobacilli belonging to the genus Brucella, which comprises 12 recognized species, all of which show varied host specificity (Godfroid et al., 2010; Khurana et al., 2021). Brucella species responsible for animal brucellosis include Brucella abortus (mainly affecting cattle), Brucella melitensis, mainly affecting goats, Brucella suis, mainly affecting pigs, and Brucella ovis, occasionally affecting sheep (Godfroid et al., 2010; Khurana et al, 2021). Human brucellosis is mainly due to Brucella melitensis, B. abortus, and B. suis. In female animals, brucellosis is characterized by late abortions in gravid animals, the birth of weak offspring, lowered fertility, retention of fetal membranes, endometritis, and reduction in milk production, while in male animals, it causes orchitis and epididymitis. Hygromas may be seen in chronic infections in both sexes (Godfroid et al., 2010; Khurana et al., 2021). Most human cases are directly or indirectly linked to animals or their products such as raw milk and milk products prepared from such milk (Godfroid et al., 2010). A proper diagnosis of brucellosis in both humans and animals is a key requirement for the control and elimination of the disease (Khan and Zahoor, 2018). Both direct and indirect approaches are used for the diagnosis of brucellosis in animals. Direct methods include the isolation and identification of Brucella organism, which is the “gold standard,” or detection of its nucleic acid from tissues or organs of infected animals using polymerase chain reaction (PCR)-based methods (Ducrotoy et al., 2018). Indirect methods involve the detection of antibodies produced by the host immune response against the bacterial immunodominant smooth lipopolysaccharide during infection (Corbel, 2006). Serological tests are crucial for laboratory diagnosis of brucellosis since most control and eradication programs rely on these methods (Senbeto, 2022). Different serological tests have been used globally to screen for and confirm brucellosis in both humans and animals (Ducrotoy et al., 2018; WOAH, 2020; Rossetti et al., 2022; Mengele et al., 2023). The most commonly used screening tests in animals include the Rose Bengal plate test (RBPT) or indirect enzyme-linked immunosorbent assay (i-ELISA), with CFT or competitive ELISA (c-ELISA) as confirmatory tests, and these are also recommended tests for trade by World Organization of Animal Health (WOAH, 2020). Various researches indicate a clear difference in the number of seropositive animals detected using different serological tests (Chota et al., 2016; Djangwani et al., 2021; Mengele et al., 2023). Most serological tests used for the diagnosis of brucellosis are, however, riddled with what is referred to as diagnostic conundrums (Moreno et al., 2022). These include technical issues surrounding their performance and interpretation, as well as being subject to complex biological, and epidemiological factors, such as differences in the quality of test reagents, the reproductive or immunological status of the animals, latent infections, and cross-reactivity with other Gram-negative bacteria (Díaz-Aparicio et al., 1994; Nielsen et al., 2004; Munoz et al., 2005; Praud et al., 2012; Moreno et al., 2022). To establish the true brucellosis status of a herd, it is recommended that at least more than one test be used, with one serving as a screening test and the other one, as a confirmatory test (Ducrotoy et al., 2018). In many countries of sub-Saharan Africa including Kenya, RBPT is the most frequently used conventional screening test for brucellosis in animals (Djangwani et al., 2021). Therefore, seroprevalence results reported from various studies in Kenya using RBPT may not represent the true status of brucellosis in the country. Kajiado County is located in the Southern part of Kenya. More than 90% of the county falls under the arid and semi-arid zones which cannot support rain-fed agriculture, hence livestock production is its economic backbone (Kajiado County Integrated Development Program 2018–2022, CIDP, 2018). The county is inhabited mainly by the Maasai community who still practice nomadic pastoralism to a large extent. Like other pastoralists, livestock plays an important central role in the Maasai's daily economic as well as ceremonial life. They depend on livestock for meat, milk, and blood, in addition to providing the principal currency for social and commercial transactions. Kajiado County is one of the most important cattle production areas in Kenya. Kajiado County boasts a livestock population comprising of 577,710 cattle comprising of 36,547 exotic dairy breeds, 56,696 exotic beef breeds and 484,467 indigenous cattle, 1,120,649 sheep, and 877,744 goats, 3,584 camels, 50,153 donkeys, and 12,390 pigs, among others (Kenya National Bureau of Statistics, 2019). In Kajiado County, the success of the livestock industry is threatened by diseases such as brucellosis. Previous studies have reported brucellosis seroprevalences of 1.3% and 12.91% in humans and livestock, respectively, in parts of Kajiado County (Nakeel et al., 2016). However, these studies were undertaken in three administrative wards of the county and, therefore, the reported seroprevalence rates may not be a true reflection of the situation in the whole county. For Kenya to mount an effective brucellosis control or eradication program, the spatial distribution of this disease in each county must be determined in addition to other epidemiological parameters. The objective of this study was to determine the current seroprevalence and spatial distribution of animal brucellosis in Kajiado County and also to compare the three serological tests, namely; RBPT, i-ELISA, and c-ELISA in the detection of anti-Brucella antibodies in animals. Materials and MethodsStudy area and designA cross-sectional study was carried out in 5 sub-counties and 13 administrative wards in Kajiado County (Fig. 1), from December 2020 to 2021. Flocks and herds were randomly selected for inclusion in the study. If a particular flock or herd was small (≤5 animals), all adult animals were targeted, if the herd/flock numbers were more than 10, then 20% of the animals were targeted for sampling. Most animals sampled were females that had given birth at least once. No history of vaccination was reported in any herd or flock included in the study.
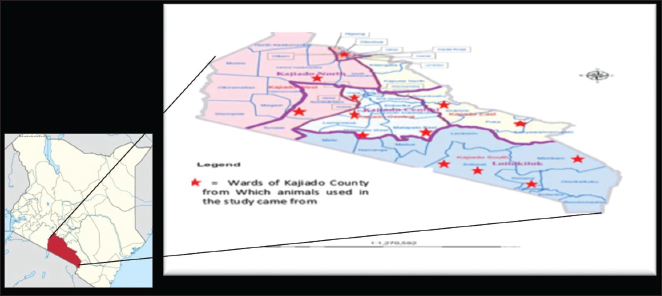
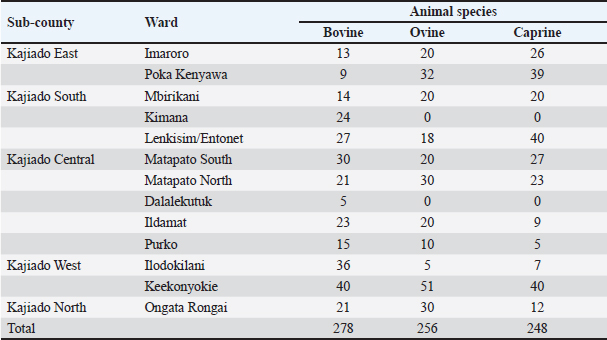
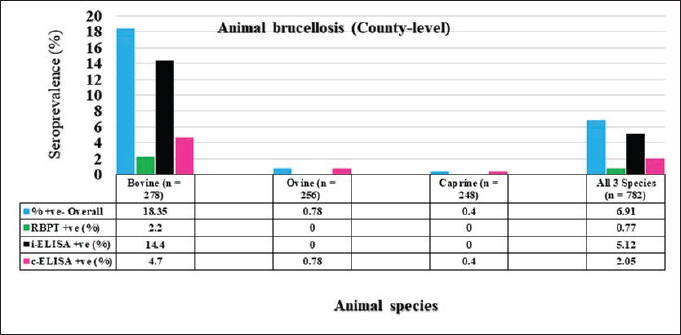
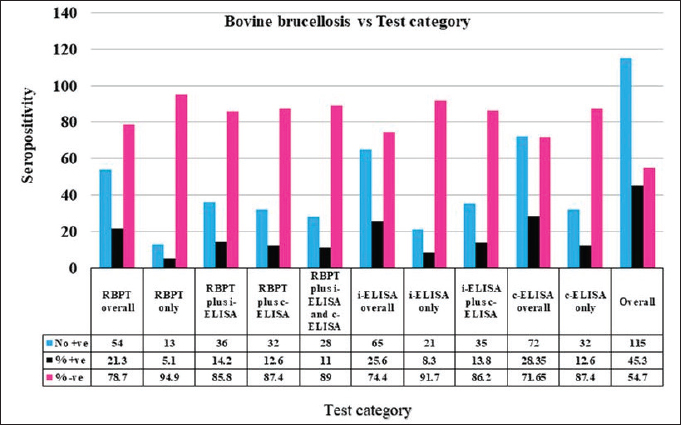
Fig. 1. Map of Kenya showing the location of Kajiado County, and respective wards and study areas (red stars) involved in the study. Sample size calculationThe sample size for animals used in the study was calculated according to the formula for cross-sectional studies by Dohoo et al. (2012). The prevalence rates previously estimated for cattle (21.92%), sheep (14.8%), and goats (11.8%) in Kajiado County (Nakeel et al., 2016) were used to calculate the sample size using the formula: n=Z2p (1-p)/d2, with a margin error (d) of 0.05. Therefore, the calculated sample sizes were: 263 bovine, 186 ovine, and 148 caprine, respectively. Blood sample collectionFive to ten milliliters of blood were collected from respective jugular veins, using a 21G needle, into a vacutainer tube without anticoagulant. The clotted blood was transported chilled on ice to the Faculty of Veterinary Medicine from where serum was harvested from the vacutainer tubes by centrifugation, transferred into sterile 2-ml tubes, and stored at −20°C until use. Serological tests carried-outRBPT was done in the Microbiology Research Laboratory of the Department of Veterinary Pathology, Microbiology, and Parasitology, whereas enzyme-linked immunosorbent assay tests were done in the Immunology Laboratory of the Department of Public Health, Pharmacology, and Toxicology, both of the Faculty of Veterinary Medicine, University of Nairobi, Kenya. All the serum samples [bovine (278); ovine (256); caprine (248)] were tested for anti-Brucella antibodies using the three serological tests. Rose Bengal plate testThe test was carried out following the procedure described in the WOAH (2020) for bovine samples. Briefly, serum samples and antigen were allowed to equilibrate at room temperature for 30 minutes first, and then, 25 μl of each serum sample were mixed with 25 μl of Rose Bengal Brucella antigen (from CITA, Spain) on a clean white porcelain plate. The mixture was then gently shaken using two hands for up to 4 minutes at ambient temperature. In a positive reaction, there was evidence of agglutination. Any amount of agglutination was scored positive regardless of the degree. All plates showing no evidence of agglutination were scored negative. Every plate test included a positive and negative serum sample as controls. For ovine and caprine samples, a modified RBPT method (Blasco et al., 1994) was used. In this method, 75 µl of serum and 25 µl of antigen were used as described above for bovine sera. Indirect enzyme-linked immunosorbent assayThe bovine brucellosis i-ELISA kit according to the manufacturer's instruction (Elabscience Biotechnology Inc, 8th edition), 2018. The test results were determined by measuring the absorbance or optical density (OD) using a Microplate reader at 450 nm wavelength. The interpretation of the results was based on the following ODs: OD reading Result ≥0.38 Positive ≤0.38 Negative The sample results were calculated as percentage positive, relative to the strong positive control serum (S/P) using the formula: S/P%=100 × [(Sample OD—negative control OD)/(Mean OD of positive control—negative control)]. A sample was considered positive if the S/P value was greater than 120%. Competitive enzyme-linked immunosorbent assayThe c-ELISA kit used in the study was the COMPELISA 160 & 140 kit from APHA Scientific (www.aphascientific.com). The test was conducted according to the instructions of the manufacturer. The microtiter plates were read in a Microtiter plate reader with the 450 nm filter. The results of the tested samples/wells were interpreted by comparing readings of the test samples to those of both positive and negative control wells. A plate was considered valid if the mean OD of the 6 negative controls at 450 nm was greater than 0.700, and the mean OD of the 6 positive controls was less than 0.100 [www.aphascientific.com]. The difference between the OD of the positive and negative controls had to be equal to or greater than 0.300. A cut-off was determined using the conjugate control, i.e., 60% of the mean OD of the four conjugate control wells (Praud et al., 2012). Any OD equal to or below the determined cut-off value was considered as being positive, while values above the cut-off were considered negative [www.aphascientific.com]. Statistical analysisData obtained from the serological tests were entered into a spreadsheet (Microsoft Excel 2010 version, Redmond, WA), and the proportions were estimated for the individual, herd, Ward, and sub-county brucellosis seroprevalence. The individual animal seroprevalence was calculated by dividing the number of RBPT- and/or c-ELISA-positive animals by the total number of animals that were tested. The herd-level seroprevalence was calculated by dividing the number of herds with at least one reactor on the RBPT and c-ELISA by the number of all the herds tested. Associations between hypothesized risk factors and the outcome variables were assessed, and statistical analysis was performed using chi-square tests and logistic regression using the statistical software STATA® version 16 (Stata Corp LLC, College Station, TX), and Epitools applications (Sim and Wright, 2005) Ethical approvalAnimal handling and sample collection were done in conformity with the principles of good clinical practice and the study was approved by the Ethical and Biosafety Committee of the Faculty of Veterinary Medicine (Ref: FVM BAUEC/2019/236) of the University of Nairobi, Kenya, as well as National Commission for Science, Technology and Innovations (NACOSTI) license No: NACOSTI/P19/951. ResultsAnimals sampled in the studyIn total, 278 bovine, 256 ovine, and 248 caprines were sampled in the study (Table 1). Animal-level brucellosis prevalence in Kajiado CountyThe results of serological testing for anti-Brucella antibodies in bovine, ovine, and caprine are given in Figure 2. At the animal level, 54/782 animals tested were positive, thus giving a county-level animal seroprevalence of 6.91% (95% CI: 5.13%–8.69%). At the animal species level, 18.35% (95% CI: 13.8%–22.9%; n=51/278), 0.78% (95% CI: 0.16%–1.4%; n=2/256), and 0.4% (95% CI: 0.004%–0.84%; n=1/248), bovine, ovine, and caprine were seropositive, respectively (Fig. 1). The seroprevalence of bovine brucellosis was significantly higher than that of either ovine or caprine (p < 0.05). There was no significant difference (>0.05) in the seroprevalences of ovine and caprine brucellosis. Brucellosis seropositivity rates in the 3 serological testsSeropositivity rates in the three serological tests were also compared; the results are shown in Figures 3 and 4. Overall, i-ELISA 40/278 (14.4%) test picked significantly more bovine reactors than either RBPT 6/278 (2.2%) or c-ELISA 14/278 (5%) (p < 0.05; p < 0.05), but there was no difference in the number of bovine reactors picked by RBPT (2.2%; 95%CI: 0.48%–3.92%), and c-ELISA (5%: 95%CI: 2.47%–7.61%). c-ELISA is the only test that picked ovine and caprine reactors, but the difference in numbers picked between the two animal species was not significant (p > 0.05); all ovine and caprine samples were negative in both RBPT and i-ELISA tests. Table 1. Number of animals sampled for brucellosis in Kajiado County.
Fig. 2. Seroprevalence of animal brucellosis in the three serological tests.
Fig. 3. Seropositivity of bovine serum samples in the three serological tests in Kajiado County. Thirty-five out of 278 (12.6%) bovines were seropositive in i-ELISA but negative in both RBPT and c-ELISA. On the other hand, 9/278 (3.24%) bovines were positive in c-ELISA but negative in RBPT and i-ELISA; and 2/278 (0.72%) bovines were positive in RBPT but negative in both i-ELISA and c-ELISA tests. There was no difference in bovine seropositivity in both RBPT and i-ELISA, RBPT and c-ELISA, i-ELISA and c-ELISA, and in all three serological tests (p > 0.05) (Fig. 3). Table 2. Agreement between the 3 serological tests.
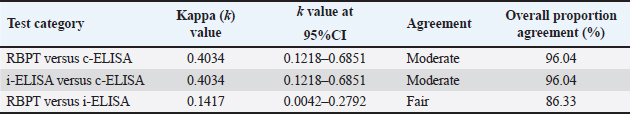
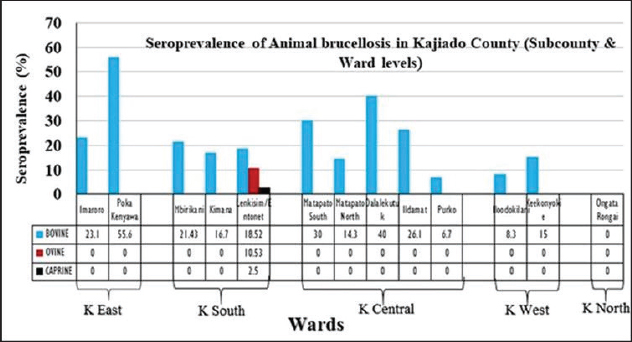
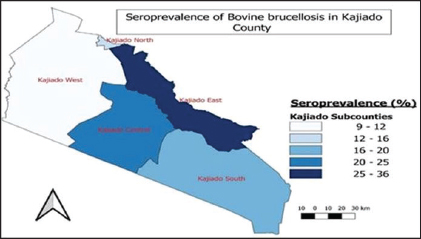
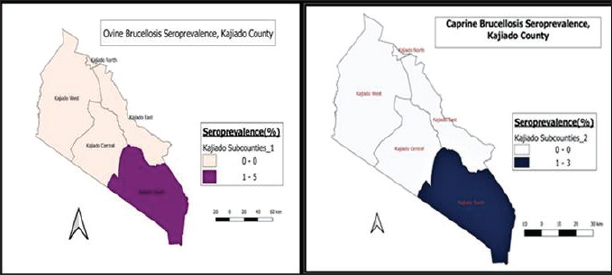
Fig. 4. Sub-county and ward seroprevalence of livestock brucellosis in Kajiado County. There was moderate agreement between RBPT and c-ELISA and between i-ELISA and c-ELISA ( k=0.4034; 95%CI: 0.1218–0.6851) with an overall proportion agreement of 96.04%. In contrast, there was a slight agreement between RBPT and the i-ELISA test ( k=0.1417; 95%CI: 0.0042–0.2792), with an overall proportion agreement of 86.33% (Table 2). Sub-county and ward-level seroprevalence of livestock brucellosis in Kajiado CountyThe seroprevalence and spread of livestock brucellosis were also compared across all 5 sub-counties and 13 wards of Kajiado County; the results are shown in Figure 4. Sub-county seroprevalences of animal brucellosis ranged from 4.76%–36.4%, 0%–5.13%, and 0%–2.6%, for bovine, ovine, and caprine, respectively. All the five sub-counties involved in the study had bovine herds with at least one seropositive animal. Kajiado East sub-county had the highest seroprevalence followed by Kajiado Central and Kajiado South. Kajiado West sub-county had moderate seroprevalence, whereas, Kajiado North had the lowest seroprevalence of bovine brucellosis in Kajiado County. There was no significant difference in seroprevalence of bovine brucellosis between the sub-counties of Kajiado County (p > 0.05), except between Kajiado East and Kajiado North, and between Kajiado East and Kajiado West (p < 0.05). For ovine and caprine flocks, the seropositive animal(s) were all from the sub-county of Kajiado South. Sub-county seroprevalence rates for ovine and caprine brucellosis ranged from 0%–5.13% and 0%–2.6%, respectively. Twelve out of 13 (92.3%) wards involved in this study had herds with at least one seropositive animal for bovine brucellosis. Ward seroprevalence rates for bovine brucellosis ranged from 0%–55.6%. High seroprevalence rates (≥30%) were in Poka Kenyawa, Dalalekutuk, and Matapato South wards, respectively; moderate seroprevalence rates (14%–29%) were in Ildamat, Mbirikani, Imaroro, Lenkisim/Entonet, Kimana, Keekonyokie, and Matapato North wards; whereas, low seroprevalence rates (≤10%) were in Purko, and Iloodokilani wards, respectively. Ongata Rongai ward had no herds with seropositive animals. There was no significant difference in the seroprevalence of bovine brucellosis between wards in the same sub-county (p > 0.05). For small ruminants, only 1/13 (9.1%) wards had at least one ovine and caprine flock with seropositive animal, respectively. All the seropositive ovine and caprine flocks were from the Lenkisim/Entonet ward. The remaining 10/11 (90.9%) wards had no seropositive animals. Ward seroprevalence rates for ovine and caprine brucellosis ranged from 0–10.53 and 0–2.5, respectively.
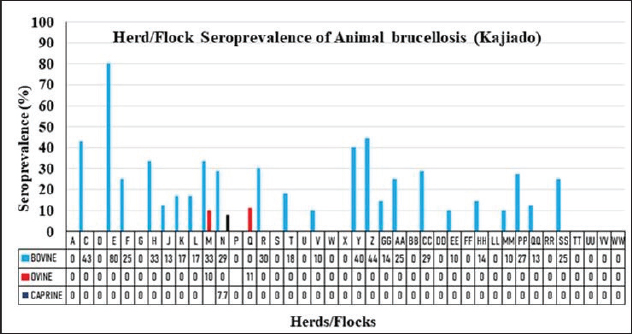
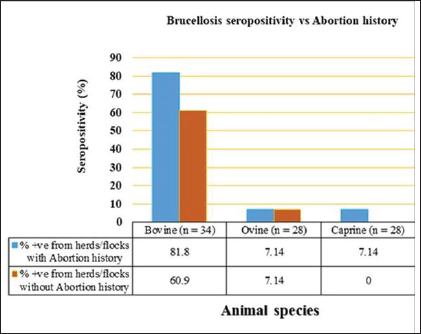
Fig. 5. Herd/flock seroprevalence of livestock brucellosis in Kajiado County. Herd and flock seroprevalence of animal brucellosis in Kajiado CountyThe results of the seroprevalence of bovine brucellosis are shown in Figure 5. Overall, 23/34 (67.65%) bovine herds had at least one seropositive animal; 11/34 (34.35%) herds had no seropositive animals. Herd seroprevalence for bovine brucellosis ranged from 0%–80%. In general, there was no significant difference in seroprevalence rates between herds in the same ward (p > 0.05). Only 2/25 ovine (8%) and 1/28 caprine (3.6%) flocks had at least one seropositive animal. Ovine and caprine animal-level seroprevalence rates within flocks ranged from 0%–11.1% and 0%–7.7%, respectively. There was no significant difference between ovine and caprine flock seroprevalence of brucellosis (p > 0.05). Animal brucellosis versus history of abortionIn the study, 11/34 (32.35%) bovine herds had a history of abortion, and of these, 9/11 (81.8%) had at least one seropositive animal (Fig. 6). On the other hand, 23/34 (67.65%) herds had no history of abortion, and of these, 14/23 (67.65%) had at least one seropositive animal. There was no significant difference in brucellosis seroprevalence between herds with abortion history and those without (p > 0.05). At the animal level, 27/98 (27.6%) of animals in herds with abortion history were seropositive, whereas 24/180 (13.3%) animals were seropositive in herds without abortion history. Overall, animal-level seroprevalence rates in bovine herds with abortion history were significantly higher than those without abortion history (p < 0.05). Only one seropositive ovine and caprine flock, respectively, had a history of abortion. The other seropositive ovine flock had no history of abortion. Spatial distribution of bovine brucellosis in Kajiado CountyThe spatial distribution of bovine brucellosis in Kajiado County is shown in Figures 7 and 8. The highest seroprevalence rate (≥30%) of bovine brucellosis was in the Kajiado East sub-county, with a moderate rate (11%–24%) in Kajiado Central, Kajiado South, and Kajiado West (Fig. 7). Kajiado North sub-county had no seropositive herds. There was a moderate seroprevalence rate (11%) of ovine, and a low seroprevalence rate (2.3%) of caprine brucellosis in Narok South sub-county, specifically in the Entonet/Lenkisim ward; other sub-counties had no detectable burdens of small ruminant brucellosis (Fig. 8). DiscussionThe objective of this study was to determine the current seroprevalence and spatial distribution of animal brucellosis in Kajiado County as well as compare the three serological tests, namely; RBPT, i-ELISA, and c-ELISA in the detection of seropositive animals. The results of the study show that there was a moderate seroprevalence in bovine (18.35%), and a low seroprevalence in ovine (0.78%) and caprine (0.4%) brucellosis in Kajiado County. The present study is the first one to carry out an extensive serological survey of livestock brucellosis in Kajiado County of Kenya. Simultaneous use of three serological tests in parallel rather than in series provided a more complete assessment of the immune response of animals to Brucella infection and also deepened the understanding of infection-immune response dynamics at the animal level.
Fig. 6. Herd/flock seropositivity of livestock brucellosis versus history of abortion in Kajiado County.
Fig. 7. Sub-county distribution of bovine brucellosis in sub-counties of Kajiado County. The animal-level seroprevalence (6.91%; n=782) found in this study is significantly lower than that previously reported for the same area (21.91%; n=209) (Nakeel et al., 2016) (p < 0.05). It is also lower than for Narok County and Baringo Counties (Enström et al., 2017; Nthiwa et al., 2019; Lokamar et al., 2022). It is, however, comparable to that reported for Marsabit County, (Osoro et al., 2015). The animal-level seroprevalence was also within the range of 0.2–43.8 reported for the East African region (Djangwani et al., 2021; Warioba et al., 2023). The similarity in animal-level seroprevalence results between countries within the East African region could be attributed to similarities in traditional grazing practices as most regions from which studies have been conducted happen to be in pastoral areas, where different animal species are mixed, and grazing pastures and watering points are shared. Globally, the prevalence detected in this study was lower than for Nigeria, Ethiopia, and Angola, respectively (Mufinda et al., 2015; Ogugua et al., 2018; Akinyemi et al., 2021; Tschopp et al., 2021). The variations in results observed in different studies may probably be attributed to several factors such as the epidemiological situation, sampling techniques, sample sizes, different diagnostic tests used, and interpretations of results. Bovine seroprevalence (18.35%) found in this study is comparable to that previously reported in one study in the same county (Nakeel et al., 2016), and also Baringo County (Lokamar et al., 2022). It was significantly higher (p < 0.05) than for Marsabit and Kiambu counties, respectively (Osoro et al., 2015) (p < 0.05), but was lower than for the Maasai Mara area in Narok County (Nthiwa et al., 2019). Bovine seroprevalence in this study was also significantly higher (p < 0.05) than the range (0%–13%) reported for the East African region and Nigeria (Akinyemi et al., 2021; Djangwani et al., 2021). It was, however, much lower than for South Sudan (Madut et al., 2018).
Fig. 8. Distribution of ovine and caprine brucellosis in sub-counties of Kajiado County. In this study, 23/34 (67.6%) bovine herds had at least one seropositive animal for brucellosis, indicating that in Kajiado County, two out of every three bovine herds are seropositive for brucellosis. The herd seroprevalence observed in this study is significantly higher than the 28% previously reported for the same county (Osoro et al., 2015). The difference between the herd seroprevalence in the current study and that by Osoro et al. (2015) could be attributed to several factors such as the extent of the geographic area covered, sampling techniques, sample sizes, different diagnostic tests used, and interpretations of results. The herd seroprevalence in the current study is, however, comparable to that reported for Marsabit County, Tanzania, and Sokoto State in Nigeria (Osoro et al., 2015; Cadmus et al., 2021; Warioba et al., 2023). It was, however, higher than that reported for Kiambu County in Kenya, and also other countries including, Uganda, Angola, and Cameroon (Mufinda et al., 2015; Mugizi et al., 2015; Osoro et al., 2015; Awah-Ndukum et al., 2018). The relatively high levels of individual and herd seroprevalence recorded in this study are indications of a high level of introduction of infected animals to herds through transhumance, and a high level of mixing of herds in communal grazing pastures, as well as livestock markets. Variation in management and hygiene practices in the farms has been associated with differences in seroprevalence rates reported in various studies (Mufinda et al., 2015; Ndazigaruye et al., 2018; Mfune et al., 2021). However, the differences in herd seroprevalence rates reported in Kajiado County of Kenya in this study, and other regions of Africa and beyond could also be attributed to the protocol adopted such as the different diagnostic tests used, and their sensitivities and interpretations. The protocol could have involved one test or more than one test carried out in series, which entails the use of a screening test followed by confirmation of positive reactors by another test, or in parallel, whereby all tests are used to test the sampled animals independently (Awah-Ndukun et al., 2018; Ducrotoy et al., 2018; Moreno et al., 2022). The differences could also be attributed to other factors including the differences in management practices in the areas studied, sampling techniques and sample sizes, and seasonal cattle movements in search of pastures during drought periods (Akinyemi et al., 2021; Mfune et al., 2021; Warioba et al., 2023). One of the clinical manifestations of animal brucellosis is abortion (Merga Sima et al., 2021; Rehman et al., 2020). In the current study, 11/34 (32.4%) bovine herds had a history of abortion, whereas 23/34 (67.6%) had no history of abortion; 9/11 (81.8%) of the herds with abortion history had at least one seropositive animal, whereas 14/23 (60.9%) without abortion history were seropositive. At the animal level, seroprevalence of bovine herds with a history of abortion had significantly higher prevalence rates of brucellosis than those without (p < 0.05). In addition, bovine herds with a history of abortion had an odds ratio of 3.54 (95% CI:0.631–19.819), implying that the chances of getting one brucellosis seropositive animal were 3.5 times more in herds with a history of abortion than those without. Other studies have also reported high brucellosis prevalence rates in herds with a history of abortion (Ndazigaruye et al., 2018; Rehman et al., 2020; Merga Sima et al., 2021). Generally, seroprevalence of small ruminant brucellosis in Kenya has fluctuated between 0% and 20% for ovine and 0%–13.8% for caprine (Djangwani et al., 2021). This study found very low levels of ovine (0.78%; n=2/256) and caprine (0.4%; n=1/248) brucellosis than seroprevalence levels reported in previous studies in Kenya, including Baringo, Tana River, Kiambu, and Marsabit counties (Djangwani et al., 2021; Mwasi, 2021; Lokamar et al., 2022). The seroprevalence rates in this study were even lower than those reported in a previous study in the same county (Nakeel et al., 2016). Ovine and caprine seroprevalences were however, within the range (0%–20%) reported for the East African region (Chota et al., 2016; Djangwani et al., 2021; Akwongo and Kakooza, 2022). Other researchers have reported variable animal seroprevalences according to regions and locations (Mugizi et al., 2015; Ndazigaruye et al., 2018; Edao et al., 2020). In this study, there was no significant difference in the seroprevalences of ovine and caprine brucellosis (p > 0.05), which may suggest common epidemiological cum management factors and aetiological agents in seropositive flocks. Taken together, these findings indicate a low prevalence of brucellosis in small ruminants in Kajiado County, Kenya. The flock seroprevalence rates for ovine (8%; n=2/25) and caprine (3.6%; n=1/25), were higher than 0.34% and 1.78% for ovine and caprine, recently reported in the same county (Mwasi, 2021). These results are in agreement with those reported in one study in the same area in which only two ovine and one caprine flock, from Entonet/Lenkisim Ward, in South Kajiado sub-county had at least one seropositive animal (Mwasi, 2021). These results suggest the possibility of there being similar management practices for small ruminants in the Kajiado South sub-county. In other studies, differences in management practices have been reported to be responsible for the observed differences in the seroprevalence rates in different locations or regions of the same country (Asiimwe et al., 2015; Tegegn et al., 2016). In this study, all ovines from Kajiado East were seronegative, which is in stark contrast with the findings of another study in which the Mashuru area in Kajiado East sub-county had the highest seroprevalence of ovine brucellosis (Nakeel et al., 2016). These differences could be explained by factors such as sampling techniques, sample sizes, different diagnostic tests used, and different testing regimes as well as test results interpretation criteria (Ducrotoy et al., 2018; Moreno et al., 2022). In this study, 16/25 (64%) ovine and 18/28 (64.3%) caprine flocks had abortion history; however, only 2/16 (12.5%) ovine and 1/18 (5.6%) caprine flocks were seropositive; all seropositive flocks were from the same Lenkisim/Entonet Ward in Kajiado South sub-county. Small ruminant flocks with a history of abortion have been reported to have higher brucellosis seroprevalences than those without (Asiimwe et al., 2015; Mwasi, 2021). In this study, abortion history was not correlated with brucellosis as only a small percentage of flocks with abortion history were seropositive. This may suggest the presence of other causes of abortion in small ruminants in this county. It is worth noting that one study found high seroprevalence rates of leptospirosis and Q fever in small ruminants in the study area (Nakeel et al., 2016). These two diseases could account for the high abortion incidences reported by farmers in the study area (Nakeel et al., 2016; Mwasi, 2021). In this study, three serological tests, namely RBPT, i-ELISA, and c-ELISA were used to test animal sera in parallel. Seropositivity was defined as any animal positive in any of the three serological tests. Overall, i-ELISA (14.4%; n=40/278) picked significantly more seropositive bovine samples than both c-ELISA (5.04%; n=14/278) and RBPT (2.2% n=6/278) (p < 0.05). The variations in the seropositivity in the three serological tests used in this study could be due to false positive and negative reactions which may occur with RBPT and i-ELISA, differences in sampling size, sensitivity and specificity of the employed serological tests, demography, and different clinical conditions of animals, and quality of the samples (Ducrotoy et al., 2018; Umar et al., 2019; El-Diasty et al., 2021). In this study, i-ELISA picked 13 seropositive bovines that were not positive in either c-ELISA or RBPT. These group of animals could also represent either false positives due to cross-reactions with closely related Gram-negative bacteria, or early cases of infection with low antibody titers, as i-ELISA is usually more sensitive than both RBPT and c-ELISA (Nielsen, 2002; Corbel, 2006; Bonfini et al., 2018; WOAH, 2020). On the other hand, c-ELISA picked nine animals that were negative in both RBPT and i-ELISA. This group of animals could represent true positives from chronic stages of infection, as this test is more specific than either i-ELISA or RBPT and also can pick all antibody isotypes (Ghodasara et al., 2010; Godfroid et al, 2010; Ducrotoy et al, 2018). RBPT picked two reactors that were negative in both i-ELISA and c-ELISA. This group of animals may be false positive reactors due to cross-reactions with other closely related Gram-negative bacteria (WOAH, 2020). In this study, no test picked all the positive animals; 230/278 (82.7%) bovines were seronegative. This could be attributed to the quality of the sample, the prozone phenomenon that occurs usually in acidified antigens as in the case of the RBPT test, or the presence of other blocking antibodies. Similar findings have been reported by other researchers (Abernethy et al., 2012; Chisi et al., 2017). In this study, seropositivity results for bovine samples in all three serological tests were analyzed using kappa statistics (Sim and Wright, 2005). There was a slight agreement between RBPT and i-ELISA ( k=0.1417; 95% CI: 0.0042–0.2792); whereas there was moderate agreement between RBPT and c-ELISA ( k=0.4034) and between i-ELISA and c-ELISA ( k=4034; 95%CI: 0.1218–0.6851), and the corresponding overall proportion agreements were: 86.33%, 96.04%, and 96.04%, respectively. These results are in contrast to those reported in one study in Pakistan where there was a near-perfect agreement between RBPT and i-ELISA tests in cattle (Jamil et al., 2020). The good overall agreement between the three serological tests indicated that any one of them could be used for screening bovines for anti-Brucella antibodies; however, the kappa values for these three tests when used in parallel, suggest that the tests may be picking different sets of animals in terms of clinical syndromes. The moderate overall agreement between RBPT and i-ELISA indicated that the two tests could have picked the same clinical set of animals and that either one of them could be used as a screening test for anti-Brucella antibodies in bovines. In this study, three tests were also compared for the detection of anti-Brucella antibodies in nonvaccinated ovine and caprine flocks of Kajiado County. Overall, c-ELISA is the only test that picked seropositive ovines and caprines; no reactors were picked by either RBPT or i-ELISA. These results differ from those of others who reported seropositivity for ovine brucellosis in both tests, with some reporting i-ELISA to be more sensitive than RBPT or vise-versa (Umar et al., 2019; Al-Marzooqi et al., 2022), while others, reported similar sensitivities for both tests (Traoré et al., 2021). One of the problems of ELISAs performed on ovine serum is the high background reactivity obtained when testing sera from brucellosis-free animals (De Bagüés et al., 1992). This problem can be circumvented by using monoclonal anti-ruminant IgG1 conjugate, which increases the specificity of ELISA tests (Díaz-Aparicio et al., 1994). The sensitivity of i-ELISAs with either protein G or monoclonal conjugates has been shown to decrease when compared to that performed using the polyclonal conjugate (Moreno et al., 2022). The lack of i-ELISA seropositive ovines in this study could be due to the quality of the reagents used, particularly the conjugate, or simply the presence of other interfering antibodies (Moreno et al., 2022). Equally, the lack of RBPT seropositivity could be due to either the prozone phenomenon or epidemiological factors not determined by the study. In this study, caprines had very low seropositivity in c-ELISA, but none at all in RBPT and i-ELISA. These results are in agreement with those of another study conducted in the same county using RBPT, in which both ovine and caprine seroprevalences were very low (Mwasi, 2021). It could be that there is genuinely low small ruminant brucellosis in Kajiado County and that the high incidences of abortions reported in this group of animals are due to other causes such as leptospirosis and Q fever, which have been shown to occur in this county at high prevalence rates (Nakeel et al., 2016). These results point to the need to use more than one serological test in parallel rather than in series, particularly when carrying out seroprevalence studies in small ruminants, as these tests seem to have different sensitivities in these animals (Rossetti et al., 2022). In this study, c-ELISA was found to be the best test for serological screening of small ruminants for anti-Brucella antibodies, and should therefore be recommended as a routine screening test in these species ConclusionThe results of this study indicate a moderate seroprevalence of brucellosis in bovines but a very low prevalence in small ruminants. The low prevalence of small ruminant brucellosis, notwithstanding high seroprevalences of other abortion-causing infections, calls for more studies to be conducted on small ruminants to establish the real cause of abortions. The results also indicated that i-ELISA was more sensitive than either RBPT or c-ELISA in detecting seropositive bovine, whereas c-ELISA was superior to both RBPT and i-ELISA in detecting seropositive ovines and caprines. Furthermore, studies need to be carried out using both cultural and molecular methods such as Multiplex PCR, multilocus variable number of tandem repeat analysis, or single nucleotide polymorphism genotyping to elucidate the Brucella species and genotypes infecting the animals and humans in Kajiado County, results of which will be crucial in formulating brucellosis control or eradication strategies for the county. AcknowledgmentThe authors are very much grateful to all the livestock owners in Kajiado County for allowing their animals to be used during sample collection, to the then County Director of Veterinary Services, Dr. Achola, and the current one, Dr. Nazaria Nyaga, for allowing the study to be conducted in Kajiado County, and Messers Alfred Mainga, Ephantus Nyaga, George Dimbu, Saitabau Sena, Hellen Naanyu, and Moreno Ole Kutesho for their technical inputs during sample collection and analysis. Conflict of interestThe authors declare that they have no conflicts of interest. FundingThe research work was supported with funds from the NRF (Leap Agri) Grant no. 65/MoEST, Kenya. Data availabilityData is available on request from the corresponding author. Authors’ contributionsMahacla Odongo collected the samples, carried out laboratory analysis and statistical analysis, and wrote the first draft; Lilly Bebora, Joseph Erume, and Lilian Waiboci acquired the funds and participated in the design and implementation of the project; Lilly Bebora and Joseph Erume also critically revised the manuscript; Bebora, Gathumbi, and Aboge guided the execution and the research, particularly sample processing and analysis. ReferencesAbernethy, D.A., Menzies, F.D., McCullough, S.J., McDowell, S.W.J., Burns, K.E., Watt, R., Gordon, A.W., Greiner, M. and Pfeiffer, D.U. 2012. Field trial of six serological tests for bovine brucellosis. Vet. J. 191, 364–370. Akinyemi, K.O., Fakorede, C.O., Amisu, K.O. and Wareth, G. 2021. Human and animal brucellosis in Nigeria: a systemic review and meta-analysis in the last twenty-one years (2001-2021). Vet. Sci. 9(8), 384. Akwongo, C.J. and Kakooza, S. 2022. Exposure to Brucella spp. in goats and sheep in Karenga District, Uganda diagnosed by modified Rose Bengal method. Zoonotic. Dis. 2, 163–171. Al-Marzooqi, W., Elshafie, E.I., Al-Toobi, A., Al-Hamrashdi, A., Al-Kharousi, K., El-Tahir, H., Jay, M., Corde, Y. and ElTahir, Y. 2022. Seroprevalence and risk factors of brucellosis in ruminants in Dhofar Province in Southern Oman. Vet. Med. Int. 2022, 3176147. Asiimwe, B.B., Kansiime, C. and Rwego, I.B. 2015. Risk factors for human brucellosis in agro-pastoralist communities of south western Uganda: a case-control study. BMC. Res. Notes. 8, 405. Awah-Ndukum, J., Mouiche, M.M.M., Bayang, H.N., Ngwa, V.N., Assana, E., Feussom, K.J.M., Manchang, T.K. and Zoli, P.A. 2018. Seroprevalence and associated risk factors of brucellosis among Indigenous cattle in the Adamawa and North Regions of Cameroon. Vet. Med. Int. 2018, 3468596. Blasco, J.M., Garin-Bastuji, B., Marín, C., Gerbier, G., Fanlo, J., Jiménez De Bagués, M. and Cau, C. 1994. Efficacy of different Rose Bengal and complement fixation antigens for the diagnosis of Brucella melitensis in sheep and goats. Vet. Rec. 134, 415–420. Bonfini, B., Chiarenza, G., Paci, V., Sacchini, F., Salini, R., Vesco, G., Villari, S., Zilli, K. and Tittarelli, M. 2018. Cross-reactivity in serological tests for brucellosis: a comparison of immune response of Escherichia coli O157:H7 and Yersinia enterocolitica O:9 vs Brucella spp. Vet. Ital. 54(2), 107–114. Cadmus, S., Salam, S.P., Adesokan, H.K., Akporube, K., Ola-Daniel, F. and Awosanya, E.J. 2021. Seroprevalence of brucellosis and Q fever infections amongst pastoralists and their cattle herds in Sokoto State, Nigeria. PLoS One 16(7), e0254530. Chisi, S.L., Marageni, Y., Naidoo, P., Zulu, G., Akol, G.W. and Van Heerden, H. 2017. An evaluation of serological tests in the diagnosis of bovine brucellosis in naturally infected cattle in KwaZulu-Natal province in South Africa. J. S. Afr. Vet. Assoc. 88, e1–e7. Chota, A., Magwisha, H., Stella, B., Bunuma, E., Shirima, G., Mugambi, J., Omwenga, S., Wesonga, H., Mbatha, P. and Gathogo, S. 2016. Prevalence of brucellosis in livestock and incidences in humans in East Africa. Afr. Crop. Sci. J. 24(1), 45. Corbel, M.J. 2006. Brucellosis in humans and animals. Geneva, Switzerland: WHO Press, World Health Organization CIDP. 2018. County integrated development plan 2018-2022. Kajiado, Kenya: County Government of Kajiado. De Bagüés, M.J., Marin, C.M., Blasco, J.M., Moriyon, I. and Gamazo, C. 1992. An ELISA with Brucella lipopolysaccharide antigen for the diagnosis of B. melitensis infection in sheep and for the evaluation of serological responses following subcutaneous or conjunctival B. melitensis strain Rev 1 vaccination. Vet. Microb. 30(2-3), 233–224. Díaz-Aparicio, E., Marín, C., Alonso-Urmeneta, B., Aragón, V., Pérez-Ortiz, S., Pardo, M., Blasco, J.M., Díaz, R. and Moriyón, I. 1994. Evaluation of serological tests for diagnosis of Brucella melitensis infection of goats. J. Clin. Microbiol. 32(5), 1159–1165. Djangwani, J., Ooko Abong', G., Gicuku Njue, L. and Kaindi, D.W.M. 2021. Brucellosis: prevalence with reference to the East African Community countries—a rapid review. Vet. Med. Sci. 7(3), 851–867. Dohoo, I.R., Martin, S.W. and Stryhn, H. 2012. Methods in epidemiologic research. Charlottetown, Canada: P.E.I.: VER, Inc. Ducrotoy, M.J., Muñoz, P.M., Conde-Álvarez, R., Blasco, J.M. and Moriyón, I. 2018. A systematic review of current immunological tests for the diagnosis of cattle brucellosis. Prev. Vet. Med. 151, 57–72. Edao, B.M., Ameni, G., Assefa, Z., Berg, S., Whatmore, A.M. and Wood, J.L.N. 2020. Brucellosis in ruminants and pastoralists in Borena, Southern Ethiopia. PLoS. Negl. Trop. Dis. 14(7), e0008461. El-Diasty, M., El-Said, R. and Abdelkhalek, A. 2021. Seroprevalence and molecular diagnosis of sheep brucellosis in Dakahlia governorate, Egypt. Ger. J. Vet. Res. 1(1), 34–39. Enström, S., Nthiwa, D., Bett, B., Karlsson, A., Alonso, S. and Lindahl, J.F. 2017. Brucella seroprevalence in cattle near a wildlife reserve in Kenya. BMC. Res. Notes. 10, 61. Ghodasara, S.N., Roy, A. and Bhanderi, B.B. 2010. Comparison of Rose Bengal plate agglutination, standard tube agglutination, and indirect ELISA tests for detection of Brucella antibodies in cows and buffaloes. 2: an insight into the viruses zoonotic aspects. Vet. World. 3(2), 61. Godfroid, J., Nielsen, K. and Saegerman, C. 2010. Diagnosis of brucellosis in livestock and wildlife. Croat. Med. J. 51(4), 296–305. Jamil, T., Melzer, F., Saqib, M., Shahzad, A., Khan Kasi, K., Hammad Hussain, M., Rashid, I., Tahir, U., Khan, I., Haleem Tayyab, M., Ullah, S., Mohsin, M., Mansoor, M.K., Schwarz, S. and Neubauer, H. 2020. Serological and molecular detection of bovine brucellosis at Institutional Livestock Farms in Punjab, Pakistan. Int. J. Environ. Res. Public. Health. 17(4), 1412. Kenya National Bureau of Statistics. 2019. Distribution of livestock population by type, fish ponds, and fish cages by County and Sub County. Kenya Population and Housing Census, Volume IV. Nairobi, Kenya: KNBS. Khan, M.Z. and Zahoor, M. 2018. An overview of brucellosis in cattle and humans, and its serological and molecular diagnosis in control strategies. Trop. Med. Infect. Dis. 3(2), 65. Khurana, S.K., Sehrawat, A., Tiwari, R., Prasad, M., Gulati, B., Shabbir, M.Z., Chhabra, R., Karthik, K., Patel, S.K., Pathak, M., Iqbal Yatoo, M., Gupta, V.K., Dhama, K., Sah, R. and Chaicumpa, W. 2021. Bovine brucellosis—a comprehensive review. Vet. Q. 41(1), 61–88. Lokamar, P.N., Kutwah, M.A., Munde, E.O., Oloo, D., Atieli, H., Gumo, S., Akoko, J.M. and Ouma, C. 2022. Prevalence of brucellosis in livestock keepers and domestic ruminants in Baringo County, Kenya. PLOS. Glob. Public. Health. 2(8), e0000682. Madut, N.A., Muwonge, A., Nasinyama, G.W., Muma, J.B., Godfroid, J., Jubara, A.S., Muleme, J. and Kankya, C. 2018. The seroprevalence of brucellosis in cattle and their herders in Bahr el Ghazal region, South Sudan. PLoS. Negl. Trop. Dis. 12(6), 1–14. Mengele, I.J., Shirima, G.M., Bronsvoort, B.M., Hernandez-Castro, L.E. and Elizabeth, A.J. Cook. 2023. Diagnostic challenges of brucellosis in humans and livestock in Tanzania: a thematic review. CABI. One. Health. 2023, 1–16; doi: 10.1079/cabionehealth.2023.0001. Merga Sima, D., Abdeta Ifa, D., Merga, A.L. and Tola, E.H. 2021. Seroprevalence of bovine brucellosis and associated risk factors in Western Ethiopia. Vet. Med. (Auckl). 12, 317–324. Mfune, R.L., Mubanga, M., Silwamba, I., Sagamiko, F., Mudenda, S., Daka, V., Godfroid, J., Hangombe, B.M. and Muma, J.B. 2021. Seroprevalence of bovine brucellosis in selected districts of Zambia. Int. J. Environ. Res. Public. Health. 18(4), 1436. Moreno, E., Blasco, J.M. and Moriyón, I. 2022. Facing the human and animal brucellosis conundrums: the forgotten lessons. Microorganisms 10, 942. Mufinda, F.C., Boinas, F. and Nunes, C. 2015. Prevalence and factors associated with cattle brucellosis in animal herds of the Namibe Province in Angola. AJVS 47(1), 7–17. Mugizi, D.R., Boqvist, S., Nasinyama, G.W., Waiswa, C., Ikwap, K., Rock, K., Lindahl, E., Magnusson, U. and Erume, J. 2015. Prevalence of and factors associated with Brucella seropositivity in cattle in urban and peri-urban Gulu and Soroti towns of Uganda. J. Vet. Med. Sci. 77, 557–564. Munoz, P.M., Marın, C.M., Monreal, D., Gonza´lez, D., Garin-Bastuji, B., Dı´az, R., Mainar-Jaime, R.C., Moriyon, I. and Blasco, J.M. 2005. Efficacy of several serological tests and antigens for diagnosis of bovine brucellosis in the presence of false-positive serological results due to Yersinia enterocolitica O:9. Clin. Diagn. Lab. Immunol. 12(1), 141–150. Mwasi, A.J. 2021. Serological and molecular detection of brucellosis and its risk factors for sheep and goat flocks in Kajiado County, Kenya. MSc Thesis, University of Nairobi, Nairobi, Kenya. Nakeel, M.J., Arimi, S.M., Kitala, P.K., Nduhiu, G., Njenga, J.M. and Wabacha, J. 2016. A seroepidemiological survey of brucellosis, Q-fever, and leptospirosis in livestock and humans and associated risk factors in Kajiado County- Kenya. J. Trop. Dis. 4(3), 1–8. Ndazigaruye, G., Mushonga, B., Kandiwa, E., Samkange, A. and Segwagwe, B.E. 2018. Prevalence and risk factors for brucellosis seropositivity in cattle in Nyagatare District, Eastern Province, Rwanda. J. S. Afr. Vet. Assoc. 89(0), e1–e8. Nielsen, K. 2002. Diagnosis of brucellosis by serology. Vet. Microb. 90, 447–459. Nielsen, K., Smith, P., Widdison, J., Gall, D., Kelly, L., Kelly, W. and Nicoletti, P. 2004. Serological relationship between cattle exposed to Brucella abortus, Yersinia enterocolitica O: 9, and Escherichia coli O157:H7. Vet. Microbiol. 100, 25–30. Nthiwa, D., Alonso, S., Odongo, D., Kenya, E. and Bett, B. 2019. Zoonotic pathogen seroprevalence in cattle in a wildlife-livestock interface, Kenya. Ecohealth 16(4), 712–725. Ogugua, A.J., Akinseye, V.O., Cadmus, E.O., Awosanya, E.A.J., Alabi, P.I., Idowu, O.S., Akinade, S.A., Dale, E.J., Perrett, L. and Taylor, A. 2018. Prevalence and risk factors associated with bovine brucellosis in herds under extensive production system in southwestern Nigeria. Trop. Anim. Health. Prod. 50, 1573–1582. Osoro, E.M., Munyua, P., Omulo, S., Ogola, E., Ade, F., Mbatha, P., Mbabu, M., Ng'ang'a, Z., Kairu, S., Maritim, M., Thumbi, S.M., Bitek, A., Gaichugi, S., Rubin, C., Njenga, K. and Guerra, M. 2015. Strong association between human and animal Brucella seropositivity in a linked study in Kenya, 2012-2013. Am. J. Trop. Med. Hyg. 93(2), 224–231. Praud, A., Champion, J.L., Corde, Y., Drapeau, A., Meyer, L. and Garin-Bastuji, B. 2012. Assessment of the diagnostic sensitivity and specificity of an indirect ELISA kit for the diagnosis of Brucella ovis infection in rams. BMC. Vet. Res. 8, 68. Rehman, A., Khan, M.R., Khalid, S., Ahmad, M.U.D., Ijaz, M. and Mushtaq, M.H. 2020. Seroprevalence of brucellosis in bovine herds using parallel diagnostic approach in the semi-arid agro-ecological zone in Pakistan. Int. J. Infect. Dis. 101(1), 537. Rossetti, C.A., Maurizio, E. and Rossi, U.A. 2022. Comparative review of brucellosis in small domestic ruminants. Front. Vet. Sci. 9, 887671. Senbeto, Y.A. 2022. Brucellosis: a review. Int. J. Adv. Res. Biol. Sci. 9(8), 146–161. Sim, J. and Wright, C.C. 2005. The kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys. Ther. 85(3), 257–268. Tegegn, A.H., Feleke, A., Adugna, W. and Melaku, S.K. 2016. Small ruminant brucellosis and public health awareness in two districts of Afar Region, Ethiopia. J. Vet. Sci. Technol. 7, 335. Traoré, S., Yapi, R.B., Coulibaly, K., Mathew, C., Fokou, G., Kazwala, R.R., Bonfoh, B. and Alambedji, R.B. 2021. Seroprevalence of brucellosis in small ruminants and related risk behaviors among humans in different husbandry systems in Mali. PLoS One 16(1), e0245283. Tschopp, R., Gebregiorgis, A., Tassachew, Y., Andualem, H., Osman, M., Waqjira, M.W., Hattendorf, J., Mohammed, A., Hamid, M., Molla, W., Mitiku, S.A., Walke, H., Negron, M., Kadzik, M. and Mamo, G. 2021. Integrated human-animal sero-surveillance of brucellosis in the pastoral Afar and Somali regions of Ethiopia. PLoS. Negl. Trop. Dis. 15(8), e00095932021. Umar, Y.M., Sundaram, S., Lakshmanan, G., Ayyasamy, E., Purushothaman, S., Kannan, P. and Sanjeevi, T. 2019. Serosurveillance of bovine brucellosis using RBPT and c-ELISA and comparative evaluation of test performance. Int. J. Curr. Microbiol. App. Sci. 8(8), 559–568. Warioba, J.P., Karimuribo, E.D., Komba, E.V.G., Kabululu, M.L., Minga, G.A. and Nonga, H.E. 2023. Occurrence and risk factors of brucellosis in commercial cattle farms from selected districts of the Eastern Coast Zone, Tanzania. Vet. Med. Int. 13, 4904931. WOAH. 2020. Chapter 2.4.3: bovine brucellosis. WOAH manual of diagnostic tests and vaccines for terrestrial animals. Paris, France: World Organization for Animal Health. Available via https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.01.04_BRUCELLOSIS.pdf (Accessed 28 March 2023). | ||
| How to Cite this Article |
| Pubmed Style Odongo MO, LCB, JKG, GOA, LWW, Erume J. Seroprevalence and spatial distribution of livestock brucellosis using three serological tests in Kajiado County, Kenya. Open Vet J. 2023; 13(12): 1583-1596. doi:10.5455/OVJ.2023.v13.i12.8 Web Style Odongo MO, LCB, JKG, GOA, LWW, Erume J. Seroprevalence and spatial distribution of livestock brucellosis using three serological tests in Kajiado County, Kenya. https://www.openveterinaryjournal.com/?mno=166136 [Access: May 13, 2024]. doi:10.5455/OVJ.2023.v13.i12.8 AMA (American Medical Association) Style Odongo MO, LCB, JKG, GOA, LWW, Erume J. Seroprevalence and spatial distribution of livestock brucellosis using three serological tests in Kajiado County, Kenya. Open Vet J. 2023; 13(12): 1583-1596. doi:10.5455/OVJ.2023.v13.i12.8 Vancouver/ICMJE Style Odongo MO, LCB, JKG, GOA, LWW, Erume J. Seroprevalence and spatial distribution of livestock brucellosis using three serological tests in Kajiado County, Kenya. Open Vet J. (2023), [cited May 13, 2024]; 13(12): 1583-1596. doi:10.5455/OVJ.2023.v13.i12.8 Harvard Style Odongo, M. O., , . L. C. B., , . J. K. G., , . G. O. A., , . L. W. W. & Erume, . J. (2023) Seroprevalence and spatial distribution of livestock brucellosis using three serological tests in Kajiado County, Kenya. Open Vet J, 13 (12), 1583-1596. doi:10.5455/OVJ.2023.v13.i12.8 Turabian Style Odongo, Mahacla Omungala, Lilly Caroline Bebora, James Kinuthia Gathumbi, Gabriel Oluga Aboge, Lillian Wangechi Waiboci, and Joseph Erume. 2023. Seroprevalence and spatial distribution of livestock brucellosis using three serological tests in Kajiado County, Kenya. Open Veterinary Journal, 13 (12), 1583-1596. doi:10.5455/OVJ.2023.v13.i12.8 Chicago Style Odongo, Mahacla Omungala, Lilly Caroline Bebora, James Kinuthia Gathumbi, Gabriel Oluga Aboge, Lillian Wangechi Waiboci, and Joseph Erume. "Seroprevalence and spatial distribution of livestock brucellosis using three serological tests in Kajiado County, Kenya." Open Veterinary Journal 13 (2023), 1583-1596. doi:10.5455/OVJ.2023.v13.i12.8 MLA (The Modern Language Association) Style Odongo, Mahacla Omungala, Lilly Caroline Bebora, James Kinuthia Gathumbi, Gabriel Oluga Aboge, Lillian Wangechi Waiboci, and Joseph Erume. "Seroprevalence and spatial distribution of livestock brucellosis using three serological tests in Kajiado County, Kenya." Open Veterinary Journal 13.12 (2023), 1583-1596. Print. doi:10.5455/OVJ.2023.v13.i12.8 APA (American Psychological Association) Style Odongo, M. O., , . L. C. B., , . J. K. G., , . G. O. A., , . L. W. W. & Erume, . J. (2023) Seroprevalence and spatial distribution of livestock brucellosis using three serological tests in Kajiado County, Kenya. Open Veterinary Journal, 13 (12), 1583-1596. doi:10.5455/OVJ.2023.v13.i12.8 |