| Research Article | ||
Open Vet J. 2023; 13(12): 1623-1630 Open Veterinary Journal, (2023), Vol. 13(12): 1623–1630 Original Research Protecting mechanism of Swietenia macrophylla ethanol extract nanoparticle on streptozotocin induced renal damage in ratRochmah Kurnijasanti1, Giftania Wardani2, Muhammad Rais Mustafa3 and Sri Agus Sudjarwo1*1Department of Basic Veterinary Medicine, Faculty of Veterinary Medicine, Airlangga University, Surabaya, Indonesia 2Program Study of Pharmacy, Faculty of Medicine, Hang Tuah University, Surabaya, Indonesia 3Department of Pharmacology, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysia *Corresponding Author: Sri Agus Sudjarwo. Department of Basic Veterinary Medicine, Faculty of Veterinary Medicine, Airlangga University, Surabaya, Indonesia. Email: ags158 [at] yahoo.com Submitted: 04/09/2023 Accepted: 18/11/2023 Published: 31/12/2023 © 2023 Open Veterinary Journal
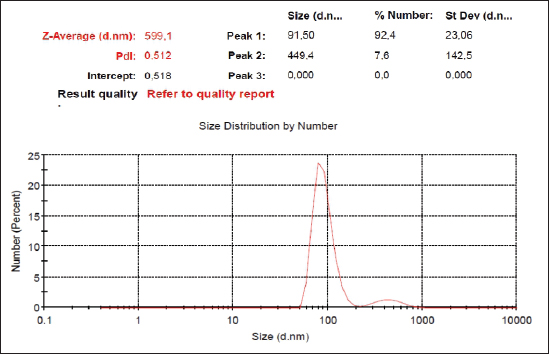
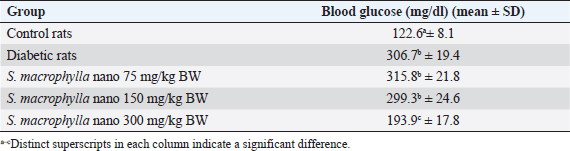
AbstractBackground: Hyperglycemia increases reactive oxygen species (ROS), which contributes to diabetic complications such as kidney cell damage. Antioxidant administration could inhibit ROS and kidney cell damage commonly seen in hyperglycemia. Aim: We want to demonstrate that the antioxidant properties of Swietenia macrophylla ethanol extract nanoparticles can prevent kidney cell damage brought on by streptozotocin (STZ) in the current investigation. Methods: This study employs high-energy ball milling to produce nanoparticles from S. macrophylla extract. Additionally, dynamic light scattering (DLS) is utilized to characterize the nanoparticle sizes of the S. macrophylla ethanol extract. Five groups, each consisting of 8 rats, were formed from 40 rats. Control rats received distilled water, the diabetic rats were administered STZ injections, while S. macrophylla rats were given S. macrophylla extract nanoparticles orally and STZ injection. After the trial, blood from a rat was drawn intracardially to check the levels of blood urea nitrogen (BUN) and creatinine. The levels of superoxide dismutase (SOD), glutathione peroxidase (GPx), and malondialdehyde (MDA) were then assessed in kidney tissue samples. Histological alterations were evaluated in kidney section samples. Results: A DLS analysis estimated the size of the S. macrophylla ethanol extract nanoparticles to be about 91.50 ± 23.06 nm. BUN and creatinine levels were significantly raised after STZ treatment. STZ significantly decreased SOD and GPx levels in kidney tissue while raising MDA levels (p < 0.05). Swietenia macrophylla ethanol extract nanoparticle caused the decreased levels of BUN and creatinine in blood to normal levels (p < 0.05), indicating that S. macrophylla ethanol extract prevented the STZ-induced kidney cell damage. Additionally, S. macrophylla nanoparticles significantly raise GPx and SOD levels in kidney tissue while lowering MDA levels (p < 0.05). These actions are thought to have prevented kidney histological alterations (degeneration and necrosis) in diabetic rats. Conclusion: According to these results, the anti-oxidative stress properties of S. macrophylla nanoparticles make them potentially effective nephroprotective therapies for STZ-induced kidney cell damage. Keywords: Swietenia macrophylla, Nanoparticles, Antioxidant, Nephroprotector, Diabetes. IntroductionDiabetes mellitus, a metabolic disorder brought on by either faulty insulin action on target organs or abnormal insulin release from pancreatic beta cells, is characterized by hyperglycemia. Hyperglycemia-induced elevated oxidative stress is a factor in developing diabetic complications, including kidney cell damage (Volpe et al., 2018; Ighodaro, 2019). Oxidative stress is brought on by an increase in reactive oxygen species (ROS) such O2, OH−, and H2O2 and a reduction in the production of antioxidant enzymes like glutathione peroxidase (GPx), superoxide dismutase (SOD), and catalase. Most diabetes-related comorbidities, notably diabetic nephropathy, impact this syndrome (Asmat et al., 2016; Oguntibeju, 2016). Common physiological activities generate ROS, which are necessary for tissue homeostasis and cell communication. However, excessive ROS generation can lead to the oxidation of DNA, lipids, and proteins, which is detrimental to cellular components and can result in necrosis and apoptosis (Farrag et al., 2019; Wardani et al., 2023). Overproduction of ROS may cause polyunsaturated fats in the cell membrane to undergo lipid peroxidation, which produces lipid peroxides or malondialdehyde (MDA). excessive MDA levels, which may result in kidney cells dying or necrosis, are a sign of excessive ROS levels (Bigagli and Lodovici, 2019; Zhang et al., 2020). MDA is used as a biomarker to assess oxidative stress in diabetic rats induced by STZ. In a diabetic rat model, STZ usage destroys kidney cells, which may increase blood levels of blood urea nitrogen (BUN) and creatinine. Consequently, BUN and creatinine may be utilized as signs of a problem with renal function (Abouzeda et al., 2020; Wardani et al., 2022). To preserve kidney cells in diabetic rats, natural product antioxidants, including black mulberry, adropinin, Syzygium aromaticum, and fucoidan are often employed as alternative exogenous antioxidants (Abouzeda et al., 2020; Abtahi-Eivari et al., 2021; Guo et al., 2021; Wardani et al., 2022). A natural substance called Swietenia macrophylla exhibits vigorous antioxidant activity and may scavenge ROS to prevent oxidative damage. Swietenia macrophylla offers pharmacological benefits such as anti-inflammatory, antifungal, antioxidant, antiviral, immunomodulatory, antibacterial, and anti-diabetic properties (Yudhani et al., 2021; Borah et al., 2022; Kurnijasanti et al., 2023). Bioavailability, solubility, absorption, and distribution are common challenges associated with natural product antioxidants. To address these issues, nanotechnology is developing to create dosed nanoparticles of natural antioxidant products. Nanobiotechnology is a scientific field focused on producing material particles with sizes ranging between 10 and 1,000 nm (Sahu et al., 2021; Sim and Wong, 2021). Nanotechnology can potentially improve therapeutic results while lowering the toxicity of natural antioxidant compounds. This study’s major objective was to show how S. macrophylla ethanol extract nanoparticles might prevent kidney cell damage in rats with diabetes brought on by streptozotocin (STZ). Its antioxidant activity and potential to prevent diabetes are the bases for this consideration. Materials and MethodsSwietenia macrophylla ethanolic extract preparationThe S. macrophylla seed was dried and powdered using a blender. The S. macrophylla seed powdered 200 g was macerated in 800 ml of 96% ethanol for 72 hours. The extract was filtered through a Whatman filter and the filtrate was collected and concentrated in a rotary evaporator at 40°C. The concentrated extract was dried under open air and stored under refrigeration until further use. The manufacturing of S. macrophylla ethanol extract nanoparticlesThe S. macrophylla extract was milled using a high-energy ball equipped with an insulating sheath and a cooling machine. The weight ratio of S. macrophylla ethanol extract to the ball (1:20) in stainless steel bottles (50 ml). The container is filled about a third of its capacity. During milling, the flask was rotated at a constant milling speed at 500 rpm for up to 5 hours. The direction of rotation of the ball mill is changed every 30 minutes. The process of ball milling is conducted at a temperature of 27°C and the temperature is maintained with the air conditioning system to prevent overheating. Then the identification particle size of S. macrophylla ethanol extract nanoparticles by dynamic light scattering (DLS) was carried out (Wardani et al., 2022; Kurnijasanti et al., 2023). Experimental of animalIn this experiment, male Wistar rats were used, where the rats used had to meet the requirements, including a body weight of 250–300 g with a rat age of between 2.5 and 3 months. These rats were obtained from the LPPT at Universitas Gajah Mada, Yogyakarta, Indonesia. In this experiment, the rats were put in a plastic cage placed in an air-conditioned room with a temperature maintained at 26°C ± 2°C, in addition, the dark and light cycles were alternated for 12 hours. The rats for this experiment were fed a standard commercial drinking water ad libitum (Wardani et al., 2022). Model of diabetic ratThe rats fasted overnight and then were injected with STZ at a single dose of 55 mg/kg BW intraperitoneal (ip) that was dissolved in citra te buffer (0.1 M; pH 4.5). Three days after STZ injection, blood samples were taken through the lateral vein of the tail and tested for blood glucose levels by the glucometer (Accu-Check, Roche Diagnostics, Pvt., Ltd.). Rats with the level of glucose in >200 mg/dl were used as experimental animals (Kurnijasanti et al., 2023; Wardani et al., 2023). Experimental designThe rats were divided into control, diabetic, and S. macrophylla group. The control group received distilled water; the diabetic group received an intraperitoneal injection of 55 mg/kg STZ; and the S. macrophylla group, rats were injected intraperitoneally with a single dose of STZ at 55 mg/kg BW, and then after 3 days, rats were given S. macrophylla ethanol extract nanoparticles in a dose of 75, 150, 300 mg/kg BW, respectively for 72 days. Blood was collected intracardially from rats to assess glucose, BUN, and creatinine levels. Subsequently, the kidneys were removed to analyze MDA, SOD, and GPx levels. Hematoxylin and eosin staining is an additional method used for histopathological assessment of the kidneys. Measurement of blood glucose levels in diabetic ratsThe ACCU-Check glucometer (made by Roche, Germany) was used to measure blood glucose levels. Biochemical estimation of serum BUN and creatinineErba Semi-auto analyzer by Erba Biochemkit (Transasia Bio-Medical, India) was used to determine BUN and creatinine in serum according to the manufacturer’s instructions. Assessment of MDA in diabetic rat kidney tissuesThe thiobarbituric acid (TBA) method is utilized to measure MDA in kidney tissues, which can assess MDA formation by TBARS assay kit chemical by using a TBARS Assay Kit (Company of Cayman Chemical, USA). The MDA-TBA complex coefficient was measured with absorbance at 532 nm with the reader of the microplate for assessing MDA levels. The MDA levels are expressed in nm/mg tissue (Kurnijasanti et al., 2023; Wardani et al., 2023). Assessment of SOD and GPx in diabetic rat kidney tissueProtein was extracted from the rat kidney to evaluate the SOD enzymatic activity inside the tissue and put through the Bradford technique. SOD inhibition was measured using spectrophotometry at 560 nm to track the drop in nitro blue tetrazolium (Sigma-Aldrich, USA) in each sample. SOD levels are shown as U/mg of protein (Kurnijasanti et al., 2023). The samples were incubated with NaN3 and H2O2 to determine the levels of GPx. The kidney tissue homogenate was treated with 0.1 ml of ethylenediaminetetraacetic acid, 0.2 ml sodium azide, and a mixture of H2O2 and phosphate buffer. At 200 rpm, the mixture was centrifuged while the TCA-stopping agent was added. A combination of DTNB (Sigma-Aldrich, USA) and disodium hydrogen phosphate was introduced to the supernatant. Following color development, the absorbance at 412 nm was measured. The GPx concentration is expressed as U/mg of protein (Kurnijasanti et al., 2023). Histopathological observationsAfter the investigation, all rat kidneys were fixed in 10% buffered formalin and subsequently paraffin-embedded. A 4 μm section of kidney tissue was stained using hematoxylin and eosin. A light microscope was employed for the histopathological examination of the kidneys, focusing on identifying renal cell injuries such as degeneration and necrosis. A scoring system was used to evaluate kidney histopathology as follows: no necrosis=0; score of necrosis=1 (necrosis <25%); score of necrosis=2 (necrosis 26%–50%); score of necrosis=3 (necrosis 51%–75%); score of necrosis=4 (necrosis >75%) (Zaaba et al., 2022). Statistical analysisThrough Statistical Package for the Social Sciences 21, a one-way analysis of variance (ANOVA) test and a Duncan multiple comparison test were used to examine all research data. For this reason, we checked t he normal distribution using the Shapiro–Wilk test and homogeneity using the Levene test. If the data is normally distributed and homogeneous, then data analysis can use the ANOVA test. Data were presented as means ± standard deviation. Ethical approvalRats were used with permission from the University of Hang Tuah Animal Care and Ethics Committee. The study process adhered to the Declaration of Helsinki and the Association for Assessment and Accreditation of Laboratory Animal Care International guidelines. ResultsThe size distribution of S. macrophylla ethanol extract nanoparticlesAccording to a DLS analysis, the distribution of sizes of chitosan nanoparticles in Figure 1 is 91.50 ± 23.06 nm. Effects of S. macrophylla ethanol extract nanoparticles on blood glucose level of diabetic ratsRats given intraperitoneal STZ had a substantial diabetogenic response as compared to the control group, as shown via a rise in blood sugar levels (Table 1). Notably, rats that received oral S. macrophylla ethanol extract nanoparticles at 300 mg/kg showed significantly lower blood glucose levels than the STZ group (p < 0.05).
Fig. 1. Size distribution of S. macrophylla extract nanoparticles. Table 1. Effects of S. macrophylla ethanol extract nanoparticles on blood glucose level of diabetic rats.
Table 2. Effect of S. macrophylla ethanol extract nanoparticles on serum BUN and creatinine levels of diabetic rats.
Table 3. Effect of S. macrophylla ethanol extract nanoparticles on the MDA, SOD, and GPx levels in kidney tissues of diabetic rats.
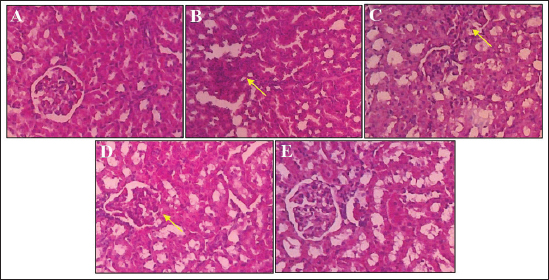
Effect of S. macrophylla ethanol extract nanoparticles on serum BUN and creatinine of diabetic ratsAt the end of the research, serum BUN and creatinine were measured for evaluating kidney function (Table 2). According to the findings, STZ-treated rats’ blood levels of BUN and creatinine were substantially higher than those of control rats (p < 0.05). Though only at a dosage of 300 mg/kg BW did the administration of S. macrophylla ethanol extract nanoparticles substantially lower blood BUN and creatinine levels when compared to the STZ (diabetic) group (p < 0.05) the increase in serum BUN and creatinine levels were suppressed. These results demonstrated that S. macrophylla ethanol extract nanoparticles can inhibit kidney function in diabetic rats. Effect of S. macrophylla ethanol extract nanoparticles on kidney tissue MDA, SOD, and GPx level of diabetic ratsThe most used ROS indicator of oxidative stress in kidney cell injury is MDA. Table 3 displays the experimental MDA kidney tissue level. MDA levels in kidney tissue were substantially greater in STZ-induced diabetic animals than in control rats (p < 0.05). Compared to diabetic rats, administering S. macrophylla ethanol extract nanoparticles considerably decreased the MDA level in renal tissue, especially at a dose of 300 mg/kg BW (p < 0.05). The kidney tissue SOD and GPx levels are shown in Table 3. At the forefront of the cellular defense against oxidative damage, SOD and GPx function as scavenger enzymes for ROS, neutralizing both superoxide and hydrogen peroxide before they combine and generate more harmful hydroxyl radicals. When compared to control rats, animals who received STZ had considerably reduced levels of SOD and GPx in their kidney tissue (p < 0.05). Nevertheless, SOD and GPx levels increased in a dose-dependent manner in rats given S. macrophylla extract nanoparticle treatment. In comparison to rats administered STZ, treatment with S. macrophylla extract nanoparticles significantly increased levels of SOD and GPx, particularly at doses of 300 mg/kg BW (p < 0.05). Effect of S. macrophylla ethanol extract nanoparticles on kidney tissue histopathological of diabetic ratsWe conducted a histopathological examination to prove the nephroprotective effect of S. macrophylla extract nanoparticles on diabetic rats (Table 4 and Figure 2). The kidney tissue histopathological of control rats showed a normal tubules and glomeruli. However, the kidney tissue histopathological of diabetic rat showed glomeruli and tubule necrosis. When compared to STZ-treated (diabetic) rats, the S. macrophylla extract nanoparticles only displayed almost normal glomeruli and tubules at a dosage of 300 mg/kg BW (p < 0.05). Table 4. Effect of S. macrophylla ethanol extract nanoparticles on histological necrosis of diabetic rats kidney cell.
Central Most of the medicinal agents originate from nature and are considered a rich source for producing drugs including modern drugs. Swietenia machrophylla is one of the medicinally important plants of plants indigenous to tropical and subtropical regions of the World. Swietenia macrophylla has been widely used in folk medicine to treat various diseases. Its chemical molecules like limonoids, phenolics, catechin, swietenine, swietenolide (a bitter compound), 8,30-epoxy-swietenine acetate, swietenolide diacetate, augustineolide, and 3β,6-dihydroxydihydrocarapin are the major constituents of S. macrophylla. There are several data in the literature indicating a great variety of pharmacological activities of S. macrophylla to possess antimicrobial, anti-inflammatory, anticancer, antidiarrhoeal, anti-infective, antiviral, antimalarial, antioxidant effects, and antidiabetic activities. In the current work, we looked at S. macrophylla’s capacity to protect kidney cells from damage brought on by STZ. According to this research, STZ may harm the beta cells in the islets of Langerhans, which would prevent the islets from producing insulin and producing high blood sugar levels. However, S. macrophylla ethanol extracts nanoparticle therapy lowered glucose levels. Swietenia macrophylla ethanolic extracts have anti-diabetic activities comparable to the synthetic drug and observed no to relatively mild toxic effect. The hypoglycemic mechanism suggested via reducing blood glucose level, restoring liver and β-cells islet function (might) blocking epinephrine function, and inhibiting of α-amylase and β-glucosidase (Wresdiyati et al., 2015).
Fig. 2. Images of rat kidney tissue under a microscope. (a) Hematoxylin and eosin staining revealed that the renal tubules and glomeruli of the control rat were normal. (b) The diabetic rat (black arrow) had necrosis of glomeruli and tubule cells. (c and d) Rats treated with S. macrophylla extract nanoparticles still exhibited considerable necrotic at dosages of 75 mg/kg BW and 150 mg/kg BW; (e) however, at doses of 300 mg/kg BW, there is an improvement in histoarchitecture comparable to that of the diabetic rats. H&E, 400×. In our study, STZ treatment dramatically raised MDA levels while lowering SOD and GPx levels, which in turn raised blood BUN and creatinine levels. This indicates impaired function and damage to kidney cells, as previously reported by Wardani et al. (2022). STZ is commonly used in experiments to induce diabetes in model animals. STZ stimulates the generation of ROS, which is crucial to the development and progression of hyperglycemia problems, according to in vitro and in vivo studies. It is well documented that the overproduction of free radicals in persistent hyperglycemia conditions is a significant targeting factor for generating/activating all pathways involved in the diabetic mellitus complication pathogenesis. Hyperglycemia not only boosts ROS production but also attenuates antioxidative mechanisms via the glycosylation of antioxidative enzymes presented as previously reported. The ROS overproduction causes lipid peroxidation of the cell membrane, DNA damage, and protein denaturation, resulting in diabetic kidney cell damage which causes an increment in serum BUN and creatinine. Increased ROS can also lead to MDA level elevation and reduced the antioxidant enzyme system such as SOD, GPx, and catalase. As mentioned before, administration of STZ induces oxidative stress, and ROS attacks tubules and glomerulus cells, and this may cause destruction in the structure, and inhibit the function of the kidney. Various kinds of research suggest that MDA and SOD are closely related to kidney cell damage in diabetes. Oxidative stress is an imbalance condition between the ROS and cellular anti-oxidative capability resulting induces the dysregulation of an endogenous antioxidant system. Thus, amelioration of imbalanced conditions by scavenging ROS and boosting the endogenous antioxidant system create a difference in the pathology of disease. Excess generation of ROS starts the disruption of nuclear factor erythroid-2 related factor-2 (Nrf2)/Kelch-like ECH associated protein-1 (KEAP1) complex that contributes to the activation of Nrf2. Nrf2 plays an important role in the regulation of antioxidant gene (SOD, GPX, and catalase) and cytoprotective triggered via oxidative stress. Additionally, STZ is susceptible to glycation of scavenging enzymes like SOD and GPX, which results in ROS overproduction and oxidative stress-related kidney cell injury (Abouzeda et al., 2020; Abtahi-Eivari et al., 2021; Guo et al., 2021; Wardani et al., 2022). This also supported, our recent finding demonstrating that STZ reduced the Kelch-like erythroid-associated factor 2 [Nrf2/protein 1 (KEAP1)] pathway which is known to have a role important to increase antioxidant enzymes such as SOD, GPx, and catalase (Wardani et al., 2022). Oxidative stress plays an important role in the expansion of diabetic nephropathy and supports the antioxidant theory contributes a significant role in the improvement of diabetes condition and related complications. In recent years, herbal antioxidant medicines have been investigated for their utility in the protection of diabetic complications such as diabetic nephropathy. One of the strong antioxidants herbal is S. macrophylla. Using the 1,1-diphenyl-2-picrylhydrazyl radical scavenging experiment, the antioxidant activity of S. macrophylla extract was examined, and it demonstrated a great antioxidant effect (Yudhani et al., 2021; Borah et al., 2022; Kurnijasanti et al., 2023). Herbal nanotechnology can be used to increase the therapeutic value by reducing toxicity and increasing bioavailability. The results of this study showed the size of S. macrophylla extract nanoparticles is 91.50 ± 23.06 nm. In the current experimental study, we have observed that S. macrophylla ethanol extract nanoparticles pretreatment can reduce BUN, creatinine, and MDA levels. Also elevated SOD, GPx which can inhibit kidney cell damage. The presence of limonoids, catechin, swietenine, and phenolic compounds in S. macrophylla extract nanoparticles, which act as antioxidants by scavenging free radicals such as ROS and shielding cells from oxidative processes, has been demonstrated. This action may contribute to the reduction of MDA levels. Additionally, S. macrophylla extract nanoparticles enhance the expression of Nrf2, a regulator and a critical factor in activating genes primarily responsible for producing antioxidant enzymes, including SOD, GPx, and catalase. The study’s results also indicated that STZ affects renal function in rats, as evidenced by significantly higher levels of BUN and creatinine than in the control group. Conversely, administering S. macrophylla ethanol extract nanoparticles to STZ-induced diabetic rats led to a dose-dependent reduction in BUN and creatinine levels. The antioxidant effect of S. macrophylla ethanol extract nanoparticle is able to remove ROS which has an important role in increasing BUN and creatinine serum levels. This suggests that S. macrophylla extract nanoparticle has the ability to inhibit kidney cell damage caused by STZ. The proximal tubule, distal tubule, and normal glomeruli were visible in photomicrographs of the control rat’s kidney slice. The kidney section in rats treated with STZ showed glomeruli and tubule necrosis. However, administration of S. macrophylla ethanol extract nanoparticle only at a dose of 300 mg/kg BW inhibited kidney changes of both glomeruli and tubules, which were able to repair these alterations to nearly normal histological features when compared with STZ (diabetic) rats. The nanoparticles of S. macrophylla ethanol extract include a phenolic substance proven to be an antioxidant by scavenging free radicals. This can decrease MDA, and increase SOD and GPx is one of the possible mechanisms to protect the kidney cell damage due to the oxidative stress produced by STZ (Borah et al., 2022; Kurnijasanti et al., 2023). Studies have indicated that limonoids, catechin, swietenine, and phenolic compounds enhance the tissue architecture of the kidneys, boost antioxidant enzyme activity, decrease lipid peroxidation, and scavenge free radicals, thereby preventing STZ-induced damage to kidney cells. ConclusionBiochemical and histopathological results of the present study showed that S. macrophylla ethanol extract nanoparticle possesses antioxidant properties and protects against STZ-induced kidney cell damage. The protective mechanism of S. macrophylla ethanol extract nanoparticle is through its ability to decrease MDA levels and to increase SOD and GPx levels. To avoid diabetes problems such as retinopathy, hepatopathy, neuropathy, atherosclerosis, and nephropathy, it is envisaged that S. macrophylla ethanol extract nanoparticle may be used. AcknowledgmentThe author thanks Airlangga University for supporting this work. Author contributionsWG, SAS, and MRM conceptualization. WG and RK contributed to the animal study. WG analyzed data and draft preparation. SAS and MRM writing review and editing. All authors have read and agreed to the published version of the manuscript. Conflict of interestAll authors declare that there is no conflict of interest. FundingThis research was funded by Airlangga University, Surabaya, Indonesia, through the Mandat Research Grant number 1408/UN3/2019. Data availabilityThe datasets used and analyzed during this study are included in the article. ReferencesAbouzeda, T.K., Sade kb, K.M. and Ghazyc, E.W. 2020. Black mulberry fruit extract alleviates streptozotocin-induced diabetic nephropathy in rats: targeting TNF-α inflammatory pathway. J. Pharm. Pharmacol. 72, 1615–1628. Abtahi-Eivari, S., Shokoohi, M., Ghorbani, M., Halimi, M., Hajizadeh, H., Pourlak, T., Bahrami, J. and Ghoreishi Z. 2021. Effects of hydroalcoholic extracts of cloves (Syzygium aromaticum) on the serum biomarkers, antioxidant status, and histopathological changes of kidneys in diabetic rats. Crescent J. Med. Biol. Sci. 8(4), 269–275. Asmat, U., Abad, K. and Ismail, K.K. 2016. Diabetes mellitus and oxidative stress—a concise review. Saudi Pharm. J. 24(5), 547–553. Bigagli, M. and Lodovici, M. 2019. Circulating oxidative stress biomarkers in clinical studies on type 2 diabetes and its complications. Oxid. Med. Cell. Longev. 2019, 5953685. Borah, A., Selvaraj, S., Holla, S.R. and De, S. 2022. Extraction and characterization of total phenolic and flavonoid contents from bark Swietenia macrophylla and their antimicrobial and antioxidant properties. Arab. J. Chem. 15, 104370. Farrag, A.R., Nassar, M. and El-Khayat, Z. 2019. Heteroxenia ghardaqensis extract protects against DNA damage in streptozotocin-induced experimental diabetes. Biomed. Pharmacol. J. 12(1), 24–39. Guo, L., Jiang, B., Li, D. and Xiao X. 2021. Nephroprotective effect of adropinin against streptozotocin-induced diabetic nephropathy in rats: inflammatory mechanism and YAP/TAZ factor. Drug Des. Dev. 15, 589–600. Ighodaro, O.M. 2019. Molecular pathways associated with oxidative stress in diabetes mellitus. Biomed. Pharmacother. 10, 656–662. Kurnijasanti, R., Wardani, G., Mustafa, M.R. and Sudjarwo, S.A. 2023. Protective mechanism pathway of Swietenia macrophylla extract nanoparticles against cardiac cell damage in diabetic rats. Pharmaceuticals 16, 973. Oguntibeju, O.O. 2016. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 11(3), 45–63. Sahu, T., Ratre, Y.K., Chauhan, S., Bhaskar, L.V.K.S., Nair, M.P. and Verma, H.K. 2021. Nanotechnology based drug delivery system: current strategies and emerging therapeutic potential for medical science. J. Drug. Deliv. Sci. Technol. 63, 102487. Sim, S. and Wong, N.K. 2021. Nanotechnology and its use in imaging and drugs delivery. Biomed. Rep. 14, 42–48. Volpe, C.M.O., Villar-Delfino, P.H. and dos Anjos, P.M.F. 2018. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell. Death Dis. 9(2), 119–131. Wardani, G., Nugraha, J., Mustafa, M.R. and Sudjarwo, S.A. 2022. Antioxidative stress and ant-inflamatory activity of fucoidan nanoparticles against nephropathy of streptozotocin-induced diabetes in rats. Evid. Based Complement. Altern. Med. 2022, 1–10. Wardani, G., Nugraha J., Kurnijasanti, R., Mustafa R.M. and Sudjarwo, S.A. 2023. Molecular mechanism of fucoidan nanoparticles as protector on endothelial cell dysfunction in diabetic rats’ aortas. Nutrients 15, 568–581. Wresdiyati, T., Sa’diah, S., Winarto, A. and Febriyani, V. 2015. Alpha-glucosidase inhibition and hypoglycemic activities of Swietenia mahagoni seed extract. Hayati J. Biosci. 22(2), 73–78. Yudhani, R.D., Nugrahaningsih, D.A.A., Sholikhak, E.N. and Mustofa, M. 2021 The molecular mechanisms of hypoglycemic properties and safety profiles of Swietenia macrophylla seeds extract: a review. Open Access Maced. J. Med. Sci. 12, 370–378. Zaaba, N.E., Beegam, S., Elzaki, O., Yasin, J., Nemmar, B.M., Ali, B.H., Adeghate, E. and Nemmar, A. 2022. The nephroprotective effects of bisabolol in cisplatin-induced acute kidney injury in mice. Biomedicines 2022, 10, 842–856. Zhang, P., Li, T., Wu, X., Nice, E.D., Huang, C. and Zhang, Y. 2020. Oxidative stress and diabetes: antioxidative strategies. Front. Med. 14, 583–600. | ||
| How to Cite this Article |
| Pubmed Style Kurnijasanti R, Wardani G, Mustafa MR, Sudjarwo SA. Protecting mechanism of Swietenia macrophylla ethanol extract nanoparticle on Streptozotocin induced renal damage in rat. Open Vet J. 2023; 13(12): 1623-1630. doi:10.5455/OVJ.2023.v13.i12.12 Web Style Kurnijasanti R, Wardani G, Mustafa MR, Sudjarwo SA. Protecting mechanism of Swietenia macrophylla ethanol extract nanoparticle on Streptozotocin induced renal damage in rat. https://www.openveterinaryjournal.com/?mno=167742 [Access: May 12, 2024]. doi:10.5455/OVJ.2023.v13.i12.12 AMA (American Medical Association) Style Kurnijasanti R, Wardani G, Mustafa MR, Sudjarwo SA. Protecting mechanism of Swietenia macrophylla ethanol extract nanoparticle on Streptozotocin induced renal damage in rat. Open Vet J. 2023; 13(12): 1623-1630. doi:10.5455/OVJ.2023.v13.i12.12 Vancouver/ICMJE Style Kurnijasanti R, Wardani G, Mustafa MR, Sudjarwo SA. Protecting mechanism of Swietenia macrophylla ethanol extract nanoparticle on Streptozotocin induced renal damage in rat. Open Vet J. (2023), [cited May 12, 2024]; 13(12): 1623-1630. doi:10.5455/OVJ.2023.v13.i12.12 Harvard Style Kurnijasanti, R., Wardani, . G., Mustafa, . M. R. & Sudjarwo, . S. A. (2023) Protecting mechanism of Swietenia macrophylla ethanol extract nanoparticle on Streptozotocin induced renal damage in rat. Open Vet J, 13 (12), 1623-1630. doi:10.5455/OVJ.2023.v13.i12.12 Turabian Style Kurnijasanti, Rochmah, Giftania Wardani, Mohammad Rais Mustafa, and Sri Agus Sudjarwo. 2023. Protecting mechanism of Swietenia macrophylla ethanol extract nanoparticle on Streptozotocin induced renal damage in rat. Open Veterinary Journal, 13 (12), 1623-1630. doi:10.5455/OVJ.2023.v13.i12.12 Chicago Style Kurnijasanti, Rochmah, Giftania Wardani, Mohammad Rais Mustafa, and Sri Agus Sudjarwo. "Protecting mechanism of Swietenia macrophylla ethanol extract nanoparticle on Streptozotocin induced renal damage in rat." Open Veterinary Journal 13 (2023), 1623-1630. doi:10.5455/OVJ.2023.v13.i12.12 MLA (The Modern Language Association) Style Kurnijasanti, Rochmah, Giftania Wardani, Mohammad Rais Mustafa, and Sri Agus Sudjarwo. "Protecting mechanism of Swietenia macrophylla ethanol extract nanoparticle on Streptozotocin induced renal damage in rat." Open Veterinary Journal 13.12 (2023), 1623-1630. Print. doi:10.5455/OVJ.2023.v13.i12.12 APA (American Psychological Association) Style Kurnijasanti, R., Wardani, . G., Mustafa, . M. R. & Sudjarwo, . S. A. (2023) Protecting mechanism of Swietenia macrophylla ethanol extract nanoparticle on Streptozotocin induced renal damage in rat. Open Veterinary Journal, 13 (12), 1623-1630. doi:10.5455/OVJ.2023.v13.i12.12 |