| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 428-437 Original Research The corrective role of superparamagnetic iron oxide nanoparticles for the genes controlling hypothalamus-pituitary-testis-axis in male obesity-associated secondary hypogonadismHanaa Refaat1*, Mohamed F. Dowidar2, Amany I. Ahmed2, Tarek Khamis3, Shehab E. Abdelhaleem4 and Samar A. Abdo21Department of Biochemistry, Faculty of Pharmacy, Zagazig University, Zagazig, Egypt 2Department of Biochemistry, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 3Department of Pharmacology and Laboratory of Biotechnology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 4Department of Medical Microbiology and Immunology, Military Medical Academy, Cairo, Egypt *Corresponding Author: Hanaa Refaat. Department of Biochemistry, Faculty of Pharmacy, Zagazig University, Zagazig, Egypt. Email: h.7mmad4288 [at] gmail.com Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
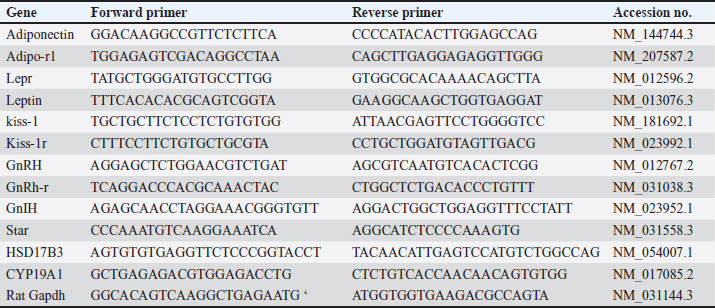
AbstractBackground: Obesity is one of the most prevalent and perilous health affairs. Male obesity-associated secondary hypogonadism (MOSH) is one of many of its complexities, which is mounting in parallel with the aggravation of obesity. Magnetic nanoparticles seem to be an advanced favorable trend in multiple biomedical fields. Aim: In this study, we explore the therapeutic effects of superparamagnetic iron oxide nanoparticles (SPIONs) coated with carboxymethyl cellulose (CMC) on an obese male rat model with MOSH syndrome, comparing their impacts with a well-known anti-obesity medication (Orlistat). Methods: 42 male albino rats split into 7 equal groups: 1-negative control: nonobese, untreated; 35 rats fed the high fat-high fructose (HFHF) diet for a period of 12 weeks. Obese rats splitted into 6 equal groups; 2-positive control: obese untreated; 3-obese given Orlistat (30 mg/kg); 4-obese given CMC-SPIONs (25 mgFe/kg); 5-obese given CMC-SPIONs (50 mgFe/kg); 6-obese given CMC-SPIONs(25 mgFe/kg) + Orlistat (30 mg/kg), 7-obese given CMC-SPIONs (50 mgFe/kg) + Orlistat (30 mg/kg); all treatments given orally for 4 weeks. During sacrifice, blood serum and sectioned hypothalamic, pituitary, testicular, and adipose tissues were collected for biochemical and biomolecular assessments. Results: The HFHF diet for 12 weeks resulted in a significant upsurge in body weight, body mass index, serum fasting glucose, insulin resistance, TAG, total cholesterol, and LDL-c; HDL-c was dropped. Serum FSH, LH, and testosterone values declined. A significant disorder in expression levels of genes regulating the hypothalamic-pituitary-testicular-axis pathway. Hypothalamic GnRH, Kisspeptin-1, Kisspeptin-r1, and Adipo-R1 values declined. GnIH and Leptin-R1 values raised up. Pituitary GnRH-R values declined. Testicular tissue STAR, HSD17B3, and CYP19A1 values declined. Adipose tissue adiponectin declined, while leptin raised up. CMC-SPIONs 25–50 mg could modulate the deranged biochemical parameters and correct the deranged expression levels of all previous genes. Co-treatments revealed highly synergistic effects on all parameters. Overall, CMC-SPIONs have significant efficiency whether alone or with Orlisat in limiting obesity and consequence subfertility. Conclusion: CMC-SPIONs act as an incoming promising contender for obesity and MOSH disorders management, and need more studies on their mechanisms. Keywords: GnRH, Leptin, Aromatase, HPT-axis, Superparamagnetic iron oxide nanoparticles. IntroductionObesity is a chronic condition associated with metabolic syndrome. Metabolic syndrome is characterized by truncal obesity, dyslipidemia, insulin resistance, endothelial dysfunction, or thrombosis, aggravated by physical inactivity, age, genetic factors, and high caloric diet. These often result in testicular dysfunction and male fertility impairment (Cignarelli et al., 2018). This disorder termed as male obesity-associated secondary hypogonadism (MOSH); a condition characterized by morbidness of hypothalamic-pituitary-testicular (HPT) axis, and testosterone blood levels are diminished, followed by symptoms of subfertility, such as diminution in erectile function, libido, and semen quality (Cignarelli et al., 2021). The convoluted organization of the HPT-axis makes it liable to flaws by acquired or genetic etiologies such as obesity, leading to variable grades of secondary hypogonadism (Genchi et al., 2022). Now, we will survey the significance of genes that are expressed in hypothalamic, pituitary, and testicular tissues and their roles in reproductive processes. At the hypothalamic level, leptin is the key mediator of energy metabolism and neuro-endocrine reproductive hormones signaling via leptin receptors (LepRs) activation. LepR is expressed highly in arcuate nucleus (ARC) neurons of the hypothalamus, where they are partially co-placed with kisspeptin, one of the most dynamic controllers of the HPT-axis (George et al., 2010). It is approved that the Kisspeptin/Kiss receptor1-GnRH/GnIH scheme works significantly in the central regulation of gonadotropins. Kisspeptin neurons release kisspeptin peptides to bind with Kiss-r1 on Gonadotropin Releasing Hormone (GnRH) neurons to promote GnRH release (Pinilla et al., 2012). Not only the GnRH but Gonadotropin Inhibiting Hormone (GnIH) is also included as a neuro-regulator of the HPT-axis. GnIH-R is situated on GnRH-1 neurons, at which GnIH acts directly and suppresses GnRH and gonadotropins release (Li et al., 2012). The standard ratio of GnRH/GnIH performs a vital physiological role in gonadotropins and androgen homeostasis. It has been demonstrated that the ratio of GnRH/GnIH in obese rats' serum was considerably less than the ratio in healthy ones, emphasizing that the stimulating effect of gonadotropin release in obese rats was suppressed (Jia et al., 2017). The gene expression of adiponectin and its receptors in the hypothalamus and pituitary is clear in humans and diverse species, proposing its significance in regulating the gene expressions and synthesis of kisspeptins, GnRH, and gonadotropins of HPT-axis (Guillod-Maximin et al., 2009). It is predominant in cerebrospinal fluid, which points to its paracrine or autocrine impacts on a hypothalamic-pituitary pivot (Kusminski et al., 2007). Thus, its defect in obesity leads to suppression in FSH and LH release (Cheng et al., 2016). At the pituitary level, the gonadotropin-releasing hormone receptor (GnRH-R) is one of the G-protein coupled receptor members (Conn et al., 2006). GnRH-R rates determine the rate-limiting factor of gonadotropin actions. Actually, appropriate levels of the receptor are required for the secretion and release of gonadotropins FSH and LH (Bedecarrats and Kaiser, 2003). At the testicular level, the equilibrium of androgens with estrogens is crucial to keep the normal reproductive function in males. The aromatase enzyme is expressed by (CYP19A1) gene in testicular somatic and germ cells (Xu et al., 2017). It regulates the transformation of androgens into estrogens. (STAR) gene normalizes the transport of cholesterol into the inner membrane of mitochondria, where it is transformed into pregnenolone which is the prime rate-limiting pace in androgen biosynthesis. Studies have demonstrated that a high-fat regime could accumulate lipids in Leydig cells in mice, inducing mitochondrial flaws and down-regulating STAR expression (Su et al., 2019). (HSD17B3) gene is expressed particularly in Leydig cells, which catalyzes the reduction of androstenedione into testosterone, the ending step in testosterone biosynthesis (Baker et al., 1997). Superparamagnetic iron oxide nanoparticles (SPIONs) have caught up great attention in medical applications. Their sensitivity to any magnetic field makes SPIONs exceptional. SPIONs are made up of a magnetic iron oxide core which is usually surface-modified via enveloping with a hydrophilic, biocompatible polymer (Dulińska-Litewka et al., 2019). The coating step of SPIONs is highly essential as it reduces nanoparticle aggregation, and modulates their dispersibility and colloidal stability. It avoids their surface oxidation. It prolongs blood circulation time. SPIONs become more biocompatible with less nonspecific interactions and toxicity (Wahajuddin and Arora, 2012). Materials and MethodsExperimental animals housingForty-two male adult albino rats were used, obtained from Central Animal House at the Faculty of Veterinary Medicine, Zagazig University, Egypt. After weighing (120–160 g), rats were adapted in standard cages of six rats each for 7 days before work to avert transport stress, under normal laboratory circumstances, reasonable temperature of (25°C ± 2°C), good ventilation, 12-hour light/dark cycle, and with availability for chow and pure water in all experiment phases. SupplementsCMC-coated SPIONsMagnetite (Fe3O4) is a type of magnetic functional nanomaterial; CMC-Fe3O4 was synthesized by covalent binding CMC with PEI- Fe3O4 (Polat and topel, 2019). TEM was preceded on JOEL JEM-2100 high resolution of transmission electron microscope at an accelerated voltage (200 kV). CMC-Fe3O4 is sized (15 ± 5 nm) in diameter, spherically shaped, crystalline structured, and highly dispersed in water. CMC-SPIONs brought from NanoTech Egypt for PhotoElectronics. OrlistatXenicalR is the brand name of the orlistat capsule from (Roche Pharmaceuticals) purchased from a local pharmacy, and dissolved in dimethyl sulfoxide (Gomaa et al., 2019) to be administered via gastric gavage tubes. Obesity inductionRats were fed on the obesogenic regime for 12 weeks, which included (per 100 g): 30 g of protein (300 cal), 26.5 g of fat (195 cal lard, 70 cal corn oil), 36.5 g of carbohydrate (106 cal corn starch and 105 cal dextran), and 17 g fructose (de Castro et al., 2013). Achievement of HFHF diet-induced obesity was proved by recording the body weight (BW) and body mass index (BMI) variations at the beginning and end of the experiment. Experimental designRats were grouped into: (1) control group: 6 healthy male rats. After obesity, a period of 36 obese male rats were split into six equal groups, according to the given treatment. (2) Positive control: obese untreated, (3) Orlistat-treated obese group: given Orlistat solution orally with dose (30 mg/kg/ml) (Gomaa et al., 2019), (4) CMC-SPIONs-treated obese group: given CMC-SPIONs suspension orally with dose (25 mg Fe/kg/ml) (Ali et al., 2020), (5) CMC-SPIONs-treated obese group: given CMC-SPIONs suspension orally with dose (50 mg Fe/kg/ml) (Ali et al., 2020), (6) CMC-SPIONs + Orlistat treated obese group: given mixture of CMC-SPIONs (25 mg Fe/kg/ml) and Orlistat (30 mg/kg/ml), and (7) CMC-SPIONs + Orlistat treated obese group: given mixture of CMC-SPIONs (50 mg Fe/kg/ml) and Orlistat (30 mg/kg/ml). All treatments have been given daily and lasted for 4 weeks. Collection of samplesAfter 4 weeks of treatments, rats refrained from diet for a night. Then they were anesthetized using ketamine (75 mg/kg) and xylazine (10 mg/kg) injected intraperitoneally to be sacrificed. Blood samples from retro-orbital veins packed in anticoagulant-free tubes, are to be centrifugated at 3,000 rpm/15 minute. The obtained serum samples were forwarded for biochemical assessment. Also, the hypothalamic, pituitary, testicular, and adipose tissues were sectioned and directly frozen in liquid nitrogen, stored at (−80°C) for extracting the total RNA for (qRT-PCR) examination. Biochemical analysisSerum fasting glucose assayed by glucose oxidase technique using (Glucose-LQ kit, Spinreact) (El-Gayar et al., 2012). Insulin in serum was assayed via rat insulin ELISA kit (Biovendor Laboratory Medicine, Brno, Czech Republic), according to the manufacturer’s procedures. Homeostatic model assessment of insulin resistance (HOMA-IR) is calculated via this equation: HOMA-IR=fasting blood glucose (mg/dl) × fasting insulin (ng/ml)/405 (Roza et al. , 2016). Total cholesterol (TC) was assessed by the CHOD-PAP-enzymatic colorimetric method (Penttilä et al., 1981), and triglycerides (TG) by the GPO-PAP-enzymatic colorimetric method (Bucolo and David, 1973). Serum HDL-c and LDL-c were assessed by direct enzymatic colorimetric liquid method (Friedman and Young, 1989), and the lipid parameters were measured using commercially accessible kits (Spinreact, Spain). Serum testosterone was assessed by Rat Testosterone ELISA, Kit Cat.No.SE120089 (Sigma Aldrich, St Louis, MO, USA). Serum FSH and LH were assessed by Rat FSH ELISA Kit Cat. No. CSB-E06869r, and Rat LH ELISA Kit Cat.No.CSB-E12654r respectively, from CUSABIO. Quantitative real-time PCR analysisExtracting the total RNA from all isolated tissues was done by using Trizol (Invitrogen; Thermo Fisher Scientific, Inc.). Quantifying RNA concentrations was done through a Nanodrop using the NanoDrop® ND-1000 Spectrophotometer (NanoDrop Technologies; Wilmington, Delaware, United States). Synthesis of cDNA was by using a High-Capacity cDNA Reverse Transcription Kit cDNA Kit (Applied Biosystems™, USA). All primers are prepared following the manufacturer’s instructions. Real-time RT-PCR was accomplished in the M × 3005P Real-Time PCR System (Agilent Stratagene, USA) by means of TOPreal™ qPCR 2 × PreMIX (SYBR Green with low ROX) (Cat.#P725or P750) (Enzynomics, Korea) following manufacturer's directions. Expression levels of the investigated assayed genes were normalized via mRNA expression of B-actin (identified housekeeping gene). The outcomes were expressed as fold-changes compared with the control group according to the 2-ΔΔCT method (Livak and Schmittgen, 2001). The primer sequences that have been used are listed in Table 1. Table 1. The primer sequences for RT-PCR (Khamis et al., 2020).
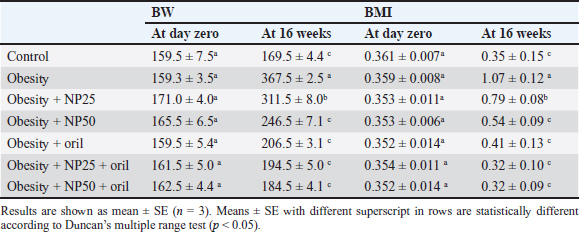
Statistical analysisThe results have been expressed as a mean ± SE (standard error of mean). The impact of the five groups of treatment on the parameters was valued by using a one-way analysis of variance (ANOVA) with Duncan multiple tests serving as a post hoc test. A value of p < 0.05 was used to point to the statistical significance. The graph pad prism version 8.0.2 (GraphPad Software, Inc) and SPSS version 28.0 (IBM Corp., NY, USA) were used for all statistical analysis and charts. Ethical approvalThe search protocol has been revised and approved by the Institutional Animal Care and Use Committee, Zagazig University, Egypt, with an approval number (ZU-IACUC/2/F/289/2023). ResultsEffect of CMC-SPIONs (25 mg) and (50 mg) or/and Orlistat on BW and BMI in obese male ratsTable 2 shows the initial BW and BMI of all rats were not significantly variable. The results presented that final BW and BMI in the obese group statistically significantly increased compared with the control group. A statistically significant decline was observed in the CMC-SPIONs (50 mg)-treated group values, while the CMC-SPIONs (25 mg)-treated group recorded nonsignificant values in comparison with the obese group. The co-treatment groups showed the highest significant results in comparison to the obese group at (p < 0.05). Table 2. Effects of CMC-SPIONs (25 mg), CMC-SPIONs (50 mg) and/or orlistat on BW and BMI in obese male rats.
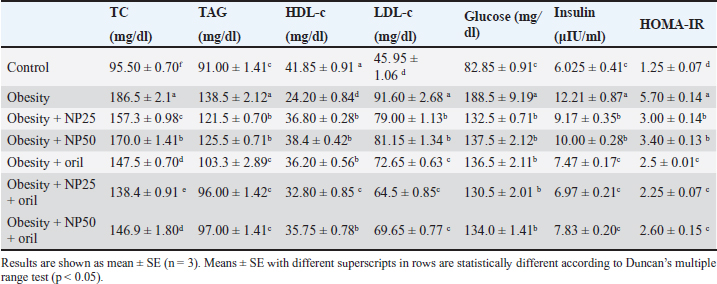
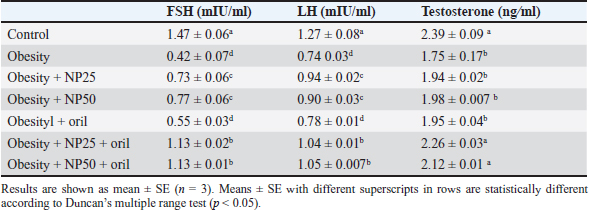
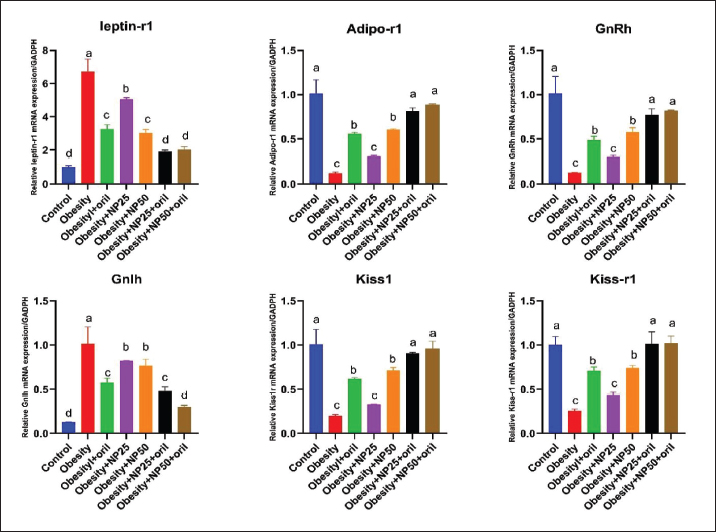
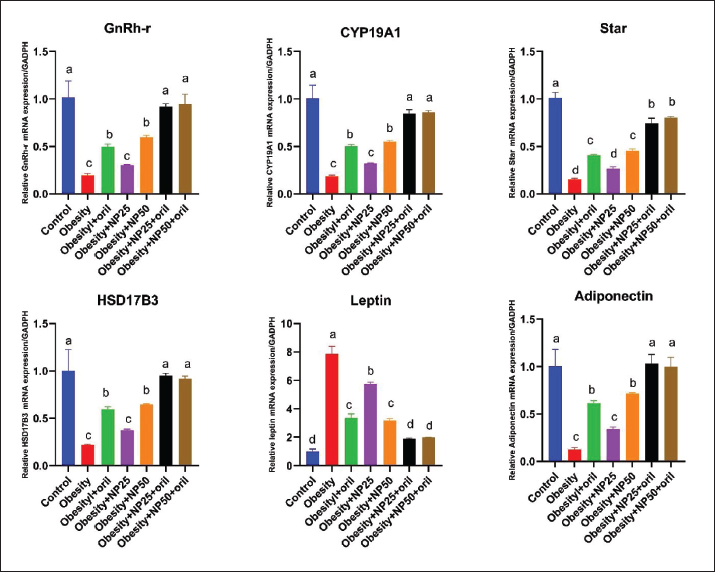
Effect of CMC-SPIONs (25 mg) and (50 mg) and/or Orlistat on serum lipid profile, glycemic index, and HOMA-IR in obese male ratsTable 3 displays a statistically significant elevation in serum values of TC, TAG, and LDL-c and a decline in HDL-c in the obese group compared with the control group. These results were reversed in CMC-SPIONs (25 mg) and (50 mg)-treated groups. The combined treated groups showed high significant values in comparison to the obese group at (p < 0.05). As exhibited in Table 3: the values of serum glucose, insulin, and HOMA-IR were highly significantly elevated in the obese group if compared to the normal group. These values were lowered in all treated groups compared to the obese group; however, all treated groups showed convergent significant results compared to the obese group at (p < 0.05). Effect of CMC-SPIONs (25 mg) and (50 mg) and/or Orlistat on serum FSH, LH, and testosterone levels in obese male ratsTable 4 exhibits the values of serum FSH, LH and testosterone in the obese group were statistically significantly declined compared to the control group. The results of CMC-SPIONs (25 mg), (50 mg), and Orlistat were not significantly variable. However, the combined-treated groups showed highly significant results in comparison to the obese group at (p < 0.05). Effect of CMC-SPIONs (25 mg) and (50 mg) and/or Orlistat on mRNA expression levels of hypothalamic (LepR1, AdipoR1, GnRH, GnIH, Kiss-1, and Kiss-r1), pituitary (GnRH-R), testicular (STAR, HSD17B3, and CYP19A1) and adipose (Leptin and Adiponectin) in obese male ratsRT-PCR revealed that the obese group exhibited statistically significant down-regulating mRNA levels of hypothalamic GnRH, Kiss-1, Kiss-r1, and Adipo-R1, while significant up-regulation in mRNA levels of GnIH and Leptin-R1 (Fig. 1). Hypophyseal GnRH-R was significantly down-regulated. Expression levels of STAR, HSD17B3, and CYP19A1 in testicular tissue were significantly down-regulated. In adipose tissue, mRNA levels of leptin were upregulated, while adiponectin was down-regulated in obese rats if compared to the normal group (Fig. 2). In contrast, mRNA levels of these genes were significantly reversed in CMC-SPIONs (25 mg) and (50 mg)-treated groups. However, the combined treated groups gave the highest statistically significant results in comparison to the obese group at (p < 0.05). Table 3. Effects of CMC-SPIONs (25 mg), CMC-SPIONs (50 mg), and/or orlistat on serum lipid profile, glycemic index, and HOMA-IR levels in obese male rats.
Table 4. Effects of CMC-SPIONs (25 mg), CMC-SPIONs (50 mg), and/or orlistat on serum FSH, LH, and testosterone levels in obese male rats.
DiscussionThe current study revealed for the first time the significant potential of CMC-SPIONs in alleviating MOSH disorder in an HFHF diet rat model. This impact may be mediated by central modulation of the disturbed expression of genes regulating the HPT-axis pathway, as well as leptin and adiponectin of adipose tissue. Our HFHF-obese rat model is considered as a traditional progress of obesity and its related complexities as rats gain more than double their initial weight. Hyperlipidemia, hyperglycemia, and insulin resistance were developed. As mentioned previously, visceral obesity is highly associated with insulin resistance and type-2 diabetes (Kapoor et al., 2008; Kelsey et al., 2016), or accompanied by exaggerated insulin resistance (Yan et al., 2015). A remarkable decline in serum levels of FSH, LH, and testosterone. Consistent studies revealed that a high-fat diet diminished serum and testicular levels of FSH, LH, and testosterone (Mody et al., 2015; Suleiman et al., 2020). A compatible study revealed diminished LH, testosterone, and sperm amount in obese C57BL/6J mice which are involved in the regulatory mechanisms at neuroendocrine levels (Lainez and Coss, 2019). In the current study, CMC-SPIONs could ably relieve the extent of glucose intolerance, insulin resistance, and hyperlipidemia, in addition, SPIONs could elevate the declined levels of circulating reproductive hormones; FSH, LH, and testosterone, particularly in co-treatment with Orlistat. In accordance with a previous study that detected the anti-obesogenic impacts of SPIONs through activation of hepatic (PGC-1α), adiponectin and correcting lipid profile levels in a dose-dependent manner, comparing with metformin (Ali et al., 2020). Agreeing with another study illustrated the anti-obesity potential of SPIONs in obese rat models may be mediated via repressing WAT expansion and activating of BAT role. (Alsenousy et al., 2022).
Figure 1. Effects of CMC-SPIONs (25 mg), CMC-SPIONs (50 mg), and/or orlistat on the mRNA levels of leptin-r1, Adipo-r1, GnRH, GnIH, Kiss1, and Kiss-r1 in the hypothalamus of obese male rats. (Data are represented as mean ± SEM according to Duncan’s multiple range test (p < 0.05)). Our HFHF-obese rat model revealed aggravated disturbance in mRNA levels of Leptin and Adiponectin expressed in adipose tissue which is highly correlated with disturbed levels of genes involved in the HPT-axis pathway. In accordance, Genchi et al. (2022) explained that the obesity-induced impaired adipocytes upregulated leptin and down-regulated adiponectin expression levels which further impaired secretion of other mediators affecting GnRH release from the hypothalamus, FSH and LH release from the pituitary which in turn worsen the testicular ability to synthesize testosterone (Genchi et al., 2022). Regardless of the adipokines' role in GnRH pathway regulations, leptin seems to directly regulate testicular functions, as it can cross the blood-testes barrier, and modulate the steroidogenic process (Tena-Sempere and Barreiro, 2002). Isidori et al. (1999) clarified that leptin up-regulation resulted in hormonal resistance and correlated with testosterone reduction, specifying leptin as the most significant hormonal indicator of decline in androgen levels in obese men (Isidori et al., 1999). Leptin has an endocrine or paracrine role in the testes inhibiting testosterone synthesis in Leydig cells (Ramos and Zamoner, 2014). Thus, multiple hormonal alterations, such as lower testosterone, higher leptin, estradiol, and insulin levels, adversely affect spermatogenesis and semen quality in obesity (Yan et al., 2015). Our biomolecular investigations on mRNA expression levels of hypothalamic-pituitary-related genes revealed that the obesogenic atmosphere signalized by accumulated visceral fat, dyslipidemia, and insulin resistance badly affects HPT-axis function in males, induced by a considerable upregulation of LepR in hypothalamus as it may bind with the excess leptin released from adipocytes, occurring central leptin resistance, which may down-regulate both of kisspeptin and its binding receptor Kiss-r1, may resulting in GnRH down-regulation and may inversely affect GnIH via up-regulation that may lead to a deranged ratio of GnRH/GnIH. At the hypophyseal level, our study revealed a significant down-regulation in GnRH-R in obese rats indicating reduced levels of the receptor expression which may highly adverse gonadotropins FSH and LH release indicated by their declined serum levels, by virtue of a previous study confirmed that sufficient levels of GnRH-R needed to regulate gonadotropins secretion (Bedecarrats and Kaiser, 2003).
Figure 2. Effects of CMC-SPIONs (25 mg), CMC-SPIONs (50 mg), and/or orlistat on the mRNA levels of GnRH-r in the pituitary, CYP19A1, Star, and HSD17B3 in the testicular tissue and Leptin and Adiponectin in the adipose tissue of obese male rats. (Data are represented as mean ± SEM according to Duncan’s multiple range test (p < 0.05)). Our findings nearly integrated with previous study clarified a down-regulation of Kiss-1 in obese rats while Kiss-r1 expression leaned to be amended but not significantly, supposed to keep the balance between stimulatory (Kisspeptin/GnRH) and inhibitory (GnIH) levels (Jia et al., 2017). Another study illustrated that these downregulations may be attributed to obesity-induced hypothalamic inflammation (Grossmann et al., 2020). As a sequel of HFHF diet-induced obesity consequences on HPT pathways, our biomolecular outcomes revealed a significant down-regulation of STAR, HSD17B3, and CYP19A1 expression levels in testicular tissue indicating a suppression in testicular steroidogenic pathway imputing the HPT-axis impairment, and declined LH levels that regulate testicular steroidogenesis and testosterone synthesis. Convergent studies elucidated repression of the testicular steroidogenic pathway due to the down-regulated expression levels of STAR, CYP17A1, HSD17B, and CYP19A1 in type-2 diabetic rats (Soliman et al., 2019; Khamis et al., 2020). However, Abd El-Hakim et al. (2020) manifested the suppression of testicular STAR and HSD17B3 expression levels, accompanied by CYP19A1 up-regulation in high-fat diet rats, signifying defective testicular steroidogenesis (Abd El-Hakim et al., 2020). Piling up findings have emphasized that steroidogenesis and spermatogenesis testicular impairment are associated with testicular oxidative stress, metabolic syndrome, and male subfertility (Premalatha et al., 2013). For the first time, our data showed that CMC-SPIONs at doses (25 mgFe/kg), (50 mgFe/kg) significantly modulated the disturbed mRNA expression levels of several HPT-axis receptors, mediators, and hormones, starting from Leptin and adiponectin in adipocytes, passing by the hypothalamus, pituitary, until testicular steroidogenesis and spermatogenesis. The co-treatments with Orlistat gave synergistic therapeutic effects. These findings could illuminate more prospects for a novel design of combinatorial preparations that can manage the consequent subfertility in obese sufferers. ConclusionThe findings of this work, for the first time, are a promising contender for obesity and its related subfertility management; CMC-SPIONs that are much closer to the traditional Orlistat in the HFHF-rat model. CMC-SPIONs positively affect the expression of genes involved in HPT pathway mechanisms, amending HPT-axis function, and improving steroidogenesis and spermatogenesis. Thus, CMC-SPIONs can serve as a treatment or co-treatment for obesity and its complexities. However, CMC-SPIONs need more clinical studies on their mechanisms and limitations to be further affirmed. AcknowledgmentNone. Conflict of interestThe authors state that they have no competing interests. FundingThis work did not obtain any award from any commercial, public, or not-for-profit sector. Author contributionsConceptualization: Samar A. Abdo, Data curation and investigation: Mohamed F. Dowidar, Amany I. Ahmed, Methodology: Tarek Khamis, Hanaa Refaat. Analysis: Tarek Khamis, Shehab Eldeen Abdelhaleem. Writing-original draft: Hanaa Refaat, Samar A. Abdo, Reviewing and editing: Mohamed F. Dowidar, Amany I. Ahmed. All authors have assembled, revised, and approved the final form of the manuscript. Data availabilityAll data which support the outcomes of this study are available within the manuscript. ReferencesAbd El-Hakim, Y.M., Abdel-Rahma n Mohamed, A., Khater, S.I., Hamed Arisha, A., Metwally, M.M., Nassan, M.A. and Hassan, M.E. 2020. Chitosan-stabilized selenium nanoparticles and metformin synergistically rescue testicular oxidative damage and steroidogenesis-related genes dysregulation in high-fat diet/streptozotocin-induced diabetic rats. Antioxidants. 10(1), 17. Ali, L.M., Shaker, S.A., Pinol, R., Millan, A., Hanafy, M.Y., Helmy, M.H. and Mahmoud, S.A. 2020. Effect of superparamagnetic iron oxide nanoparticles on glucose homeostasis on type 2 diabetes experimental model. Life. Sci. 245, 117361. Alsenousy, A.H., El-Tahan, R.A. , Ghazal, N.A., Piñol, R., Millán, A., Ali, L.M. and Kamel, M.A. 2022. The Anti-obesity potential of superparamagnetic iron oxide nanoparticles against high-fat diet-induced obesity in rats: possible involvement of mitochondrial biogenesis in the adipose tissues. Pharmaceutics. 14(10), 2134. Baker, P., Sha, J. and O'Shaughne ssy, P. 1997. Localisation and regulation of 17β-hydroxysteroid dehydrogenase type 3 mRNA during development in the mouse testis. Mol. Cell. Endocrinol. 133(2), 127–133. Bedecarrats, G.Y. and Kaiser, U.B. 2003. Differential regulation of gonadotropin subunit gene promoter activity by pulsatile gonadotropin-releasing hormone (GnRH) in perifused LβT2 cells: role of GnRH receptor concentration. Endocr. J. 144(5), 1802–1811. Bucolo, G. and David, H. 1973. Quanti tative determination of serum triglycerides by the use of enzymes. Clin. Chem. 19(5), 476–482. Cheng, L., Shi, H., Jin, Y., Li, X., P an, J., Lai, Y. and Zhao, A. 2016. Adiponectin deficiency leads to female subfertility and ovarian dysfunctions in mice. Endocr. J. 157(12), 4875–4887. Cignarelli, A., Genchi, V.A., D’Oria, R ., Giordano, F., Caruso, I., Perrini, S. and Giorgino, F. 2021. Role of glucose-lowering medications in erectile dysfunction. J. Clin. Med. 10(11), 2501. Conn, P.M., Knollman, P.E., Brothers, S. P. and Janovick, J.A. 2006. Protein folding as posttranslational regulation: evolution of a mechanism for controlled plasma membrane expression of a G protein-coupled receptor. Mol. Endocrinol. 20(12), 3035–3041. de Castro, U.G.M., dos Santos, R.A.S.A.S. , Silva, M.E., De Lima, W.G., Campagnole-Santos, M.J. and Alzamora, A.C. 2013. Age-dependent effect of high-fructose and high-fat diets on lipid metabolism and lipid accumulation in liver and kidney of rats. Lipids Health Dis. 12, 1–11. Dulińska-Litewka, J., Łazarczyk, A., Hałub iec, P., Szafrański, O., Karnas, K. and Karewicz, A. 2019. Superparamagnetic iron oxide nanoparticles-Current and prospective medical applications. Materials. 12(4), 617. El-Gayar, K.E., Ibrahim, M.A., Mohamed, S.H., Zakaria, Z. and Alhameed, A. 2012. Application of extracted peroxidase enzyme from turnip roots (BRASSICA NAPUS) in clinical diagnostic kit. Int. J. Cur. Res. Rev. 4(21), 17–22. Friedman, R.B. and Young, D.S. 1989. Clin. Chem . (Vol. 24): New York, NY: Columbia University Press. Genchi, V.A., Rossi, E., Lauriola, C., D’Oria, R., Palma, G., Borrelli, A. and Cignarelli, A. 2022. Adipose tissue dysfunction and obesity-related male hypogonadism. Int. J. Mol. Sci. 23(15), 8194. George, J.T., Millar, R.P. and Anderson, R.A. 2010. Hypothesis: kisspeptin mediates male hypogonadism in obesity and type 2 diabetes. Neuroendocrinology 91(4), 302–307. Gomaa, A.A., El-Sers, D.A., Al-Zokeim, N.I. and G omaa, M.A. 2019. Amelioration of experimental metabolic syndrome induced in rats by orlistat and Corchorus olitorius leaf extract; role of adipo/cytokines. J. Pharm. Pharmacol. 71(2), 281–291. Grossmann, M., Ng Tang Fui, M. and Cheung, A.S. 2020. Late-onset hypogonadism: metabolic impact. J. Androl. 8(6), 1519–1529. Guillod-Maximin, E., Roy, A.F., Vacher, C.M., Aubour g, A., Bailleux, V., Lorsignol, A. and Taouis, M. 2009. Adiponectin receptors are expressed in hypothalamus and colocalized with proopiomelanocortin and neuropeptide Y in rodent arcuate neurons. J. Endocrinol. l200(1), 93. Isidori, A.M., Caprio, M., Strollo, F., Moretti, C., Frajese, G., Isidori, A. and Fabbri, A. 1999. Leptin and androgens in male obesity: evidence for leptin contribution to reduced androgen levels. J. Clin. Endocrinol. Metab. 84(10), 3673–3680. Jia, Y.F., Guo, Y., Zhou, F., Zhang, K.S., Wang, X.W. , Lu, W.H. and Gu, Y.Q. 2017. Expression of Kisspeptin-GnRH system is down-regulated in hypothalamic arcuate nucleus of male rats with high-fat diet. Int. J. Clin. Exp. Pathol. 10(5), 6099–6107. Kapoor, D., Channer, K.S. and Jones, T.H. 2008. Rosigl itazone increases bioactive testosterone and reduces waist circumference in hypogonadal men with type 2 diabetes. Diab. Vasc. Dis. Res. 5(2), 135–137. Kelsey, M.M., Bjornstad, P., McFann, K. and Nadeau, K. 2016. Testosterone concentration and insulin sensitivity in young men with type 1 and type 2 diabetes. Pediatr. Diabetes. 17(3), 184–190. 22 Khamis, T., Abdelalim, A.F., Abdallah, S.H., Saeed, A.A. , Edress, N.M., and Arisha, A.H. 2020. Early intervention with breast milk mesenchymal stem cells attenuates the development of diabetic-induced testicular dysfunction via hypothalamic Kisspeptin/Kiss1r-GnRH/GnIH system in male rats. Biochim. Biophys. Acta. Mol. Basis. Dis. 1866(1), 165577. Kusminski, C., McTernan, P.G., Schraw, T., Kos, K., O’Ha re, J.P., Ahima, R. and Scherer, P. 2007. Adiponectin complexes in human cerebrospinal fluid: distinct complex distribution from serum. Diabetologia. 50, 634–642. Lainez, N.M. and Coss, D. 2019. Obesity, neuroinflammation , and reproductive function. Endocr. J. 160(11), 2719–2736. Li, X., Su, J., Lei, Z., Zhao, Y., Jin, M., Fang, R. and Ji ao, Y. 2012. Gonadotropin-inhibitory hormone (GnIH) and its receptor in the female pig: cDNA cloning, expression in tissues and expression pattern in the reproductive axis during the estrous cycle. Peptides. 36(2), 176–185. Livak, K.J. and Schmittgen, T.D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 25(4), 402–408. Miceli, D.D., Vidal, P.N., Batter, M.F.C., Pignataro, O. and Cas tillo, V.A. 2018. Metformin reduces insulin resistance and the tendency toward hyperglycaemia and dyslipidaemia in dogs with hyperadrenocorticism. Open. Vet. J. 8(2), 193–199. Mody, A., White, D., Kanwal, F. and Garcia, J.M. 2015. Relevance of low testosterone to nonalcoholic fatty liver disease. Cardiovasc. Endocrinol. Metab. 4(3), 83–89. Penttilä, I., Voutilainen, E., Laitinen, P. and Juutilainen, P. 1981. Comparison of different analytical and precipitation methods for direct estimation of serum high-density lipoprotein cholesterol. Scand. J. Clin. Lab. 41(4), 353–360. Pinilla, L., Aguilar, E., Dieguez, C., Millar, R.P. and Tena-Sempere , M. 2012. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol. Rev. 92(3), 1235–1316. Polat, T.G. and Topel, S.D. 2019. pH-responsive carboxymethyl cellulo se conjugated superparamagnetic iron oxide nanocarriers. J. Sci. Perspect. 3(2), 99-110. Premalatha, R., Jubendradass, R., Rani, S.J.A., Srikumar, K. and Math ur, P.P. 2013. A phytooxysterol, 28-homobrassinolide modulates rat testicular steroidogenesis in normal and diabetic rats. Reprod. Sci. 20(5), 589–596. Ramos, C.F. and Zamoner, A. 2014. Thyroid hormone and leptin in the te stis. Front. Endocrinol. 5, 198. Roza, N.A., Possignolo, L.F., Palanch, A.C. and Gontijo, J.A. 2016. Ef fect of long-term high-fat diet intake on peripheral insulin sensibility, blood pressure, and renal function in female rats. Food. Nutr. Res. 60(1), 28536. Soliman, G.A., Saeedan, A.S., Abdel-Rahman, R.F., Ogaly, H.A., Abd-Els alam, R.M. and Abdel-Kader, M.S. 2019. Olive leaves extract attenuates type II diabetes mellitus-induced testicular damage in rats: Molecular and biochemical study. Saudi. Pharm. J. 27(3), 326–340. Su, X., Lin, D., Luo, D., Sun, M., Wang, X., Ye, J. and Yu, C. 2019. Cy clophilin D participates in the inhibitory effect of high-fat diet on the expression of steroidogenic acute regulatory protein. J. Cell. Mol. Med. 23(10), 6859–6871. Suleiman, J.B., Nna, V.U., Othman, Z.A., Zakaria, Z., Bakar, A.B.A. and M ohamed, M. 2020. Orlistat attenuates obesity-induced decline in steroidogenesis and spermatogenesis by up-regulating steroidogenic genes. J. Androl. 8(5), 1471–1485. Tena-Sempere, M. and Barreiro, M. 2002. Leptin in male reproduction: the tes tis paradigm. Mol. Cell. Endocrinol. 188(1-2), 9–13. Wahajuddin, N. and Arora, S. 2012. Superparamagnetic iron oxide nanoparticles : magnetic nanoplatforms as drug carriers. Int. J. Nanomedicine. 3445–3471. Xu, X., Sun, M., Ye, J., Luo, D., Su, X., Zheng, D. and Guan, Q. 2017. The eff ect of aromatase on the reproductive function of obese males. Horm. Metab. Res. 49(08), 572–579. Yan, W.J., Mu, Y., Yu, N., Yi, T.-l., Zhang, Y., Pang, X.-l. and Yang, J. 2015a . Protective effects of metformin on reproductive function in obese male rats induced by high-fat diet. J. Assist. Reprod. 32(7), 1097–1104. | ||
| How to Cite this Article |
| Pubmed Style Refaat H, Dowidar MF, Ahmed AI, Khamis T, Abdelhaleem SE, Abdo SA, . The Corrective role of superparamagnetic iron oxide nanoparticles for the genes controlling hypothalamus-pituitary-testis-axis in male obesity associated secondary hypogonadism. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 428-437. doi:10.5455/OVJ.2024.v14.i1.39 Web Style Refaat H, Dowidar MF, Ahmed AI, Khamis T, Abdelhaleem SE, Abdo SA, . The Corrective role of superparamagnetic iron oxide nanoparticles for the genes controlling hypothalamus-pituitary-testis-axis in male obesity associated secondary hypogonadism. https://www.openveterinaryjournal.com/?mno=181957 [Access: May 10, 2024]. doi:10.5455/OVJ.2024.v14.i1.39 AMA (American Medical Association) Style Refaat H, Dowidar MF, Ahmed AI, Khamis T, Abdelhaleem SE, Abdo SA, . The Corrective role of superparamagnetic iron oxide nanoparticles for the genes controlling hypothalamus-pituitary-testis-axis in male obesity associated secondary hypogonadism. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 428-437. doi:10.5455/OVJ.2024.v14.i1.39 Vancouver/ICMJE Style Refaat H, Dowidar MF, Ahmed AI, Khamis T, Abdelhaleem SE, Abdo SA, . The Corrective role of superparamagnetic iron oxide nanoparticles for the genes controlling hypothalamus-pituitary-testis-axis in male obesity associated secondary hypogonadism. Open Vet J. (2024), [cited May 10, 2024]; 14((1) (Zagazig Veterinary Conference)): 428-437. doi:10.5455/OVJ.2024.v14.i1.39 Harvard Style Refaat, H., Dowidar, M. F., Ahmed, A. I., Khamis, T., Abdelhaleem, S. E., Abdo, S. A. & (2024) The Corrective role of superparamagnetic iron oxide nanoparticles for the genes controlling hypothalamus-pituitary-testis-axis in male obesity associated secondary hypogonadism. Open Vet J, 14 ((1) (Zagazig Veterinary Conference)), 428-437. doi:10.5455/OVJ.2024.v14.i1.39 Turabian Style Refaat, Hanaa, Mohamed F. Dowidar, Amany I. Ahmed, Tarek Khamis, Shehab E. Abdelhaleem, Samar A. Abdo, and . 2024. The Corrective role of superparamagnetic iron oxide nanoparticles for the genes controlling hypothalamus-pituitary-testis-axis in male obesity associated secondary hypogonadism. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 428-437. doi:10.5455/OVJ.2024.v14.i1.39 Chicago Style Refaat, Hanaa, Mohamed F. Dowidar, Amany I. Ahmed, Tarek Khamis, Shehab E. Abdelhaleem, Samar A. Abdo, and . "The Corrective role of superparamagnetic iron oxide nanoparticles for the genes controlling hypothalamus-pituitary-testis-axis in male obesity associated secondary hypogonadism." Open Veterinary Journal 14 (2024), 428-437. doi:10.5455/OVJ.2024.v14.i1.39 MLA (The Modern Language Association) Style Refaat, Hanaa, Mohamed F. Dowidar, Amany I. Ahmed, Tarek Khamis, Shehab E. Abdelhaleem, Samar A. Abdo, and . "The Corrective role of superparamagnetic iron oxide nanoparticles for the genes controlling hypothalamus-pituitary-testis-axis in male obesity associated secondary hypogonadism." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 428-437. Print. doi:10.5455/OVJ.2024.v14.i1.39 APA (American Psychological Association) Style Refaat, H., Dowidar, M. F., Ahmed, A. I., Khamis, T., Abdelhaleem, S. E., Abdo, S. A. & (2024) The Corrective role of superparamagnetic iron oxide nanoparticles for the genes controlling hypothalamus-pituitary-testis-axis in male obesity associated secondary hypogonadism. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 428-437. doi:10.5455/OVJ.2024.v14.i1.39 |