| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 481-499 Original Research Pathological, histochemical, and immune-histochemical studies on some canine-skin neoplasm at Sharkia province, EgyptHatem A. Zwai1,2*, Al-Sayed R. Al-Attar1, Abdulla E. Morsi1 and Moustafa S. Abou El-Fetouh11Department of Pathology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 2Libyan Authority for Scientific Research, Tripoli, Libya *Corresponding Author: Hatem Ali Zwai. Department of Pathology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. Email: hatemali26 [at] yahoo.com Submitted: 01/10/2023 Accepted: 15/12/2023 Published: 31/01/2024 © 2024 Open Veterinary Journal
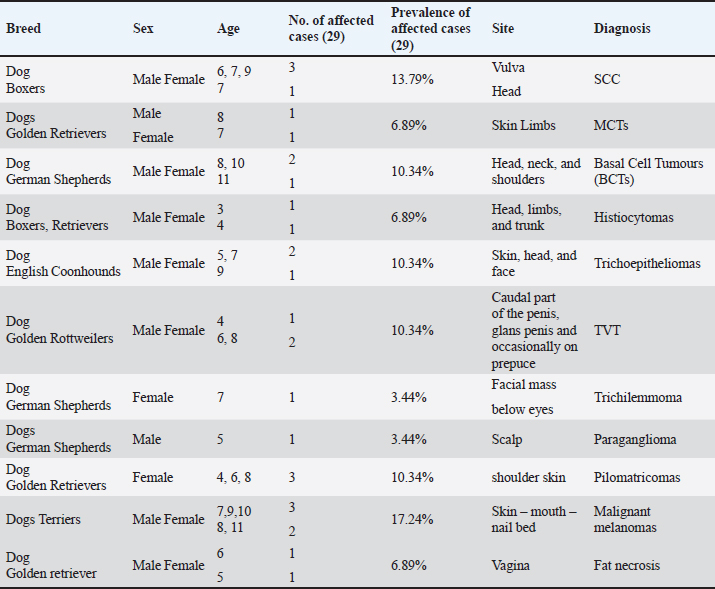
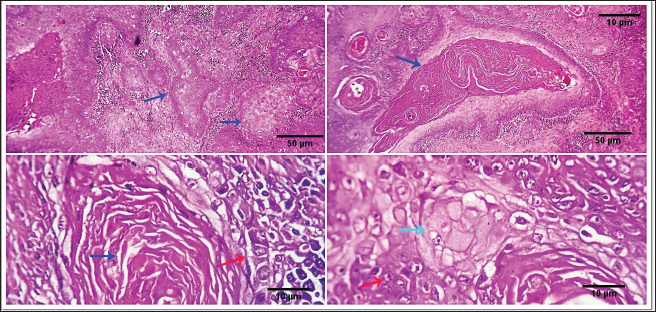
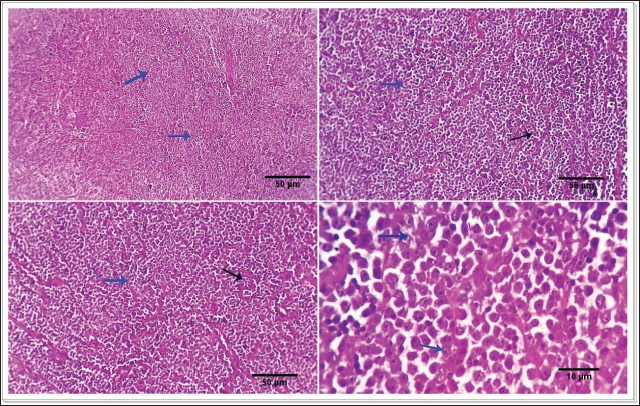
AbstractBackground: Cutaneous neoplastic disorders are often observed in small mammal pets, such as dogs, regardless of their gender. Aim: An important objective of this work was to give a full account of the clinical, pathological, and immune-histochemical features of several skin tumors in dogs. Methods: This study was a case series in the hospital clinic, Faculty of Veterinary Medicine, Zagazig University, Egypt. Twenty-five dogs (14 males and 11 females) were examined clinically during the period from March 2022 to October 2023. The skin swelling was collected from affected animals and then subjected to a detailed histopathological study to record the different gross and microscopic findings and confirm the diagnosis by immunohistochemistry. Results: Skin neoplasia in dogs was exposed to various clinical signs, and the dogs' ages ranged between 3 and 11 years. Concerning tumor features, the majority of neoplasms were malignant (65.52%) more than benign (34.48%). The study revealed the presence of 29 cases of dogs showed neoplasia with different prevalence rates including squamous cell carcinoma (13.79%), mast cell tumor (6.89%), basal cell tumors (10.34%), histiocytoma (6.89%), trichoepithelioma (10.34%), transmissible venereal tumor (10.34%), trichilemmoma (3.44%), scalp paraganglioma (3.44%), pilomatricoma (10.34%), malignant melanomas (17.24%), and miscellaneous cases as fat necrosis (6.89%), in males and females dogs with different histopathological lesions and immunohistochemistry expressions for pan-cytokeratin (CK), melanocyte-differentiation antigens (S100 protein), and synaptophysin. Conclusion: Malignant melanomas (17.24%) are the extremely common cutaneous tumors diagnosed in this study. Meanwhile, benign tumors such as trichilemmoma, trichoepithelioma, pilomatricoma, and paraganglioma are less frequent in dogs. Keywords: Dogs, Skin tumors, Trichoepithelioma, Paraganglioma, Melanoma. IntroductionVarious small mammal species are extremely popular as companion pets and as such require expert veterinary care, including appropriate diagnosis and treatment protocols. Similar to human history, the prevalence of neoplastic illnesses in companion animals has increased over time (Otrocka-Domagała et al., 2022). Adnexa and the subcutis are the two other skin regions where cancers can spread. Dogs and cats are commonly shown to have skin tumors, which encompass a broad range of benign and malignant neoplasms (Wiener, 2021). It is essential to comprehend the characteristics and nature of tumors in small animals to develop and determine prognostic and predictive criteria for oncologic treatment. Small mammals can also be used as markers of environmental and public health issues. This is because, according to Kok et al. (2019), they are subjected to the same environmental elements that may cause cancer in other animals, both domestic and wild. As a result, our understanding of small domestic animal malignancies is continually changing. According to recent studies, a significant percentage of all cancers seen in dogs, cats, guinea pigs, and pet rabbits are cutaneous and subcutaneous tumors (Mahajan et al., 2019; Bertram et al., 2021). Nonetheless, data about specific cutaneous and subcutaneous tumor forms in pets with small mammals have been studied extensively, but the results seem to fluctuate over time (Bertram et al., 2021; Otrocka-Domagała et al., 2022). Because skin tumors are so apparent, owners frequently bring them to the veterinarian's attention. Common diagnostic procedures include grading the tumor, searching for metastases for further clinical staging, and cellular investigation of skin cancer by cytology and histology. The most effective method for treating cutaneous tumors is still surgical excision. However, the type of neoplasm, together with its grade, stage, and location, all play a role in this decision (Kok et al., 2019; Martins et al., 2022). The most common tumors in small pets are skin tumors, which include basal cell carcinoma (BCC), keratoacanthoma, paraganglioma, histiocytoma, mast cell tumor, sebaceous and sweat gland adenocarcinoma, cutaneous lymphoma, and trichoepithelioma (Goldschmidt and Goldschmidt, 2017; Kok et al., 2019; Wiener, 2021; Martins et al., 2022). In both dogs and cats, tricholemmomas are uncommon (Wiener, 2021). Squamous cell carcinoma (SQCC) is one of the most common malignancies in dogs. Although the skin, mouth cavity, and digits are the most commonly affected sites, this type of cancer is primarily caused by squamous epithelial cells found in the skin, kidneys, reproductive system, intestines (oral to rectal), and mucosal surfaces of most organs (Yamashita-Kawanishi et al., 2021; Altamura et al., 2022). Using a multimodal approach, 51 samples of canine and feline skin and subcutaneous cancers, lipomas, soft tissue sarcomas, and mast cell tumors were examined in a unique veterinary oncology study (Tamošiūnas et al., 2022). When an animal's skin tumor is more than 1 cm in diameter, causes self-inflicted harm, or causes discomfort, it may be detected early. A final diagnosis requires histological confirmation to rule out other tumor-like or non-neoplastic lesions that commonly occur in the skin. The morphological appearance (central ulcer, baldness, and so on) or localization implies a diagnosis based on clinical examination (Abramo et al., 1999). Thus, the purpose of this study was to examine the immunohistochemical and histological characteristics of a few cutaneous and subcutaneous tumors and their incidence in dogs. Materials and MethodsStudy animalsTwenty-five dogs (14 males and 11 females) were referred to the veterinary hospital, 25 dogs (14 males and 11 females) were examined clinically during the period from March 2022 to October 2023. Clinical examination and case historyAge, sex, breed, clinical symptoms, and the location of skin tumors on the body were all disclosed in the case histories of all the dogs that were evaluated for the study. The animals under examination ranged in age from 3 to 11 years. Any potential severe abnormalities were noted on the specimens, which were then gathered for additional analysis. Gathering of biopsy specimensBefore surgery, the owners of the dogs were asked for their informal consent. A veterinary specialty surgeon removed the suspicious lumps by routine surgical procedures. The tissue samples were fixed in a neutral solution containing 10% before being examined. Histopathological examinationFollowing a thorough gross inspection, tissue samples from a variety of canine neoplasms and neoplasm-like cutaneous lesions were quickly removed and placed in 10% formalin for analysis using histopathology and immunohistochemistry. The tissues were preserved by immersing them in 10% buffered formalin for 48 hours, followed by a 30-minutes rinse in distilled water to remove the fixative. Subsequently, the tissue underwent a dehydration process consisting of 120 minutes at 70% alcohol, 90 minutes at 90% alcohol, and two cycles of 100% alcohol, each lasting an hour. After that, the specimens were repeatedly cleaned with xylene. The tissue was submerged in a 50% alcohol and 50% xylene solution for 1 hour, and then it was left in pure xylene for the remaining 1.5 hours. Samples were embedded and blocked out using paraffin wax that had been heated to its melting point. According to Suvarna et al. (2013), paraffin slices (4–5 m) were stained using hematoxylin and eosin (H&S) stains. To evaluate cellular morphological changes, proliferative indices, circulatory problems, inflammation, degeneration, apoptosis, necrosis, and any further pathological modifications, the stained sections were examined. Investigation using immunohistochemistryThe tissue portions were treated using a microwave. By using a two-step procedure, immunostaining was used to find antigens in the histology specimens. The matching antigen was first bound by the primary antibody, and the reaction was then seen using a biotin-streptavidin method (Hsu et al., 1981). 3, 30 Hematoxylin was used for counterstaining, and diamethybenzidine (DAB) was used for the permanent preparation. Glass slides with a positive charge were coated with five-micron-thick paraffin sections (Biogenex, Freemont, CA). After a prolonged immersion in xylene, the paraffin sections were exposed to a succession of ethanol solutions containing 100%, 95%, 75%, and 50% concentrations. The slides were dried after the excess buffer was blotted off. The histological sections were carefully treated with a single droplet of incredibly sensitive primary monoclonal antibodies against pan-cytokeratin (CK), melanocyte-differentiation antigens (S100 protein), and synaptophysin. The histopathology slides were incubated for 60 minutes, and then rinsed in phosphate-buffered saline (PBS) for 5 minutes. The sample was given two drops of DAKO EnVision for 20 minutes, and then it was gently rinsed with phosphate-buffered saline (PBS). The slides were exposed to the DAB chromogen for 10 to 20 minutes, or until the required brown color was obtained. To get rid of the DAB residue, the slides were then put through a buffer wash. In the histopathological sections, nuclear counterstaining was done using Mayer's hematoxylin (MH). The histological sections were submerged in MH solution for 3 to 5 minutes, depending on the level of nuclear staining. After being rinsed with tap water, they were separated in alcohol and acid and then rinsed again with tap water. Carefully mounted histopathology slides that had air dried were made using Canada balsam. Analysis of morphometricsImage analysis slides were digitized for the investigation using an Olympus digital camera and an Olympus microscope. Then, using Video Test Morphology 5.2 on an Intel® Core I3®-based computer, the images were examined morphometrically. The percentage of cellular area that was stained positive for synaptophysin and pan-CK was measured by the program. A total of five tissue slices were taken, 200 μm apart. The image analysis program JID801D was used to randomly select five visions/slices for the evaluation of positive cells. The mean grayscale value of the positively stained cells was calculated automatically (Hashish and Kamal, 2015). Statistical analysisThe study employed one-way analysis of variance together with a post-hoc test to determine the relevance of immunohistochemical marker expression. P less than 0.05 was deemed significant. The statistical program SPSS ver. 17.0 (SPSS Inc., Chicago, IL) was used for all analyses. Ethical approvalThe experimental research was in accord with the strategies of the Agency of Animal Usage in Research Committee at Zagazig University (ZU-IACUC/2/F/379/2023). ResultsClinical findingsSkin tumor-afflicted dogs showed a range of symptoms. Depending on where the tumor was and how far it had spread, these symptoms varied. Around the head, neck, chest, back, abdomen, tail, and shoulders, as well as the fore and rear limbs, the tumors appeared as elevated, hairless masses. Additional signs and symptoms included skin redness, pigment loss, scaliness, itching, sores or ulcers around the mouth, nose or eyes bleeding, swollen lymph nodes, and delayed wound healing. Coughing, anorexia, sadness, and a low fever were among the systemic clinical symptoms. Breed, age, and sex of the impacted dogsThe animals ranged in age from just three years old to eleven years old. The age group >4 years had the highest percentage of affected animals (82.12%), whereas the age group <5 years had the lowest percentage (17.86%). Compared to female dogs, more male dogs' tumor samples were submitted (51.72% vs. 48.28%). Breed-wise, Golden Retrievers accounted for 39.29% of cases of skin cancers, followed by Terriers (17.86%), Boxers (17.86%), German Shepherds (14.29%), and English Coonhounds (10.71%). Gross characteristics of skin tumorsBased on histopathological and IHC examination, our results diagnosed skin tumors in 29 cases of dogs, 19 cases of malignant tumors (65.52%) including: SQCC (4), malignant melanoma (5), BCC (3), TVT (3), histiocytoma (2), mastocytoma (2), and 10 cases of benign tumours (34.48%) including: trichoepithelioma (3), Trichilemmoma (1), pilomatricoma (3), miscellaneous (2), and Paraganglioma (1) (Table 1). Squamous cell carcinomaSQCC is an infrequent manifestation of cutaneous malignancy observed in dogs. Tumors are commonly observed with higher frequency in regions of the skin that have light pigmentation, a lack of hair, or poor hair coverage. The majority of cutaneous SQCCs often manifest as solid, elevated, and frequently ulcerated patches and growths. Certain instances involving female canines exhibit the presence of nodular or erosive lesions, as well as a red, hard plaque situated upon a cauliflower-like ulcerated mass within the vulva. Tumors frequently have an expansive growth pattern, extending outward to form substantial masses and displaying a surface morphology reminiscent of a wart. Histopathologically, the neoplasm is represented in the infiltrating cords and trabeculae of malignant epithelial cells with the formation of central keratinized structures. Malignant features are easily seen in the tumor cells and occasional necrosis and inflammatory cellular infiltrations are detected (Fig. 1). Mast cell tumorsClinical examination of the afflicted dog showed normal heart rate, breathing frequency, and body temperature. The most distinctive symptoms included itching, rubbing the body against hard surfaces, and skin ulceration with bloody mixed serous exudation, especially from the affected areas. A solid, small lump develops on the skin or limbs; there may be licking or scratching at the mass, as well as hair loss surrounding it. The animal had a mild melancholy mood. The tumor's macroscopic dimensions were 5 × 3 × 2.5 cm. It had an uneven form, weighed between 130 and 150 g, was hard, grayish–white, encapsulated, and had a granular cut surface. Under a microscope, the tumor was made up of spherical, enlarged vesicular hyperchromatic nuclei with one or more nucleoli arranged in cords or sheets of neoplastic cells. Eighty percent of the cells had coarse basophilic granules in their cytoplasm. Giemsa stain provided a distinctive demonstration of these granules. There were some different-sized cells. The mitotic figures (about 2–4/HPF) were modest. Tumor morphology and biological behaviors are intimately related to one another and aid in tumor diagnosis. The genetic code for ribosomal RNA, which is necessary for a cell to produce proteins, is found in the neuron-derived orphan receptor (NORs) (rDNA). Silver reveals the activated rDNA and stains proteins linked to NORs. Consequently, the proliferation potential of a particular cell or tumor is covered by the AgNOR staining. Our findings demonstrate the value of the AgNORs staining technique in the diagnosis of mast cell tumours (MCTs). We employed two different negative control tissues in the vicinity of the tumor: fibroma and normal connective tissue from the same animal. Table 1. Tumors in the skin of dogs.
The average number of NORs per cell in the fibroma was 2.4, compared to 2.03 in the animal's control tissue, and 3.13 in the mastocytoma cells. Mast cell tumors have a middle-grade capability for proliferation, albeit it is obviously higher than that of control tissues. There were enormous cells with several nuclei. Tumor cells go deep into adipose tissue and blood vessels under the skin, possibly creating a stage between grades II and III, mostly stage III because it comes back multiple times and affects blood vessels. The latter, particularly capillaries, were expanded and filled with neutrophils and eosinophils. Furthermore, the tumor cells might sometimes infiltrate them. In addition, multifocal hemorrhages were observed. Since mast cells are proportionate to vascularization, mast cells could promote angiogenesis, which in turn might contribute to tumor growth. Although the exact etiology of this tumor is unknown, it is possible that an infectious agent or a genetic component (a mutation in the C-Kit gene) is at play. In addition, topical skin irritating treatments and persistent dermatitis are likely contributing factors (Figs. 2 and 3). BCCBCCs are a pr evalent kind of cutaneous malignancy observed in dogs. BCC, alternatively known as basosquamous cell carcinoma, represents the malignancy of this condition, constituting around 10% of the total occurrences of basal cell cases in dogs. The neoplastic growth referred to as a malignant tumor has a typically indolent progression and has the potential to manifest in several locations on the integumentary system. The manifestation of this condition is commonly observed as either a flattened or elevated region on the epidermal layer. The metastasis of this malignancy may occur in several anatomical sites, including the lymph nodes and lungs, while exhibiting limited involvement with the dog's internal organs. BCC is a commonly seen neoplastic condition in dogs of medium to advanced age. BCC should not be mistaken for BCCs, which are benign cutaneous neoplasms arising from the basal cells of the epidermis. Histopathologically, BCCs typically originate as benign lesions and exhibit a low propensity for malignant transformation, with rare occurrences of malignancy. In contrast, BCC manifests as a canine skin neoplasm that initially presents as malignant and has the potential to disseminate or metastasize to distant anatomical sites in the absence of prompt intervention (Fig. 4).
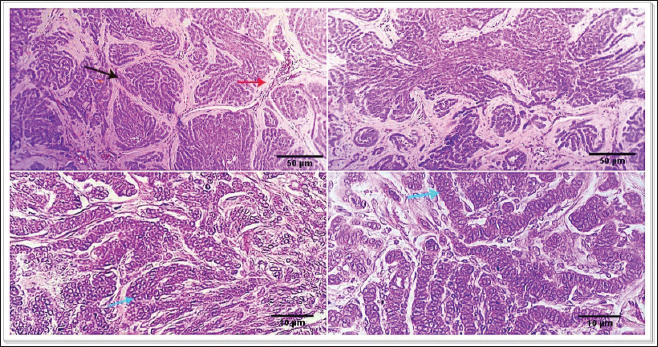
Fig. 1. Photomicrograph showing infiltrating cords and trabeculae of malignant epithelial cells with the formation of central keratinized structures (dark blue arrows). Malignant features are easily seen in the tumor cells (light blue and red arrows). Scale bars 10, 50 µm.
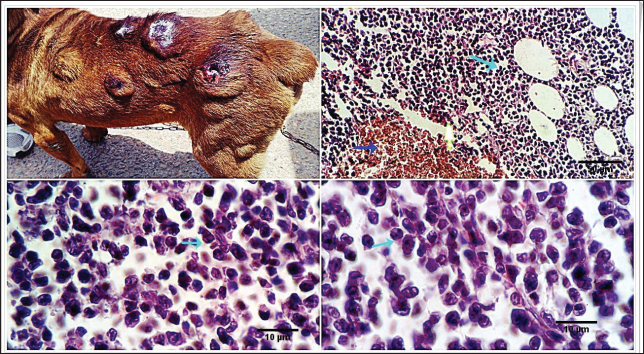
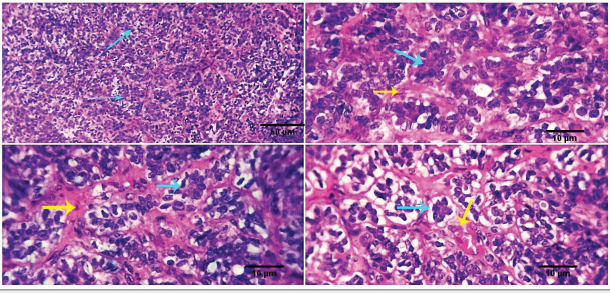
Fig. 2. 1-The mast cells are arranged in a cord (light blue arrow). 2-The mast cells appear sheet (light blue arrow). 3-Basophilic granules are scattered in the mast cells cytoplasm, Giemsa stain (light blue arrow). 4-The mast cells are showing a moderate mitotic—activity (light blue arrow). Scale bars 10, 50 µm.
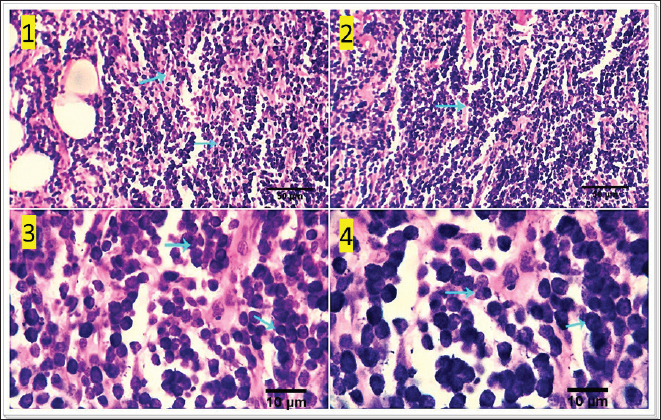
Fig. 3. 1-High magnification to show the mitotic activity (dark blue arrow). 2-Nuclear organizing regions, for fibroma, appear as intranuclear black dot—AgNOR stain (black arrow). 3-Nuclear organizing regions, for normal connective tissue of the same animals, appear as black dots. AgNOR stain (black arrow). 4-Nuclear organizing regions, for mast cell tumors, appear as black dots for an average number (3.13) in the nuclei AgNOR stain (red arrow). Scale bars 10, 50 µm. Malignant histiocy tomaNeoplasms encompass cutaneous histiocytoma (CCH) as well as localized and diffuse histiocytic sarcoma. CCH is characterized by the presence of a single, raised, firm mass on the skin. The mass is typically small, less than 2.5 cm in diameter, and hairless, red, pink, or brown in color, itchy. These neoplasms exhibit diverse biological characteristics, although malignancies frequently have an unfavorable prognosis. Immunohistochemistry serves a crucial function in distinguishing histiocytic tumors from other neoplasms that may exhibit comparable histological features. This enables the establishment of a conclusive diagnosis and offers a more precise prognosis. The histopathological examination reveals that tumors belonging to the histiocytic sarcoma complex have indistinct boundaries, and the excessive growth of cells often leads to the obliteration of the normal structure of the surrounding tissue. Typically, there exists a population of big, vacuolated round cells, which are commonly found with plump mesenchymal cells. The presence of unusually multinucleated giant cells is also a notable observation. Typically, the presence of significant anisokaryosis and anisocytosis, were accompanied by a high mitotic rate. In addition, it is not unusual to observe the identification of phagocytosis of neutrophils and red blood cells or cell fragments by the cancer cells (Fig. 5). TrichoepitheliomaA trichoepithelio ma is a kind of skin tumor that is often benign. Basaloid epithelial islands, which are very small, can be seen as cords and strands at the tumor's base and border. A great deal of calcification, horn cysts, and foreign-body granulomas can be seen. In general, cells have little mitotic activity and lack any distinguishing features. Diseases having a sclerosing growth pattern, such as syringoma and microcystic adnexal carcinoma, are possible misdiagnoses. Trichoblastoma is a deeper kind of trichoepithelioma (Fig. 6). Transmissible venereal tumorThe canine transmi ssible venereal tumor (CTVT) is a contagious neoplasm that is frequently found in dogs who exhibit unrestrained sexual behavior, such as those living on the streets or in shelters. CTVT is an exceptional form of naturally occurring infectious neoplasm in which the mutated cancer cell functions as the etiological factor and persists as a parasite allograft within the recipient organism. TVT is a benign tumor of the reticuloendothelial system in canines. It mostly affects the external genitalia and the internal genitalia rarely. This disorder is known by several names, including sticker tumor, infectious sarcoma, venereal granuloma, transmissible lymphosarcoma, and infectious lymphosarcoma. The transmission of this condition typically takes place during sexual intercourse, mostly affecting individuals in their youth who have reached sexual maturity. Clinical history, the animal was slightly depressed and showed a mass on the genitals, licking at the genitals, difficulty urinating or defecating, pain at pines, redness, swelling, and discharge. The gross morphology of a dog with TVT showed pinkish nodular masses in the gland penis (Fig. 7). Histopathologically, TVTs display a homogeneous tissue composition characterized by a densely packed cluster of mesenchymal-derived cells displaying indistinct borders. There is a consistent presence of lymphocytic, plasmacytic, and macrophagic infiltrates. TVTs would be distinguished from mastocytomas, histiocytomas, or malignant lymphomas (Fig. 8).
Fig. 4. Photomicrograph showing malignant cells organized in masses, cords, and sheets of basaloid cells (black arrow) with peripheral palisading (light blue arrows) and distinct stroma (red arrow). Scale bars 10, 50 µm.
Fig. 5. Photomicrograph showing multiple subcutaneous irregulars, and ulcerated nodules which are represented microscopically by a population of large, vacuolated round cells, in combination with plump mesenchymal cells (light blue arrows). Tumor cells show high mitosis and focal hemorrhages (dark blue arrow). Scale bars 10, 50 µm.
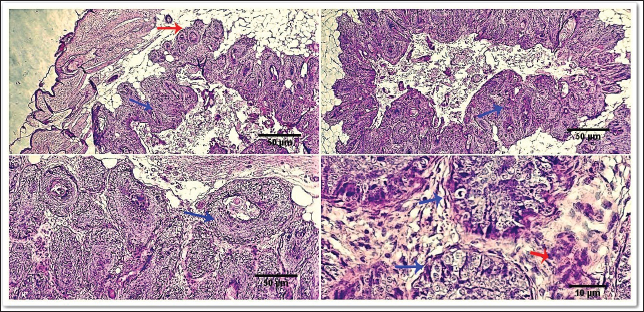
Fig. 6. Photomicrograph showing small islands of basaloid epithelium (dark blue arrows), which form cords and strands toward the base and periphery (red arrows). Scale bars 10, 50 µm.
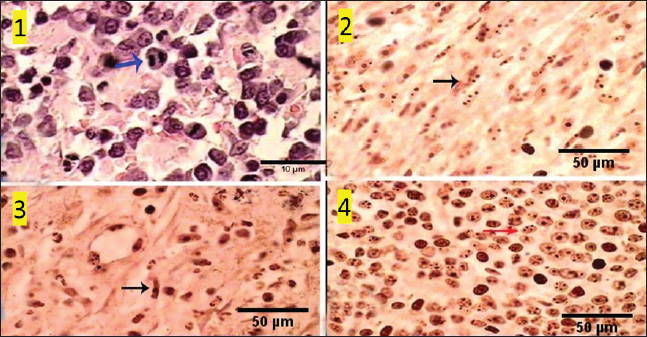
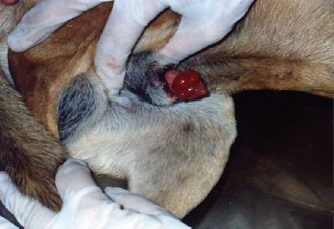
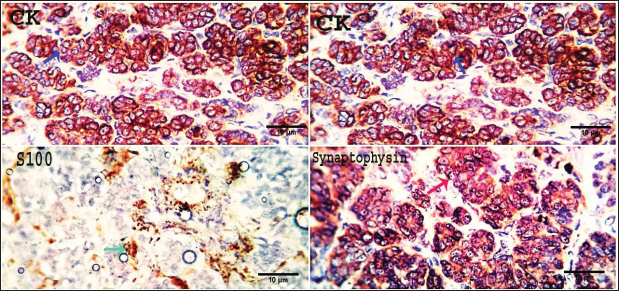
Fig. 7. Gross morphology of TVT in a dog, showing pinkish nodular masses involving gland penis. Trichilemmoma versus scalp paragangliomaTrichilemmoma is characterized by the presence of lobular clusters, including tiny cells that are organized into islands, nests, and fragile trabeculae. The cells located in the outer layer of tumor nests exhibit cytoplasm that is transparent in appearance, and there is a noticeable presence of peripheral palisading. The pale appearance of these cells can be attributed to the presence of cytoplasmic glycogen storage, which is detected by periodic acid-Schiff (PAS) staining. This glycogen storage indicates the differentiation of these cells into the outer root sheath (ORS) cells located in the hair bulb. In proximity to the central region, cells exhibit a heightened degree of eosinophilia inside their cytoplasm. The basement membrane around each tumor cluster has a substantial thickness and displays eosinophilia, appearing glassy in nature. This characteristic is reminiscent of the vitreous sheath found in the normal hair follicle. Trichilemmal keratinization can manifest as small foci, accompanied by a slightly mucinous surrounding matrix. The overlaying epidermis lacks contiguity. The gross morphol ogy of paraganglioma showed a round to oval shape and a well-demarcated, raised, firm mass on the scalp, painless or painful. In some cases, the mass may rupture and bleed. The most significant diagnostic feature observed in the histological analysis of cutaneous paragangliomas was the presence of a "Zellballen" appearance, characterized by clusters of neoplastic cells arranged in nests with spherical to irregular morphology. The tumor infiltrates the scalp skin with a very narrow margin. The fibrovascular stroma separates clusters or nests of pleomorphic epithelioid cells with varying shapes into polygonal tumor cells, which are enclosed by delicate trabeculae composed of fibrous tissue. Anaplastic features and mitotic index were very low (Fig. 9). Immunohistochemically, pan-CK and synaptophysin strongly reacted and most of the tumor cells were positively labeled. Tumor cells were negatively labeled by S100, although the interstitial cells were positively reacted (Fig. 10). PilomatricomaPilomatricoma is a distinct neoplasm located within the dermal and/or subcutaneous layers, characterized by the presence of many cystic structures of varying dimensions. These cystic structures are bordered by tiny, basaloid cells. Grossly, well-circumscribed, and firm masses located just under the skin, the nodules may vary in color, but they are typically skin colored to slightly bluish or reddish. At the microscopic level, tiny structures inside the cyst wall that look like dermal papillae can be seen. The nuclei exhibit significant size and hyperchromatism, whereas the nucleoli are inconspicuous. Trichohyalin granules, which serve as an indication of differentiation in inner root sheath cells (IRS), are typically observed. With very few instances, the tumor is typically categorized as benign even though the cells frequently show strong mitotic activity. The cyst-like formations exhibit a sudden keratinization without a covering of granular cells. Throughout the lumina of cysts, there are a lot of ghost cells, also known as shadow cells, which are identified by an abundance of eosinophilic cytoplasm and a central empty space in place of a nucleus, which symbolizes matrical differentiation (Fig. 11).
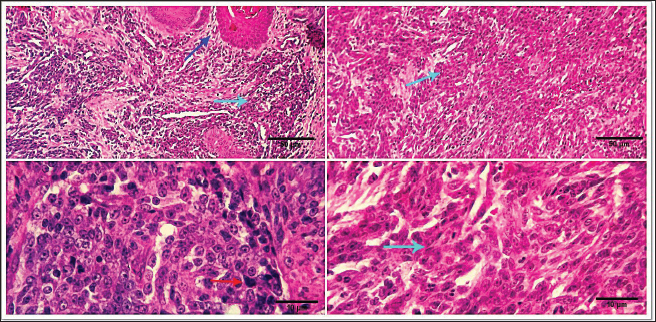
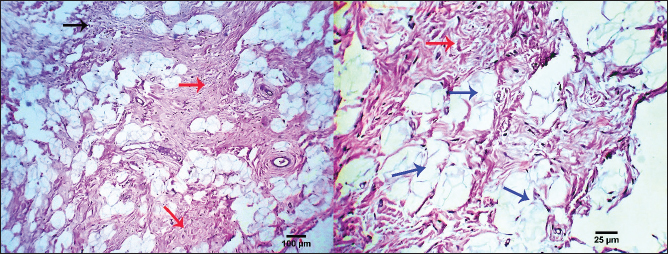
Fig. 8. Photomicrograph showing homogenous tissue with a compact mass of cells that are mesenchymal in origin and the borders of which cannot easily be differentiated (dark blue arrows). There is frequently an infiltration of lymphocytes, plasma cells, and macrophages (dark blue arrows). Small strands of fibrous stroma intervene in tumor cells (black arrows). Scale bars 10, 50 µm. Malignant melanomaMalignant melanoma s in canines are neoplastic formations or tumors that have cancerous characteristics, primarily manifesting on the epidermis, oral cavity, or ungual apparatus. These neoplasms are observed with a higher frequency (17.24%) in geriatric male canines and specific canine breeds, notably Terriers. The frequently observed form of melanoma manifests as an elevated neoplasm on the dermis, exhibiting a reddish or blackish hue, and an ulcerated nodular structure displaying a darker shade compared to the adjacent integument. However, it is noteworthy that certain melanomas lack pigmentation, a condition referred to as amelanotic. Malignant melanomas represent a highly aggressive form of neoplasms that commonly exhibit metastatic behavior, disseminating to various anatomical sites within the organism. Malignant melanoma frequently exhibits a high fatality rate within a span of twelve months, despite the implementation of therapeutic interventions aimed at eradicating neoplastic growth. The mean survival duration for this ailment is approximately 10 months, although canines have been observed to exhibit a maximum lifespan of 36 months upon expeditious excision of the melanoma through surgical intervention. Malignant melanoma manifests in four distinct stages, with each stage exhibiting a unique duration of survival. Stage one (less than 2 cm in size), approximately twelve months, stage two (2–4 cm), approximately eight months in duration, stage three (greater than 4 cm or a neoplasm that has metastasized), approximately four months, and stage IV (extensive metastasis), approximately one month. At the microscopic level, the tumor consists of submucosal or subcutaneous layers composed of malignant spindle cells (Fig. 12). Miscellaneous casesFat necrosisThe clinical history and gross pathology of the current case revealed a necrotizing dermatological condition that was characterized by the presence of numerous sizable, red–purple, nonpruritic skin lesions. The necropsy findings revealed the presence of diffuse fat necrosis and pancreatic nodular hyperplasia. The larger lesions had substantial dimensions and featured soft, highly pigmented cores that harbored an aseptic, thick, hemoglobin-infused substance. The histopathological analysis unveiled the presence of necrotizing panniculitis, characterized by the necrosis of subcutaneous fat tissue. In addition, there was observed capillary dilation, marked infiltration of leukocytes, and localized proliferation of mesenchymal cells (Fig. 13).
Fig. 9. Photomicrograph showing Fibrovascular stroma divides packets or nests of variable pleomorphic epithelioid to polygonal tumoral cells (light blue arrows) and is surrounded by thin trabeculae of fibrous tissue (yellow arrow). Scale bars 10, 50 µm.
Fig. 10. Photomicrograph showing pan-CK (dark blue arrow) and synaptophysin (red arrow) appear strongly reacted and most of the tumor cells are positively labeled. Tumor cells appear negatively labeled by S100, although the interstitial cells were reacted positively (light blue arrow). Scale bars 10 µm. DiscussionThe skin is a primary organ with epithelial, mesenchymal, and local immune cells that maintain balance and protect against external stimuli. It accounts for 9.5%–51.5% of all canine tumors and is one of the supreme sites for cancers because of its constant exposure to physical, chemical, and environmental risk factors (Bronden, et al., 2010). The leading cause of death for companion animals is cancer, which is a significant problem in veterinary medicine across the globe. As a result, throughout the last few decades, a large number of papers have covered this subject (Burrai et al., 2023). This study compared the clinical, macroscopic, and microscopic results of tumors on different parts of the skin in dogs of different ages, breeds, and sexes in Sharkia Province. It also offers important new information about the common histotypes of cutaneous cancers. Despite the small sample size, the findings aid in our understanding of these components (Graf et al., 2018).
Fig. 11. Photomicrograph of the epidermis of a dog's shoulder reveals numerous cystic structures of varying sizes, lined by microscopic basaloid cells (green arrows), and large, hyperchromatic nuclei with inconspicuous nucleoli. Small structures resembling dermal papillae may exist within the cyst wall (black arrow). Trichohyalin granules indicating IRS differentiation (IRS) are present (light blue arrow). Scale bars 10 cm, 50 µm.
Fig. 12. Photomicrograph showing subcutaneous sheets of malignant spindle, round, or ovoid cells (dark blue arrow), anaplastic pleomorphic changes (light blue arrows) with the occasional presence of intracellular melanin granules (a melanotic variant) (red arrow). Scale bars 10, 50 µm. According to the current study, the percentage of malignant tumors was frequently higher than the percentage of benign tumors (65.52% vs. 34.48%), which is consistent with findings from other researchers (Pakhrin et al., 2007; Kok et al., 2019). Due to the fact that referral veterinary clinics have gathered the majority of the patients, some benign tumors may go unnoticed. Furthermore, there has not been a regular histological assessment or surgical resection of benign tumors. According to earlier reports, more than half of the tumors diagnosed in dogs between the ages of 3 and 11 are linked to an increased risk of canine skin tumor development in later life (Graf et al., 2018; Kok et al., 2019). Male dog tumor sample submissions exceeded female dog tumor sample submissions (51.72% vs. 48.28%). The majority of earlier research findings (Bronden et al., 2010; Hassan et al., 2022) are in agreement with these results. SCCs and MCTs are the most prevalent canine lesions, with a prevalence of 13.79% and 6.89%, respectively, according to earlier research. Because cutaneous SCC and MCT occur often and have a variety of biological characteristics, multiple histologic grading systems have been developed to assess their prognosis (Pakhrin et al., 2007; Martins et al., 2022). Dogs have a very high incidence of skin neoplasms, which could be caused by both species-specific variables such as genetic predisposition (Lima et al., 2018) and tutor-related characteristics such as ease of lesion observation (Goldschmidt and Goldschmidt, 2017). In addition, skin is directly exposed to carcinogenic conditions (Martinez et al., 2006), has a high rate of cell renewal (Murphy, 2006), and is comprised of multiple components in its structure (Bastos et al., 2017).
Fig. 13. Photomicrograph showing necrotizing panniculitis (subcutaneous fat necrosis), capillary dilation (dark blue arrows), intense leucocytic infiltration (black arrow), and focal mesenchymal proliferation (red arrows). Scale bars 25, 100 µm. SCCs typically occur in clusters and are more common in middle-aged and older dogs. On the other hand, individual lesions have been observed on the belly, limbs, and head (Goldschmidt and Goldschmidt, 2017). Three cases of SCC, all involving male canines, have been diagnosed, and this information is noteworthy. When compared to other skin places, this particular tumor's prognosis is different (Belluco et al., 2013). The subsequent authors demonstrated that although canine digital SCC has a greater incidence rate and multicentric development, it seldom metastasizes. Within the current investigation, there were four instances of SCCs with a multicentric distribution, two of which were subungual SCCs. This study's findings, which show that two of the three dogs with subungual SCC were mixed-breed dogs and the third was a Labrador retriever, are consistent with previous research showing that these two breeds were among the most affected by this histotype. Histopathologically, the SCC tumor is depicted as malignant epithelial cell trabeculae and infiltrating cords that have formed core keratinized structures. The tumor cells have easily observable malignant characteristics, and sporadic necrosis and inflammatory cellular infiltrations are noted. Previous studies (Gross et al. 2008; Nemec et al. 2012; Auler et al. 2014; Ortloff et al. 2020) produced comparable outcomes. Previous research states that MCTs are the third most common tumor subtype in dogs and account for 11% of cases of skin cancer (de Nardi et al., 2022). More than two-thirds of the cutaneous MCT cases assessed using Kiupel's grading technique were classified as low grade, whereas less than one-third were classified as high grade, according to the current study, which is consistent with previous findings (Sabattini et al., 2015). In contrast to other studies and the Patnaik categorization system, the current study revealed that grades II and III were more common than grade I (Murphy et al., 2004; Murphy et al., 2006). Compared to the subcutaneous variety, the cutaneous form of MCT was more prevalent. Both Labrador retrievers and boxers were revealed to have exceptionally high rates of breed occurrence, which is consistent with several studies (Warland and Dobson, 2013). There were more occurrences of golden retrievers classified as Patnaik's grades I and II and as Kiupel's low grades (Martins et al., 2022). Our results aligned with previous research on various dog breeds that had either MCCs (Mochizuki et al., 2017; Kiupel and Camus, 2019; Avallone et al. 2021) or SCCs (Sierra Matiz et al., 2019; Łojszczyk et al., 2021). Because high-grade MCT frequently grows quickly, lacks demarcation from surrounding tissue, has tumors larger than 3 cm in diameter, can cause irritation or ulceration, has satellite lesions present, or is linked to paraneoplastic symptoms such as wheal formation or gastric ulceration, their gross appearance may, to some extent, be correlated with their clinical behavior. Under a microscope, the mast cell tumor was made up of spherical, enlarged, vesicular hyperchromatic nuclei that had one or more nucleoli arranged in cords or sheets. Eighty percent of the cells had coarse basophilic granules in their cytoplasm. A prior study observed similar results (Stefanello et al., 2015; Bellamy and Berlato, 2022). Roughly 12% of canine tumors are malignant BCCs. A type of skin cancer known as BCCs is common in dogs. This cancerous growth can appear anywhere on your skin and develop gradually. These results were in line with earlier researchers' findings (Hassan et al., 2022) and contradicted those of another study (Stockhaus et al., 2001). Larger scale studies are required to confirm whether German shepherd dogs have a hereditary propensity to BCC. This study revealed that the head, neck, and shoulders were the most common locations for BCC, which is in line with other findings (Goldschmidt and Hendrick, 2002; Goldschmidt and Goldschmidt, 2017). The present results indicated that males were more predisposed to BCC than females; these findings are consistent with Hassan et al. (2022) and differ from a prior study (Goldschmidt and Hendrick, 2002). The presence of malignant cells arranged in masses, cords, and sheets of basaloid cells with peripheral palisading was used in the current investigation to classify BCC under the microscope. According to previous research (Lee Gross et al., 2005; Ho et al., 2018), these findings were consistent. According to Sano et al. (2019), the BCC mass consisted of tubular structures mixed with stratified neoplastic basaloid cells that were grouped in nests and connected islands. Moreover, the histology shows tumor growth with modest infiltration, with eosinophilic thick basal lamina material around the glandular structures and multi-stratified glandular structures of the basaloid and innermost laminal neoplastic cells. According to Sakai et al. (2017), both varieties of neoplastic cells showed moderate anisokaryosis and mitotic figures similar to those of basaloid neoplastic cells. A common skin tumor in dogs, CCH is characterized by the clonal growth of Langerhans cells (Delcour et al., 2013). In dogs and cats, interstitial dendritic cells, which are found in most bodily tissues, are the source of histiocytic sarcomas. The disease starts as localized lesions that spread quickly to numerous organs, including the spleen, lungs, skin, brain (meninges), lymph nodes, and bone marrow in addition to the synovial tissues of the limbs (Moore, 2014). Based only on histology, it can be challenging to distinguish CCH from plasmacytomas, mast cell tumors, and cutaneous lymphomas due to its morphologic similarity to other round-cell tumors (Fernandez et al., 2005; Araújo et al., 2012). According to previous research (Schwens et al., 2011; Moore, 2014), the majority of the assessed CCHs were discovered in dogs under the age of five and were situated in the head, neck, limbs, or trunk. Mutts, Boxers, and Yorkshire Terriers were among the hardest affected, but this was probably more a result of their widespread appeal than of any innate animosity toward particular breeds. Still, Boxers and Retrievers are viewed as vulnerable breeds (Moore, 2014). The description of CCH morphology provided by this study is consistent with previous research (Lee Gross et al., 2005; Moore, 2014). According to this study, CCH is histologically defined by a substantial population of circular, vacuolated cells with either central or eccentric nuclei. Unusual multinucleated gigantic cells are another noteworthy discovery. There is a noticeably high mitotic rate anisokaryosis and anisocytosis. Previous records of similar histologic findings exist (Paździor-Czapula et al., 2015). When compared to other cutaneous tumors in dogs, CCH exhibits the highest mitotic index in the current study. According to earlier observations, the mitotic activity in CCH is either very varied but frequently high (Martin de Las Mulas et al., 1999; Moore, 2014) or moderate (Lee Gross et al., 2005). In the current work, trichoepitheliomas are made up of smaller basaloid epithelial islets that form cords and strands near the base and periphery. It is possible to observe calcification, foreign body granulomas, and horn cysts—often numerous. The cells have a dull appearance, and there is typically very little mitotic activity. It might be challenging to differentiate this lesion from syringoma or occasionally microcystic adnexal carcinoma due to its sclerosing development pattern. Similar findings were found in another investigation (Goldschmidt and Goldschmidt, 2016). Furthermore, there is some similarity between the benign and malignant forms of trichoepithelioma in terms of their histological features. Nonetheless, the malignant form's frequent invasiveness and the ensuing poor delineation from adjacent tissues are its primary histologically distinctive characteristics. The general appearance of dogs with TVT include pinkish nodular masses involving the penis gland, licking at the genitalia, discomfort at the pines, redness, swelling, and discharge. From a histological perspective, TVTs are made up of the same kind of tightly packed cells that start off in mesenchymal tissue. It is difficult to tell where these cellular boundaries are. Infiltrations of lymphocytes, macrophages, and plasma cells are frequent. Previous studies (Das and Das, 2000; Nak et al., 2004) reported similar results. In a 2005 study, Nak et al. found that dogs with TVT were exhibiting severely serosanguinous hemorrhagic exudate that was dripping from the vulva in females and the preputium in males. The masses emerged from the vulva in females. The lumps caused the vulva to protrude outward in most cases. The masses were found on the penis and lamina interna of the preputium in male dogs, and in the posterior vagina in female dogs. Tumors exhibited the typical look of TVTs, which are cauliflower-shaped nodules with ulcerated surfaces and varying diameters (0.5–5 cm). Under the microscope, TVT cells have round to ovoid, hyperchromatic nuclei, marginal chromatin, and massive nucleoli positioned in the center. There was a moderate amount of eosinophilic cytoplasm. Tumor smears exhibiting superficial bleeding and ulceration revealed the presence of neutrophils, erythrocytes, and bacteria. Subsequent investigation revealed that untreated TVT was primarily composed of sheets or bundles of spherical cells with large, highly basophilic nuclei and noticeable, highly basophilic nucleolae (Singh et al., 1996). Regarding trichilemmoma in this study, it exhibits lobular aggregates with microscopic cells arranged in nests, islands, and fine trabeculae. The exterior layer of the tumor nests' cells clearly displays cytoplasm, and there is also noticeable peripheral palisading. The cytoplasm of the central cells is more strongly eosinophilic. A thick, glassy, eosinophilic basement membrane encircling each tumor cluster contains tiny foci of trichilemmal keratinization. The current results were related to investigations conducted by Schweiger et al. (2004) and Cabral and Cassarino (2007). In addition, the tumor cells of trichilemmoma were arranged into distinct cords, nests, and lobules. Dog tumors usually included small lobules made up of a few cells encased in dense, eosinophilic hyaline mantles, together with conspicuous desmoplastic stromal tissues; however, the lobular structure eventually vanished as it approached the tumor's center. The tumor cells exhibited mild degrees of anisokaryosis and anisocytosis and varied in shape from ovoid to polygonal. They also included a sizable amount of granular eosinophilic cytoplasm. The cells were arranged in irregular cords and often seemed spindle-shaped. The nucleus was spherical to oval, positioned in the center of the cell, and featured coarse chromatin and a less visible nucleolus (Kok et al., 2017). As of right now, nests of round to polygonal neoplastic cells giving rise to a "Zellballen" look are the most common histological diagnostic features found in cutaneous paragangliomas. The edge of the tumor's infiltration into the scalp skin is quite thin. Thin trabeculae of fibrous tissue around the fibrovascular stroma, which splits packages or nests of varied pleomorphic epithelioid to polygonal tumoral cells. The mitotic index and anaplastic characteristics were extremely low. Nearly similar outcomes regarding cutaneous paraganglioma were documented in cats by Friedlein et al. (2017) and in dogs by Mai et al. (2015), Gombert et al. (2022). In addition, Rodrigues et al. (2020) researched paraganglioma in a chow dog's tongue and discovered that the lingual mass was extremely cellular, with cells packed in discrete spherical nests (known as "Zellballen" appearance) surrounded by a thin fibrovascular stroma. The cells ranged in shape from round to polygonal. The malignant cells exhibited notable anisocytosis and anisokaryosis, along with sporadic visible single nucleoli. In every 10 high-power fields (2.37 mm2) of tumor mass, two mitotic figures were observed. The cytoplasm had a little granular appearance, was eosinophilic, and was PAS-negative. There were several locations where the tumor had invaded the muscular tissue, and the surgical margins were very small. The current study confirms that dogs with paragangliomas, both benign and malignant, have a confirmed low mitotic index (Fischer et al., 2018). The mitotic index, however, was noticeably higher in a cat specimen suffering from malignant renal paraganglioma (Friedlein et al., 2017). Among the pathological features of paragangliomas that could make it challenging to establish the criteria of malignancy include tumor size, infiltration grade, and mitotic index (Rodrigues et al., 2020). The paraganglioma tumor cells in this investigation had robust reactions to pan-CK and synaptophysin, with the majority of the cancer cells being positively labeled, according to IHC data. S100 tagged tumor cells negatively, while interstitial cells responded positively. The observed histopathological results showed how valuable IHC is as an additional tool for pathologists evaluating cutaneous tumors. It is imperative to recognize that IHC contributes significantly to histopathologic features. The final diagnosis ought to be based on the assessment of IHC results in combination with the cellular characteristics noted in the histology. Our IHC results are consistent with previously published research in cats (Friedlein et al. 2017) and dogs (Fischer et al., 2018). The results of the IHC assay were quite comparable to those of another researcher (Galac and Korpershoek, 2017) who reported that the majority of dogs with paragangliomas do not have anti-S100 protein. A prior investigation found that the neuroendocrine markers synaptophysin, chromogranin A, and vimentin, as well as moderate glial fibrillary acidic protein positivity and remarkable sustentacular cell immunostaining for S100 protein, were strongly immunolabeled in tumor samples from a dog case of paraganglioma (Rodrigues et al., 2020). Currently, 10.34% of skin tumors are pilomatricomas, which are most commonly identified in 4- to 8-year-old dogs, which is consistent with findings from Goldschmidt and Hendrick (2002). It appears as hard, well-circumscribed lumps immediately beneath the skin. The nodules can be any color, although they usually have a flesh-colored to slightly bluish or reddish tint. Under a microscope, the cyst wall may show microscopic features resembling dermal papillae. The nucleoli are rarely visible, and the nuclei are large and hyperchromatic. Despite this, high mitotic activity in the cells is typical, leading to the assumption that the tumor is benign. There is no granular cell layer and the cystic forms keratinize very quickly. Ghost cells, also known as shadow cells, are often characterized by matrical differentiation, large amounts of eosinophilic cytoplasm, and the absence of a nucleus in the center of the cyst lumina. According to a previous study (Lee Gross et al., 2005), histologic examination can be used to differentiate malignant pilomatricoma from other follicular tumors, such as benign pilomatricoma and malignant trichoepithelioma. Inadequate circumscription with infiltration, increased mitotic activity, abnormal nuclear and mitotic features in the basaloid cells, and a greater proportion of basaloid cells than keratinized ghost cells are common characteristics of malignant pilomatricomas. Furthermore, it can be challenging to differentiate malignant pilomatricomas from malignant trichoepitheliomas; nonetheless, the former are typically characterized by greater zones of ghost cells, larger epithelial aggregates, and a notable prevalence of atypical matrical cells. Trichohyalin granules and significant focal inner and outer root sheath differentiation are features of malignant trichoepitheliomas, which often lack calcification or ossification. According to Carroll et al. (2010), keratinizing basal cell carcinoma is distinct from malignant pilomatricoma due to its superficial plaque-like morphology and proximity to the epidermis. In the past, islands and trabeculae of neoplastic cells invaded the distal portion of the dog's femur, encircling lakes of eosinophilic material to produce structures resembling cysts. According to Carroll et al. (2010), the neoplastic cells were polygonal, had four mitotic figures per ten fields at 400×, little cytoplasm, hyperchromatic oval nuclei, moderate anisocytosis, and mild anisokaryosis. Numerous investigations have confirmed that the histopathological features of pilomatricoma comprise lobulated, asymmetrically formed islands that house two distinct cell populations: basaloid cells and keratinized embryonic hair cells known as shadow or ghost cells. A sudden change in keratinization within the lobules from basaloid to shadow cells characterizes this neoplasia (McKee, 1999). Our results were in agreement with Treggiari and Elliott (2013). Furthermore, under a microscope, the bulk seemed to be made up of several small and big lobules. Basophilic basaloid cells, which varied in shape from circular to elliptical, were seen around the lobules. The basophilic cells quickly become keratinized. A plethora of ghostly cells could be seen at the center of the bulk. The ghost cells were filled with eosinophilic cytoplasm and lacked a nucleus. Atypical mitotic structures were abundant in basophilic basaloid cells. According to Lee et al. (2016), pleomorphism and cellularity were both remarkable. Malignant melanomas on the skin, lips, or toenails were seen in this study. These tumors affect 17.24% of older male dogs and some breeds, including Terriers. Compared to the other forms, it has the highest occurrence rate and is the most frequently observed tumor in our study. Although some melanomas are amelanotic, or not pigmented, it is generally identified by an elevated red or black mass on the skin or an ulcerated nodule that is darker than the surrounding skin. Under a microscope, the tumor is composed of malignant spindle, round, or ovoid cells arranged in submucous or subcutaneous sheets. These cells exhibit anaplastic pleomorphic alterations, and intracellular melanin granules, a melanotic variety, are occasionally seen. In the melanotic variety, most of the cytoplasm is replaced by a high number of coarse and fine melanin granules. It is rare to see necrosis and bleeding. Only for cutaneous tumors are histologically determined ulceration and macroscopically determined tumor thickness indicative of prognosis (Silvestri et al., 2019). Furthermore, fusiform melanoma, which is characterized by elongated spindloid cells grouped in streaming bundles and occasionally observed intracytoplasmic melanin granules, large nests of neoplastic melanocytes infiltrate the dermis, and numerous melanomacrophages infiltrate the periphery of the neoplasm in a dog with cutaneous melanoma are among the observed histopathological features (Stevenson et al., 2023). Other types of oral melanoma are characterized by vast sheets of neoplastic cells that obscure the propria mucosa and tiny nests of neoplastic melanocytes that penetrate the mucosal epithelium. Brown intracytoplasmic granules, which are probably melanin, are a characteristic of the neoplasm. As previously noted, the mouth cavity, skin, mucocutaneous junctions, paws (nail junction and cushions), and, less frequently, the eyes and meninges are the major locations for malignant melanomas (van der Weyden et al., 2020). Some authors (Smedley et al., 2011; Smedley et al., 2022) have shown that nuclear atypia, mitotic count (MC), degree of pigmentation, amount of infiltration/invasion, and vascular invasion are statistically significant histologic criteria for prognosis prediction of cutaneous and oral melanocytic neoplasms in dogs. Ultimately, the clinical history and macroscopical pathology of the present individuals pointed to a necrotizing dermatological illness characterized by several large, reddish–purple, nonpruritic lesions. A necropsy revealed diffuse fat necrosis and pancreatic nodular hyperplasia. The larger lesions had mushy, highly discolored centers that were filled with a blood-tinged, viscous, sterile fluid. Necrotizing panniculitis (subcutaneous fat necrosis), capillary dilatation, strong leucocytic infiltration, and localized mesenchymal growth were all found during the histopathological examination. The results of this investigation were consistent with the results of the research that were previously cited (DeManuelle and Stannard, 1998; Contreary et al., 2015). A variety of inflammatory diseases affecting the subcutaneous adipose tissue (panniculus adiposus) caused by the death of adipocytes by physical, chemical, infectious, or immunological stressors are together referred to as panniculitis. It has previously been reported that sterile nodular panniculitis is a cutaneous sign of a systemic underlying illness. Clinical investigations demonstrated inflammation of the falciform, mesenteric, and subcutaneous fat. The reported pathological results aligned with the previously cited studies (German et al., 2003; Muller et al., 2022). In addition, Kim et al. (2011) demonstrated distinct soft nodules or well-circumscribed firm nodules in several dog cases. Pleomorphic mesenchymal cells were found in all of the well-circumscribed firm nodules; in contrast, soft nodules showed a large number of inflammatory and adipose cells, suggesting that firm nodules in panniculitis may be mistakenly classified as tumors. Finally, the current work elucidated the epidemiology of canine cutaneous tumors in the animal data retrieved from the Clinic hospital and shows the relative prevalence of the different forms of tumors in dogs at Sharkia Governorate. Malignant (65.52%) neoplastic tumors accounted for the great majority of skin tumors. Benign tumors, however, account for 34.48%. In male dogs (51.72%) and female dogs (48.28%), the most common tumor types were malignant melanomas (17.24%), SQCC (13.79%), basal cell tumors (10.34%), trichoepithelioma (10.34%), transmissible venereal tumor (10.34%), pilomatricoma (10.34%), mast cell tumor (6.89%), histiocytoma (6.89%), and other cases such as fat necrosis (6.89%), trichilemmoma (3.44%), and scalp paraganglioma (3.44%). ConclusionIn conclusion, the current work illustrates the relative prevalence of the various tumor's forms in dogs at Sharkia Governorate and explains the epidemiology of canine cutaneous tumors in the animal data recovered from the Clinic hospital. The vast majority of neoplastic tumors on the skin were malignant (65.52%). However, benign tumors represent 34.48%. The most common types of tumors were malignant melanomas (17.24%), SQCC (13.79%), basal cell tumors (10.34%), trichoepithelioma (10.34%), transmissible venereal tumor (10.34%), pilomatricoma (10.34%), mast cell tumor (6.89%), histiocytoma (6.89%), miscellaneous cases as fat necrosis (6.89%), trichilemmoma (3.44%), and scalp paraganglioma (3.44%) in males (51.72%) and females (48.28%) dogs. The differences observed between some previously published results and the outcomes obtained here could be attributed to varying aetiologies and environmental factors that may contribute to the development of these neoplasms in the different geographical regions under consideration. AcknowledgmentsThe authors thank the Surgery Department in the Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt, for using the facilities and for the help. Conflict of interestThe authors declare that they have no conflict of interest. Author contributionsHZ performed a clinical examination and wrote the manuscript. AA, AEM, and MSA supervised the work, conducted a histopathological examination, and revised the final version of the manuscript. FundingThis research received no specific grant. Data availabilityThe data supporting the conclusions of this study are available upon reasonable request from the corresponding author. ReferencesAbramo, F., Pratesi, F., Cantile, C., Sozzi, S. and Poli, A. 1999. Survey of canine and feline follicular tumours and tumour-like lesions in central Italy. J. Small Anim. Pract. 40, 479–481. Altamura, G., Cuccaro, B., Eleni, C., Strohmayer, C., Brandt S. and Borzacchiello G. 2022. Investigation of multiple Felis catus papillomavirus types (-1/-2/-3/-4/-5/-6) DNAs in feline oral squamous cell carcinoma: a multicentric study. J. Vet. Med. Sci. 84, 881–884. Araújo, M.R., Preis, I.S., LavalleII, G.E., Cassali, G.D. and Ecco, R. 2012. Histomorphological and immunohistochemical characterization of 172 cutaneous round cell tumours in dogs. Small Animal Diseases. Pesq. Vet. Bras. 32, 772–780. Auler, P., Gamba, C., Horta, R., Lavalle, G. and Cassali, G. 2014. Metastatic well differentiated squamous cell carcinoma in the prepuce of a dog: a report of clinicopathological, immunophenotypic and therapeutic approach. Arq. Bras. Med. Vet. e Zootec. 66, 1317–1322. Avallone, G., Rasotto, R., Chambers, J.K., Miller, A.D., Behling-Kelly, E., Monti, P., Berlato, D., Valenti, P. and Roccabianca, P. 2021. Review of histological grading systems in veterinary medicine. Vet. Pathol. 58, 809–828. Bastos, R.S.C., Farias, K.M., Lopes C.E.B., Pacheco, A.C.L. and Viana, D.A. 2017. Estudo retrospectivo de neoplasias cutâneas em cães da região metropolitana de Fortaleza. Revta Bras. Hig. San. Anim. 11, 39–53. Bellamy, E. and Berlato, D. 2022. Canine cutaneous and subcutaneous mast cell tumours, a narrative review. J. Small Anim. Pract. 63, 497–511. Belluco, S., Brisebard E., Watrelot, D., Pillet, E., Marchal, T. and Ponce, F. 2013. Digital squamous cell carcinoma in dogs: epidemiological, histological, and imunohistochemical study. Vet. Pathol. 50, 1078–1082. Bertram, C.A., Bertram, B., Bartel, A., Ewringmann, A., Fragoso-Garcia, M.A., Erickson, N.A., Müller, K. and Klopfleisch, R. 2021. Neoplasia and tumor-like lesions in pet rabbits (Oryctolagus cuniculus): a retrospective analysis of cases between 1995 and 2019. Vet. Pathol. 58, 901–911. Bronden, L.B., Eriksen, T. and Kristensen, A.T. 2010. Mast cell tumours and other skin neoplasia in danish dogs--data from the danish veterinary cancer registry. Acta Vet. Scand. 52, 6. Burrai, G.P., Gabrieli, A., Polinas, M., Murgia, C., Becchere, M.P., Demontis P. and Antuofermo, E. 2023. Canine mammary tumor histopathological image classification via computer-aided pathology: an available dataset for imaging analysis. Animals (Basel) 13, 1563. Cabral, E.S. and Cassarino, D.S. 2007. Desmoplastic tricholemmoma of the eyelid misdiagnosed as sebaceous carcinoma, a potential diagnostic pitfall. J. Cutan. Pathol. 1, 22–25. Carroll, E.E., Fossey, S.L., Mangus, L.M., Carsillo, M.E., Rush, L.J., McLeod, C.G. and Johnson, T.O. 2010. Malignant pilomatricoma in 3 dogs. Vet. Pathol. 47, 937–943. Contreary, C.L., Outerbridge, C.A., Affolter, V.K., Kass, P.H. and White, S.D. 2015. Canine sterile nodular panniculitis, a retrospective study of 39 dogs. Vet. Dermatol. 26, 451–458. Das, U. and Das, A.K. 2000. Review of canine transmissible venereal sarcoma. Vet. Res. Commun. 24, 545–546. de Las Mulas, J.M., Millan, Y., Ruiz-Villamor, E., Bautista, M.J., Rollon, E. and De Los Monteros, A.E. 1999. Apoptosis and mitosis in tumours of the skin and subcutaneous tissues of the dog. Res. Vet. Sci. 66, 139–146. de Nardi, A.B., Dos-Santos-Horta, R., Fonseca-Alves, C.E., de Paiva, F.N., Linhares, L.C.M., Firmo, B.F., Ruiz Sueiro, F.A., de Oliveira, K.D., Lourenço, S.V., De Francisco Strefezzi, R. and Brunner, C.H.M. 2022. Diagnosis, prognosis and treatment of canine cutaneous and subcutaneous mast cell tumors. Cells 11, 618. Delcour, N.M., Klopfleisch, R., Gruber, A.D. and Weiss, A.T.A. 2013. Canine cutaneous histiocytomas are clonal lesions as defined by X-linked clonality testing. J. Comp. Pathol. 149, 192–198. DeManuelle, T.C. and Stannard, A.A. 1998. Difficult dermatologic diagnosis. Sterile nodular panniculitis. J. Am. Vet. Med. Assoc. 213, 356–357. Fernandez, N.J., West, K.H., Jackson, M.L. and Kidney, B.A. 2005. Immunohistochemical and histochemical stains for differentiating canine cutaneous round cell tumors. Vet. Pathol. 42, 437–445. Fischer, M.C., Taeymans, O.N., Monti, P., Scurrell, E.J., Eddicks, L., Matiasek, K. and Busse, C. 2018. Orbital paraganglioma in a dog. Tierarztl Prax Ausg K Kleintiere Heimtiere. 46, 410–415. Friedlein, R.B., Carter, A.J., Last, R.D. and Clift, S. 2017. The diagnosis of bilateral primary renal paragangliomas in a cat. J. S. Afr. Vet. Assoc. 88, e1–6. Galac, S. and Korpershoek, E. 2017. Pheochromocytoma s and paragangliomas in humans and dogs. Vet. Comp. Oncol. 15, 1158–1170. German, A.J., Foster, A.P., Holden, D., Hotston Moore, A., Day, M.J. and Hall, E.J. 2003. Sterile nodular panniculitis and pansteatitis in three weimaraners. J. Small Anim. Pract. 44, 449–455. Goldschmidt, M.H. and Goldschmidt, K.H. 2016. Epithelial and melanocytic tumors of the skin. Tumors in domestic animals, 5th ed. John Wiley & Sons., pp: 88–141. Goldschmidt, M.H. and Goldschmidt, K.H. 2017. Epithelial and melanocytic tumors of the skin. In Tumors in domestic animals. Ed. Meuten D.J.,. 5th ed. Ames, IA: Wiley & Sons, Inc., pp: 88–141, 976–978. Goldschmidt, M.H. and Hendrick, MJ. 2002. Tumors of the skin and soft tissues. In Tumors in domestic animals. Ed. Meuten D.J., 4th ed., Ames, Iowa: Iowa State University Press, pp: 45–117. Gombert, A., Diana, A., Hecht, S., Nicoli, S., Fracassi, F., Mortier, J., Reyes-Gomez, E. and Pey, P. 2022. Imaging features of retroperitoneal extra-adrenal paragangliomas in 10 dogs. Vet. Radiol. Ultrasound. 63, 393–402. Graf, R., Pospischil, A., Guscetti, F., Meier, D., Welle, M. and Dettwiler, M. 2018. Cutaneous tumors in swiss dogs: retrospective data from the swiss canine cancer registry, 2008–2013. Vet. Pathol. 55, 809–820. Gross, T.L., Ihrke, P.J., Walder, E.J. and Affolter, V.K. 2008. Skin diseases of the dog and cat: clinical and histopathologic diagnosis, 2nd ed. John Wiley & Sons., pp: 562–603. Hashish, H. and Kamal, R. 2015. Effect of curcumin on the expression of Caspase-3 and Bcl-2 in the spleen of diabetic rats. J. Exp. Clin. Anat. 14, 18–23. Hassan, B., Al-Mokaddem, A., Abdelrahman, H., Samir, A. and Mousa, M. 2022. Cutaneous tumors in dogs: a retrospective epidemiological and histological study of 112 cases. Adv. Anim. Vet. Sci. 10, 170–182. Hsu, S.-M., Raine, L. and Fanger, H. 1981. A comparative study of the peroxidase-antiperoxidase method and an avidin-biotin complex method for studying polypeptide hormones with radioimmunoassay antibodies. Am. J. Clin. Pathol. 75, 734–738. Kim, H.J., Kang, M.H., Kim, J.H., Kim, D. and Park, H.M. 2011. Sterile panniculitis in dogs: new diagnostic findings and alternative treatments. Vet. Dermatol. 22, 352–359. Kiupel, M. and Camus, M. 2019. Diagnosis and prognosis of canine cutaneous mast cell tumors. Vet. Clin. North Am. Small Anim. Pract. 49, 819–836. Kok, M.K., Chambers, J.K., Dohata, A., Uchida, K., Nishimura, R. and Nakayama, H. 2017. Desmoplastic tricholemmoma in a dog. J. Vet. Med. Sci. 79, 984–987. Kok, M.K., Chambers, J.K., Tsuboi, M., Nishimura, R., Tsujimoto, H., Uchida, K. and Nakayama, H. 2019. Retrospective study of canine cutaneous tumors in Japan, 2008–2017. J. Vet. Med. Sci. 81, 1133–1143. Lee Gross, T.L., Ihrke, P.J., Walder, E.J. and Affolter, V.K. 2005. Skin diseases of the dog and cat, clinical and histopathologic diagnosis, 2nd ed. Oxford, UK: Blackwell, pp: 624–625, 637–638. Lee, E., Kim, A., Lee, E., Park, J. and Jeong, K. 2016. Malignant pilomatricoma in a young dog. Acta Vet.-Beograd 66, 556–561. Lima, S.R., Stocco, M.B., Rondelli, L.A.S., Silva, G.S., Lopes, R.S., Furlan, F.H., Colodel, E.M. and Pescador, C.A. 2018. Neoplasmas cutâneas em cães , 656 casos (2007–2014) em Cuiabá, MT. Pesq. Vet. Bras. 38, 1405–1411. Łojszczyk, A., Łopuszyński, W., Szadkowski, M., Orzelski, M. and Twardowski, P. 2021. Aggressive squamous cell carcinoma of the cranium of a dog. BMC Vet. Res. 17, 1–8. Mahajan, S., Barker, C.A., Singh, B. and Pandit-Taskar, N. 2019. Clinical value of 18F-FDG-PET/CT in staging cutaneous squamous cell carcinoma. Nucl. Med. Commun. 40, 744– 751. Mai, W., Seiler, G.S., Lindl-Bylicki, B.J. and Zwingenberger, A.L. 2015. CT and MRI features of carotid body paragangliomas in 16 Dogs. Vet. Radiol. Ultrasound. 56, 374–383. Martinez, M.A.R., Francisco, G., Cabral, L.S., Ruiz, R.G. and Festa-Neto, C. 2006. Genética molecular aplicada ao câncer cutâneo não melanoma. Anais Bras. Dermatol. 81, 405–419. Martins, A.L., Canadas-Sousa, A., Mesquita, J.R., Dias-Pereira, P., Amorim, I. and Gärtner, F. 2022. Retrospective study of canine cutaneous tumors submitted to a diagnostic pathology laboratory in Northern Portugal (2014–2020). Canine Med. Genet. 9(1), 2. McKee, P. 1999. Essential skin pathology. 2nd edition. London: Mosby International Ltd. Mochizuki, H., Thomas, R., Moroff, S. and Breen, M. 2017. Genomic profiling of canine mast cell tumors identifies DNA copy number aberrations associated with KIT mutations and high histological grade. Chromosome Res. 25, 129–143. Moore, P.F. 2014. A review of histiocytic diseases of dogs and cats. Vet. Pathol. 51, 167–184. Muller, C., Guaguere, E., Muller, A., Guaguere, J. and Degorce-Rubiales, F. 2022. Canine sterile nodular panniculitis associated with digestive disease: a report of 4 cases. Rev. Vét. Clin. 57, 109–117. Murphy, S. 2006. Skin neoplasia in small animals. 1. Principles of diagnosis and management. Clin. Pract. 28, 266–277. Murphy, S., Sparkes, A.H., Blunden, A.S., Brearley, M.J. and Smith, K.C. 2006. Effects of stage and number of tumours on prognosis of dogs with cutaneous mast cell tumours. Vet. Rec. 158, 287–291. Murphy, S., Sparkes, A.H., Smith, K.C., Blunden, A.S. and Brearley, M.J. 2004. Relationships between the histological grade of cutaneous mast cell tumours in dogs, their survival, and the efficacy of surgical resection. Vet. Rec. 154, 743–746. Nak, D., Misirlioglu, D., Nak, Y., Seyrek-Intas, K. and Tek, H.B. 2004. Transmissible venereal tumor with mammary gland metastase in a bitch. Vet. Bilimleri Derg. 20, 99–102. Nak, D., Nak, Y., Cangul, I.T. and Tuna, B. 2005. A Clinico-pathological study on the effect of vincristine on transmissible venereal tumour in dogs. J. Vet. Med. A Physiol. Pathol. Clin. Med. 52, 366–370. Nemec, A., Murphy, B., Kass P. and Verstraete F. 2012. Histological subtypes of oral non-tonsillar squamous cell carcinoma in dogs. J. Comp. Pathol. 147, 111–120. Ortloff, A., Bustamante, F., Molina, L., Ojeda, J., Figueroa, C. and Ehrenfeld, P. 2020. Kallikrein-related peptidase 5 (KLK5) expression and distribution in canine cutaneous squamous cell carcinoma. J. Comp. Pathol. 174, 113–119. Otrocka-Domagała, I., Paździor-Czapula, K., Fiedorowizc J, Mikiewicz, M., Piotrowska, A. and Gesek, M. 2022. Cutaneous and subcutaneous tumours of small pet mammals-retrospective study of 256 Cases (2014–2021). Animals (Basel) 12, 965. Pakhrin, B., Kang, M.S., Bae, I.H., Park, M.S., Jee, H., You, M.H., Kim, J.H., Yoon, B.I., Choi, Y.K. and Kim, D.Y. 2007. Retrospective study of canine cutaneous tumors in Korea. J. Vet. Sci. 8, 229–236. Paździor-Czapula, K., Rotkiewicz, T., Otrocka-Domagała, I., Gesek, M. and Śmiech A. 2015 Morphology and immunophenotype of canine cutaneous histiocytic tumours with particular emphasis on diagnostic application. Vet. Res. Commun. 39, 7–17. Rodrigues, F.R.N., da Silva Freire, J.M., Fidelis, L.A.P., Pereira, A.A.B.G., de Sousa, D.E.R., Wilson, T.M., Soto-Blanco, B. and de Castro, M.B. 2020. Paraganglioma of the tongue in a chow chow dog: a comparison with the human counterpart and literature review. Front. Vet. Sci. 27, 422. Sabattini, S., Scarpa, F., Berlato, D. and Bettini, G. 2015. Histologic grading of canine mast cell tumor: is 2 better than 3? Vet. Pathol. 52, 70–73. Sakai, H., Goto, M. and Komatsu, T. 2017. Basal cell adenocarcinoma in the gland of the third eyelid of a brown bear (Ursus arctos). J. Vet. Med. Sci. 79, 1348–1351. Sano, Y., Miyazaki, M., Yaegashi, R., Okamoto, M., Masuko, A., Maehara, S. and Matsuda, K. 2019. Basal cell adenocarcinoma on bulbar conjunctiva of third eyelid in a dog. J. Vet. Med. Sci. 81, 30–34. Schweiger, E., Spann, C.T., Weinberg, J.M. and Ross, B. 2004. A case of desmoplastic trichilemmoma of the lip treated with Mohs surgery. Dermatol. Surg. 30, 1062–1064. Schwens, C., Thom, N. and Moritz, A. 2011. Reaktive und neoplastische histiozytäre Erkrankungen beim Hund. Tierarztl Prax K. 3, 176–190. Sierra Matiz, O.R., Viera, R.B., Jark, P.C., Dos Anjos, D.S., Monteiro, J.E.H., Matsui, A., Vasconcelos, R.O., Sueiro, F.R., Calazans, S.G. and Tinucci-Costa, M. 2019. Squamous cell carcinoma of unknown primary origin in a dog presenting with bone metastasis. J. Vet. Med. Sci. 81, 1177–1181. Silvestri, S., Porcellato, I., Mechelli, L., Menchetti, L., Rapastella, S. and Brachelente, C. 2019. Tumor thickness and modified Clark level in canine cutaneous melanocytic tumors. Vet. Pathol. 56, 180–188. Singh, J., Rana, J.S., Sood, N., Pangawkar, G.R. and Gupta, P.P. 1996. Clinico-pathological studies on the effect of different anti-neoplastic chemotherapy regimens on transmissible venereal tumours in dogs. Vet. Res. Commun. 20, 71–81. Smedley, R.C., Bongiovanni, L., Bacmeister, C., Clifford, C.A., Christensen, N., Dreyfus, J.M., Gary, J.M., Pavuk, A., Rowland, P.H., Swanson, C. and Tripp, C. 2022. Diagnosis and histopathologic prognostication of canine melanocytic neoplasms: a consensus of the oncology-pathology working group. Vet. Comp. Oncol. 20, 739–751. Smedley, R.C., Spangler, W.L., Esplin, D.G., Kitchell, B.E., Bergman, P.J., Ho, H.Y., Bergin, I.L. and Kiupel, M. 2011. Prognostic markers for canine melanocytic neoplasms: a comparative review of the literature and goals for future investigation. Vet. Pathol. 48, 54–72. Stefanello, D., Buracco, P., Sabattini, S., Finotello, R., Giudice, C., Grieco, V., Iussich, S., Tursi, M., Scase, T., Di Palma, S. and Bettini, G. 2015. Comparison of 2- and 3-category histologic grading systems for predicting the presence of me`tastasis at the time of initial evaluation in dogs with cutaneous mast cell tumors, 386 cases (2009–2014). J. Am. Vet. Med. Assoc. 246, 765–769. Stevenson, V.B., Klahn, S., LeRoith, T. and Huckle, W.R. 2023. Canine melanoma, a review of diagnostics and comparative mechanisms of disease and immunotolerance in the era of the immunotherapies. Front. Vet. Sci. 9, 1046636. Stockhaus, C., Teske, E., Rudolph, R. and Werner, H.G. 2001. Assessment of cytological criteria for diagnosing basal cell tumours in the dog and cat. J. Small Anim. Pract. 42, 582–586. Suvarna, S.K., Layton, C. and Bancroft, J.D. 2013. Bancroft's theory and practice of histological techniques, 8th ed. Churchill Livingstone Elsevier., pp: 126–138. Tamošiūnas, M., Čiževskis, O., Viškere, D., Melderis, M., Rubins, U. and Cugmas B. 2022. Multimodal approach of optical coherence tomography and raman spectroscopy can improve differentiating benign and malignant skin tumors in animal patients. Cancers (Basel) 14, 2820. Treggiari, E. and Elliott, J.W. 2017. Malignant pilomatricoma in a dog with local and distant metastases treated with chemotherapy and bisphosphonates. Open Vet. J. 7, 208–213. van der Weyden, L., Brenn, T., Patton, E.E., Wood, G.A. and Adams, D.J. 2020. Spontaneously occurring melanoma in animals and their relevance to human melanoma. J. Pathol. 252, 4–21. Warland, J. and Dobson, J. 2013. Breed predispositions in canine mast cell tumour: a single centre experience in the United Kingdom. Vet. J. 197, 496–498. Wiener, D.J. 2021. Histologic features of hair follicle neoplasms and cysts in dogs and cats, a diagnostic guide. J. Vet. Diagn. Invest. 33, 479–497. Yamashita-Kawanishi, N., Chang, C.Y., Chambers, J.K., Uchida, K., Sugiura, K., Kukimoto, I., Chang, H.W. and Haga, T. 2021. Comparison of prevalence of Felis catus papillomavirus type 2 in squamous cell carcinomas in cats between Taiwan and Japan. J. Vet. Med. Sci. 83, 1229–1233. | ||
| How to Cite this Article |
| Pubmed Style Zwai HA, Al-Attar AR, Morsi AE, Abou-El-Fetouh MS. Pathological, histochemical, and immune-histochemical studies on some canine-skin neoplasm at Sharkia province, Egypt. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 481-499. doi:10.5455/OVJ.2024.v14.i1.44 Web Style Zwai HA, Al-Attar AR, Morsi AE, Abou-El-Fetouh MS. Pathological, histochemical, and immune-histochemical studies on some canine-skin neoplasm at Sharkia province, Egypt. https://www.openveterinaryjournal.com/?mno=182392 [Access: October 06, 2024]. doi:10.5455/OVJ.2024.v14.i1.44 AMA (American Medical Association) Style Zwai HA, Al-Attar AR, Morsi AE, Abou-El-Fetouh MS. Pathological, histochemical, and immune-histochemical studies on some canine-skin neoplasm at Sharkia province, Egypt. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 481-499. doi:10.5455/OVJ.2024.v14.i1.44 Vancouver/ICMJE Style Zwai HA, Al-Attar AR, Morsi AE, Abou-El-Fetouh MS. Pathological, histochemical, and immune-histochemical studies on some canine-skin neoplasm at Sharkia province, Egypt. Open Vet J. (2024), [cited October 06, 2024]; 14((1) (Zagazig Veterinary Conference)): 481-499. doi:10.5455/OVJ.2024.v14.i1.44 Harvard Style Zwai, H. A., Al-Attar, . A. R., Morsi, . A. E. & Abou-El-Fetouh, . M. S. (2024) Pathological, histochemical, and immune-histochemical studies on some canine-skin neoplasm at Sharkia province, Egypt. Open Vet J, 14 ((1) (Zagazig Veterinary Conference)), 481-499. doi:10.5455/OVJ.2024.v14.i1.44 Turabian Style Zwai, Hatem A., Al-Sayed R. Al-Attar, Abdulla E. Morsi, and Moustafa S. Abou-El-Fetouh. 2024. Pathological, histochemical, and immune-histochemical studies on some canine-skin neoplasm at Sharkia province, Egypt. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 481-499. doi:10.5455/OVJ.2024.v14.i1.44 Chicago Style Zwai, Hatem A., Al-Sayed R. Al-Attar, Abdulla E. Morsi, and Moustafa S. Abou-El-Fetouh. "Pathological, histochemical, and immune-histochemical studies on some canine-skin neoplasm at Sharkia province, Egypt." Open Veterinary Journal 14 (2024), 481-499. doi:10.5455/OVJ.2024.v14.i1.44 MLA (The Modern Language Association) Style Zwai, Hatem A., Al-Sayed R. Al-Attar, Abdulla E. Morsi, and Moustafa S. Abou-El-Fetouh. "Pathological, histochemical, and immune-histochemical studies on some canine-skin neoplasm at Sharkia province, Egypt." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 481-499. Print. doi:10.5455/OVJ.2024.v14.i1.44 APA (American Psychological Association) Style Zwai, H. A., Al-Attar, . A. R., Morsi, . A. E. & Abou-El-Fetouh, . M. S. (2024) Pathological, histochemical, and immune-histochemical studies on some canine-skin neoplasm at Sharkia province, Egypt. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 481-499. doi:10.5455/OVJ.2024.v14.i1.44 |