| Original Article | ||
Open Vet J. 2022; 12(5): 632-638 Open Veterinary Journal, (2022), Vol. 12(5): 632–638 Original Research Investigation of botulism in free-range duck farming in the Mekong Delta, VietnamDuc-Hien Nguyen1*, Thu-Tam Nguyen1 and Huu-Thanh Nguyen2,31Department of Veterinary Medicine, Can Tho University, Can Tho, Vietnam 2An Giang University, An Giang, Vietnam 3Vietnam National University of Ho Chi Minh City, Ho Chi Minh City, Vietnam *Corresponding Author: Duc-Hien Nguyen. Department of Veterinary Medicine, Can Tho University, Can Tho, Vietnam. Email: ndhien [at] ctu.edu.vn Submitted: 25/04/2022 Accepted: 10/08/2022 Published: 09/09/2022 © 2022 Open Veterinary Journal
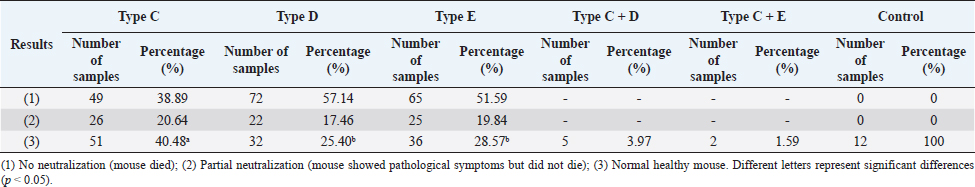
AbstractBackground: One of the most common diseases in free-range ducks in the Mekong Delta is botulism. Botulism is a poultry disease caused by botulinum exotoxin of Clostridium botulinum. Aim: To evaluate the prevalence of botulism in free-range ducks in the Mekong Delta and the risk of infection by determining the presence of C. botulinum in the farming environment. Methods: Research was carried out on 200 duck flocks with 187,050 individuals raised freely in the fields in the provinces of the Mekong Delta, including An Giang, Can Tho, Hau Giang, and Kien Giang. The ducks were diagnosed with botulism based on clinical symptoms. To demonstrate the presence of botulinum neurotoxins and identify serotype, samples of serum and/or gut were analyzed by mouse bioassay. Samples of soil (n=600), water (n=600), crabs (n=216), and snails (n=400) were taken from the grazing regions for C. botulinum analysis by PCR assay. Results: There were 1.19% (2,235/187,050) free-range ducks in the Mekong Delta positive for botulism. Clinical symptoms of botulism including limberneck, drooping eyelids–enlarged pupils, and leg paralysis were prevalent across free-range ducks, with the frequency of 87.92% (1,965/2,235), 90.07% (2,013/2,235), and 79.78% (1,783/2,235), respectively. The lesions of pulmonary edema–hemorrhage, hemorrhagic liver, and gas-producing intestines were common, accounting for 86.19% (362/420), 95.48% (401/420), and 92.14% (387/420), respectively. Botulin toxin type C was found in a considerable number of serum samples, accounting for 40.48% (51/126). Meanwhile, the percentage of serum samples containing botulin toxin types E and D was 28.57% (36/126) and 25.40% (32/126), respectively. Clostridium botulinum was detected in the farming environment specifically 17.5% (105/600) in soil, 19.67% (118/600) in water, 8.33% (18/216) in crabs, and 3.00% (12/400) in snails. Conclusion: The free-range ducks in the Mekong Delta were at high risk of botulism because of the latent presence of C. botulinum in the farming environment. Keywords: Botulism, Clostridium botulinum, Free-range ducks, Limberneck, The Mekong Delta. IntroductionThe Mekong Delta is the region in southwestern Vietnam where the Mekong River approaches and empties into the sea through a network of distributaries. The Mekong Delta has an interlaced river system and rich aquatic flora and fauna. The provinces of An Giang, Can Tho, Hau Giang, and Kien Giang belong to the Mekong Delta, are known for its large rice-growing area, and has favorable conditions for free-range duck farming. Ducks are allowed to graze and scavenge on newly harvested rice fields. They forage for food such as rice grains falling in the fields, crabs, and snails in the ponds and ditches. This farming method has the advantage of exploiting available natural foods such as crabs and snails in the ponds as well as scattering rice grains, which significantly lowers the farming expenditures. However, this farming practice is a lurking risk to veterinary supervision due to difficulties in controlling the grazing environment. Therefore, there is a high probability of disease outbreaks. One of the most common diseases among the free-range ducks in the Mekong Delta is “limberneck” or “flu” according to the local naming scheme. Limberneck known as botulism is a poultry disease caused by botulinum exotoxin of Clostridium botulinum (Harrigan, 2008). Clostridium botulinum is an absolutely anaerobic and spore-forming bacterium. Clostridium botulinum is commonly found in soil, especially in sedimentary areas, carcasses of mollusks, and in the intestines of both terrestrial and aquatic animals. Clostridium botulinum produces botulinum neurotoxins (BoNTs)—extremely poisonous protein toxins that can completely destroy the central nervous system (Harris, 2016). Ducks mistakenly consuming this toxin will develop symptoms such as soft neck paralysis, eyelid paralysis, wing and leg paralysis, and high mortality, resulting in significant economic losses for farmers (Le Maréchal et al., 2016). Currently, there have been many studies on botulism in animals (Souillard et al., 2014; Silva et al., 2016). However, the research and information on botulism in duck in the Mekong Delta are still quite limited. Therefore, this study investigated the status of botulism in the duck farming environment to offer relatively extensive information on the disease caused by C. botulinum in free-range ducks in the Mekong Delta. Materials and MethodsSample collectionFrom 2016 to 2020, a total of 200 duck flocks with 187,050 individuals raised freely in the fields in the provinces of the Mekong Delta, including An Giang, Can Tho, Hau Giang, and Kien Giang, were investigated (Fig. 1). The ducks were diagnosed with clinical botulism based on typical signs such as neck paralysis, leg paralysis, wing paralysis, etc. Samples of soil, water, and shellfish as a naturally available food source for ducks at the grazing regions were sampled for analysis of the infection of C. botulinum. Determination of BoNTsBoNTs were detected using the method previously described by Seyboldt et al. (2015). Two mice were injected intraperitoneally with 0.5 ml of serum and/or intestinal extracts in gelatin buffer (2 g/l gelatin, 4 g/l Na2HPO4). Two more mice were injected with the trypsin-treated extract to activate certain types of potentially nonactivated BoNTs. Another two mice received the extract which was heated to 100°C for 10 minutes to inactivate BoNTs as control (Cook et al., 1998). The mice were found to develop BoNTs-specific symptoms such as wasp waist, labored abdominal breathing, paralysis, and death within about 4 days. In the case of BoNTs-specific symptoms and death, mouse bioassay seroclassification must be abided to demonstrate the presence of BoNTs and subsequently identify the serotype according to existing procedures (CDC, 1998). Mice that received a sample of the neurotoxin and a specific antitoxin (A, B, C, D, E, or F) survived. Determination of C. botulinumAmounts of at least 20 g for soil, at least 100 ml for water samples, and 10–15 crabs or snails (washed with detergent, rinsed, and crushed) were analyzed. The washing step was carried out to ensure that any C. botulinum detected was present inside the crabs and snails. Culture conditions and DNA extraction were performed according to Souillard et al. (2014). Samples were diluted 1/10 in pre-reduced trypticase–peptone–glucose–yeast extract broth and cultured at 37°C ± 1°C for 4 days under anaerobic conditions. After incubation, 1 ml of each enrichment broth was collected. Cells were pelleted by centrifugation at 6,500 g for 10 minutes and subjected to DNA extraction using GeneJET Genomic DNA Purification K0721, K0722 (Thermo Scientific) according to the manufacturer’s instructions. The occurrence of Clostridium spp. was detected using primers Clos58-f (5′-AAAGGAAGATTAATACCGCATA-3′) and Clos780-r (5′-ATCTTGCGACCGTACTCCCC-3) (Amit-Romach et al., 2004). PCR method for C. botulinum serotype detection was carried out as described by Lindström et al. (2001) and Nakamura et al. (2013).
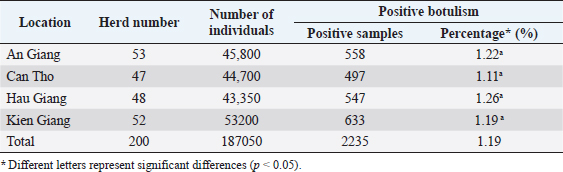
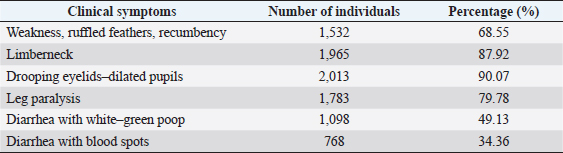
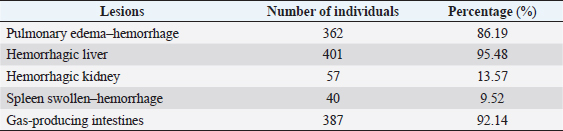
Fig. 1. Location map of the surveyed regions in the Mekong Delta. Statistical analysisThe data were processed using Excel and Minitab 16.0 software. The chi-squared test was used to compare the bacterial infection rates in different localities and different types of samples (p < 0.05). Ethical approvalEthical approval is not required for this study. ResultsFree-range ducks affected by botulismBotulism was observed in free-range ducks in the surveyed areas of the Mekong Delta (Table 1). The survey showed that, in Hau Giang province, the highest rate of botulism in free-range ducks was 1.26% (547/43,350). However, there was no statistically significant variation in the percentage of botulism-positive ducks in the surveyed areas (p > 0.05). Clinical symptoms and gross lesions of botulism in the botulism-positive ducksThe occurring frequency of clinical symptoms in the botulism-positive ducks is presented in Table 2. The findings showed that the symptoms of limberneck, drooping eyelids–dilated pupils, and leg paralysis appeared at a relatively high frequency of 87.92% (1,965/2,235), 90.07% (2,013/2,235), and 79.78% (1,783/2,235), respectively. The hemorrhagic liver and lung lesions accounted for 95.48% (401/420) and 86.19% (362/420) of gross lesions in the botulism-positive ducks, respectively. In addition, gastritis also constituted a high rate of 92.14% (387/420) (Table 3). Determination of BoNTsThe serological reaction for BoNTs type determination by standard antitoxin is the standard method for accurate results due to the specificity of the reaction. A total of 126 samples that were lethal to mice were determined BoNTs type via standard antitoxin neutralization and presented in Table 4. It can be seen that the percentage of serum samples containing BoNTs type C was relatively high, accounting for 40.48% (51/126), followed by the serum sample containing botulin toxin type E with the rate of 28.57% (36/126), and the lowest was the serum sample containing botulin toxin type D with 25.40% (32/126). In particular, the experimental results also showed that the combined presence of type C + D was 3.97% (5/126) and type C + E was 1.59% (2/126). This concludes that botulism in free-range ducks in the Mekong Delta is mainly caused by C. botulinum virulence types C, D, and E. In particular, the experimental results also showed the combined presence among type C + D and among type C + E. Table 1. Prevalence of botulism in free-range ducks in the Mekong Delta.
Table 2. Frequency of clinical symptoms in the botulism-positive ducks (n=2,235).
Table 3. Frequency of appearance of gross lesions in botulism-positive ducks (n=420).
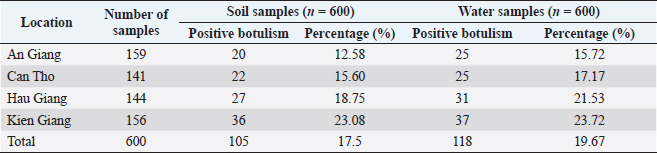
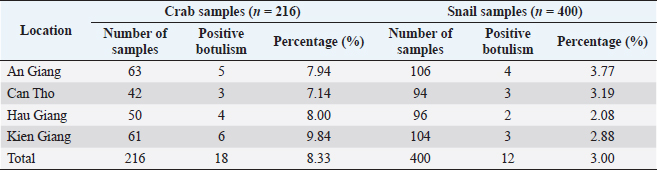
Prevalence of C. botulinum in the environment of free-range duck farmingRegarding the infection of C. botulinum, the samples of soil, water, crabs, and snails were collected to determine C. botulinum DNA. Table 5 shows that there are C. botulinum DNA existing in 105/600 farmland samples tested, accounting for 17.5%. Meanwhile, 118/600 samples of field water were observed to contain C. botulinum DNA, accounting for 19.67%. The analysis results also indicated that the presence of C. botulinum DNA in crab and snail samples was 8.33% (18/216) and 3.00% (12/400), respectively, (Table 6). DiscussionStatus of botulism in free-range ducksClostridium botulinum is widely distributed in the natural environment. It can be present in plant and animal carcasses, in soil, especially in sedimentary soils, swamps, and flooded poultry farming areas (Uzal et al., 2016). The Mekong Delta has natural characteristics and a favorable climate for the survival and growth of C. botulinum. Free-range duck farming in the fields usually takes place in the dry season after the rice harvest. The amount of water in the lakes and fields where ducks grazed is low at this time of year. When combined with the increased temperature, it accelerates the decomposition of plant and animal carcasses, creating an ideal habitat for C. botulinum to thrive. Supporting this hypothesis, botulism in free-range ducks was found in all surveyed areas. It could be suggested that the free-range ducks in the Mekong Delta are likely to be infected with botulism caused by C. botulinum. BoNTs is a naturally occurring protein toxin that is highly virulent. It is a neurotoxin with an affinity for motor neurons. Once absorbed, the toxin binds to the presynaptic membranes of cholinergic nerve endings. The toxin enters cells and blocks the release of acetylcholine across neuromuscular junctions, leading to paralysis and eventual death for heart and respiratory failure (Dohms and Cloud, 1982). The current study found that the common clinical symptoms of botulism in free-range ducks were limberneck, drooping eyelids–dilated pupils, and leg paralysis. Shin et al. (2010) also described a botulism outbreak in wild waterbirds in Incheon (Korea), through a survey of 2,000 individuals with the majority of the sick birds showing the symptoms of flaccid neck paralysis and inner eyelid paralysis. The authors believed that BoNTs destroy the central nervous system and inhibit neuromuscular and motor neurons, causing ducks to become not only weak, but also paralyzed. The symptoms of weakness, ruffled feathers, and recumbency accounted for 68.55%. Leg weakness and paralysis may be the only sign of mild poisoning or early-stage disease. Gastrointestinal symptoms resulting in white–green diarrhea and bloody diarrhea occurred with the rates of 49.13% and 34.36%, respectively. Our findings are comparable to those of Shin et al. (2010), who said that the symptoms of bloody diarrhea and some ducks with mucus coming out of their mouth accounted for 48.10% of botulism ducks. Table 4. Determination of BoNTs type (n=126).
Table 5. Prevalence of C. botulinum infection in soil and water samples.
Table 6. Prevalence of C. botulinum infection in crab and snail samples.
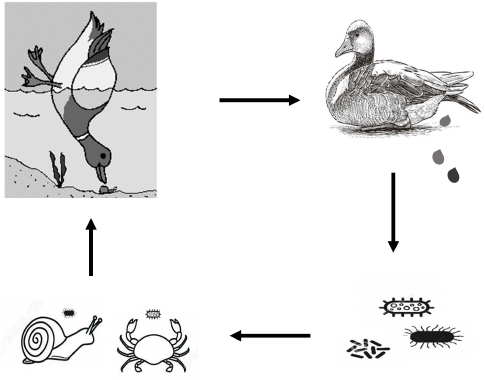
BoNTs of C. botulinum are highly virulent. Ducks could be exposed to BoNTs present in their feed or habitat. Because it is hardly damaged by gastric juice, destroying it by gastric juices is difficult. BoNTs penetrate quickly into the blood and disperse throughout the body. BoNTs enter the cells of various tissues beginning with those of the central nervous system, resulting in clinical manifestations arising from the medulla oblongata. If the incubation period is from 6 to 8 hours, ducks can die suddenly with little appearance of lesions. In case the incubation period is longer (8–10 days), lesions appear commonly in the internal organs. We found the lesions of pulmonary edema–hemorrhage, hemorrhagic liver, and gas-producing intestines accounted for a high proportion in free-range ducks positive for botulism. Jensen and Duncan (1980) also found that mostly the mallard duck (Anas platyrhynchos) died by botulinum toxicity due to respiratory paralysis, pulmonary and hepatic hemorrhage, and pulmonary edema. Analysis showed that botulinum toxin type C accounted for the highest percentage in serum samples, followed by types E and D. These results were consistent with previous reports. Botulism are commonly associated with BoNTs type C, type C + D, or type E in waterfowl and seabirds (Lafrancois et al., 2011; Vidal et al., 2011). Type C botulism is believed to have caused the deaths of more than 10,000 seabirds on Herring Gulls, an island in the Blekinge archipelago in southeastern Sweden (Neimanis et al., 2007). It is also associated with about 2,000 species of wild aquatic birds in Incheon, Korea (Woo et al., 2010). This suggested that infection with botulinum toxin type C is dominant in poultry. In this study, it can be seen that the presence of C. botulinum in soil, water, crabs, and snails was quite high. The high presence of C. botulinum in the soil and water may be due to this bacterium having accumulated in the environment for years. The presence of C. botulinum in soil and water samples from farming areas has been documented (Souillard et al., 2014; Le Maréchal et al., 2018). The current study also found C. botulinum in snails and crabs samples collected in duck grazing areas. Crustaceans species could harbor C. botulinum in their intestines. Furthermore, Clostridium spp. often presents in sedimentary lands, swamps, regularly flooded areas. This could possibly affect the bacterial infection rate in snails and crabs (U.S. Food & Drug Administration, 2019). The existence of C. botulinum in farming areas warns of a very high risk of BoNTs poisoning for free-range ducks.
Fig. 2. Circulatory risk of BoNTs toxicity in free-range ducks. Clostridium botulinum in duck-farming environmentThe favorable natural conditions for agricultural production in the Mekong Delta have had many important influences on traditional poultry production in the region. Based on agricultural farming, poultry production systems have been applied, especially the combined duck–rice production method in the Mekong Delta. In this system, during the rice harvest, the grazers enter the duck fields to take advantage of the scattered rice and all the aquatic animals that the ducks may devour. Due to the high prevalence of C. botulinum in crabs and snails, the potential risk of BoNTs poisoning is possible. In addition, free-range ducks could also become a source of disease spreading from place to place due to the characteristics of free-range farming. Droppings from sick ducks that contain C. botulinum are excreted into the environment. These bacteria infect water supplies, crabs, and snails. Then, healthy ducks may be ingest these infected crabs and snails. As a result, they will be infected with BoNTs (Fig. 2). The current study only analysed environmental samples in contact with and/or eaten by free-range ducks. However, in environmental samples BoNTs presence was not investigated. The detection of gene-producing BoNTs, but without the confirmation that the toxins were effectively produced, meaning a possible relationship can certainly be hypothesized. Therefore, it is also necessary to have further studies to confirm this relationship with more certainty. ConclusionBoNTs toxin of C. botulinum was the main cause of limberneck in free-range ducks in the Mekong Delta. The neutralization reaction between the toxin in the serum of sick ducks with the standard antitoxin identified the highest presence of BoNTs type C, accounting for about 40.48% (51/126). Type E and D toxins were also detected with the rate of 28.57% (36/126) and 25.40% (32/126), respectively. In particular, sera from infected ducks were found to be positive for type C + D at 3.97% (5/126) and type C + E at 1.59% (2/126). When examining the samples from the duck grazing environment, the presence of C. botulinum in soil, water, crabs and snails was detected at the rate of 17.5% (105/600), 19.67% (118/600), 8.33% (18/216), and 3.00% (12/400), respectively. The results showed that the free-range ducks in the Mekong Delta were at high risk of botulism because of the latent presence of C. botulinum in the farming environment. AcknowledgmentsThe authors are grateful to the free-range duck farmers and veterinarians for their participation in the study. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ ContributionsConceptualization, T.-T.N., D.-H.N., and H.-T.N.; methodology, T.-T.N. and D.-H.N.; formal analysis, H.-T.N.; investigation, T.-T.N. and D.-H.N.; data curation, T.-T.N. and D.-H.N.; writing-original draft preparation, T.-T.N. and H.-T.N.; writing-review and editing, H.-T.N.; supervision, D.-H.N. and D.-H.N. All authors have read and agreed to the published version of the manuscript. ReferencesAmit-Romach, E., Sklan, D. and Uni, Z. 2004. Microflora ecology of the chicken intestine using 16S ribosomal DNA primers. Poul. Sci. 83, 1093–1098. CDC. 1998. Botulism in the United States, 1899-1996 [microform]: handbook for epidemiologists, clinicians, and laboratory workers. Atlanta, GA: U.S. Dept. of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Center for Infectious Diseases, Division of Bacterial and Mycotic Diseases. Cook, L.V., Lee, W.H., Lattuada, C.P. and Ransom, G.M. 1998. Methods for the detection of Clostridium botulinum toxins in meat and poultry products. In Microbiology laboratory guidebook. Eds., Dey, B.P. and Lattuada, C.P. Washington, DC: USDA/FSIS. Dohms, J.E. and Cloud, S.S. 1982. Susceptibility of broiler chickens to Clostridium botulinum type C toxin. Avian. Dis. 26, 89–96. Harrigan, K. 2008. Botulism in broiler chickens. Aust. Vet. J. 56, 603–605. Harris, A. 2016. Clostridium botulinum. In Encyclopedia of food and health. Eds., Caballero, B., Finglas P.M. and Toldrá, F. Oxford, UK: Academic Press, pp: 141–145. Jensen, W.I. and Duncan, R.M. 1980. The susceptibility of the mallard duck (Anas platyrhynchos) to Clostridium botulinum C2 toxin. Jap. J. Med. Sci. Biol. 33, 81–86. Lafrancois, B.M., Riley, S.C., Blehert, D.S. and Ballmann, A.E. 2011. Links between type E botulism outbreaks, lake levels, and surface water temperatures in Lake Michigan, 1963–2008. J. Great. Lakes. Res. 37, 86–91. Le Maréchal, C., Fourour, S., Ballan, V., Rouxel, S., Souillard, R. and Chemaly, M. 2018. Detection of Clostridium botulinum group III in environmental samples from farms by real-time PCR using four commercial DNA extraction kits. BMC. Res. Notes. 11, 441. Le Maréchal, C., Woudstra, C. and Fach, P. 2016. Botulism. In Clostridial diseases of animals. Eds., Uzal, F.A., Songer, J.G., Prescott, J.F. and Popoff, M.R. pp: 303–330. Lindström, M., Keto, R., Markkula, A., Nevas, M., Hielm, S. and Korkeala, H. 2001. Multiplex PCR assay for detection and identification of Clostridium botulinum types A, B, E, and F in food and fecal material. Appl. Environ. Microbiol. 67, 5694–5699. Nakamura, K., Kohda, T., Seto, Y., Mukamoto, M., and Kozaki, S. 2013. Improved detection methods by genetic and immunological techniques for botulinum C/D and D/C mosaic neurotoxins. Vet. Microbiol. 162(2), 881–890. Neimanis, A., Gavier-Widén, D., Leighton, F., Bollinger, T., Rocke, T. and Mörner, T. 2007. An outbreak of type C botulism in herring gulls (Larus argentatus) in Southeastern Sweden. J. Wildl. Dis. 43, 327–336. Seyboldt, C., Discher, S., Jordan, E., Neubauer, H., Jensen, K.C., Campe, A., Kreienbrock, L., Scheu, T., Wichern, A., Gundling, F., DoDuc, P., Fohler, S., Abdulmawjood, A., Klein, G. and Hoedemaker, M. 2015. Occurrence of Clostridium botulinum neurotoxin in chronic disease of dairy cows. Vet. Microbiol. 177(3), 398–402. Shin, N.R., Byun, S.H., Chun, J.H., Shin, J.H., Kim, Y.J., Kim, J.H., Rhie, G.H., Chung, H.M., Mo, I.P. and Yoo, C.K. 2010. An outbreak of type C botulism in waterbirds: Incheon, Korea. J. Wildl. Dis. 46, 912-917. Silva, R.O., Oliveira, C., Aramuni Gonçalves, L. and Lobato, F. 2016. Botulism in ruminants in Brazil. Cienc. Rural. 46, 1411–1417. Souillard, R., Woudstra, C., Le Maréchal, C., Dia, M., Bayon-Auboyer, M., Chemaly, M. and Le Bouquin, S. 2014. Investigation of Clostridium botulinum in commercial poultry farms in France between 2011 and 2013. Avian. Pathol. 43, 1–22. U.S. Food & Drug Administration. 2019. Clostridium botulinum toxin formation. College Park, MA: Center for Food Safety and Applied Nutrition. Uzal, F.A., Songer, J.G., Prescott, J.F. and Popoff, M. 2016. Clostridial diseases of animals. Hoboken, NJ: John Wiley & Sons, Inc. Vidal, D., Taggart, M., Badiola, I. and Mateo, R. 2011. Real-time polymerase chain reaction for the detection of toxigenic Clostridium botulinum type C1 in waterbird and sediment samples: comparison with other PCR techniques. J. Vet. Diagn. Invest. 23, 942–946. Woo, G.H., Kim, H.Y., Bae, Y.C., Jean, Y.H., Yoon, S.S., Bak, E.J., Hwang, E.Y. and Joo, Y.S. 2010. Outbreak of botulism (Clostridium botulinum type C) in wild waterfowl: Seoul, Korea. J. Wildl. Dis. 46, 951–955. | ||
| How to Cite this Article |
| Pubmed Style DN, Nguyen T, Nguyen H. Investigation of botulism in free-range ducks farming in the Mekong Delta, Vietnam. Open Vet J. 2022; 12(5): 632-638. doi:10.5455/OVJ.2022.v12.i5.7 Web Style DN, Nguyen T, Nguyen H. Investigation of botulism in free-range ducks farming in the Mekong Delta, Vietnam. https://www.openveterinaryjournal.com/?mno=25579 [Access: November 08, 2024]. doi:10.5455/OVJ.2022.v12.i5.7 AMA (American Medical Association) Style DN, Nguyen T, Nguyen H. Investigation of botulism in free-range ducks farming in the Mekong Delta, Vietnam. Open Vet J. 2022; 12(5): 632-638. doi:10.5455/OVJ.2022.v12.i5.7 Vancouver/ICMJE Style DN, Nguyen T, Nguyen H. Investigation of botulism in free-range ducks farming in the Mekong Delta, Vietnam. Open Vet J. (2022), [cited November 08, 2024]; 12(5): 632-638. doi:10.5455/OVJ.2022.v12.i5.7 Harvard Style , D. N., Nguyen, . T. & Nguyen, . H. (2022) Investigation of botulism in free-range ducks farming in the Mekong Delta, Vietnam. Open Vet J, 12 (5), 632-638. doi:10.5455/OVJ.2022.v12.i5.7 Turabian Style , Duc-Hien Nguyen, Thu-Tam Nguyen, and Huu-Thanh Nguyen. 2022. Investigation of botulism in free-range ducks farming in the Mekong Delta, Vietnam. Open Veterinary Journal, 12 (5), 632-638. doi:10.5455/OVJ.2022.v12.i5.7 Chicago Style , Duc-Hien Nguyen, Thu-Tam Nguyen, and Huu-Thanh Nguyen. "Investigation of botulism in free-range ducks farming in the Mekong Delta, Vietnam." Open Veterinary Journal 12 (2022), 632-638. doi:10.5455/OVJ.2022.v12.i5.7 MLA (The Modern Language Association) Style , Duc-Hien Nguyen, Thu-Tam Nguyen, and Huu-Thanh Nguyen. "Investigation of botulism in free-range ducks farming in the Mekong Delta, Vietnam." Open Veterinary Journal 12.5 (2022), 632-638. Print. doi:10.5455/OVJ.2022.v12.i5.7 APA (American Psychological Association) Style , D. N., Nguyen, . T. & Nguyen, . H. (2022) Investigation of botulism in free-range ducks farming in the Mekong Delta, Vietnam. Open Veterinary Journal, 12 (5), 632-638. doi:10.5455/OVJ.2022.v12.i5.7 |