| Original Article | ||
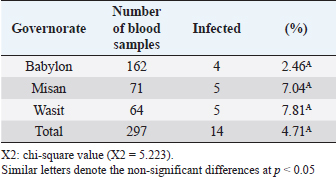
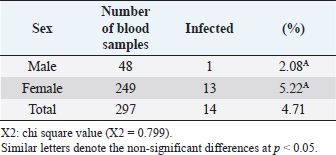
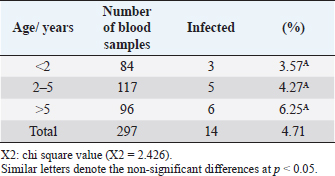
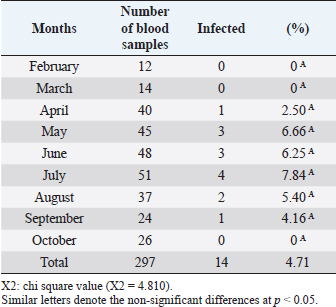
Open Vet J. 2019; 9(3): 238-245 doi: 10.4314/ovj.v9i3.8 Open Veterinary Journal, (2019), Vol. 9(3): 238–245 Original Research DOI: http://dx.doi.org/10.4314/ovj.v9i3.8 Molecular prevalence of Anaplasma phagocytophilum in sheep from IraqKarrar Jasim Hamzah1* and Saleem Amin Hasso21Department of Veterinary Internal and Preventive Medicine, College of Veterinary Medicine, Al-Qasim Green University, Babylon, Iraq 2Department of Veterinary Internal and Preventive Medicine, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq *Corresponding Author: Karrar J. Hamzah. College of Veterinary Medicine, Al-Qasim Green University, Babylon, Iraq. Email: karraraljanabi [at] vet.uoqasim.edu.iq; dr.karraraljanabi41 [at] gmail.com Submitted: 05/03/2019 Accepted: 17/07/2019 Published: 25/08/2019 © 2019 Open Veterinary Journal AbstractBackground: Tick-borne diseases are widely distributed among animal populations and are responsible for significant economic losses. However, little attention has been offered for screening such infections world widely. Anaplasma phagocytophilum is among those neglected tick-borne pathogens, particularly in the developing countries. Aim: This study was conducted to detect A. phagocytophilum infection among sheep in three governorates of Iraq (Babylon, Wasit, and Missan) and try to identify the potential tick vector responsible for A. phagocytophilum transmission among sheep in these analyzed regions. Methods: A total of 297 blood samples and 103 ticks were collected and examined for A. phagocytophilum by polymerase chain reaction using specific primers amplifying partial sequence for msp4 gene. Results: The results showed that about 14 out of 297 tested sheep were positive for A. phagocytophilum. There was no difference between A. phygocytophilum prevalence according to animal gender, age, and sampling period. Furthermore, our analysis showed that the main vectors of A. phagocytophilum: Ixodes scapularis, I. pacificus, or I. ricinus were not identified in three regions of Iraq (Rhipicephalus turanicus, Hyalomma anatolicum, and Hyalomma turanicum were identified). Conclusion: These results highlight the importance of the survey of the tick-borne bacterial infections in Iraq and in the Middle East region in general. Keywords: Anaplasma phagocytophilum, Iraq, Prevalence, Sheep, Ticks. IntroductionAnaplasma phagocytophilum infection is recorded mainly in sheep and a wide range of domestic animals. Anaplasma phagocytophilum also considered as a zoonotic for humans as well. Anaplasma phagocytophilum infection is a tick-borne transmitted disease mainly caused by ixodid tick species (Ogden et al., 2003; Ben Said et al., 2018). The number of clinical cases of human granulocytic anaplasmosis is increasing in Europe and Asia (Park et al., 2003; Ruscio and Cinco, 2003; Ohashi et al., 2005). However, the distribution of A. phagocytophilum is determined by the population of tick vectors and reservoir host species (Stuen et al., 2013). In North America, the common vectors are Ixodes scapularis and I. pacificus (Teglas and Foley, 2006). However, in Europe, the I. ricinus is the main tick vector for A. phagocytophilum (Woldehiwet, 2006). In the Middle East, the Ixodes ricinus is reported as the main possible tick vector for transmitting A. phagocytophilum in Iran and —Turkey (Gokce et al., 2008; Noaman and Shayan, 2009). The detection of A. phagocytophilum using molecular methods like the polymerase chain reaction (PCR) is considering as an effective and sensitive method (Smrdel et al., 2015; Stuen, 2016; Yang et al., 2016). Tick-borne fever (TBF) caused by the bacterium A. phagocytophilum transmitted by the tick I. ricinus is one of the main challenges for sheep farming industry during the grazing season (Grøva et al., 2011). The infection with A. phagocytophilum is widespread in the USA (Foley et al., 2008), in Europe (Woldehiwet, 2006), and in Asia (Jilintai et al., 2009; Yang et al., 2013). Anaplasma infection has been also reported in some Middle East countries like Turkey (Gokce et al., 2008) and in Iran (Noaman and Shayan, 2009). This study was aimed to detect A. phagocytophilum in sheep located from three regions of Iraq (Babylon, Wasit, and Missan). Furthermore, this study tried to detect and identify the tick vector responsible for A. phagocytophilum transmission in these three areas. Materials and MethodsAnimals and data collectionAbout 400 samples divided into 297 sheep and 103 ticks were involved in this study. Sheep were mixed in gender and the ages were ranging from less than 1 year to more than 5 years. They were randomly selected from three different areas of Iraq (Babylon, Wasit, and Missan) during the period from February to October 2018. Collection of blood samplesThe blood samples were collected from the jugular vein of sheep using EDTA-vacutainer tube. All samples were transferred using cooling boxes. All the laboratory tests were performed at the laboratories of the College of Veterinary Medicine—Al-Qasim Green University. Collection of ticksTicks were collected during the period from February to October 2018. A total of 103 ticks were collected and isolated from the same sheep that were involved in this study from the three aforementioned areas. After collection, the ticks were stored separately in 70% ethanol for classification purposes and for the molecular detection of A. phagocytophilum carriage. A stereomicroscope was used to identify the ticks based on their morphological features, such as mouthparts, scutum, color of legs, festoons, interstitial punctuations, presence or absence of adanal shields, posterior groove, and marginal spots (Hoogstraal, 1956; Hosseini-Chegeni et al., 2013). DNA extractionThe genomic DNA of A. phagocytophilum from sheep blood samples and ticks was extracted using a DNA mini extraction kit (Geneaid Biotech Ltd, Taiwan) according to the company’s instructions. Extracted genomic DNA of A. phagocytophilum was analyzed for a given concentration range of 15–60 ng/μl. The purity of extracted DNA was analyzed using a Nanodrop spectrophotometer at (260/280 nm). Polymerase chain reaction (PCR)The PCR technique was performed for the detection of A. phagocytophilum in the blood samples and ticks according to the method described by Yang et al. (2013). The two versions of extracted DNA (from the sheep blood or ticks) were tested for the presence of A. phagocytophilum using PCR by the amplification of the major surface protein 4 coding gene (msp4). Specific primers were designed and used: the forward primer is F: 5ʹ-ATGCGTCTGATGTTAGCGGT-3ʹ and the reverse primer: R: 5ʹ-AAAACTCGCCCCTAACCCAG-3ʹ. The product size is about 503 bp. DNA sequencingIn order to confirm that the positive PCR products (503-bp band) belong to the A. phagocytophilum genome and to test the specificity of the primers for detecting A. phagocytophilum in sheep blood and ticks, the products of three positive PCRs were sequenced using Sanger’s sequencing method at Macrogen, Korea. The evolutionary distances were computed using the Maximum Composite Likelihood method by phylogenetic tree UPGMA (MEGA 6.0 version). Statistical analysisThe results were analyzed using SPSS statistical software. A Chi-square test was used to assess the association between the variable results. p-value of ≤0.05 was considered to be significant. ResultsDetection of A. phagocytophilum DNA in sheep bloodPCR results showed that 14 out of 297 samples (4.71%) were positive to A. phagocytophilum (Fig. 1). Furthermore, the infection rate was higher in Wasit and Missan (7.81% (5/64) and 7.04% (5/71), respectively) compared to that in Babylon region (2.46% (4/162) (Table 1 and Fig. 2). However, the results also showed that the infection rate of A. phagocytophilum was not affected by the gender of the examined sheep (Table 2). Indeed, the infection rate was barely equal in both males (2.08%) and females (5.22%) of sheep (Table 2). Interestingly, results showed that the highest rate of infection was recorded in older sheep (aged 5 years or above) with a rate of 6.25% (Table 3). In contrast, the lowest infection rate was recorded at age less than 2 years (3.57%, Table 3). Seasonal variation analysis showed that the highest infection rate of Anaplasma phagocytophilum was recorded during the summer season of Iraq (May, June, and July: 3/45 (6.66%), 3/48 (6.25 %), and 4/51 (7.84%), respectively) (Table 4 and Fig. 3). However, there were non-significant variations among these months. On the other hand, all samples that were collected during February, March, and October were negative for A. phagocytophilum (Table 4 and Fig. 3).
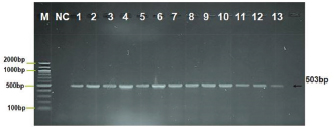
Fig. 1. Agarose gel electrophoresis image showing PCR product analysis of the major surface protein (MSP4) gene in A. phagocytophilum positive isolates. Where: M: marker (100–2,000 bp), lane (1–13) positive A. phagocytophilum at (503) bp PCR product, NC negative control. Table 1. Infection rate of A. phagocytophilum by PCR technique.
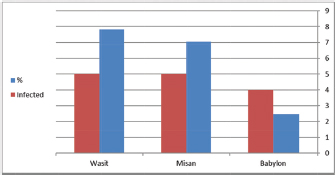
Fig. 2. Prevalence of A. phagocytophilum according to regions. Babylon 4/162, Misan 5/71, and Wasit 5/64. Red color representative numbers of infected sheep with A. phagocytophilum, % representative the percentages of infection. Table 2. Infection and gender relationship of blood samples examined by PCR technique.
Table 3. Infection rates of A. phagocytophilum and age of sheep examined by PCR technique.
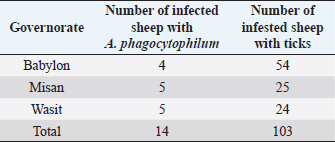
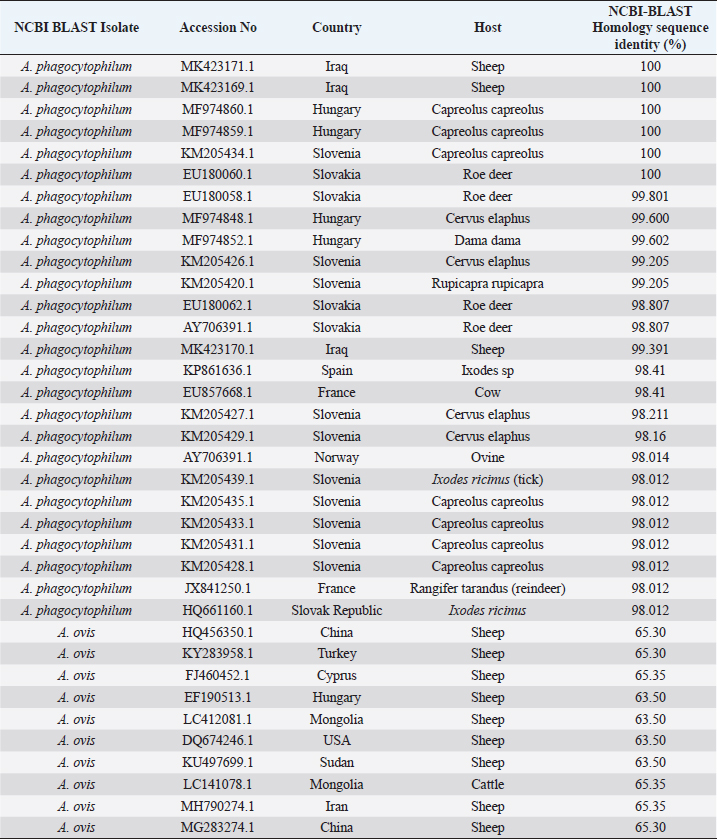
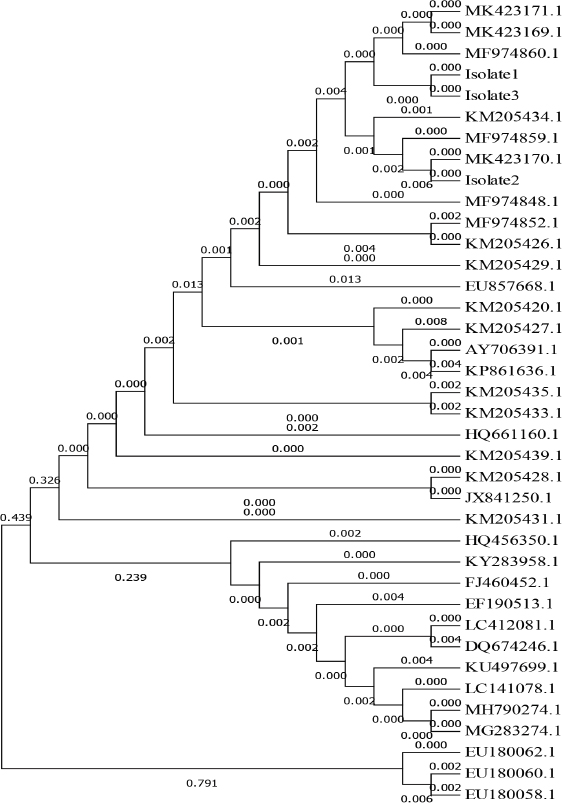
Survey of A. phagocytophilum in ticksPCR result indicated that collected ticks were identified as Rhipicephalus turanicus, Hyalomma anatolicum, and Hyalomma turanicum and none of these examined ticks (total 103) was carrying A. phagocytophilum (Table 5). Confirmation of PCR products by sequencingThe results of A. phagocytophilum sequencing were analyzed and annotated according to A. phagocytophilum genomes that banked in NCBI GenBank database using Basic Local Alignment Search Tool (BLAST). Phylogenetic analysis of A. phagocytophilumTo investigate the possible genetic relationship between A. phagocytophilum strains detected in the current study and A. phagocytophilum available in GenBank database at the NCBI, a simple phylogenetic tree was generated based on the partial sequences of major surface protein 4 coding gene (msp4). The partial sequences of msp4 gene of three positive A. phagocytophilum samples (GenBank accession numbers MK423169, MK423170, and MK423171) were shown to be closely related to A. phagocytophilum isolates found in GenBank (Table 6 and Fig. 4). Table 4. Infection rate of A. phagocytophilum according to months of study by PCR examination.
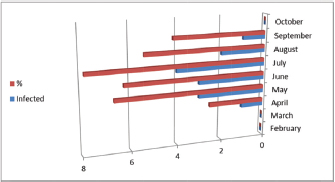
Fig. 3. Infection rate according to months. Red color representative numbers of infected sheep with A. phagocytophilum, % representative the percentages of infection. February 0/12, March 0/14, April 1/40, May 3/45, June 3/48, July 4/51, August 2/37, September 1/24, and October 0/26. Table 5. Relationships between infection of A. phagocytophilum with tick infestation.
Table 6. NCBI-BLAST homology sequence identity (%) for msp4 gene in local A. phagocytophilum and NCBI-Blast A. phagocytophilum.
DiscussionThis study targeted the detection of A. phagocytophilum in sheep blood and infesting ticks by amplifying the major surface protein 4 coding gene (msp4). This gene is reported to be conserved for A. phagocytophilum (de la Fuente et al., 2005a; Silaghi et al., 2011; Öter et al., 2015). This consistent infection rates result with what has been found in previous studies conducted by Derdáková et al. (2011)
Fig. 4. Phylogenetic tree of A. phagocytophilum by Maximum Likelihood method based on partial sequence major surface protein 4 (MSP4) and relating the local strain of A. phagocytophilum Gene Bank accession numbers sequence MK423169, MK423170, and MK423171 to other isolated strains, the Gene Bank accession numbers of EF190513.1, LC141078.1, KU497699.1, MH790274.1, LC412081.1, MG283274.1, KY283958.1, HQ456350.1, FJ460452.1, and DQ674246.1 were related to Anaplasma ovis. Furthermore, Ben Said et al. (2017) demonstrated that in Tunisia, prevalence rates of strains genetically related to A. phagocytophilum in sheep was 3.9%. Using PCR method, Scharf et al. (2011) mentioned that the infection rate of A. phagocytophilum was 4% in sheep. However, Kiilerich et al. (2009) reported that the infection rates of A. phagocytophilum in Denmark, Italy, and Turkey were higher than those of the current study and of Derdáková et al. (2011), Ben Said et al. (2017), Scharf et al. (2011). Indeed, the infection rates have been reported to be variable between countries and could even vary considerably between neighboring farms (Stuen et al., 2002b). The prevalence of A. phagocytophilum infection in sheep has been reported to range from 0.51% to 80% worldwide (Stuen and Bergström, 2001; de la Fuente et al., 2005b). Our results showed that infection rates slightly vary according to the sex and conclude that the female sheep have a higher infection rate than males. This could be due to the fact that females are more exposed to different physiological changes such as stresses caused by pregnancies, parturition, and lactation (Weiss and Wardrop, 2011). The age group of more than 5 years showed the highest infection rates, while the sheep aged from 2 to 5 years recorded the lowest rates. These results are compatible with Chaudhry et al. (2010) who mentioned that the highest rate of infection in adult animals could be due to the chronicity of infection. Ben Said et al. (2015) also reported that the infection rate with A. phagocytophilum-like strains was higher in adult compared to young sheep. Extensive study by (Egenvall et al., 2000) showed that higher infection rates were significant in older ages. These results also agreed with Radostits et al. (2006). Poitout et al. (2005) discussed the seasonality and geographical distribution of A. phagocytophilum infections and found that this due to the feeding habits of each particular tick vector. Most cases were observed between April and July, with fewer cases observed in October. In Berlin (Germany), Kohn et al. (2008) reported that most cases occurred between April and September, and the seasonal distribution of the disease most likely reflects periods of peak tick activities. This finding is in agreement with Beall et al. (2008). The common consequence of the infection with A. phagocytophilum in sheep is the direct and indirect losses and causes immunosuppression status that may lead to secondary infections. A. phagocytophilum infection causes direct losses and mortality rates of 30% lamb in the flock (Stuen and Kjølleberg, 2000). The extent of indirect production loss due to TBF decreases the bodyweight of lamb (Stuen et al., 2002a). Jensen et al. (2007) indicated that a high infestation with ticks could increase the incidence of A. phagocytophilum infection compared to those with a low rate of tick infestation. Kohn et al. (2011) also mentioned that A. phagocytophilum infection is higher in the months of tick activity. The present results are also supported by Chvostáč et al. (2018) and Grøva (2011) who reported that the seasonal dynamics of tick activity was the highest in April, May, June, and July when correlated with the highest prevalence of A. phagocytophilum infection. Overall, this study is a pioneering study regarding molecular detection of A. phagocytophilum in local Iraqi sheep from three different regions of Iraq. To our knowledge, this study is the first study that reported A. phagocytophilum infection in Iraqi sheep. ConclusionPCR method complemented by sequencing step seems to be accurate and confirmative for the detection and the genetic characterization of A. phagocytophilum in sheep. Furthermore, our results suggested that other tick species might be responsible for the transmission of A. phagocytophilum to Iraqi sheep. AcknowledgmentWe thank our colleagues and the technicians in the laboratory of Veterinary clinical pathology at the College of Veterinary Medicine—Al-Qasim Green University in Iraq for their technical support. Special thanks for Maan M Neamah from Faculty of Veterinary Medicine at the University of Kufa, Iraq, for his kind reviewing and editing of this manuscript. Finally, thanks to Dr.Asaad Mohan, Dr. Adnan Mansour, Dr. Qasim AL-Jebori, Dr. Ameer Dirwal, and Mr. Mohammed Rahman for their great help during the field samples collection. This research is a self-funded project as part of a partially-funded Ph.D. study. No specific grant was obtained from any public, commercial, or not-for-profit funding agencies. Conflict of interestWe declare no conflicts of interest in relation to this paper. Author ContributionsKarrar Jasim Hamzah and Saleem Amin Hasso conceived and wrote the study. All authors read and approved the final manuscript. ReferencesBeall, M.J., Chandrashekar, R., Eberts, M.D., Cyr, K.E., Diniz, P.P.V., Mainville, C., Hegarty, B.C., Crawford, J.M. and Breitschwerdt, E.B. 2008. Serological and molecular prevalence of Borrelia burgdorferi, Anaplasma phagocytophilum, and Ehrlichia species in dogs from Minnesota. Vector Borne Zoonotic Dis. 8(4), 455–464. Ben Said, M.B., Belkahia, H., Alberti, A., Zobba, R., Bousrih, M., Yahiaoui, M., Daaloul-Jedidi, M., Mamlouk, A., Gharbi, M. and Messadi, L. 2015. Molecular survey of Anaplasma species in small ruminants reveals the presence of novel strains closely related to A. phagocytophilum in Tunisia. Vector Borne Zoonotic Dis. 15(10), 580–590. Ben Said, M.B., Belkahia, H., El Mabrouk, N., Saidani, M., Hassen, M.B., Alberti, A., Zobba, R., Bouattour, S., Bouattour, A. and Messadi, L. 2017. Molecular typing and diagnosis of Anaplasma spp. closely related to Anaplasma phagocytophilum in ruminants from Tunisia. Ticks Tick Borne Dis. 8(3), 412–422. Ben Said, M. B., Belkahia, H. and Messadi, L. 2018. Anaplasma spp. in North Africa: a review on molecular epidemiology, associated risk factors and genetic characteristics. Vector Borne Zoonotic Dis. 9(3), 543–555. Chaudhry, Z., Suleman, M., Younus, M. and Aslim, A. 2010. Molecular detection of Babesia bigemina and Babesia bovis in crossbred carrier cattle through PCR. Pak J. Zool. 42(2), 201–204. Chvostáč, M., Špitalská, E., Václav, R., Vaculová, T., Minichová, L. and Derdáková, M. 2018. Seasonal Patterns in the Prevalence and Diversity of Tick-Borne Borrelia burgdorferi Sensu Lato, Anaplasma phagocytophilum and Rickettsia spp. in an Urban Temperate Forest in South Western Slovakia. Int. J. Environ. Res. Public Health 15(5), 994. De La Fuente, J., Massung, R.F., Wong, S.J., Chu, F.K., Lutz, H., Meli, M., Von Loewenich, F.D., Grzeszczuk, A., Torina, A. and Caracappa, S. 2005a. Sequence analysis of the msp4 gene of Anaplasma phagocytophilum strains. J. Clin. Microbiol. 43(3), 1309–1317. De La Fuente, J., Naranjo, V., Ruiz-Fons, F., Höfle, U., Fernández De Mera, I.G., Villanúa, D., Almazán, C., Torina, A., Caracappa, S. and Kocan, K.M. 2005b. Potential vertebrate reservoir hosts and invertebrate vectors of Anaplasma marginale and A. phagocytophilum in central Spain. Vector Borne Zoonotic Dis. 5(4), 390–401. Derdáková, M., Štefančíková, A., Špitalská, E., Tarageľová, V., Košťálová, T., Hrkľová, G., Kybicová, K., Schánilec, P., Majláthová, V. and Várady, M. 2011. Emergence and genetic variability of Anaplasma species in small ruminants and ticks from Central Europe. Vet. Microbiol. 153(3-4), 293–298. Egenvall, A., Bonnett, B.N., Gunnarsson, A., Hedhammar, Å., Shoukri, M., Bornstein, S. and Artursson, K. 2000. Sero-prevalence of granulocytic Ehrlichia spp. and Borrelia burgdorferi sensu lato in Swedish dogs 1991-94. Scand. J. Infect. Dis. 32(1), 19–25. Foley, J.E., Clueit, S.B. and Brown, R.N. 2008. Differential exposure to Anaplasma phagocytophilum in rodent species in northern California. Vector Borne Zoonotic Dis. 8(1), 49–56. Gokce, H., Genc, O., Akca, A., Vatansever, Z., Unver, A. and Erdogan, H. 2008. Molecular and serological evidence of Anaplasma phagocytophilum infection of farm animals in the Black Sea Region of Turkey. Acta Vet. Hung. 56(3), 281–292. Grøva, L. 2011. Tick-borne fever in sheep: production loss and preventive measures. Ph.D. Thesis, Norwegian University of Life Sciences, Norway. Grøva, L., Olesen, I., Steinshamn, H. and Stuen, S. 2011. Prevalence of Anaplasma phagocytophilum infection and effect on lamb growth. Acta Vet. Scand. 53(1), 30. Hoogstraal, H. 1956. Ticks of the Sudan,(with Special Reference to Equatoria Province and with Preliminary Reviews of the Genera Boophilus, Margaropus, and Hyallomma), United States Naval Medical Research Unit No. 3. Hosseini-Chegeni, A., Hosseini, R., Tavakoli, M., Telmadarraiy, Z. and Abdigoudarzi, M. 2013. The Iranian Hyalomma (Acari: Ixodidae) with a key to the identification of male species. Persian J. Acarol. 2(3). Jensen, J., Simon, D., Escobar, H.M., Soller, J., Bullerdiek, J., Beelitz, P., Pfister, K. and Nolte, I. 2007. Anaplasma phagocytophilum in dogs in Germany. Zoonoses Public Health 54(2), 94–101. Jilintai, S.N., Hayakawa, D., Suzuki, M., Hata, H., Kondo, S., Matsumoto, K., Yokoyama, N. and Inokuma, H. 2009. Molecular survey for Anaplasma bovis and Anaplasma phagocytophilum infection in cattle in a pastureland where sika deer appear in Hokkaido, Japan. Jpn. J. Infect. Dis. 62(1), 73–75. Kiilerich, A.M., Christensen, H. and Thamsborg, S.M. 2009. Anaplasma phagocytophilum in Danish sheep: confirmation by DNA sequencing. Acta Vet. Scand. 51(1), 55. Kohn, B., Galke, D., Beelitz, P. and Pfister, K. 2008. Clinical features of canine granulocytic anaplasmosis in 18 naturally infected dogs. J. Vet. Intern. Med. 22(6), 1289–1295. Kohn, B., Silaghi, C., Galke, D., Arndt, G. and Pfister, K. 2011. Infections with Anaplasma phagocytophilum in dogs in Germany. Res. Vet. Sci. 91(1), 71–76. Noaman, V. and Shayan, P. 2009. Molecular detection of Anaplasma phagocytophilum in carrier cattle of Iran-first documented report. Iran J. Microbiol. 1(2), 37–42. Ogden, N., Casey, A., Woldehiwet, Z. and French, N. 2003. Transmission of Anaplasma phagocytophilum to Ixodes ricinus ticks from sheep in the acute and post-acute phases of infection. Infect. Immun. 71(4), 2071–2078. Ohashi, N., Inayoshi, M., Kitamura, K., Kawamori, F., Kawaguchi, D., Nishimura, Y., Naitou, H., Hiroi, M. and Masuzawa, T. 2005. Anaplasma phagocytophilum–infected ticks, Japan. Emerg. Infect. Dis. 11(11), 1780. öter, K., Cetinkaya, H., VURUŞANER, C., Toparlak, M. and Ergünay, K. 2015. Molecular detection and typing of Anaplasma species in small ruminants in Thrace region of Turkey. Kafkas Univ. Vet. Fak. Derg. 22, 133–138. Park, J.-h., Heo, E.-J., Choi, K.-S., Dumler, J.S. and Chae, J.-S. 2003. Detection of antibodies to Anaplasma phagocytophilum and Ehrlichia chaffeensis antigens in sera of Korean patients by western immunoblotting and indirect immunofluorescence assays. Clin. Diagn. Lab. Immunol. 10(6), 1059–1064. Poitout, F.M., Shinozaki, J.K., Stockwell, P.J., Holland, C.J. and Shukla, S.K. 2005. Genetic variants of Anaplasma phagocytophilum infecting dogs in Western Washington State. J. Clin. Microbiol. 43(2), 796–801. Radostits, O.M., Gay, C.C., Hinchcliff, K.W. and Constable, P.D. 2006. Veterinary Medicine E-Book: a textbook of the diseases of cattle, horses, sheep, pigs and goats, Elsevier Health Sciences. Ruscio, M. and Cinco, M. 2003. Human granulocytic ehrlichiosis in Italy. Ann. N. Y. Acad. Sci. 990(1), 350–352. Scharf, W., Schauer, S., Freyburger, F., Petrovec, M., Schaarschmidt-Kiener, D., Liebisch, G., Runge, M., Ganter, M., Kehl, A. and Dumler, J.S. 2011. Distinct host species correlate with Anaplasma phagocytophilum ankA gene clusters. J. Clin. Microbiol. 49(3), 790–796. Silaghi, C., Hamel, D., Thiel, C., Pfister, K., Passos, L.M.F. and Rehbein, S. 2011. Genetic variants of Anaplasma phagocytophilum in wild caprine and cervid ungulates from the Alps in Tyrol, Austria. Vector Borne Zoonotic Dis. 11(4), 355–362. Smrdel, K.S., von Loewenich, F.D., Petrovec, M. and Županc, T.A. 2015. Diversity of ankA and msp4 genes of Anaplasma phagocytophilum in Slovenia. Ticks Tick Borne Dis. 6(2), 164–166. Stuen, S. 2016. Tick-Borne Fever (Anaplasma phagocytophilum Infection) in Sheep—a review. J. Vet. Med. Res. 3(5), 1062. Stuen, S. and Bergström, K. 2001. Serological investigation of granulocytic Ehrlichia infection in sheep in Norway. Acta Vet. Scand. 42(3), 331. Stuen, S., Bergström, K. and Palmer, E. 2002a. Reduced weight gain due to subclinical Anaplasma phagocytophilum (formerly Ehrlichia phagocytophila) infection. Exp. Appl. Acarol. 28(1–4), 209–215. Stuen, S., Granquist, E.G. and Silaghi, C. 2013. Anaplasma phagocytophilum—a widespread multi-host pathogen with highly adaptive strategies. Front Cell Infect. Microbiol. 3, 31. Stuen, S. & Kjølleberg, K. (2000). An investigation of lamb deaths on tick pastures in Norway. In Proceedings of the third International Conference on Ticks and Tick-borne pathogens: into the 21st century, 2000. Slovak Academy of Sciences, Bratislava, 115. Stuen, S., Van De Pol, I., Bergström, K. and Schouls, L.M. 2002b. Identification of Anaplasma phagocytophila (formerly Ehrlichia phagocytophila) variants in blood from sheep in Norway. J. Clin. Microbiol. 40(9), 3192–3197. Teglas, M.B. and Foley, J. 2006. Differences in the transmissibility of two Anaplasma phagocytophilum strains by the North American tick vector species, Ixodes pacificus and Ixodes scapularis (Acari: Ixodidae). Exp. Appl. Acarol. 38(1), 47–58. Weiss, D.J. and Wardrop, K.J. 2011. Schalm’s veterinary hematology, John Wiley & Sons. Woldehiwet, Z. 2006. Anaplasma phagocytophilum in ruminants in Europe. Ann. N. Y. Acad. Sci. 1078(1), 446–460. Yang, J., Liu, Z., Guan, G., Liu, Q., Li, Y., Chen, Z., Ma, M., Liu, A., Ren, Q. and Luo, J. 2013. Prevalence of Anaplasma phagocytophilum in ruminants, rodents and ticks in Gansu, north-western China. J. Med. Microbiol. 62(2), 254–258. Yang, J., Liu, Z., Niu, Q., Liu, J., Xie, J., Chen, Q., Chen, Z., Guan, G., Liu, G. and Luo, J. 2016. Evaluation of different nested PCRs for detection of Anaplasma phagocytophilum in ruminants and ticks. BMC Vet. Res. 12(1), 35. | ||
| How to Cite this Article |
| Pubmed Style Hamzah KJ, Hasso SA, . Molecular prevalence of Anaplasma phagocytophilum in sheep from Iraq.. Open Vet J. 2019; 9(3): 238-245. doi:10.4314/ovj.v9i3.8 Web Style Hamzah KJ, Hasso SA, . Molecular prevalence of Anaplasma phagocytophilum in sheep from Iraq.. https://www.openveterinaryjournal.com/?mno=35334 [Access: April 29, 2024]. doi:10.4314/ovj.v9i3.8 AMA (American Medical Association) Style Hamzah KJ, Hasso SA, . Molecular prevalence of Anaplasma phagocytophilum in sheep from Iraq.. Open Vet J. 2019; 9(3): 238-245. doi:10.4314/ovj.v9i3.8 Vancouver/ICMJE Style Hamzah KJ, Hasso SA, . Molecular prevalence of Anaplasma phagocytophilum in sheep from Iraq.. Open Vet J. (2019), [cited April 29, 2024]; 9(3): 238-245. doi:10.4314/ovj.v9i3.8 Harvard Style Hamzah, K. J., Hasso, S. A. & (2019) Molecular prevalence of Anaplasma phagocytophilum in sheep from Iraq.. Open Vet J, 9 (3), 238-245. doi:10.4314/ovj.v9i3.8 Turabian Style Hamzah, Karrar Jasim, Saleem Amin Hasso, and . 2019. Molecular prevalence of Anaplasma phagocytophilum in sheep from Iraq.. Open Veterinary Journal, 9 (3), 238-245. doi:10.4314/ovj.v9i3.8 Chicago Style Hamzah, Karrar Jasim, Saleem Amin Hasso, and . "Molecular prevalence of Anaplasma phagocytophilum in sheep from Iraq.." Open Veterinary Journal 9 (2019), 238-245. doi:10.4314/ovj.v9i3.8 MLA (The Modern Language Association) Style Hamzah, Karrar Jasim, Saleem Amin Hasso, and . "Molecular prevalence of Anaplasma phagocytophilum in sheep from Iraq.." Open Veterinary Journal 9.3 (2019), 238-245. Print. doi:10.4314/ovj.v9i3.8 APA (American Psychological Association) Style Hamzah, K. J., Hasso, S. A. & (2019) Molecular prevalence of Anaplasma phagocytophilum in sheep from Iraq.. Open Veterinary Journal, 9 (3), 238-245. doi:10.4314/ovj.v9i3.8 |