| Original Article | ||
Open Vet J. 2022; 12(5): 697-708 Open Veterinary Journal, (2022), Vol. 12(5): 697–708 Original Research Serum proteomic analysis reveals the differential dose effects of crocodile oil from Crocodylus siamensis on energy metabolism in ratsKongphop Parunyakul1, Krittika Srisuksai1, Pitchaya Santativongchai2, Sawanya Charoenlappanit3, Narumon Phaonakrop3, Sittiruk Roytrakul3, Phitsanu Tulayakul4, and Wirasak Fungfuang1*1Department of Zoology, Faculty of Science, Kasetsart University, Bangkok, Thailand 2Faculty of Veterinary Medicine, Kasetsart University, Bangkok, Thailand 3Functional Ingredients and Food Innovation Research Group, National Center for Genetic Engineering and Biotechnology (BIOTEC), National Science and Technology Development Agency, Pathum Thani, Thailand 4Department of Veterinary Public Health, Faculty of Veterinary Medicine, Kasetsart University, Nakhon Pathom, Thailand *Corresponding Author: Wirasak Fungfuang. Department of Zoology, Faculty of Science, Kasetsart University, Bangkok, Thailand. Email: fsciwsf [at] ku.ac.th Submitted: 27/05/2022 Accepted: 18/08/2022 Published: 18/09/2022 © 2022 Open Veterinary Journal
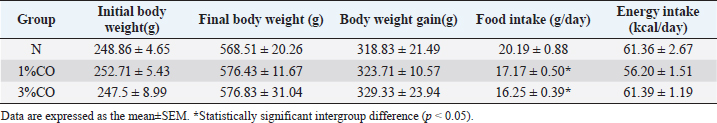
AbstractBackground: Dietary fat composition is a potential major factor affecting energy metabolism. Crocodile oil (CO) is rich in mono- and poly-unsaturated fatty acids exhibiting anti-inflammatory and healing properties. Aim: This study investigated different levels of CO consumption on alterations and expression of proteins involved in energy metabolism in rats. Methods: Twenty-one male Sprague-Dawley rats were divided into three groups and administered sterile water (N) or different doses of CO [1% or 3% (v/w) CO] orally once daily for 8 weeks. Body weight gain, food intake, energy intake, blood lipid profiles, and serum energy-related metabolites were determined. The serum proteome was analyzed using shotgun proteomics, and the functions of several candidate proteins were classified using PANTHER software. Results: There were no significant differences in body weight or energy intake were observed between groups. However, both CO-treated groups showed significantly decreased serum triglyceride (TG) levels (p < 0.05). Moreover, post-treatment serum TG levels in the 1%CO group were significantly lower than pre-treatment compared with other groups. The serum oxaloacetate level was also significantly higher in both CO groups than in the N group. The proteomic analysis classified 4,525 serum proteins and revealed more unique proteins involved in cellular metabolic activity in both CO-treated groups than in the N group. Self-organizing tree algorithm clustering of 295 shared differentially expressed proteins in both CO-treatment groups showed that upregulated hyper-expressed protein clusters in both CO groups were associated with catalytic activity and molecular activity on the same levels. Conclusion: CO simultaneously enhances energy metabolism and improves lipid profiles. Keywords: Crocodile oil, Energy metabolism, Serum, Proteomics, Rat. IntroductionDietary nutrition is an important factor that affects metabolic markers associated with energy metabolism (Binia et al., 2017). Oils and fats are important dietary nutrients that serve as dense sources of energy. However, high-fat consumption can lead to obesity, increased inflammatory markers, and insulin resistance (Herieka and Erridge, 2014; Hernández et al., 2017; Laguna-Camacho, 2017). In addition, high-fat diets rich in saturated fatty acids (SFAs) are associated with reduced expression of energy-related enzymes associated with the tricarboxylic acid (TCA) cycle (e.g., citrate synthase) and the mitochondrial electron transport chain (i.e., complexes I and II) (Echeverria et al., 2019). The protective effects of dietary oils are determined by fatty acid composition, daily intake, and consumption duration (Siddiq et al., 2019). Other studies have reported that consumption of unsaturated fatty acids, including mono-unsaturated fatty acids (MUFAs) or poly-unsaturated fatty acids (PUFAs), as dietary fat supplements may promote glucose and lipid metabolism, metabolic inflammation, and hepatic metabolism (Silva Figueiredo et al., 2017). MUFAs help prevent the development of metabolic syndrome by modulating blood lipids, insulin sensitivity, oxidation, and metabolism (Gillingham et al., 2011). Likewise, another previous study revealed that increased consumption of PUFAs can alter the expression of genes in metabolic pathways associated with increased fat oxidation and energy expenditure (Howe and Buckley, 2014). Thus, consumption of dietary oils containing different proportions and types of fats can help regulate energy metabolism and may prevent many chronic diseases. Crocodylus siamensis, commonly known as the Siamese crocodile, is a freshwater crocodile native to Southeast Asia, including Thailand. Crocodile oil (CO), which is extracted from the abdominal fat of C. siamensis, is naturally richer in MUFAs (oleic acid) and PUFAs (linoleic acid) (Gunstone and Russell, 1954) compared with oils of other animals. A previous study reported that CO exhibits both antimicrobial and anti-inflammatory activities (Buthelezi et al., 2012). However, the effect of consumption of CO on metabolism remains unclear. Nevertheless, some studies have examined the regulatory effects of bioactive compounds isolated from Siamese crocodiles, they revealed that several bioactive compounds from hemoglobin hydrolysate, crude leukocyte extract, and plasma of C. siamensis have demonstrated beneficial effects against diseases associated with inflammation and oxidative stress (Phosri et al., 2014; Lueangsakulthai et al., 2018). With regard to the fatty acid composition of CO, gene expression profiling and pathway analyses found that oils rich in PUFAs (e.g., krill oil) regulate a diverse array of genes associated with hepatic energy metabolism, including glucose metabolism and mitochondrial respiration (Burri et al., 2011). Thus, the fatty acid composition of CO may exhibit similar effects in regulating energy metabolism and preventing disease due to its essential bioactive components. The present study investigated the effect of CO consumption on health status and the expression of proteins related to energy metabolism in a rat model. The mechanism underlying the effects and molecular physiology of dietary CO were examined using a quantitative proteomic mass spectrometry approach. Changes in the serum proteome response to different doses of CO were evaluated to elucidate the mechanisms by which CO regulates energy metabolism, thereby providing new insights into the health effect of CO. Materials and MethodsCO preparationCO was extracted from abdominal fats of slaughtered 3- to 5-year-old C. siamensis according to Santativongchai et al. (2020) at the time the meat was trimmed out and prepared. Crocodilian fat samples were obtained from a crocodile farm as by-products in Nakhon Pathom Province, Thailand. The samples were pressed through two layers of filter cloth with distilled water at a proportion of 1:1 (w/v). Subsequently, the resulting filtered solution was left undisturbed until the mixture separated well. The upper clear oil fraction was then collected, evaporated, and stored in a sealed container at room temperature. Animals and experimental designTwenty-one male Sprague-Dawley rats (7 weeks old) were obtained from Nomura Siam International Co. Ltd. (Samutprakan Province, Thailand). The animals were individually housed in metabolic cages at 25 ± 2°C on a 12-h light/12-h dark cycle with ad libitum access to standard chow and drinking water. Rats were randomly assigned to three groups (n=7/group): Group 1: rats received sterile water (N), Group 2: rats received 1% (v/w) CO (1%CO), and Group 3: rats received 3% (v/w) CO (3%CO). Water, 1%CO, or 3%CO was administered orally once daily (11.00–12.00 AM) for 8 weeks. Measurement of food intake, energy intake, and body weightFood intake was measured daily by weighing the leftover chow between 11.00 and 11.30 AM. and energy intake was then calculated from the daily food intake determination. Body weight was measured weekly throughout the experiment. Sample collection and determination of energy metabolism–related intermediatesBefore the first session of the experiment, blood samples were collected to determine the pre-treatment blood lipid profile. After 8 weeks of the experiment, all animals were euthanized with 60 mg/ml pentobarbital sodium. Blood samples were collected and centrifuged at 2,200 g for 15 minutes at 4°C, and then the serum was stored at −20°C until further analysis. The serum lipid profile parameters examined included triglycerides, cholesterol, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) and were determined using a Hitachi 7080 analyzer (Hitachi, Tokyo, Japan). In the case of metabolomic analysis for the determination of energy-related intermediates, serum was centrifuged at 2,000 g for 20 minutes at 4°C. The resulting supernatants were stored at −80°C until further analysis. High-performance liquid chromatography (HPLC) was used to determine the energy-related intermediates according to Lillefosse et al. (2014). The frozen supernatants (after 20-fold dilution) were mixed with methanol at a ratio of 1:4 (v/v) and centrifuged at 20,000 g for 20 minutes at 4°C, after which the supernatants were removed and evaporated using a freeze dryer at −80°C. The intermediate-containing residues were then redissolved in 500 µl of HPLC buffer and 5-µl samples were then analyzed via HPLC. Chromatography was performed with an injection volume of 5 µl and a column temperature at 40°C. A 5-µm InertSustain C18 column (150 × 4.6 mm) was used to separate a number of individual compounds (mobile phase consisting of 8% 1 N sulfuric acid). Gradient elution was carried out at a flow rate of 1 ml/minute. Serum protein quantitation and identification by liquid chromatography–mass spectrometry (LC-MS)LC-MS was used to analyze serum proteins. Serum supernatants (after 20-fold dilution) were mixed with acetone at a 2:1 (v/v) ratio and centrifuged at 10,000 g for 10 minutes. The resulting pellets were suspended in lysis buffer [0.25% (w/v) sodium dodecyl sulfate, 50 mM Tris-HCl, pH 9.0], and then the protein concentration was measured via Lowry et al.’s method (1951) using bovine serum albumin as the protein concentration standard. All pooled protein samples were prepared by mixing equal amounts of protein from individual serum protein samples. To reduce disulfide bonds, protein samples (5 µg) were reduced using 5 mM dithiothreitol in 10 mM ammonium bicarbonate at 60°C for 1 hour. Sulfhydryl groups were alkylated by incubation in 15 mM iodoacetamide in 10 mM ammonium bicarbonate for 45 minutes in the dark at room temperature. Subsequently, the protein samples were mixed with sequencing-grade trypsin (Promega, Mannheim, Germany) at a ratio of 1:20 and incubated at 37°C overnight. The resulting tryptic peptides were dried and protonated using 0.1% formic acid and then injected into an Ultimate 3000 Nano/Capillary LC system (Dionex Ltd., UK) coupled to an HCTUltra mass spectrometer (Bruker Daltonics, Billerica, MA), and peptides were analyzed by electrospray ionization at a flow rate of 300 nl/minute using a 100-mm PepSwift monolithic nanocolumn with 50-mm internal diameter. Mobile phase solvent A consisted of 0.1% formic acid, and solvent B consisted of 80% acetonitrile and 0.1% formic acid. Peptides were eluted using a linear gradient of 4%–70% solvent B over the period 0–20 minutes (the time point of retention), followed by 90% solvent B from 20 to 25 minutes to remove all peptides retained on the column. A final elution was performed using 10% solvent B from 25 to 40 minutes to remove any remaining salts. Mass spectra of the peptide fragments were acquired in data-dependent AutoMS (2) mode over the scanning range 300–1,500 m/z. Three averages were taken, and up to five precursor ions were selected over the MS scan range 50−3,000 m/z. DeCyder MS Differential Analysis software (DeCyderMS, GE Healthcare) was used to quantify the proteins in individual samples, and the Mascot search engine was used to correlate the MS/MS spectra to the Macaca protein database maintained by UniProt (Johansson et al., 2006; Thorsell et al., 2007). Mascot’s standard settings were used, which included a maximum of three missed cleavages, a peptide tolerance of 1.2 Da, an MS/MS tolerance of 0.6 Da, trypsin as the digesting enzyme, cysteine carbamidomethylation as the fixed modification, methionine oxidation as the variable modification, and peptide charge states. Protein levels in each sample were expressed as log2 values. Data analysis and statistical methodsVenn diagrams were used to count and compare the lists of proteins in each group (Bardou et al., 2014). Jvenn software was used to visualize protein expression data as Venn diagrams and provide statistical charts to assess the homogeneity of the list size and compare the compactness of multiple Venn diagrams. Candidate proteins were classified according to function, which related the protein molecular junctions and biological processes at the organism level, using the Protein Analysis Through Evolutionary Relationships (or PANTHER) classification system (available at: http://www.pantherdb.org) (Mi et al., 2017). Multiexperiment Viewer, version 4.9 was used to generate protein expression heatmaps and for hierarchical and Self-organizing tree algorithm (SOTA) clustering analyses. Data are expressed as the mean ± SEM. Statistical analyses were performed via one-way analysis of variance, followed by Tukey’s post hoc test using the R project statistical computing package (R Core Team, 2019). p < 0.05 was considered statistically significant. Ethical approvalThe study was conducted according to the Guidelines for the Care and Use of Laboratory Animals. The ethics committee of Kasetsart University Research and Development Institute, Kasetsart University, Thailand, approved this study (approval no. ACKU61-VET-088). ResultsEffect of different CO doses on initial body weight, final body weight, body weight gain, food intake, energy intake, and lipid profilesThere were no differences among treatment groups in terms of Initial body weight, final body weight, body weight gain, or energy intake. However, both the 1%CO and 3%CO treatments resulted in a significant decrease in food intake over the 8-weeks study (Table 1). As shown in Figure 1, there were no differences in cholesterol, HDL, and LDL levels between the three groups at the end of treatment. In contrast, serum triglyceride levels were lower in both CO-treated groups than in the N group. We compared the effect of different doses of CO on pre- and post-treatment lipid profiles among three groups. Our results showed that serum triglyceride levels were increased in the N group after 8 weeks of treatment. However, 1%CO treatment resulted in a significant decline in serum triglyceride post-treatment when compared with pre-treatment, and a trend lower post-treatment triglyceride level was observed in the 3%CO group. Table 1. Effect of different doses of dietary crocodile oil on final body weight, body weight gain, average food intake, and average energy intake of rats over an 8-week period.
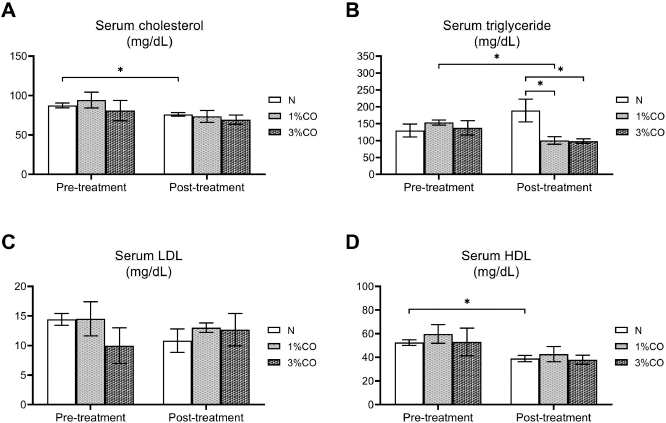
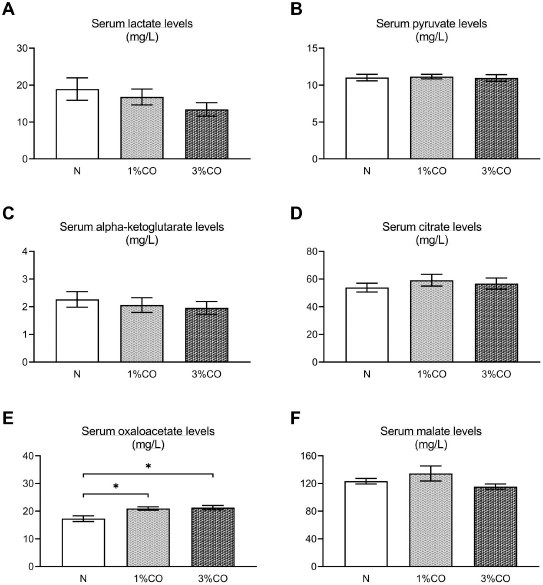
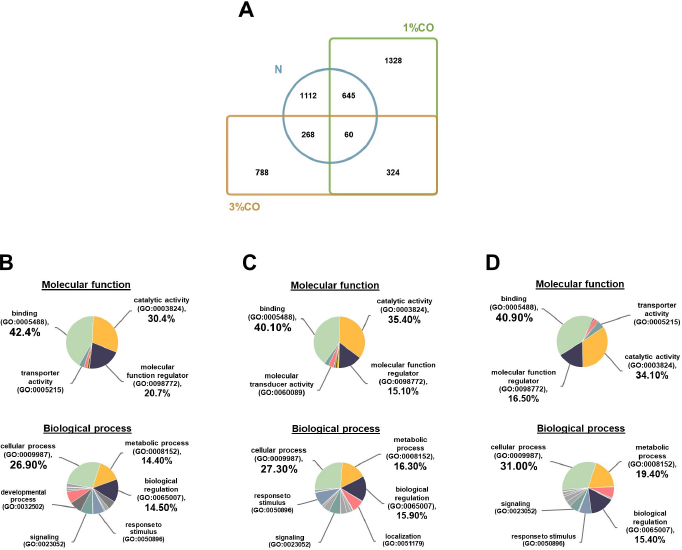
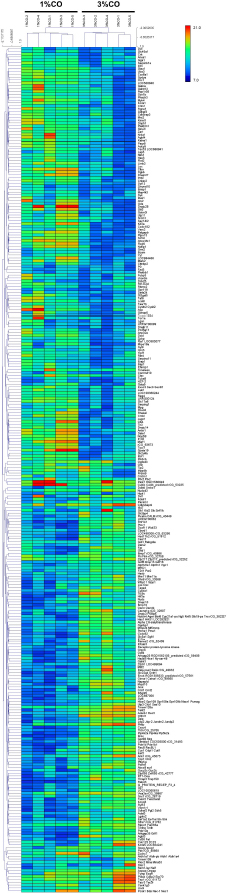
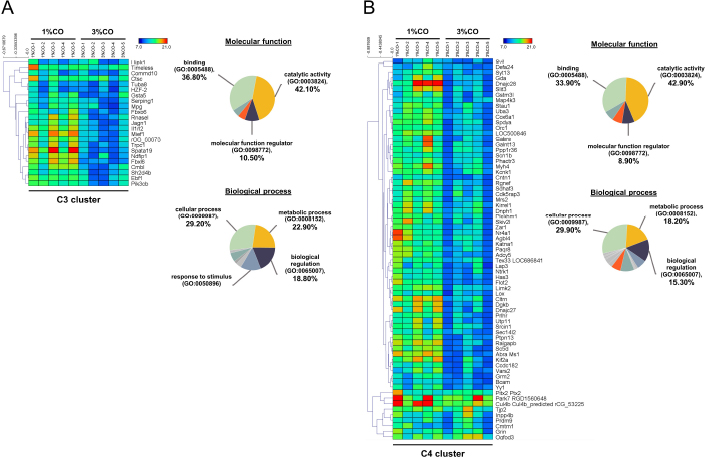
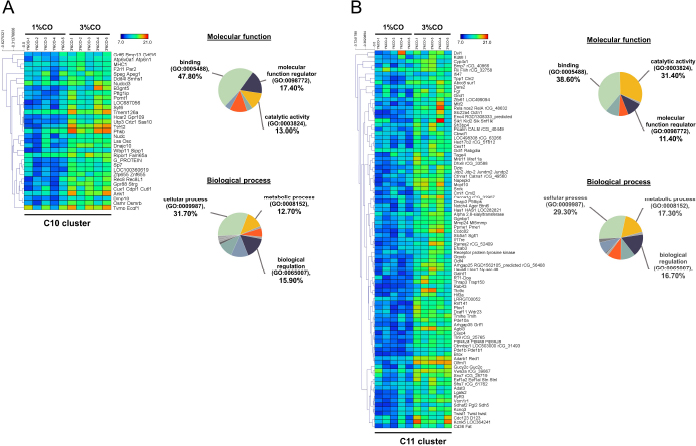
Fig. 1. Effect of different doses of dietary crocodile oil on serum (pre- and post-treatment) (A) cholesterol, (B) triglycerides, (C) LDL, and (D) HDL levels of rats over an 8-week period. Data are expressed as the mean±SEM. *Statistically significant intergroup differences (p < 0.05). Effect of different CO doses on serum energy-related metabolic intermediatesThere were no differences among the three groups in serum levels of lactate, pyruvate, citrate, alpha-ketoglutarate, or malate (Fig. 2). However, the 1%CO- and 3%CO-treated groups exhibited significantly increased serum oxaloacetate levels when compared with the N group. Interestingly, in the 3%CO group, serum lactate exhibited a trend toward lower levels when compared with the N group. Effect of different CO doses on serum protein expressionAll 4,525 serum proteins identified are shown by a Venn diagram in Figure 3A. The diagram shows the number of differentially expressed proteins among each of the three groups. A total of 1,112 proteins were expressed only in the N group, whereas 1,328 proteins were expressed only in the 1%CO-treated group, and 788 proteins were expressed only in the 3%CO-treated group. We focused on the effect of different doses of CO on changes in the expression of serum biomarkers associated with metabolic activity and catalytic processes. The 1,112 unique proteins in the N group, 1,328 unique proteins in the 1%CO group, and 788 unique proteins in the 3%CO group were categorized based on molecular function and biological process using PANTHER software (Fig. 3B and C). The PANTHER analysis revealed that when the differentially expressed proteins were grouped by molecular junction, a similar proportion of proteins associated with catalytic activity was observed in both CO-treated groups (35.4% vs. 34.1%, respectively), and this proportion was larger than that of the N group (30.4%). Analysis based on biological process indicated that compared with the N group, a greater proportion of unique proteins from both the 1%CO- and 3%CO-treated groups was primarily associated with cellular and metabolic processes. To obtain a better understanding of the impact of CO dose on the metabolic mechanisms regulating serum protein expression, 295 proteins shared between the 1%CO- and 3%CO-treatment groups were analyzed using a heatmap and SOTA clustering, as shown in Figures 4–6. The SOTA analysis yielded 11 expression clusters exhibiting opposing expression pattern trend. The most abundant protein clusters from the 3%CO group when compared with the 1%CO group were clusters 10 and 11 (C10 and C11); proteins in both clusters were up-regulated in the 3%CO group (Fig. 6A and B). C11 contained proteins primarily involved in binding (38.6%) and catalytic activity (31.4%) of molecular junctions, and these proteins were then further categorized according to biological process, which were classified as cellular process (29.3%) and metabolic process (17.3%). C10 included proteins involved in binding (47.3%), molecular function regulation (13%), and catalytic activity (13%) based on molecular function and cellular processes (31.7%), biological regulation (15.9%), and metabolic processes (12.7%) based on biological processes. However, other high abundance groups were clusters 3 and 4 (C3 and C4). As shown in Figure 5A and B, C3, and C4 consisted of proteins more highly upregulated in the 1%CO-treated group than the 3%CO-treated group. Most proteins isolated in C4 were involved in catalytic activity (42.9%) and binding (33.9%) in molecular function and cellular processes (29.9%) and metabolic processes (18.2%) in relation to biological processes. C3 consisted mostly of proteins involved in catalytic activity (42.1%), binding (36.8%) in molecular function, and cellular processes (29.2%), and metabolic processes (22.9%) in biological processes.
Fig. 2. Effect of different concentrations of dietary crocodile oil on serum energy metabolism-related metabolite levels: (A) lactate, (B) pyruvate, (C) alpha-ketoglutarate, (D) citrate, (E) oxaloacetate, and (F) malate [In (F), RO group should be revised into N group in the (F) (see the revised figure 2]. Data are expressed as the mean±SEM. *Statistically significant intergroup difference (p < 0.05). Our proteomic molecular function classification results suggest that more of the proteins upregulated in the 3%CO-treated group were associated with binding activity when compared with the 1%CO-treated group. In comparison with the 3%CO-treated group, a higher percentage of upregulated proteins in 1%CO treated group were associated with catalytic activity. Most upregulated proteins in both CO-treated groups were associated with cellular and metabolic processes.
Fig. 3. (A) Venn diagram of proteins differentially expressed in the Normal control group (N), normal rats treated with 1% (v/w) CO (1%CO), or normal rats treated with 3% (v/w) (3%CO). (B) Unique protein classification of the N group. (C) Unique protein classification of the 1%CO group. (D) Unique protein classification of the 3%CO group. Candidate proteins were classified according to molecular function and biological process using the PANTHER system. DiscussionThis is the first study to reveal the effects of different concentrations of CO on energy metabolism in rats. We demonstrated that administration of CO at two different doses (1%CO or 3%CO) significantly reduced food intake and serum triglyceride levels by the end of the experiment. Our results have indicated that both dosages of CO increased in the serum level of the energy-related intermediate oxaloacetic acid, but there was no difference between the CO groups. In the current study, we used a label-free quantitative shotgun proteomic approach to investigate the impact of different doses of CO on energy metabolic activity in the serum. The proteome of whole serum is affected by a variety of physiological processes. Our proteomic results showed that CO supplements might enhances energy metabolic activity, although there was no dose-dependent effect on metabolic changes in the serum. The primary finding of our study is that CO administration (1%CO or 3%CO) did not affect final body weight, body weight gain, or the energy content of food consumed. Normally, in a positive energy balance, energy intake is greater than energy expenditure, which contributes to overweight and obesity. Many previous studies have reported that high amounts of oils/fats in the diet lead to the development of overweight and obesity in rodents (Lauterio et al., 1994; Levin et al., 1997). Dietary oil/fat consumption is the main contributor to positive energy balance status and metabolic changes that may adversely affect long-term energy homeostasis. Considerable research indicates that an increased proportion of oils/fats in the diet results in increased hunger and energy intake and decreased sensation of fullness (Tremblay et al., 1991; Clegg et al., 2011). Another previous study found that consumption of oleic acid containing foods leads to increase sensation of fullness and a significant decrease in hunger (Naughton et al., 2018). Alfenas and Mattes (2003) also revealed that consumption of meals containing fat sources rich in oleic acid (canola oil and peanut oil) results in greater sense of fullness and lower hunger ratings 30, 60, and 120 minutes after feeding. A metabolic study indicated that a diet high in PUFAs is more metabolically beneficial compared with a diet high in SFAs in terms of energy expenditure and body weight maintenance (Krishnan and Cooper, 2014). In the present study, CO administration did not lead to a high level of body weight gain and energy intake, even though we provided both low-dose and high-dose CO supplementation. Our results suggest that a high levels of CO consumption (3%CO) are not associated with changes in body weight and energy balance when compared with lower levels of CO. Likewise, CO, an oil rich in MUFAs and PUFAs, decreased food intake at both doses. This finding indicates that MUFAs (e.g., oleic acid) and PUFAs (e.g., linoleic acid) in CO are important contributors to lowering food intake and help maintains body weight and energy balance by regulating the levels of satiety and hunger.
Fig. 4. Hierarchical clustering and heat maps of the 295 shared differentially expressed proteins of the 1%CO and 3%CO groups. Generally, lipid profile changes are associated with dietary oil patterns in the serum, especially with high-fat products. Our current results indicate that after 8 weeks of CO consumption, both low and high doses of CO decreased the serum triglyceride level when compared with the control group. Moreover, comparing pre- and post-treatment, 1%CO-treatment resulted in a significant decline in serum triglyceride levels, and a trend toward lower port-treatment triglyceride levels was observed in the 3%CO group. CO also maintained the serum HDL level in the post-treatment period, whereas the normal diet resulted in a lower HDL level. Even though CO contains high amounts of SFAs, CO has high levels of oleic acid and linoleic acid as a good combination mixture of MUFAs and PUFAs, respectively. Rice bran oil, which is rich in MUFAs and PUFAs (Orsavova et al., 2015), has been shown to improve blood lipid profiles (Lichtenstein et al., 1994; Shakib et al., 2014; Bumrungpert et al., 2019). Further studies also reported that canola oil, which is rich in MUFAs and PUFAs, decreases serum triglyceride levels when compared with diets high in dairy products (high amounts of SFAs) (Iggman et al., 2011). In this regard, others studies have proposed that unsaturated fat sources (MUFAs and PUFAs) reduce serum triglyceride levels by enhancing insulin function, regulating lipoprotein lipase activity, and repressing lipogenic enzymes (Nigam et al., 2014; Salar et al., 2016). Our data suggest that CO exerts beneficial effects on serum triglycerides and that CO also helps maintain HDL levels when consumed longer than 8 weeks.
Fig. 5. Expression patterns of the selected upregulated 1%CO-protein cluster 3 (C3) (A) and cluster 4 (C4) (B) corresponding to SOTA clustering. Candidate proteins in each cluster were classified according to molecular function and biological process using the PANTHER system. In our study, an increase in serum oxaloacetate, the key energy metabolic intermediate in the TCA cycle, was detected in both CO administration groups when compared with normal control rats. Nevertheless, the oxaloacetate level was not affected by the degree of CO consumption in our study. Oxaloacetate plays important roles in the oxidation of carbohydrates and fats involved in the progression of metabolic syndrome. However, the mechanisms by which CO or dietary oils enriched in MUFAs and PUFAs affect energy metabolic pathways are complex and still incompletely understood. Gillinghamet al. (2011) found that dietary MUFAs decrease the risk of metabolic syndrome by favorably altering blood lipids and insulin sensitivity. Jones et al. (2008) compared the effects of three fatty acids (oleic acid, linoleic acid, and linolenic acid) on postprandial energy expenditure and macronutrient oxidation in healthy men and revealed that olive oils rich in oleic acid (MUFA) may offer increased oxidation compared with linoleic acid (PUFA). Kien and Bunn (2008) demonstrated that consumption of a high-MUFA diet increases the fatty acid oxidation rate in women compared with a control diet, and they also reported that high-MUFA diets increase daily energy expenditure compared to a diet high in SFAs. Moreover, Jones and Schoeller (1988) indicated that high-PUFA diets tend to increase total fatty acid oxidation to a greater degree than high-SFA diets. Interestingly, CO exhibited a trend toward decreased serum lactate levels with increasing CO concentration. The serum lactate level is a valuable criterion for monitoring critically stressed patients. Increased lactate levels are associated with increased morbidity and mortality rates (Shapiro et al., 2005). Medical research examining the association between diabetes and elevated lactate concentrations reported serious changes in glucose metabolism, including diminished glycogen synthesis and decreased glucose oxidative metabolism (Wu et al., 2016). Further studies investigating the potential effects of omega-3 PUFAs as modulators of targets of aerobic glycolysis reported that n-3 PUFAs decrease the expression of lactate dehydrogenase, which can subsequently lead to reduced lactate levels (Manzi et al., 2015). Thus, CO improves whole-body energy metabolism by increasing level of key metabolites of TCA cycle and regulating energy-related metabolite production, which lowers serum levels of potentially toxic lactate. Our data indicate that the major component of CO plays a key role in energy expenditure, which translates into increased energy-related metabolite production.
Fig. 6. Expression patterns of the selected upregulated 3%CO-protein cluster 10 (C10) (A) and cluster 11 (C11) (B) corresponding to SOTA clustering. Candidate proteins in each cluster were classified according to molecular function and biological process using the PANTHER system. Serum proteome analyses showed that different doses of CO, which is enriched in MUFAs and PUFAs, affects the expression of proteins responsible for energy-related metabolic activity. In physiology, energy metabolism generally refers to synthesis of ATP as a molecular catalyst. The CO-dose dependent expression of various unique proteins in this study compared with the control group suggests that dietary CO upregulates cellular and metabolic processes. Zheng et al. (2008) investigated that MUFAs intake activates pathways responsible for rapid catabolism of triglyceride-rich lipoproteins. Using indirect calorimetry, Paniagua et al. (2007) found that consuming a diet rich in MUFAs lead to a higher rate of fat oxidation compared with consumption of carbohydrate- and SFA-rich diets. Further research reported that dietary PUFAs activate the expression of peroxisome proliferator-activated receptor-α (PPAR-α) and PPAR-γ (target key metabolic genes), resulting in increased lipid oxidation and leading to a reduction in serum triglycerides (Rodríguez-Cruz et al., 2005). Activation of PPAR-α reduces triglyceride levels and is involved in the regulation of energy homeostasis (Tyagi et al., 2011). Similarly, Moradi et al. (2021) discovered that omega-3 PUFA supplementation leads to upregulation of PPAR-γ and uncoupling protein_2 (UCP2) expression as indicators of energy metabolism in healthy athletes. We concluded that in rats, dietary CO upregulates the expression of proteins involved in energy metabolism, thereby affecting energy-related metabolite production and improving the lipid profile. Moreover, hierarchical and SOTA clustering of the 295 shared differentially expressed proteins in the low- and high-dose CO groups revealed 11 clusters based on expressed protein levels. PANTHER analyses indicated that the upregulated proteins in the clusters of the 1%CO-treated group (C3 and C4) and 3%CO-treated group (C10 and C11) could be categorized according to primary molecular and biological functions. It is not surprising that cellular and metabolic processes represented the predominant biological junction in both groups, whereas more proteins in the upregulated cluster of the 1%CO group were linked to catalytic activity compared with the upregulated cluster of the 3%CO group. However, the difference in expression of catalytic activity-related proteins between groups did not affect other energy metabolism parameters in the current study. The upregulation of catalytic activity and related metabolic pathways could affect processes that are involved in controlling cellular energy metabolism. Pahlavani et al. (2020) investigated the effects of consumption of 9, 18, and 36 g/kg of omega-3 PUFA enriched fish oil for 14 weeks on energy metabolism using indirect calorimetry measurements. In agreement with our results, they found that total energy expenditure at all omega-3 PUFA doses was significantly increased when compared with high-fat diet group. The same study found no difference in the level of energy use among the three study groups. Moreover, a proteomic and PANTHER analysis of the therapeutic effects of a diet rich in omega-3 PUFAs on the retina of aged mice in eyecups revealed that the majority of differentially expressed proteins were functionally associated with catalytic activity (including isomerases, ligases, acetyltransferases, and decarboxylases) (Prokopiou et al., 2019). In our experiments, CO consumption did not show a dose-response effect on the expression of proteins involved in energy metabolism (catabolic and total metabolic processes). However, our study had some limitations, including the examination of only two doses of CO. As such, it is possible that higher doses of CO could have a greater impact on metabolism via the regulation of serum protein expression. This study provides evidence regarding the potential dose-dependent effects of dietary CO on energy metabolism in rats. The present study demonstrated that rats supplemented with CO at two doses (1%CO or 3%CO) maintained bodyweight and energy intake and exhibited beneficial lipid profile changes in serum triglycerides and HDL and increased energy-related metabolite production (oxaloacetate of the TCA cycle). Moreover, serum proteomic analysis revealed unique proteins and shared differentially proteins in the upregulated clusters in both CO treatment groups. These proteins are involved in energy metabolism processes, including catalytic activity and cellular metabolic processes. However, no dose-dependent effect on metabolism was observed at the two CO supplementation doses examined in this study. Further studies to provide a better understanding of the dose-response relationship of dietary CO are thus needed. Finally, our findings suggest that CO is a suitable alternative fat source that simultaneously enhances energy metabolism and provides a healthier profile. CO may be a good natural and inexpensive dietary fat source. AcknowledgmentsThis research was supported by the Department of Zoology and Faculty of Science and partially supported by the Faculty of Veterinary Medicine, Kasetsart University, Thailand and the Science Achievement Scholarship of Thailand (SAST). Conflict of interest The authors declare that there is no conflict of interest. Author contributionsKP performed the experiments, analyzed the proteomic data, and wrote the manuscript. KS and PS performed the experiments. SC and NP performed the proteomic experiments. SR performed the proteomic analyses and edited the manuscript. PT conceived and designed the study and edited the manuscript. WF conceived, designed and supervised the study and edited the manuscript. ReferencesAlfenas, R.C. and Mattes, R.D. 2003. Effect of fat sources on satiety. Obes. Res. 11, 183–7. Bardou, P., Mariette, J., Escudié, F., Djemiel, C. and Klopp, C. 2014. jvenn: an interactive Venn diagram viewer. BMC. Bioinformatics. 15, 293. Binia, A., Vargas-Martínez, C., Ancira-Moreno, M., Gosoniu, L.M., Montoliu, I., Gámez-Valdez, E., Soria-Contreras, D.C., Angeles-Quezada, A., Gonzalez-Alberto, R., Fernández, S., Martínez-Conde, D., Hernández-Morán, B., Ramírez-Solano, M., Pérez-Ortega, C., Rodríguez-Carmona, Y., Castan, I., Rubio-Aliaga, I., Vadillo-Ortega, F., Márquez-Velasco, R., Bojalil, R., López-Alvarenga, J.C., Valet, P., Kussmann, M., Silva-Zolezzi, I. and Tejero, M.E. 2017. Improvement of cardiometabolic markers after fish oil intervention in young Mexican adults and the role of PPARα L162V and PPARγ2 P12A. J. Nutr. Biochem. 43, 98–106. Bumrungpert, A., Chongsuwat, R., Phosat, C. and Butacnum, A. 2019. Rice bran oil containing gamma-oryzanol improves lipid profiles and antioxidant status in hyperlipidemic subjects: A randomized double-blind controlled trial. J. Altern. Complement. Med. 25, 353–358. Burri, L., Berge, K., Wibrand, K., Berge, R.K, and Barger, J.L. 2011. Differential effects of krill oil and fish oil on the hepatic transcriptome in mice. Front. Genet. 2, 45. Buthelezi, S., Southway, C., Govinden, U., Bodenstein, J. and du Toit, K. 2012. An investigation of the antimicrobial and anti-inflammatory activities of crocodile oil. J. Ethnopharmacol. 143, 325–330. Clegg, M.E., McKenna, P., McClean, C., Davison, G.W., Trinick, T., Duly, E. and Shafat, A. 2011. Gastrointestinal transit, post-prandial lipaemia and satiety following 3 days high-fat diet in men. Eur. J. Clin. Nutr. 65, 240–246. Echeverria, F., Valenzuela, R., Bustamante, Álvarez, D., Ortiz, M., Espinosa, A., Illesca, P., Gonzalez-Mañan, D. and Videla, L.A. 2019. High-fat diet induces mouse liver steatosis with a concomitant decline in energy metabolism: attenuation by eicosapentaenoic acid (EPA) or hydroxytyrosol (HT) supplementation and the additive effects upon EPA and HT co-administration. Food. Funct. 10(9), 6170–6183. Gillingham, L.G., Harris-Janz, S. and Jones, P.J. 2011. Dietary monounsaturated fatty acids are protective against metabolic syndrome and cardiovascular disease risk factors. Lipids. 46(3), 209–228. Gunstone, F. and Russell, W. 1954. Animal fats. The component acids of crocodile fat. Biochem. J. 57, 462–465. Herieka, M. and Erridge, C. 2014. High-fat meal induced postprandial inflammation. Mol. Nutr. Food. Res. 58, 136–146. Hernández, E.Á., Kahl, S., Seelig, A., Begovatz, P., Irmler, M., Kupriyanova, Y., Nowotny, B., Nowotny, P., Herder, C., Barosa, C., Carvalho, F., Rozman, J., Neschen, S., Jones, J.G., Beckers, J., de Angelis, M.H. and Roden, M. 2017. Acute dietary fat intake initiates alterations in energy metabolism and insulin resistance. J. Clin. Invest. 127(2), 695–708. Howe, P. and Buckley, J. 2014. Metabolic health benefits of long-chain omega-3 polyunsaturated fatty acids. Mil. Med. 179,138–143. Iggman, D., Gustafsson, I.B., Berglund, L., Vessby, B., Marckmann, P. and Risérus, U. 2011. Replacing dairy fat with rapeseed oil causes rapid improvement of hyperlipidaemia: a randomized controlled study. J. Intern. Med. 270, 356–364. Johansson, C., Samskog, J., Sundstrom, L., Wadensten, H., Björkesten, L. and Flensburg, J. 2006. Differential expression analysis of Escherichia coli proteins using a novel software for relative quantitation of LC-MS/MS data. Proteomics. 6, 4475–4485. Jones, P.J., Jew, S. and AbuMweis, S. 2008. The effect of dietary oleic, linoleic, and linolenic acids on fat oxidation and energy expenditure in healthy men. Metabolism 57, 1198–1203. Jones, P.J.H. and Schoeller, D.A. 1988. Polyunsaturated:saturated ratio of diet fat influences energy substrate utilization in the human. Metabolism. 37, 145–151. Kien, C.L. and Bunn, J.Y. 2008. Gender alters the effects of palmitate and oleate on fat oxidation and energy expenditure. Obesity. (Silver Spring). 16, 29–33. Krishnan, S. and Cooper, J.A. 2014. Effect of dietary fatty acid composition on substrate utilization and body weight maintenance in humans. Eur. J. Nutr. 53, 691–710. Laguna-Camacho, A. 2017. Influence on adiposity and atherogenic lipaemia of fatty meals and snacks in daily life. J. Lipids. 2017, n.p. Lauterio, T.J., Bond, J.P. and Ulman, E.A. 1994. Development and characterization of a purified diet to identify obesity-susceptible and resistant rat populations. J. Nutr. 124, 2172–2178. Levin, B.E., Dunn-Meynell, A.A., Balkan, B. and Keesey R.E. 1997. Selective breeding for diet induced obesity and resistance in Sprague-Dawley rats. Am. J. Physiol. 273, R725–R730. Lichtenstein, A.H., Ausman, L.M., Carrasco, W., Gualtieri, L.J., Jenner, J.L., Ordovas, J.M., Nicolosi, R.J., Goldin, B.R. and Schaefer, E.J. 1994. Rice bran oil consumption and plasma lipid levels in moderately hypercholesterolemic humans. Arterioscler. Thromb. 14, 549–556. Lillefosse, H.H., Clausen, M.R., Yde, C.C., Ditlev, D.B., Zhang, X., Du, Z.Y., Bertram, H.C., Madsen, L., Kristiansen, K. and Liaset, B. 2014. Urinary loss of tricarboxylic acid cycle intermediates as revealed by metabolomics studies: an underlying mechanism to reduce lipid accretion by whey protein ingestion? J. Proteome. Res. 13, 2560–2570. Lowry, O., Rosebrough, N., Farr, A.L. and Randall R.J. 1951. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193, 265–275. Lueangsakulthai, J., Phosri, S., Theansungnoen, T., Jangpromma, N., Temsiripong, T., Mckendrick, J.E., Khunkitti, W. and Klaynongsruang, S. 2018. Novel antioxidant and anti-inflammatory peptides from the Siamese crocodile (Crocodylus siamensis) hemoglobin hydrolysate. Biotechnol. Appl. Biochem. 65, 455–466. Manzi, L., Costantini, L., Molinari, R. and Merendino, N. 2015. Effect of dietary ω-3 polyunsaturated fatty acid DHA on glycolytic enzymes and Warburg phenotypes in cancer. BioMed. Res. Int. 2015, 137097. Mi, H., Huang, X., Muruganujan, A., Tang, H., Mills, C., Kang, D. and Thomas, P.D. 2017. PANTHER version 11: expanded annotation data from Gene Ontology and Reactome pathways, and data analysis tool enhancements. Nucleic. Acids. Res. 45, D183–D189. Moradi, S., Alivand, M., KhajeBishak, Y., AsghariJafarabadi, M., Alipour, M., Chilibeck, P.D. and Alipour, B. 2021. The effect of omega3 fatty acid supplementation on PPARγ and UCP2 expressions, resting energy expenditure, and appetite in athletes. BMC. Sports. Sci. Med. Rehabil. 13(1), 48. Naughton, S.S., Hanson, E.D., Mathai, M.L. and McAinch, A.J. 2018. The acute effect of oleic-or linoleic acid-containing meals on appetite and metabolic markers; a pilot study in overweight or obese individuals. Nutrients. 10, 1376. Nigam, P., Bhatt, S., Misra, A., Chadha, D.S., Vaidya, M., Dasgupta, J. and Pasha, Q.M. 2014. Effect of a 6-month intervention with cooking oils containing a high concentration of monounsaturated fatty acids (olive and canola oils) compared with control oil in male Asian Indians with nonalcoholic fatty liver disease. Diabetes. Technol. Ther. 16, 255–261. Orsavova, J., Misurcova, L., Ambrozova, J.V., Vicha, R. and Mlcek, J. 2015. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int. J. Mol. Sci. 16, 12871–12890. Pahlavani, M., Ramalingam, L., Miller, E.K., Davis, H., Scoggin, S. and Moustaid-Moussa, N. 2020. Discordant dose-dependent metabolic effects of eicosapentanoic acid in diet-induced obese mice. Nutrients. 12, 1342. Paniagua, J.A., Gallego de la Sacristana, A., Romero, I., Vidal-Puig, A., Latre, J.M., Sanchez, E., Perez-Martinez, P., Lopez-Miranda, J. and Perez-Jimenez, F. 2007. Monounsaturated fat-rich diet prevents central body fat distribution and decreases postprandial adiponectin expression induced by a carbohydrate-rich diet in insulin-resistant subjects. Diabetes. Care. 30(7), 1717–1723. Phosri, S., Mahakunakorn, P., Lueangsakulthai, J., Jangpromma, N., Swatsitang, P., Daduang, S., Dhiravisit, A. and Thammasirirak, S. 2014. An investigation of antioxidant and anti-inflammatory activities from blood components of Crocodile (Crocodylus siamensis). Protein. J. 33, 484–492. Prokopiou, E., Kolovos, P., Georgiou, C., Kalogerou, M., Potamiti, L., Sokratous, K., Kyriacou, K. and Georgiou, T. 2019. Omega-3 fatty acids supplementation protects the retina from age-associated degeneration in aged C57BL/6J mice. BMJ. Open. Ophthalmol. 4, e000326. Rodríguez-Cruz, M., Tovar, A.R., del Prado, M. and Torres, N. 2005. Molecular mechanisms of action and health benefits of polyunsaturated fatty acids. Rev. Invest. Clin. 57, 457–472. Salar, A., Faghih, S. and Pishdad, G.R. 2016. Rice bran oil and canola oil improve blood lipids compared to sunflower oil in women with type 2 diabetes: a randomized, single-blind, controlled trial. J. Clin. Lipidol. 10, 299–305. Santativongchai, P., Fungfuang, W., Boonyawiwat, V., Pongchairerk, U., and Tulayakul, P. 2020. Comparison of physicochemical properties and fatty acid composition of crocodile Oil (Crocodylus siamensis) extracted by using various extraction methods. Int. J. Food. Prop. 23, 1465–1474. Shakib, M.C., Gabrial, S. and Gabrial, G. 2014. Rice bran oil compared to atorvastatin for treatment of dyslipidemia in patients with type 2 diabetes. Maced. J. Med. Sci. 2, 95–102. Shapiro, N.I., Howell, M.D., Talmor, D., Nathanson, L.A., Lisbon, A., Wolfe, R.E. and Weiss, J.W. 2005. Serum lactate as a predictor of mortality in emergency department patients with infection. Ann. Emerg. Med. 45, 524–528. Siddiq, A., Ambreen, G., Hussain, K. and Baig S.G. 2019. Oxidative stress and lipid per-oxidation with repeatedly heated mix vegetable oils in different doses in comparison with single time heated vegetable oils. Pak. J. Pharm. Sci. 32, 2099–2105. Silva Figueiredo, P., Carla Inada, A., Marcelino, G., Maiara Lopes Cardozo, C., de Cássia Freitas, K., de Cássia Avellaneda Guimarães, R., Pereira de Castro, A., Aragão do Nascimento, V. and Aiko Hiane, P. 2017. Fatty acids consumption: the role metabolic aspects involved in obesity and its associated disorders. Nutrients. 9, 1158. Thorsell, A., Portelius, E., Blennow, K. and Westman-Brinkmalm, A. 2007. Evaluation of sample fractionation using micro-scale liquid-phase isoelectric focusing on mass spectrometric identification and quantitation of proteins in a SILAC experiment. Rapid. Commun. Mass. Spectrom. 21, 771–778. Tremblay, A., Lavallée, N., Alméras, N., Allard, L., Després, J.P. and Bouchard, C. 1991. Nutritional determinants of the increase in energy intake associated with a high-fat diet. Am. J. Clin. Nutr. 53, 1134–1137. Tyagi, S., Gupta, P., Saini, A.S., Kaushal, C. and Sharma, S. 2011. The peroxisome proliferator activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2, 236–240. Wu, Y., Dong, Y., Atefi, M., Liu, Y., Elshimali, Y. and Vadgama, J.V. 2016. Lactate, a neglected factor for diabetes and cancer interaction. Mediators. Inflamm. 2016, 6456018. Zheng, C., Khoo, C., Furtado, J., Ikewaki, K. and Sacks, F.M. 2008. Dietary monounsaturated fat activates metabolic pathways for triglyceride-rich lipoproteins that involve apolipoproteins E and C-III. Am. J. Clin. Nutr. 88, 272–281. | ||
| How to Cite this Article |
| Pubmed Style Fungfuang W, . Serum proteomic analysis reveals the differential dose effects of crocodile oil from Crocodylus siamensis on energy metabolism in rats. Open Vet J. 2022; 12(5): 697-708. doi:10.5455/OVJ.2022.v12.i5.15 Web Style Fungfuang W, . Serum proteomic analysis reveals the differential dose effects of crocodile oil from Crocodylus siamensis on energy metabolism in rats. https://www.openveterinaryjournal.com/?mno=45178 [Access: July 27, 2024]. doi:10.5455/OVJ.2022.v12.i5.15 AMA (American Medical Association) Style Fungfuang W, . Serum proteomic analysis reveals the differential dose effects of crocodile oil from Crocodylus siamensis on energy metabolism in rats. Open Vet J. 2022; 12(5): 697-708. doi:10.5455/OVJ.2022.v12.i5.15 Vancouver/ICMJE Style Fungfuang W, . Serum proteomic analysis reveals the differential dose effects of crocodile oil from Crocodylus siamensis on energy metabolism in rats. Open Vet J. (2022), [cited July 27, 2024]; 12(5): 697-708. doi:10.5455/OVJ.2022.v12.i5.15 Harvard Style Fungfuang, W. & (2022) Serum proteomic analysis reveals the differential dose effects of crocodile oil from Crocodylus siamensis on energy metabolism in rats. Open Vet J, 12 (5), 697-708. doi:10.5455/OVJ.2022.v12.i5.15 Turabian Style Fungfuang, Wirasak, and . 2022. Serum proteomic analysis reveals the differential dose effects of crocodile oil from Crocodylus siamensis on energy metabolism in rats. Open Veterinary Journal, 12 (5), 697-708. doi:10.5455/OVJ.2022.v12.i5.15 Chicago Style Fungfuang, Wirasak, and . "Serum proteomic analysis reveals the differential dose effects of crocodile oil from Crocodylus siamensis on energy metabolism in rats." Open Veterinary Journal 12 (2022), 697-708. doi:10.5455/OVJ.2022.v12.i5.15 MLA (The Modern Language Association) Style Fungfuang, Wirasak, and . "Serum proteomic analysis reveals the differential dose effects of crocodile oil from Crocodylus siamensis on energy metabolism in rats." Open Veterinary Journal 12.5 (2022), 697-708. Print. doi:10.5455/OVJ.2022.v12.i5.15 APA (American Psychological Association) Style Fungfuang, W. & (2022) Serum proteomic analysis reveals the differential dose effects of crocodile oil from Crocodylus siamensis on energy metabolism in rats. Open Veterinary Journal, 12 (5), 697-708. doi:10.5455/OVJ.2022.v12.i5.15 |