| Case Report | ||
Open Vet J. 2023; 13(2): 241-246 Open Veterinary Journal, (2023), Vol. 13(2): 241–246 Case Report Giant and potentially malignant bullae in a dogCaroline Augusto Roque*, Bruno Roque Lima, Guillermo Veiga de Oliveira and Letícia Martins NascimentoOmega Imagem Veterinária, Av. Washington Luis, Vila Matias, Brazil *Corresponding Author: Caroline Augusto Roque. Omega Imagem Veterinária, Av. Washington Luis, Vila Matias, Brazil. Email: carolimagemvet [at] gmail.com Submitted: 09/06/2022 Accepted: 06/01/2023 Published: 22/02/2023 © 2023 Open Veterinary Journal
AbstractBackground: Primary lung neoplasms are, frequently represented by solid, solitary, or multiple formations. However, malignant cavitary lesions may be presented as lung adenocarcinomas. Those malignant lesions differ from benignant bullae by the thickness heterogeneity of its surrounding shape. Case Description: The present clinical case reports a 14-year-old female dog, of mixed breed, with an increase in the coughs frequency, fatigue, and exercise intolerance. A chest X-ray was taken, a large emphysematous cystic area was found, with thickened and irregular walls located in the left caudal pulmonary lobe, which measured 8 × 7.5 × 3 cm, and rejected the bronchial branch corresponding to the left caudal pulmonary lobe, in addition to thickening of the bronchial walls, compatible with bronchopathy. The tomographic examination of the cavity showed an air content structure, oval to round in shape, with irregular thick hyperattenuating walls measuring approximately 0.4 cm in thickness, occupying more than 30% of the left hemithorax, and pulmonary lobectomy was chosen. Histopathology confirmed the diagnosis of bronchoalveolar adenocarcinoma, with the presence of sparse areas of necrosis and dystrophic calcification. Conclusion: The present case successfully diagnosed a malignant bulae, after a surgical remove. The tomographic finds although not confirmatory, suggest a malignant component by the shape and thickness of its wall. The tomographic exam is of great importance, because only through it, it is possible to evaluate if there is lymph node or pleural involvement or the presence of small metastasis foci. There is indication for surgery and histopathological examination of the piece for a definitive diagnosis. Keywords: Malignant bullae, Dog, Computer tomography, Surgery. IntroductionPrimary lung tumors are rare in dogs, accounting for approximately 1% of all malignancies in these animals. This low rate may be explained by a lower exposure to risk factors, when compared to humans (Ogilvie et al., 1989; Aydin et al., 1997; Polton et al., 2008; Parry et al., 2021). Pulmonary carcinomas affect dogs of 11 years old on average (Brodey and Craig, 1965), with reported cases ranging from 9.7 to 17.6 years of age (Hahn and Muggenburg, 1996). Normally, these carcinomas appear as solid formations, with single or multiple lesions (Monnet, 2012). In a recent 2014 study, the prevalence of cavitary lesions in primary lung neoplasms in dogs was 11% (Gadkowski and Stout, 2008). According to World Health Organization (WHO) classification, pulmonary malignancies in pets are classified as bronchioalveolar carcinomas, squamous cell carcinomas, adenocarcinomas, sarcomas, neuroendocrine tumors, and lymphoma (Dungworth et al., 1999). Some pulmonary disorders can be characterized by cavitary lesions, containing air or fluid, surrounded by thin walls, called blebs, bullae, or cysts (Silverman et al., 1976; Murphy and Fishman, 1988; Park et al., 2016). The names cysts, blebs, and bullae create some confusion since they are used indiscriminately in histological examination. Indeed, they are all characterized by an unusual extension of airways distal to the bronchioles, with destruction of alveolar walls, called pulmonary emphysema (Lesur et al., 1990, Park et al., 2016). Blebs and bullae are considered pseudocysts because they lack the respiratory epithelium lining and their walls thickness are less than 1 mm (Klingman et al., 1991; Hansell et al., 2008). Blebs are smaller than bullae, they are under 1 cm in diameter while bullae are over 1 cm in diameter (Murphy and Fishman, 1988; Lesur et al., 1990; Lipscomb et al., 2003; Park et al., 2016). A pulmonary bulla is defined as a gas-filled space within a zone of pulmonary consolidation or within a mass or nodule. There are infectious and neoplastic etiologies for cavitary lesions, including neoplasms, granulomas, cysts, and abscesses (Gafoor et al., 2018). The proposed etiology behind these lesions in neoplastic desease is thickness and irregularity of the bulla’s wall, vascular heterogeneity, and central cavitation (Parry et al., 2021). Some reports suggest that bullae increase the risk of malignancy (Richardson et al., 1996, Hanaoka et al., 2002, Yoshikawa et al., 2011). This might be explained by the poor ventilation inside the bullae, which may facilitate the build-up of carcinogens, which in turn may cause metaplastic transformation of the epithelial cells in the inner lining (Hanaoka et al., 2002). It is difficult to definitively diagnose malignancies caused by pulmonary bullae by radiography or tomography tests because bullae do not display typical malignancy characteristics (Lipscomb et al., 2003; Nemanic et al., 2006; Yoshikawa et al., 2011). It has been observed, by radiography, that neoplastic bullae show thick and irregular inner walls (Lamb and Neiger, 2000). Using tomography, another study reported that giant bullae are regions of low attenuation, with destroyed or distorted blood vessels around the hypoattenuated region (Au et al., 2006). However, neither observation can confirm bullae malignancy. Although cavitary lesions can be seen on chest X-rays, due to increased accessibility to computed tomography (CT) in veterinary practice, these cases are often submitted to CT scans to better characterize the lesions. Nonetheless, tomography tests can assess nodal, pleural damage, and identify the presence of small metastatic foci (Park et al., 2016). In one study, two elderly dogs previously diagnosed with solitary emphysematous bulla showed nonspecific clinical signs. At presentation, lung auscultation was normal. In both cases, thoracic CT showed the transformation of cystic airspace lesions, characterized by progressive increase of the solid component and reduction of the air component. Surgical excision followed by histopathology confirmed lung carcinoma in both cases (Bello et al., 2021). Pre-operative pathological exams are also challenging, because the procedure to obtain the sample increases the risk of rupturing the bullae (Allison, 1947; Yoshikawa et al., 2011). Histological examination after surgical removal is still the gold standard test for a definitive diagnosis (Polton et al., 2008). For giant bullae that occupy more than 30% of the hemithorax, the recommendation is to surgically remove it (Godwin et al., 1980; Klingman et al., 1991; Richardson et al., 1996). Giant bullae increase the risk of complications such as infection, coagulopathy, subcutaneous emphysema, bleeding, and pneumothorax, with subsequent compression of pulmonary lobes, leading to dyspnea (Allison, 1947; Klingman et al., 1991; Bonath 1996; Walsh et al., 1999; Monnet, 2012; Park et al., 2016). A little over 30% of cases are asymptomatic and are accidentally diagnosed during examination (Rebhun and Culp, 2013). The prognosis of carcinoma associated with bullae and that associated with other factors is the same (Hanaoka, et al., 2002; Yoshikawa et al., 2011). There have been few reports of pulmonary malignancies associated with giant bullae (Stogdale et al., 1982; Park et al., 2016). A retrospective study evaluated 64 cases of spontaneous pneumothorax in dogs, from which only two cases were malignancies associated with giant bullae (a bronchoalveolar carcinoma and an anaplastic carcinoma) (Puerto et al., 2002). Another report detailed that a 12-year-old Shih Tzu with giant pulmonary bullae, without pneumothorax, was diagnosed with papilliferous bronchoalveolar carcinoma after the bullae were surgically removed (Park et al., 2016). The goals of this case report were to describe the clinical and tomographic features of a dog with cavitary lung adenocarcinoma and to compare tomographic features with histopathological findings. Case DetailsA 15-year-old mixed breed bitch, castrated, with approximately 14 kg, had sporadic coughing. Approximately 1 month before the appointment, the coughing became more frequent, she was tired easily and intolerant to physical exercise. The bitch was examined in a diagnosis center and a thoracic radiography was performed, in which we observed a large cystic emphysematous area, with thick and irregular walls, located in the left caudal pulmonary lobe (Fig. 1). The lesion measured 8.0 × 7.5 × 3.0 cm and pressed the bronchial branching of the caudal left lobe. We also observed thickening of bronchial walls, compatible with bronchopathy (Fig. 1). Even though the cardiac silhouette looked normal in the radiography, the dog was diagnosed with moderate mitral insufficiency by echocardiography. The patient was referred to a tomographic examination of the thoracic cavity. The CT images were acquired with a 1-row detector helical CT unit (Somatom Spirit, Siemens; 1,5 pitch, 1s rotation time, 130 de Kv and 70 de mAs). We observed thickening of the bronchial walls and an extensive structure, filled with air, oval to round in shape, with hyperattenuated, thick and irregular walls, approximately 0.4 cm thick (Fig. 2). The CT after intravenous contrast has not been performed, because the owner did not allow it, so the solid part of the tumor could not be contrasted. The structure was located in the perihilar region of the left caudal lung lobe, and it was in contact with its bronchial branches and air bronchogram. It measured approximately 6.6 × 4.5 × 5.9 cm; compatible with aberrant bronchiectasis or a low-grade pneumatocele (Fig. 2). This structure created a medial displacement and luminal compression of the main bronchus of the left caudal pulmonary lobe. In the bifurcation of this main bronchus, its branches moved medially and followed the medioventral and mediodorsal walls of the bullae, respectively, with the ventral branch slipping into the lumen of the bullae. We did not observe lymphomegalies. With the identification of the giant bullae, which took more than 30% of the left hemithorax, we opted for performing a pulmonary lobectomy. The patient was submitted to inhalation anesthesia with mechanical ventilation (anesthesia: 0.05 mg/kg morphine associated acepromazine, MPA; 3 mg/kg propofol induction; and kept in anesthesia with isoflurane).
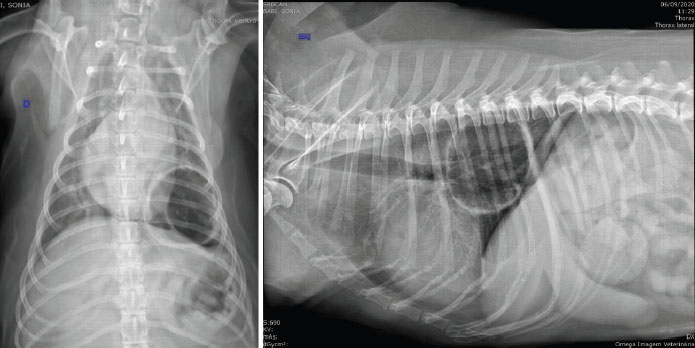
Fig. 1. Thoracic radiographic images in ventrodorsal (left) and lateral (right) views, in which we observe a big cystic emphysematous area. This area has thick and irregular walls and is located in the left caudal lung lobe. It is pressing the bronchial branching of the left caudal lung lobe. We also observe thickening of the bronchial walls.
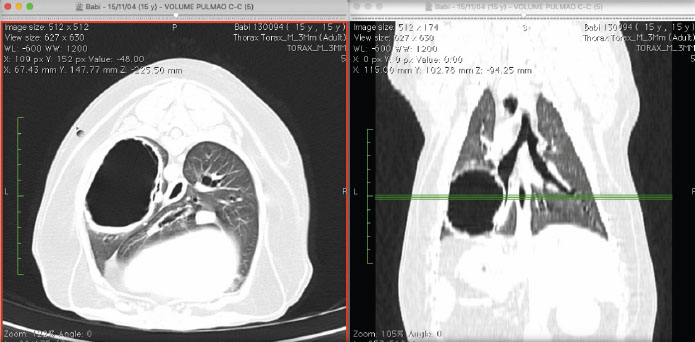
Fig. 2. Tomographic images in axial (left) and dorsal (right) views, in which we observe the thickening of the bronchial walls. Moreover, we can observe a structure filled with air, of round shape, with hyperattenuated, thick and irregular walls. It is located in the perihilar region of the left caudal pulmonary lobe in contact with bronchial branches and air bronchograms. The thoracotomy was conducted on the left sixth intercostal space. There were neither pleural adhesions nor fluid deposition. The left caudal lobe was completely removed. It had a big air-filled saccule with thin walls and was highly vascularized (Fig. 3). The bandage was performed with a linear thoracoabdominal stapler (ethicon®). There were no bleeding or other events. The histopathologist confirmed the diagnosis of bronchioalveolar adenocarcinoma, with necrotic areas, with areas of sparse dystrophic calcification, and with multifocal areas of neutrophil infiltration.
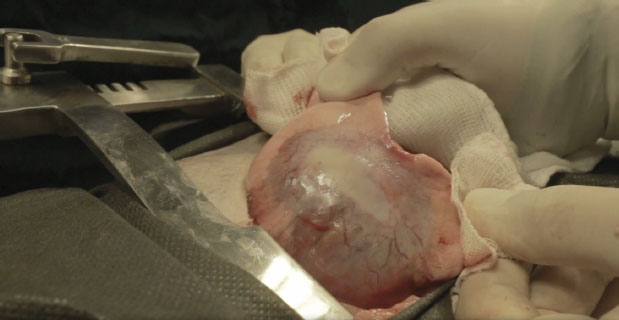
Fig. 3. Surgically removed left caudal lung lobe. We can observe the saccule with a highly vascularized wall. The patient was kept in Intensive Care Unit (ICU) for 24 hours. The patient recovered well and was discharged but had to return daily for clinical evaluation. Four days after the surgery, the patient decompensated from its previous cardiopathy, leading to a severe cardiogenic pulmonary edema. The patient was brought to the ICU but did not survive. DiscussionThis study reports the case of a bitch with a large pulmonary emphysemic cyst and a bronchioalveolar adenocarcinoma, a rare case in the veterinary literature. We could not determine if the build-up of air was due to the malignancy or if the malignancy was secondary to the bulla. The bulla may cause the malignancy because of its poor internal ventilation, which can facilitate the deposition of some carcinogens, as stated before (Hanaoka et al., 2002; Bello et al., 2021). As the patient presented a setting of bronchopathy, we suspect the bulla is secondary to the bronchitis. The radiography and tomography findings corroborate the description of pulmonary bullae by other authors (Godwin et al., 1980; Lipscomb et al., 2003; Park et al., 2016; Bello et al., 2021), who have observed sizes larger than 1 cm and complete destruction of the pulmonary tissue. In this particular patient, the bulla had thick and irregular walls, a characteristic seldomly described (Lamb and Neiger, 2000), and which may not be characterized as a malignant lesion. The bulla was located in the left caudal lobo, as also reported by Park et al. (2016). In this study, we neither observe nodal or pleural damage, nor the presence of small metastatic foci. As such, we ruled out the diagnosis of cancer (Park et al., 2016). In a macroscopic analysis, we detected neovascularization, both in the internal and external cyst walls (Park et al., 2016). The imaging results were not informative for determining a diagnosis of pulmonary bulla-associated malignancy, despite the higher resolution of the tomography (Waters et al., 1998; Nemanic et al., 2006). Pathological examinations before the surgery were not performed because of the risk they could cause the rupture the bulla, as reported before (Allison, 1947; Yoshikawa et al., 2011). We chose to surgically remove the bulla because of its large size (Godwin et al., 1980; Klingman et al., 1991; Richardson, et al., 1996). Afterwards, the removed material was sent for histopathological analysis, which determined the diagnosis of bronchioalveolar carcinoma. Other dogs presenting similar clinical characteristics were diagnosed with bronchioalveolar carcinoma before (Puerto et al., 2002; Park et al., 2016). Regarding the histological pattern, acinar and papillary adenocarcinomas are the most common ones (Caprioli et al., 2018). Caprioli et al. (2018) reported that solid tumors are considered rare in pets. A previous study done on 18 primary pulmonary adenocarcinomas in dogs, reported that only 1 had a solid pattern (Caprioli et al., 2018). Other histopathologic findings included granulation tissue with airway dilation, granulomas, pleuritis, thromboembolism, and pneumonia, all of which have been reported as underlying causes of spontaneous pneumothorax in dogs (Au et al., 2006). Several authors state that prognosis of solid and bullous carcinomas is similar and that the presence of a pulmonary bulla, when not neoplastic, can be an important risk factor for the development of pulmonary carcinoma (Hanaoka et al., 2002; Yoshikawa et al., 2011). ConclusionMalignancy should be described as a differential diagnosis in cases of giant bullae, even though it cannot be diagnosed by imaging techniques. Tomographic examination plays an important role because it is the only technique where we can assess nodal and pleural damage and small metastatic foci. There is indication for surgery in cases of giant bullae and histopathological examinations of the removed region should be performed for a definitive diagnosis. Conflict of interestThe Authors declares that there is no conflict of interest. ReferencesAllison, P.R. 1947. Giant bullous cysts of the lung. Thorax. 2, 169–175. Au, J.J., Weisman, D.L., Stefanacci, J.D. and Palmisano, M.P. 2006. Use of computed tomography for evaluation of lung lesions associated with spontaneous pneumothorax in dogs: 12 cases (1999–2002). J Am Vet Med Assoc. 228(5), 733–737. Aydin, Y., Toplu, N. and Alkan, Z. 1997. Squamous cell carcinoma of the lung in a dog. Aust. Vet. J. 757, 488–490. Bello, A.M., Anselmi, C., Frau, M., Berman, K.G., Novellas, R., Espada, Y., Longley, M.J. and Dhumeaux, M.P. 2021. Pulmonary carcinoma associated with cystic airspaces in two dogs. J. Small Anim. Pract. 63, 486–491. Bonath, K.H. 1996. Thoracic wall closure. In Complications in Small Animal Surgery. Diagnosis, Management, Prevention. Eds., Lipowitz, J. M, Caywood, D, Newton, C.D. and Schwartz, A. Baltimore, MD: Williams and Wilkins, pp: 229–243. Brodey, R.S. and Craig, P.H. 1965. Primary pulmonary neoplasms in the dog: a review of 29 cases. J. Am. Vet. Med. Assoc. 147, 1628–1643. Caprioli, R.A., Argenta, F.F., Hammerschmitt, M.E., Pereira, P.R., Lorenzo, C., Pavarini, S.P., Driemeier, D. and Sonne, L. 2018. Achados patológicos e imuno-histoquímicos de neoplasmas pulmonares primários em caninos na região metropolitana de Porto Alegre, Rio Grande do Sul. Pesq. Veterin. Bras. 38(6), 1151–1158. Dungworth, D.L., Hauser, F.F., Hahn, D.W., Wilson, T.T. and Harkema, J.R. 1999. Histological classification of tumors of the respiratory system of domestic animals, Washington, DC: WHO 2nd series, pp: 69. Gadkowski, L.B. and Stout, J.E. 2008. Cavitary pulmonary disease. Clin. Microbiol. Rev. 21, 305–333. Gafoor, K., Patel, S., Girvin, F., Gupta, N., Naidich, D., Machnicki, S., Brown, K.K., Mehta, A., Husta, B., Ryu, J.H., Sarosi, G.A., Franquet, T., Verschakelen, J., Johkoh, T., Travis, W. and Raoof, S. 2018. Cavitary lung diseases. Chest 153, 1443–1465. Godwin, J.D, Webb, W.R, Savoca, C.J., Gamsu, G. and Goodman, P.C. 1980. Multiple, thin-walled cystic lesions of the lung. Am. J. Roentgenol. 135, 593-–604. Hanaoka, N., Tanaka, F., Otake, Y., Yanagihara, K., Nakagawa, T., Kawano, Y., Miyahara, R., Li, M. and Wada, H. 2002. Primary lung carcinoma arising from emphysematous bullae. Lung Cancer 38, 185–191. Hahn, F.F., Muggenburg, B.A. and Griffith, W.C. 1996. Primary lung malignancy in a beagle colony. Vet. Pathol. 33, 633–638. Hansell, D.M., Bankier, A.A., MacMahon, H., McLoud, T.C., Muller, N.L. and Remy, J. 2008. Fleischner society: glossary of terms for thoracic imaging. Radiology 246, 697–722. Klingman, R.R., Angelillo, V.A. and DeMeester, T.R. 1991. Cystic and bullous lung disease. Ann. Thorac. Surg. 52(3), 576–580. Lamb, C.R. and Neiger, R. 2000. Radiology corner differential diagnosis of pulmonary cavitary lesions. Veter. Radiol. Ultras. 41(4), 340–341. Lesur, O., Delorme, N., Fromaget, J.M., Bernadac, P., Polu, J.M. 1990. Computed tomography in the etiologic assessment of idiopathic spontaneous pneumothorax. Chest. 98, 341–347. Lipscomb, V.J., Hardie, R.J. and Dubielzig, R.R. 2003. Spontaneous pneumothorax caused by pulmonary blebs and bullae in 12 dogs. J. Am. Anim. Hosp. Assoc. 39, 435–445. Monnet, E. 2012. Lungs. In Veterinary surgery: small animal. Eds., Tobias, K.M. and Johnston, S.A St. Louis, MO: Elsevier, pp: 1752–1768. Murphy, D.M. and Fishman, A.P. 1988. Bullous disease of the lung. Pulmonary diseases and disorders. 2nd ed. New York, McGraw-Hill,pp: 1219–2793. Nemanic, S., London, C. A. and Wisner, E. R. 2006. Comparison of thoracic radiographs and single breath-hold helical CT for the detection of pulmonary nodules in dogs with metastatic malignancy. J. Vet. Int. Med. 20, 508–515. Ogilvie, G.K., Haschek, W.M., Withrow, S.J., Richardson, R.C., Harvey, H.J., Henderson, R.A. and McCaw, D. 1989. Classification of primary lung tumors in dogs: 210 cases (1975-1985). J. Am. Vet. Med. Assoc. 195(1), 106–108. Park, J., Lee, H.B. and Jeong, S.M. 2016. Treatment of a giant pulmonary emphysematous cyst with primary bronchoalveolar papillary carcinoma in a Shih Tzu dog. Vet. Surg. 46(1), 158–164. Parry, M., Selmic, L.E., Lumbrezer-Johnson, S., Lapsley, J., Wavreille, V.A. and Hostnik, E. 2021. Computed tomographic characteristics of cavitary pulmonary adenocarcinoma in 3 dogs and 2 cats. Can. Vet. J. 62(7), 719–724. Polton, G.A., Brearley, M.J., Powell, S.M. and Burton, C.A. 2008. Impact of primary tumour stage on survival in dogs with solitary lung tumours. J. Small Anim. Pract. 49, 66–71. Puerto, D.A., Brockman, D.J., Lindquist, C. and Drobatz, K. 2002. Surgical and nonsurgical management of and selected risk factors for spontaneous pneumothorax in dogs: 64 cases (1986–1999). J. Am. Vet. Med. Assoc. 220(11), 1670–1674. Rebhun, R.B. and Culp, W. 2013. Pulmonary malignancy. In Withrow and MacEwen’s small animal clinical oncology, 5th ed. Eds., Withrow, S.J., Vail, D.M., and Page, R.L. St. Louis, MO: Elsevier, pp: 453–462. Richardson, M.S.A., Reddy, V.D. and Read, C.A. 1996. New air-fluid levels in bullous lung disease: a reevaluation. J. Natl. Med. Assoc. 88(3), 185–187. Silverman, S., Poulos, P.W. and Suter, P.F. 1976. Cavitary pulmonary lesions in animals. Vet. Radiol. Ultras. 17, 134–146. Stogdale, L., O´Connor, C.D., Williams, M.C., Smuts, M.M.S. 1982. Recurrent pneumothorax associated with a pulmonary emphysematous bulla in a dog: surgical correction and proposed pathogenesis. Can. Vet. J. 23, 281–287. Walsh, P.J., Remedios, A.M., Ferguson, J.F., Walker, D.D., Cantwell, S. and Duke, T. 1999. Thoracoscopic versus open partial pericardiectomy in dogs: comparison of postoperative pain and morbidity. Vet. Surg. 28, 472–479. Waters, D.J., Coakley, F.V., Cohen, M.D., Davis, M.M., Karmazyn, B., Gonin, R., Hanna, M.P., Knapp, D.W. and Heifetz, S.A. 1998. The detection of pulmonary metastases by helical CT: a clinicopathologic study in dogs. J. Comput. Assist. Tomogr. 22(2), 235–240. Yoshikawa, T., Misao, T. and Aoe, M. 2011. Primary lung cancer arising from the wall of a giant bulla in which positron emission tomography was useful for preoperative diagnosis. General Thorac. Cardiovasc. Surg. 59, 137–140. | ||
| How to Cite this Article |
| Pubmed Style Roque CA, Lima BR, Oliveira GV, Nascimento LM. Giant and potentially malignant bullae in a dog. Open Vet J. 2023; 13(2): 241-246. doi:10.5455/OVJ.2023.v13.i2.13 Web Style Roque CA, Lima BR, Oliveira GV, Nascimento LM. Giant and potentially malignant bullae in a dog. https://www.openveterinaryjournal.com/?mno=46944 [Access: July 27, 2024]. doi:10.5455/OVJ.2023.v13.i2.13 AMA (American Medical Association) Style Roque CA, Lima BR, Oliveira GV, Nascimento LM. Giant and potentially malignant bullae in a dog. Open Vet J. 2023; 13(2): 241-246. doi:10.5455/OVJ.2023.v13.i2.13 Vancouver/ICMJE Style Roque CA, Lima BR, Oliveira GV, Nascimento LM. Giant and potentially malignant bullae in a dog. Open Vet J. (2023), [cited July 27, 2024]; 13(2): 241-246. doi:10.5455/OVJ.2023.v13.i2.13 Harvard Style Roque, C. A., Lima, . B. R., Oliveira, . G. V. & Nascimento, . L. M. (2023) Giant and potentially malignant bullae in a dog. Open Vet J, 13 (2), 241-246. doi:10.5455/OVJ.2023.v13.i2.13 Turabian Style Roque, Caroline Augusto, Bruno Roque Lima, Guillermo Veiga Oliveira, and Letícia Martins Nascimento. 2023. Giant and potentially malignant bullae in a dog. Open Veterinary Journal, 13 (2), 241-246. doi:10.5455/OVJ.2023.v13.i2.13 Chicago Style Roque, Caroline Augusto, Bruno Roque Lima, Guillermo Veiga Oliveira, and Letícia Martins Nascimento. "Giant and potentially malignant bullae in a dog." Open Veterinary Journal 13 (2023), 241-246. doi:10.5455/OVJ.2023.v13.i2.13 MLA (The Modern Language Association) Style Roque, Caroline Augusto, Bruno Roque Lima, Guillermo Veiga Oliveira, and Letícia Martins Nascimento. "Giant and potentially malignant bullae in a dog." Open Veterinary Journal 13.2 (2023), 241-246. Print. doi:10.5455/OVJ.2023.v13.i2.13 APA (American Psychological Association) Style Roque, C. A., Lima, . B. R., Oliveira, . G. V. & Nascimento, . L. M. (2023) Giant and potentially malignant bullae in a dog. Open Veterinary Journal, 13 (2), 241-246. doi:10.5455/OVJ.2023.v13.i2.13 |