| Original Article | ||
Open Vet J. 2021; 11(2): 277-282 Open Veterinary Journal, (2021), Vol. 11(2): 277–282 Original Article Effects of fake and original perfumes on the presence, numbers, and distribution of mast cells in selected tissues in ratsWael M. Hananeh1*, Fatima Al Ghbari1, Raida Al Rukibat1, Mohammad Al Zghoul2 and Zuhair Bani Ismail31Department of Veterinary Pathology and Public Health. Faculty of Veterinary Medicine, Jordan University of Science and Technology, Irbid, Jordan 2Department of Basic Veterinary Medical Sciences, Faculty of Veterinary Medicine, Jordan University of Science and Technology, Irbid, Jordan 3Department of Veterinary Clinical Sciences, Faculty of Veterinary Medicine, Jordan University of Science and Technology, Irbid, Jordan *Corresponding Author: Wael Hananeh. Department of Veterinary Pathology and Public Health. Faculty of Veterinary Medicine, Jordan University of Science and Technology, Irbid, Jordan. Email: whananeh [at] just.edu.jo Submitted: 21/02/2021 Accepted: 14/05/2021 Published: 06/06/2021 © 2021 Open Veterinary Journal
AbstractBackground: Perfumes, whether original or fake, are considered of great economic value. However, overzealous use of perfumes could be associated with local or systemic side effects. Aim: This study was conducted to investigate the effects of daily use of original and fake perfumes on numbers and distribution of mast cells in several organs and tissues of Wistar rats. Methods: Three different kinds of original perfumes coded as O1, O2, and O3 and their fake counterparts coded as F1, F2, and F3 were applied once daily directly on the skin of rats for 28 consecutive days. At the end of the study, representative tissue samples were taken and processed for histopathological examination using Hematoxylin and Eosin, toluidine blue, and Wright’s stains. Results: A significant (p < 0.05) elevation in mast cell count was observed in F3, O3, F1, and F2 compared to the control group. The majority of mast cells were distributed in the hepatic perivascular tissues, peribronchial and pleural tissues. There was a significant (p < 0.05) elevation in mast cell count in along the interalveolar wall, peribronchial area, and pleura tissues in F1 and O3 groups. Conclusion: Results of this study indicated that repeated use of both original and fake perfumes was associated with an increased number of mast cells in different body organs and tissues. Keywords: Perfumes, Fake, Original, Mast cells, Histopathology. IntroductionPerfume is one of the main products that have a great economic significance in the world. It contains many components of natural and/or synthetic fragrances (Mata et al., 2005; Abdel Hafez, 2019). The origin of the “Perfume” word comes from the Latin word “per fumum”, which means through smoke (Mata et al., 2005). Perfume production started about 4,000 years ago in ancient Mesopotamia and Egypt, further reiterated by the Persians and Romans (Van Der Burg et al., 2008). Nowadays, the great demand for affordable perfumes have led to the booming market of fake perfumes. Fake perfumes usually contain low-quality raw ingredients with imperfect concentrations, the final product of which looks similar to their original counterpart brands (Marques et al., 2006). This type of perfumes not only negatively affects the sales of original perfumes but also may pose significant public health risks (Thyssen et al., 2009). Clinical studies have shown that some ingredients in fake perfumes may cause skin allergy and dermatitis (Thyssen et al., 2009; Cuesta et al., 2010; Arribas et al., 2013). In fact, advanced analytical methods have exposed banned compounds present in the finished products of some perfumes such as musks, phthalates, and parabens (Ford et al., 1990; Chisvert et al., 2013; Adefolaju et al., 2016). Mast cells play critical roles in inflammatory and hypersensitivity reactions and immune-mediated diseases (Brown et al., 2008; da Silva et al., 2014). The widespread distribution of mast cells in body organs and tissues provides them with the advantage to become the first responders in harmful situations (da Silva et al., 2014). There are no scientific studies that could be cited in recent literature that investigated the effects of fake perfumes on the numbers and distribution of mast cells in various body organs and tissues in animals. In this study, we hypothesize that the frequent daily application of original and fake perfumes results in increased number and distribution of mast cells locally (skin) and different body organs in rats. Therefore, this study was conducted to investigate the local and systemic effects of selected fake and original perfumes on the numbers and distribution of mast cells in different organs of Wistar rats. Materials and MethodsAnimals and ethical approvalsAll procedures performed in this study were reviewed and approved by the institutional committee of animal care and were used in research for Jordan University of Science and Technology (ACUC Number 20160151). A total of 84 adult Wistar rats were used in this study. The rats were obtained from the Animal House of Jordan University of Science and Technology. The rats were kept in clear-sided cages in groups of five rats per cage. Wood shaving was used as cage bedding. The rats were housed in a temperature (22oC–23oC) and humidity (55°–65°) controlled rooms and were subjected to 12/12 hours light to dark cycle. The rats were offered locally produced rat pellets and fresh drinking water ad libitum. Rats were acclimatized for 1 week before the study began. The rats were subjected to multiple daily observation periods to make sure they are healthy and exhibiting normal behaviors. Experimental designA total of 84 adult Wistar rats were randomly allocated into seven different groups (n=12; 6 males and six females) and were assigned to a different treatment using three original (O1, O2, and O3), and three corresponding fake perfumes (F1, F2, and F3). One group of rats received no treatment and were used as control. Original and fake perfumes were selected and purchased from local markets randomly. Perfumes were applied once per day for 28 consecutive days on a shaved 2 × 2 cm square area located on the dorsal inter-scapular region. The perfume was applied in two or three puffs so that the area gets completely covered with perfume liquid. Care was exercised to avoid the application of excessive perfume on the adjacent skin areas. At the end of the experiments, all animals were sacrificed, and the hearts, lungs, livers, kidneys, and skin were harvested and fixed in 10% formalin. Fixed tissues were transferred to new cups containing fresh formalin after 12 hours, and histopathological processing was performed 24 hours after the animals were sacrificed. Histopathological examinationFormalin-fixed tissues were cut and processed routinely in an automatic processor for histopathological examination, according to Bancroft and Gamble (2008). Three slides were prepared from each selected tissue with 4–5 μm thickness and were stained by three different stains: Hematoxylin and Eosin stain (H&E), Wright’s stain, and toluidine blue stain. The prepared glass slides were examined blindly by a certified veterinary pathologist. In the Wright stain, the mast cells were characterized by intracytoplasmic densely basophilic granules (Fig. 1). In contrast, in the toluidine blue stain, the mast cell granules exhibited purple metachromatic granules (Fig. 2). Mast cell countingThe total number of mast cells was counted in five high power fields per section under 40× magnification using a light microscope. Results were expressed as the average number of mast cells per mm2.
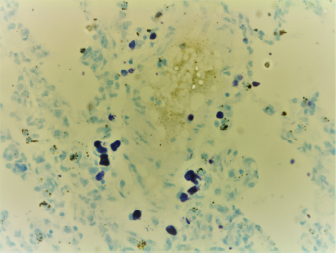
Fig. 1. Random distribution of the mast cells around blood vessel that were characterized by intracytoplasmic densely basophilic granules in the lung of the rat (Wright stain, 40×).
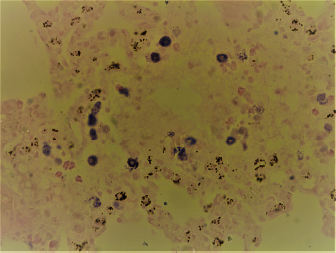
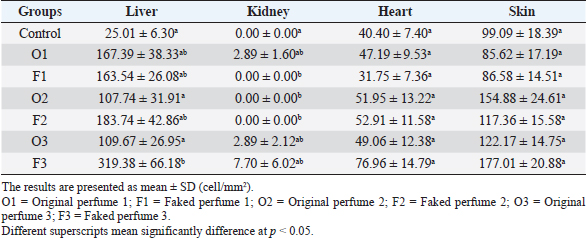
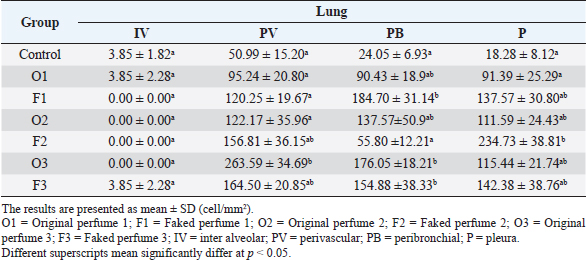
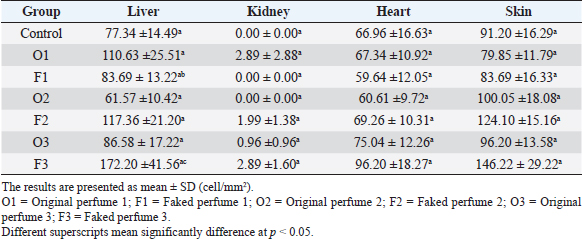
Fig. 2. Widespread distribution of the mast cells in the interstitium that were characterized by purple metachromatic granules in the lung of the rat (Toluidine blue stain, 40×). Statistical analysisAll recorded data were expressed as mean ± SD. Data were analyzed using one-way analysis of variance. Statistical analysis was performed using the IBM Statistical Package for the Social Sciences Software program (version 23). Statistical significance between variables was considered at p < 0.05. ResultsAll H&E tissue sections were examined for any significant histopathological changes. No evidence of pathological or infectious processes was detected in any of the examined tissue sections. Table 1 shows the means ± SD (cell/mm2) of mast cells in different body tissues. There was a significant (p < 0.05) elevation in the mean values of mast cell counts in the F3 group compared to O2, O3, and in control groups for the liver. No significant difference in mast cell counts was present in the tissues obtained from the skin and heart in all treatment groups and their control counterparts. Table 2 shows the means ± SD of mast cells (cell/mm2) in interalveolar wall, perivascular tissues, peribronchial tissues, and pleura. In the interalveolar wall, results showed no significant differences in all groups when compared with the control group. In the perivascular tissues, results showed a significant elevation (p < 0.05) of mast cell counts in the O3 group compared with the control group. There were also significant differences (p < 0.05) between O3 and O1, F1, O2 groups. In the peribronchial tissues, results showed a significant elevation (p < 0.05) in mast cell counts in F1, O3, and F3 groups compared with the control group. A significant elevation (p < 0.05) in the F2 group compared with O1 and control groups was noticed in the pleura. Table 3 shows the means ± SD of mast cell counts (cells/mm2) in different tissues stained with Wright’s stain. In the liver, kidney, heart, and skin tissues, the results showed no significant differences in mast cell count upon comparing it with the control group. Table 4 shows the means ± SD of mast cell counts (cells/mm2) in the interalveolar wall, perivascular tissues, peribronchial tissues, and the pleura stained with Wright’s stain. In the interalveolar wall, results showed significant elevation (p < 0.05) of mast cell count in O3 compared to control, O1, F1, and F3 groups. Results showed no significant differences in mast cell counts in all groups in the perivascular area compared to the control group. However, a significant difference (p < 0.05) was observed in the mast cells count between the O1, F1 and O2 groups. In the peribronchial area, results showed a significant elevation (p < 0.05) in mast cell counts in the F1 group compared to the control, O1, and O2 groups. In the pleura, a significant elevation (p < 0.05) in mast cell counts was observed in the F1 group compared to the control group. Table 1. Mast cell count in liver, kidney, heart, and skin tissues stained by toluidine blue in rats after once daily exposure to original and faked perfumes on the skin for 28 days.
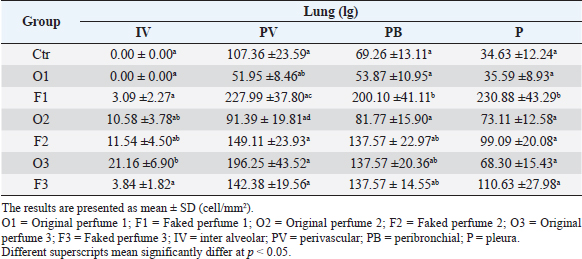
Table 2. Mast cell count in intraaleveolar walls, perivascular, peribronchial and pleural tissues stained by toluidine blue in rats after once daily exposure to original and faked perfumes on the skin for 28 days.
Table 3. Mast cell count in liver, kidney, heart, and skin tissues stained by Wright’s stain in rats after once daily exposure to original and faked perfumes on the skin for 28 days.
Table 4. Mast cell count in interalveolar walls, perivascular, peribronchial and pleural tissues stained by Wright’s stain in rats after once daily exposure to original and faked perfumes on the skin for 28 days.
DiscussionMast cells are specialized immune cells that originate in the bone marrow and are commonly found in connective tissues throughout the body (Krystel-Whittemore et al., 2016). Demonstration of mast cells in tissues requires special stains such as Wrights stain or toluidine blue but not routine H&E stain (Krystel-Whittemore et al., 2016; Ribatti, 2018). However, H&E stain was used in this study to detect any other ongoing pathological conditions not related to mast cell activation and degranulation (Krystel-Whittemore et al., 2016; Ribatti, 2018). Although in this study, frequent application of both original and fake perfumes was demonstrated to result in mast cell aggregation, no evidence of local or systemic allergic or inflammatory reactions were detected in any of the examined organs. Therefore, a direct causal link between increased mast cell density and an allergic process was not established. However, the critical role of mast cells in the development of allergic reactions has been reported previously (Theoharides et al., 2019). Mast cells are stimulated by IgE-dependent allergens (Theoharides et al., 2019). Other triggers of mast cells include pathogenic bacteria, viruses, and fungi, chemicals and drugs, certain foods, as well as certain neuropeptides and substance P (Theoharides et al., 2019). In this study, significant elevation in mast cell numbers was observed in the liver and lungs after daily application of perfumes in test subjects compared to the controls. It has been indicated that mast cell numbers increase substantially at sites of tissue injuries (Amin, 2012). This could be attributed to the migration of progenitor cells to the site of injury or proliferation of local mast cell precursors (Amin, 2012). In fact, in human beings, high numbers of mast cells have been found in asthmatic lungs and the gastrointestinal tract with chronic inflammatory bowel disease (Amin, 2012). In one study, mast cells were distributed mainly in the periportal tissues in rats (Wilhelm et al., 1978). Similarly, the study results showed that mast cells were present mainly in the periportal region of the examined livers regardless of the treatment group. In rats, it has been reported that a significant number of a heterogeneous population of mast cells are distributed mainly in the portal tract of normal rats, and they were called hepatic mast cells (Chan et al., 2001). In humans, it was found that small numbers of mast cells were located in the portal tracts and sinusoids of normal livers (Farrell et al., 1995). The same study reported an increased number of mast cells in progressive chronic liver disease correlated with liver fibrosis (Farrell et al., 1995). Similarly, several studies reported that small numbers of mast cells normally accumulate along the portal tracts in normal rodents and human livers (Farrell et al., 1995; Jones et al., 2016; Jarido et al., 2017). In the current study, the results indicate that the rats exposed to fake perfumes have significantly more mast cells in the periportal tissues compared to rats exposed to original perfumes and control rats. Mast cells are present in normal lung tissues where they are distributed in the alveolar walls, peribronchial, and pleura tissues (Wilhelm et al., 1978). Results obtained in this study are in complete agreement with previously reported mast cell distribution in the pulmonary tissues (Kanter et al., 2004). In the pulmonary tissues, further distribution of mast cells was reported in intrabronchial lamina propria, subpleural, alveolar wall, and septa (Kanter et al., 2004). Previous reports have indicated that the number of mast cells/mm2 in Wistar albino adult rats has increased significantly after long-term exposure to biomass smoke (Kanter et al., 2004). However, in that study, the distribution of mast cells in specific anatomical sites of the lungs was not reported. In the study reported here, the distribution of the mast cells was recorded in each anatomical site of the lungs. The number of mast cells was higher than those reported previously (Kanter et al., 2004). Furthermore, the number of mast cells was increased significantly in treated groups exposed to fake perfumes. These results are similar to previously reported studies in which the average number of mast cells was significantly increased after exposure to chronic allergen compared to their numbers under physiological conditions in human beings (Fox et al., 1981; Ikeda et al., 2003). In the skin, this study showed that mast cells were located mainly in the dermal layer. Fewer mast cells were also found in the epidermis. These results are similar to previously reported data (Wilhelm et al., 1978). The study reported here that exposure to original and faked perfumes had no significant effects on the numbers of dermal mast cells than the control groups. These results are in complete agreement with previous results where dermal injection of certain substances had no significant effect on mast cell numbers in the skin (Czarnetzki and Mecklenburg, 1991). There was no significant difference in mast cell counts between the treatment groups and their normal counterparts in the heart. In the heart tissues, mast cells were located mainly within the interstitium with no apparent pathological lesions. These results agreed with previously reported data on the distribution of mast cells in the heart of growing rats (Rakusan et al., 1990). The kidneys reported that newborn rats had many mast cells located perivascularly that disappeared later in life (Wilhelm et al., 1978). In fact, normal kidneys were devoid of mast cells in adult rats (Majeed, 1994). These results are in total agreement with the current study where no or a few mast cells were present in kidneys of the control group. However, in treated groups, variable number of mast cells were present. Similar findings were reported in humans where mast cells were reported in kidneys affected with fibrosis but not in normal kidneys (Roberts and Brenchley, 2000). In conclusion, although there was no detected evidence of local or systemic allergic or inflammatory reactions involving any of the examined organs, this study shed some light on the effects of daily exposure to original and fake perfumes on the number and distribution of mast cells in different body organs and tissues. However, further studies are warranted to demonstrate whether this may result in local or systemic allergic reactions or serious tissue damage in people. AcknowledgementsThis work was financed by the Deanship of Research, Jordan University of Science and Technology. Conflict of interestThe authors declare no conflict of interest. ReferencesAbdel Hafez, S.M.N. 2019. Age related changes in the dermal mast cells and the associated changes in the dermal collagen and cells: a histological and electron microscopy study. Acta Histochem. 121(5), 619–627. Adefolaju, G.A., Falana, B.A. and Ajao, M.S. 2016. Effects of exposure to two fragrances on the gene expression of Ckm and Ckmt2 and total CK activity in the hearts of wistar rats. Zahedan J. Res. Med. Sci. 18(1), e5876; doi:10.17795/zjrms-5876. Amin, K. 2012. The role of mast cells in allergic inflammation. Respir. Med. 106(1), 9–14. Arribas, M.P., Soro, P. and Silvestre, J.F. 2013. Allergic contact dermatitis to fragrances: part 2. Actas Dermosifiliogr. 104(1), 29–37; doi:10.1016/j.ad.2012.03.005. Bancroft, J.D. and Gamble, M. 2008. Theory and practice of histological techniques. 6th ed, Churchill Livingstone, London, UK. Brown, J., Wilson, T. and Metcalfe, D. 2008. The mast cell and allergic diseases: role in pathogenesis and implications for therapy. Clin. Exp. Allergy. 38(1), 4–18. Chan, A., Cooley, M.A. and Collins, A.M. 2001. Mast cells in the rat liver are phenotypically heterogeneous and exhibit features of immaturity. Immunol. Cell Biol. 79(1), 35–40. Chisvert, A., López-Nogueroles M. and Salvador A. 2013. Essential oils: analytical methods to control the quality of perfumes. In Natural products. Eds., Ramawat, K. and Mérillon, J.M. Springer, Berlin, Germany; doi:10.1007/978-3-642-22144-6_142. Cuesta, L., Silvestre, J.F., Toledo, F., Lucas, A., Pérez-Crespo, M. and Ballester, I. 2010. Fragrance contact allergy: a 4-year retrospective study. Contact Dermatitis. 63(2), 77–84. Czarnetzki, B.M. and Mecklenburg, G. 1991. In vivo studies of factors influencing mast cell numbers in rat skin. J. Dermatol. Sci. 2(1), 1–8. da Silva, E.Z., Jamur, M.C. and Oliver, C. 2014. Mast cell function: a new vision of an old cell. J. Histochem. Cytochem. 62(10), 698–738. Farrell, D.J., Hines, J.E., Walls, A.F., Kelly, P.J., Bennett, M.K. and Burt, A.D. 1995. Intrahepatic mast cells in chronic liver diseases. Hepatology 22(4), 1175–1181. Ford, R., Api, A. and Newberne, P. 1990. 90-day dermal toxicity study and neurotoxicity evaluation of nitromusks in the albino rat. Food Chem. Toxicol. 28(1), 55–61. Fox, B., Bull, T. and Guz, A. 1981. Mast cells in the human alveolar wall: an electronmicroscopic study. J. Clin. Pathol. 34(12), 1333–1342. Ikeda, R.K., Miller, M., Nayar, J., Walker, L., Cho, J.Y., McElwain, K., McElwain, S., Raz, E. and Broide, D.H. 2003. Accumulation of peribronchial mast cells in a mouse model of ovalbumin allergen induced chronic airway inflammation: modulation by immunostimulatory DNA sequences. J. Immunol. 171(9), 4860–7. Jarido, V., Kennedy, L., Hargrove, L., Demieville, J., Thomson, J., Stephenson, K. and Francis, H. 2017. The emerging role of mast cells in liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 313(2), G89–101. Jones, H., Hargrove, L., Kennedy, L., Meng, F., Graf-Eaton, A., Owens, J., Alpini, G., Johnson, C., Bernuzzi, F. and Demieville, J. 2016. Inhibition of mast cell-secreted histamine decreases biliary proliferation and fibrosis in primary sclerosing cholangitis Mdr2−/− mice. Hepatology 64(4), 1202–16. Kanter, M., Yörük, M., Özbay, B., Karaca, T., Acar, S. and Coskun, O. 2004. Distribution of mast cells in lung tissues of rats exposed to biomass smoke. Scand. J. Lab. Anim. Sci. 31(2), 67–72. Krystel-Whittemore, M., Dileepan, K.N. and Wood, J.G. 2016. Mast cell: a multi-functional master cell. Front. Immunol. 6, 620. doi:10.3389/fimmu.2015.00620. Majeed, S.K. 1994. Mast cell distribution in rats. Arzneimittelforschung. 44(3), 370–374. Marques, L.A., Catharino, R.R., Bruns, R.E. and Eberlin, M.N. 2006. Electrospray ionization mass spectrometry fingerprinting of perfumes: rapid classification and counterfeit detection. Rapid Commun. Mass Spectrom. 20(24), 3654–3658. Mata, V.G., Gomes, P.B. and Rodrigues, A.E. 2005. Engineering perfumes. AIChE J. 51(10), 2834–2852. Rakusan, K., Sarkar, K., Turek, Z. and Wicker, P. 1990. Mast cells in the rat heart during normal growth and in cardiac hypertrophy. Circ. Res. 66(2), 511–516. Ribatti, D. 2018. The staining of mast cells: a historical overview. Int. Arch. Allergy Immunol. 176(1), 55–60. Roberts, I. and Brenchley, P. 2000. Mast cells: the forgotten cells of renal fibrosis. J. Clin. Pathol. 53(11), 858–862. Theoharides, T.C., Tsilioni, I. and Ren, H. 2019. Recent advances in our understanding of mast cell activation – or should it be mast cell mediator disorders? Expert Rev. Clin. Immunol. 15(6), 639–656. Thyssen, J., Menné, T., Linneberg, A. and Johansen, J. 2009. Contact sensitization to fragrances in the general population: a Koch’s approach may reveal the burden of disease. Br. J. Dermatol. 160(4), 729–735. Van Der Burg, B., Schreurs, R., Van Der Linden, S., Seinen, W., Brouwer, A. and Sonneveld, E. 2008. Endocrine effects of polycyclic musks: do we smell a rat? Int. J. Androl. 31(2), 188–193. Wilhelm, D., Yong, L. and Watkins, S. 1978. The mast cell: distribution and maturation in the rat. Agents Actions. 8(1–2), 146–152. | ||
| How to Cite this Article |
| Pubmed Style Hananeh W, Ghbari FA, Rukibat RA, Zghoul MA, Ismail ZB. Effects of fake and original perfumes on the presence, numbers, and distribution of mast cells in selected tissues in rats. Open Vet J. 2021; 11(2): 277-282. doi:10.5455/OVJ.2021.v11.i2.11 Web Style Hananeh W, Ghbari FA, Rukibat RA, Zghoul MA, Ismail ZB. Effects of fake and original perfumes on the presence, numbers, and distribution of mast cells in selected tissues in rats. https://www.openveterinaryjournal.com/?mno=57836 [Access: October 06, 2024]. doi:10.5455/OVJ.2021.v11.i2.11 AMA (American Medical Association) Style Hananeh W, Ghbari FA, Rukibat RA, Zghoul MA, Ismail ZB. Effects of fake and original perfumes on the presence, numbers, and distribution of mast cells in selected tissues in rats. Open Vet J. 2021; 11(2): 277-282. doi:10.5455/OVJ.2021.v11.i2.11 Vancouver/ICMJE Style Hananeh W, Ghbari FA, Rukibat RA, Zghoul MA, Ismail ZB. Effects of fake and original perfumes on the presence, numbers, and distribution of mast cells in selected tissues in rats. Open Vet J. (2021), [cited October 06, 2024]; 11(2): 277-282. doi:10.5455/OVJ.2021.v11.i2.11 Harvard Style Hananeh, W., Ghbari, . F. A., Rukibat, . R. A., Zghoul, . M. A. & Ismail, . Z. B. (2021) Effects of fake and original perfumes on the presence, numbers, and distribution of mast cells in selected tissues in rats. Open Vet J, 11 (2), 277-282. doi:10.5455/OVJ.2021.v11.i2.11 Turabian Style Hananeh, Wael, Fatima Al Ghbari, Raida Al Rukibat, Mohammad Al Zghoul, and Zuhair Bani Ismail. 2021. Effects of fake and original perfumes on the presence, numbers, and distribution of mast cells in selected tissues in rats. Open Veterinary Journal, 11 (2), 277-282. doi:10.5455/OVJ.2021.v11.i2.11 Chicago Style Hananeh, Wael, Fatima Al Ghbari, Raida Al Rukibat, Mohammad Al Zghoul, and Zuhair Bani Ismail. "Effects of fake and original perfumes on the presence, numbers, and distribution of mast cells in selected tissues in rats." Open Veterinary Journal 11 (2021), 277-282. doi:10.5455/OVJ.2021.v11.i2.11 MLA (The Modern Language Association) Style Hananeh, Wael, Fatima Al Ghbari, Raida Al Rukibat, Mohammad Al Zghoul, and Zuhair Bani Ismail. "Effects of fake and original perfumes on the presence, numbers, and distribution of mast cells in selected tissues in rats." Open Veterinary Journal 11.2 (2021), 277-282. Print. doi:10.5455/OVJ.2021.v11.i2.11 APA (American Psychological Association) Style Hananeh, W., Ghbari, . F. A., Rukibat, . R. A., Zghoul, . M. A. & Ismail, . Z. B. (2021) Effects of fake and original perfumes on the presence, numbers, and distribution of mast cells in selected tissues in rats. Open Veterinary Journal, 11 (2), 277-282. doi:10.5455/OVJ.2021.v11.i2.11 |