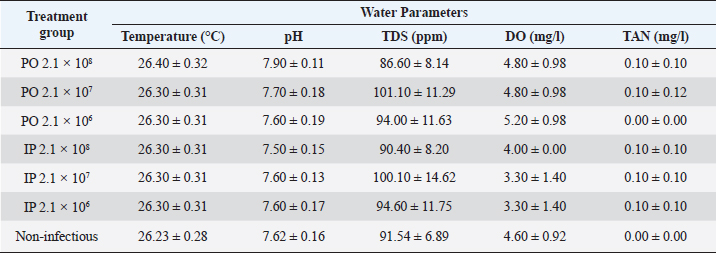
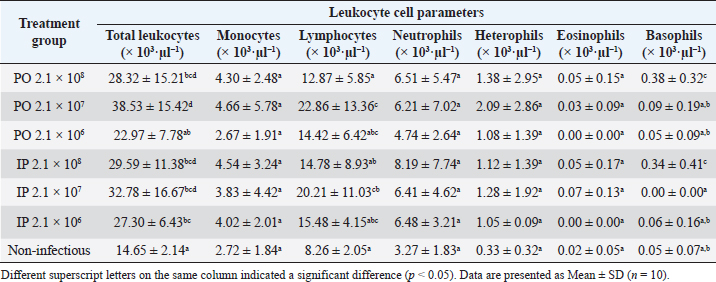
| Original Article | ||
Open Vet J. 2021; 11(2): 309-318 Open Veterinary Journal, (2021), Vol. 11(2): 309–318 Original Research Experimental infection of Enterococcus faecalis in red tilapia (Oreochromis hybrid) revealed low pathogenicity to cause streptococcosisRifky Rizkiantino1, Fachriyan Hasmi Pasaribu1, Retno Damajanti Soejoedono1, Sucitya Purnama1, Danny Bagus Wibowo2 and I Wayan Teguh Wibawan1*1Division of Medical Microbiology, Department of Animal Infectious Diseases and Veterinary Public Health, Faculty of Veterinary Medicine, Bogor Agricultural University (IPB University), Bogor, Indonesia 2Undergraduate Program, Faculty of Veterinary Medicine, Bogor Agricultural University (IPB University), Bogor, Indonesia *Corresponding Author: I Wayan Teguh Wibawan. Division of Medical Microbiology, Department of Animal Infectious Diseases and Veterinary Public Health, Faculty of Veterinary Medicine, Bogor Agricultural University (IPB University), Bogor, Indonesia. Email: teguhwibawan [at] yahoo.co.id Submitted: 02/03/2021 Accepted: 29/05/2021 Published: 28/06/2021 © 2021 Open Veterinary Journal
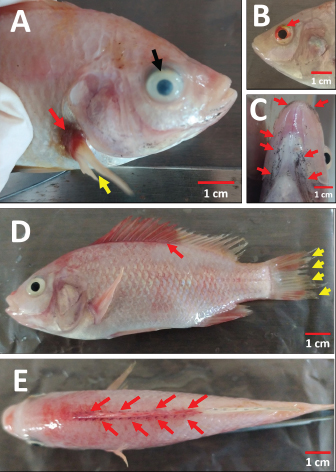
AbstractBackground: Streptococcosis, as a bacterial disease with broad tropism in fish and one of the causes of septicemia. Enterococcus faecalis is one of the causative agents of streptococcosis that can be isolated in tilapia. Aim: This study was undertaken to complete the reporting gap on the pathogenicity profile and clinical symptoms of E. faecalis bacterial infection in red tilapia (Oreochromis hybrid). The study is expected to provide enriching information regarding recognizable clinical signs in the field that can lead to the diagnosis of streptococcosis caused by E. faecalis, especially in the Indonesian aquaculture environment. Methods: The method used in this artificial infection study using red tilapia, which were divided into two types of route groups infection, namely intraperitoneal (IP) and peroral (PO) with bacterial concentrations given for each route of infection to be 2.1 × 108 CFU ml−1; 2.1 × 107 CFU ml−1; and 2.1 × 106 CFU ml−1. One group was given brain heart infusion broth media sterile as a non-infectious control. Clinical symptoms, changes in swimming habits and consuming feed, external and internal organ lesion, and leukocytes profile changes were observed during the observation period along 14 days to evaluate the infectious effect of each treated fish group. The lethal dose 50 (LD50) was estimated with the Spearman–Kärber method. The evaluation of the leukocyte profile was performed to find leukocytosis as the clinical sign of infection. Results: The results showed variations in clinical symptoms inflicted on fish through death or the moribund stage. The highest mortality occurred in the treatment group of 2.1 × 108 CFU ml−1 with the PO route. The bacterial concentration of 2.1 × 107 CFU ml−1 given either as PO or IP can cause mild infection symptoms but did not cause mortality. The LD50 of the PO and IP route was obtained at 1.99 × 108 CFU ml−1 and 0.79 × 108 CFU ml−1, respectively. The total leukocytes in the infected fish group increased significantly (p < 0.05) by twofold when compared with the non-infectious group. The bacteria’s discovery on the blood smear examination was taken from fresh dead fish or moribund fish in the treatment group of 2.1 × 108 CFU ml−1, for both PO and IP. Conclusion: Enterococcus faecalis with low pathogenicity can lead to septicemia, characterized by a total increase in leukocytes, bacteria’s discovery on the blood smear examination, and various clinical symptoms systemically found in the treated fish. Keywords: Enterococcus faecalis, Experimental infection, Red tilapia, Septicemia, Streptococcosis. IntroductionStreptococcosis is a bacterial disease that can cause septicemia in fish (Ferguson et al., 1994). This disease has a broad tropism level because it can infect various freshwater and seawater fish, both cultivated and feral. One kind of fish that is very susceptible to this disease is tilapia. Several causative agents of streptococcosis have been isolated from various fish worldwide, including Enterococcus faecium, Lactococcus lactis, Streptococcus mutans, Streptococcus phocae, Vagococcus salmoninarum (Toranzo et al., 2005; Austin and Austin, 2012), Streptococcus agalactiae, Streptococcus iniae, Streptococcus dysgalactiae, Lactococcus garvieae (Evans et al., 2006; Netto et al., 2011; Taukhid and Purwaningsih, 2011; Anshary et al., 2014), Streptococcus parauberis (Kwon et al., 2017), and Enterococcus faecalis (Khafagy et al., 2009; Fawzy et al., 2014; Rahman et al., 2017; Rizkiantino et al., 2020). According to Lancefield Group, Enterococcus faecalis is a streptococcal bacterium classified into Group D Streptococcus based on bacterial serological groups and other species, such as E. faecium and Streptococcus bovis. This bacterium is Gram-positive, catalase-negative, and γ hemolytic if cultured on a 5% sheep blood agar medium (Haslam and Geme III, 2018). Enterococcus faecalis lives commensally in the digestive tract of humans or mammals. Still, sometimes it can cause nosocomial infection in hospitals (Ryan and Ray, 2004). This bacterium is also abundant in humans reported as a common cause of sepsis and can be cultured from blood in patients who died from severe sepsis and septic shock (Linden, 2003). This bacterium in fish is one of the causative pathogens for the incidence of streptococcosis. Enterococcus faecalis can also grow at a temperature of 10°C–45°C and up to 60°C for 30 minutes. This bacterium is tolerant and can grow at alkaline conditions with pH 9.6; high salt content reaching up to 6.5%, the presence of bile salts, heavy metals, ethanol, and azides. Enterococcus faecalis is a non-motile bacterium, ferments glucose without producing gas and catabolizes glycerol, lactate, malic acid, citrate, arginine, agmatine, and ketone acids for energy sources (Stuart et al., 2006). The clinical symptoms of streptococcosis are varied and determined mainly by the bacterium’s virulence that infects a host. Rahman et al. (2017) stated that the virulence of E. faecalis in tilapia varies widely. Virulence properties can be high, moderate, low, and avirulent. The clinical symptoms of streptococcosis are commonly seen as abnormalities in swimming if it causes meningoencephalitis lesion; decreased appetite; lesions of the eye which can lead to opacity and hemorrhage of the eye, cloudy cornea, panophthalmitis, and exophthalmos; there are hemorrhagic on the operculum, around the mouth, anus, and fins (Siti-Zahrah et al., 2008; Amal and Zamri-Saad, 2011; Austin and Austin, 2012). However, the clinical symptoms of streptococcosis caused by E. faecalis have not been widely reported as a comparison between cases of streptococcosis caused by other streptococcal bacteria. Therefore, this study was conducted to complement the gap reported on the pathogenicity profile and clinical symptoms of E. faecalis bacterial infection in red tilapia (Oreochromis hybrid). This study is expected to provide information related to the clinical signs that can be identified as symptoms in the field, leading to the diagnosis of streptococcosis caused by E. faecalis, especially in the Indonesian aquaculture environment. Materials and MethodsPreparation of bacteriaThe study began preparing the E. faecalis field isolate strain 7INB (accession no. MT105346) from Bogor, Indonesia, isolated from the liver of red tilapia (Oreochromis hybrid) with clinical signs of streptococcosis in the field (Rizkiantino et al., 2020). Isolate confirmed conventionally and molecularly before use in the study. The isolate was cultured on selective-differential media using Streptococcus KF agar (Merck KgaA, Darmstadt, Germany). Enterococcus faecalis colonies will be colored red and change the media from purple to yellow. Confirmation was achieved using Gram staining, a catalase test, and a growth test on a 6.5% NaCl agar medium. Molecular confirmation was performed using polymerase chain reaction (PCR). Extraction of bacterial DNA using the boiling method (Rizkiantino et al., 2020). The specific forward primer used Efac F1 (5′-CGTTAGTAACTGAACGTC-3′) and 1492R universal reverse primer (5′-GGATACCTTGTTACGACTT-3′) with a target amplicon length of 1,022 bp. The initial denaturation process was carried out at 94°C for 5 minutes; process denaturation at 94°C for 1 minute, annealing at 57°C for 40 seconds, and elongation at 72°C for 1 minute (35 cycles). The final extension stage was carried out at a temperature of 72°C for 10 minutes (Rahman et al., 2017). DNA Ladder (Promega, Madison, WI) of 100 bp was used as a standard molecular length. Experimental infection in lethal dose 50 (LD50) study of E. faecalis to red tilapia (Oreochromis hybrid)A total of seventy red tilapia fingerlings (Oreochromis hybrid) with an average bodyweight of 18–24 g (Taufek et al., 2020; Wan-Mohtar et al., 2021) were used in the LD50 study with artificial infection. Treated fish were acclimatized for 14 days to observe for clinical symptoms of streptococcosis before use in the study. Fish were divided into seven groups with ten fish each and were maintained in an aquarium with dimensions 80 × 40 × 40 cm. Fish were divided into two groups based on the route of infection, namely intraperitoneal (IP) and peroral (PO) as much as 0.5 ml bacterial suspension in brain heart infusion broth (BHIB) medium using a 1 ml syringe. The concentration of bacteria assigned to each infection route was 2.1 × 108, 2.1 × 107, and 2.1 × 106 CFU ml−1. One group was given sterile BHIB medium as a non-infectious control. The observation period was for 14 days. During the observation period, control and examination of water quality were performed in temperature, pH, total dissolved solids (TDS), dissolved oxygen (DO), and total ammonia nitrogen (TAN) to avoid data bias in finding the cause of death or moribund in the treated fish. Providing aeration, feed with a frequency of three times a day (±3% of the bodyweight), and water was changed once in two days during the observation period. The LD50 of the PO and IP route was estimated with the Spearman–Kärber method (Hamilton et al., 1977) modified from Dias et al. (2016). Anatomical pathology examination and bacterial re-isolationNecropsy was performed to evaluate external changes in the body and internal organs of the artificially infected fish. Swimming and feed-consuming behavior was also observed in those infected fish. Necropsy was performed on the fish that showed clinical signs, and the blood was collected for bacterial re-isolation. Re-isolation and re-culture on selective-differential Streptococcus KF agar (MerckKgaA, Darmstadt, Germany) and molecular confirmation were also carried out from organ samples showing pathological anatomical changes confirmed by the presence of the bacterium. The fish was euthanized using the low-temperature method by soaking the fish in ice water slowly until the fish reached the stage of medullary collapse characterized by the gills’ movement, which was entirely out of sight as the fish went into cardiac arrest (Stoskopf, 2010). Evaluating the leukocyte profileBlood from all individuals in each group was collected through a caudal vein as much as 0.1 ml and mixed with 0.1 ml of anticoagulant 10% ethylenediaminetetraacetic acid. The leukocytes profile was evaluated in total and differential leukocytes using the Rosenfeld (1947) method. The total leukocyte count was carried out by diluting the blood using a 0.65% sodium chloride solution containing 1% crystal violet. The result of the blood dilution was then counted on a Neubauer hemocytometer. Differential leukocytes were conducted by making a blood smear and stained with 10% Giemsa staining and counted per hundred cells to get a percentage of each leukocyte type (modification from Martins et al., 2004). Examination results were then analyzed statistically with the analysis of variance method with a 5% confidence interval. If there is a significant difference, it will be followed by the least significant difference test to see if there are any significant differences between the treated fish groups. Ethical approvalMaintenance, euthanasia, and invasive methods in treated fish were carried out by following the protocols approved by the Animal Care and Use Committee, The Institute of Research and Community Empowerment of IPB University with approval ethic number 171-2019 IPB. ResultsExperimental infection in LD50 study of E. faecalis to red tilapia (Oreochromis hybrid)Based on the experimental infection in the LD50 study, it was found that in the group of fish infected by PO with a bacterial concentration of 2.1 × 108 CFU ml−1 showed that as many as 8 out of 10 treated fish (80%) go through fresh death or in the moribund stage with a wide variety of clinical symptoms especially in the appearance of each fish. In IP group-infected fish with a bacterial concentration of 2.1 × 108 CFU ml−1 as many as 4 out of 10 treated fish (40%) go through fresh death or in the moribund stage, variations were observed for clinical symptoms of each individual. Fish that were in the moribund stage swimming vertically with the mouth facing up the water surface. The treated fish group with a bacterial concentration of 2.1 × 108 CFU ml−1, both PO and IP route, showed a decrease in speed during the qualitative feed on day 2 of post-infection when compared with the non-infectious group. There was no mortality in both non-infectious and infectious fish groups with bacterial concentrations of 2.1 × 107 and 2.1 × 106 CFU ml−1, both of PO or IP route. There was no significant change in feed consumption and swimming behavior in these groups. The LD50 of PO and IP route of E. faecalis strain 7INB for red tilapia was obtained at 1.99 × 108 and 0.79 × 108 CFU ml−1, respectively. Water quality results indicated that the water’s physical and chemical conditions were still expected to be optimal for red tilapia (Table 1). Anatomical pathology examination and bacterial re-isolationBased on the results of the examination on the external body of the fish at necropsy, the majority of lesions were obtained from the fish which were infected with a bacterial concentration of 2.1 × 108 CFU ml−1; both PO and IP were hemorrhaging at the base of the dorsal and pectoral fins, erosion on the caudal (tail) and pectoral fins, unilateral and bilateral hemorrhage of the eye, cloudy eye or opacity of the eye was both unilateral and bilateral. There were black spots on the ventral mouth and gill areas. The appearance of clinical symptoms in these groups started on the 2nd day post-infection. Fish which were infected with a bacterial concentration of 2.1 × 107 CFU ml−1; both PO and IP did not go through death. However, 4–5 individuals in each group showed black spots on the ventral mouth and gill areas, which appeared on the 7th day post-infection (Fig. 1). Table 1. Water quality parameters (Mean ± SD) during the 14 day observation period of artificial infection in red tilapia (Oreochromis hybrid) with the E. faecalis strain 7INB, Bogor, Indonesia.
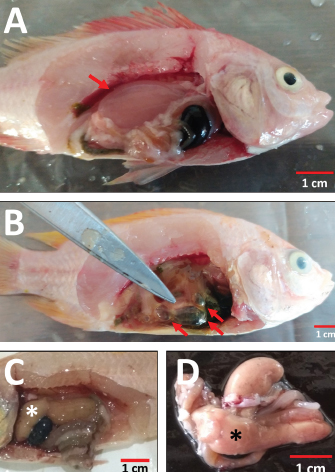
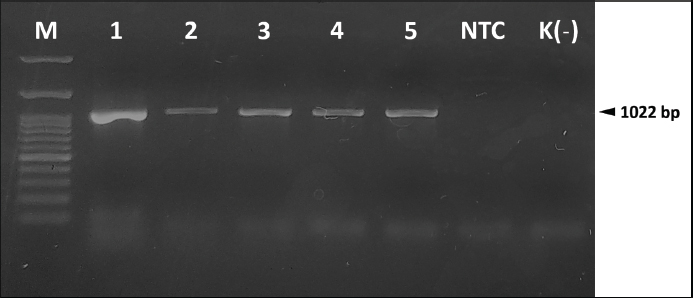
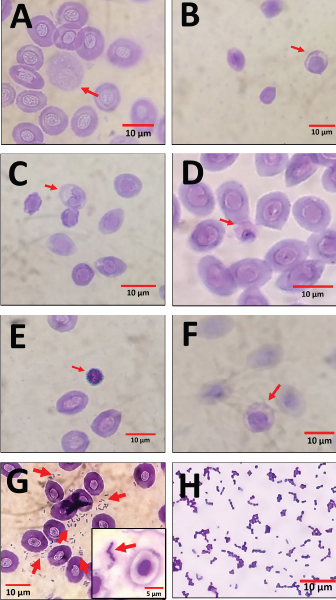
Organ examination results in internal lesions indicated a pale liver with a brittle texture on all the dead or moribund fish. Fluid in the stomach (1 out of 8 dead or moribund fish) and gas accumulation in the intestine (2 out of 8 dead or moribund fish) of PO-infected fish with a concentration of 2.1 × 108 CFU ml−1 can also be found (Fig. 2). Two fish were randomly selected for postmortem examination and bacterial re-isolation in the treatment group of non-infectious, 2.1 × 107 CFU ml−1, and 2.1 × 106 CFU ml−1; both PO and IP. No abnormality in the internal organs was observed in the infected fish with a bacterial concentration of 2.1 × 107 and 2.1 × 106 CFU ml−1; both PO and IP routes. There was no pathological lesion in all fish’s gills organ. The result of the re-isolation of bacterium demonstrated that liver and blood cultured on selective-different media showed positive signs of colonies characterizing E. faecalis of all fish through the death or moribund from 2.1 × 108 CFU ml−1; both PO and IP. Molecular confirmation results also showed a specific band at 1,022 bp from pure isolates from liver and blood (Fig. 3). In comparison, no E. faecalis colonies grew from blood and liver cultured from fish in the treatment group of 2.1 × 107 and 2.1 × 106 CFU ml−1; both PO and IP. Evaluating the leukocyte profileThe leukocyte profile examination results showed a significant increase (p < 0.05) in leukocytes in artificially infected fish groups when compared with non-infectious groups. Lymphocytes are the type of leukocytes high in each treatment group (Table 2). The blood smear examination results on the dead or moribund fish in the treatment group of 2.1 × 108 CFU ml−1, both PO and IP, were found the bacterium in the form of a coccus-chain formation, which refers to E. faecalis (Fig. 4), which was not found in fish blood smears that survived the end of the observation period. DiscussionEnterococcus faecalis is one of the causative agents of streptococcosis in fish. However, the leading cause of concern in the tilapia industry is from the species of S. iniae, S. agalactiae, S. dysgalactiae, and Lactococcus garvieae (Evans et al., 2006; Netto et al., 2011), but a case report on streptococcosis caused by E. faecalis bacterium has also been found in several countries, such as in Egypt (Khafagy et al., 2009; Fawzy et al., 2014), Bangladesh (Rahman et al., 2017), Indonesia (Rizkiantino et al., 2020), and Brazil (Martins et al., 2008). There is a lack of pathogenicity profile and clinical symptoms caused by E. faecalis infection in fish which makes this study very significant to complement the studies that have been reported. Based on the pathogenicity results found during the study, it was found that the concentration of E. faecalis strain 7INB from Bogor, Indonesia, amounting to 2.1 × 108 CFU ml−1 was able to cause death in treated fish. When compared between infection routes, oral infection is capable of causing the death of 80% of the total number of treated fish. While the IP injection only caused death by 40% of the total number of treated fish at a concentration of 2.1 × 108 CFU ml−1 shows that the E. faecalis strain 7INB from Bogor, Indonesia, can cause 80% mortality in treated fish, which amounted to 2.1 × 108 CFU ml−1, the route of infection via PO. Bacterial concentrations were 2.1 × 107 and 2.1 × 106 CFU ml−1, respectively; PO or IP did not show any mortality. A few surviving fish infected by 2.1 × 107 CFU ml−1, both by PO and IP route showed mild lesions in the form of black spots like hemorrhage marks around the ventral area of mouth and gill (Fig. 1c). This indicates that at this concentration, the bacteria are capable of causing mild lesions changes; however, physiologically, it is suspected that the fish can neutralize the infectious process started by the bacterium so that the infection does not develop towards critical, which can cause death. The concentration of E. faecalis 7INB strain from Bogor, Indonesia, amounted to 2.1 × 107 CFU ml−1 by route of infection by PO or IP can cause at least 50% of mild clinical symptoms in the treated fish. Table 2. Profile of red tilapia (Oreochromis hybrid) leukocytes that are artificially infected with E. faecalis strain 7INB, Bogor, Indonesia.
Fig. 1. The external anatomical pathology examination of the red tilapia body (Oreochromis hybrid) is artificially infected by E. faecalis strain 7INB, Bogor, Indonesia. (A) Hemorrhage at the base of the pectoral fin (red arrow), erosion in pectoral fin (yellow arrows), and cloudy eye (opacity on the eye) (black arrow). (B) Hemorrhage of the eye (red arrow). (C) Black spots in the ventral mouth and gill areas (red arrows). (D) Hemorrhage at the base of the dorsal fin (red arrows) and erosion on the caudal fin (tail) (yellow arrows). (E) Dorsal visualization of the hemorrhage at the base of the dorsal fin (red arrows). The use of concentrations of E. faecalis starting at 2.1 × 106, 2.1 × 107, and 2.1 × 108 CFU ml−1 with the PO infection route was based on the explorative study because there is still no pathogenicity study across this range of E. faecalis infection via the oral cavity and digestive tract. The PO route is analogous to an infection route through the cannibalistic behavior of fish that is easily found in intensive aquaculture ponds, so this study is expected to be able to provide a measure of the high concentration of E. faecalis needed to be able to cause clinical symptoms or death of more than 50%. This provides new information to initiate an infective dose in trials that can be used in other studies, such as developing the streptococcosis vaccine caused by E. faecalis and the development of other therapeutic applications. While the selection of the concentration of E. faecalis starting at 2.1 × 106, 2.1 × 107, and 2.1 × 108 CFU ml−1 using the IP infection route was based on the results of a study reported by El-Sayed and El-Gheit (2005) where E. faecalis can cause mortality of only 1 out of 10 fish at a concentration of 1 × 105 CFU ml-1, 3 out of 10 fish at a concentration of 1 × 106 CFU ml−1, and 3 out of 10 at a concentration of 1 × 107 CFU ml−1. While at these concentrations, there was still no evidence of fish mortality that reached 50%, increasing it one level to 108 to explore it further. Fawzy et al. (2014) reported that the concentration of E. faecalis bacterium was isolated from Egypt and infected artificially in the laboratory at a concentration of 3 × 107 CFU ml−1. However, it did not show any mortality in the treated fish. Rahman et al. (2017) reported that by artificial infection, the route of immersion during 15 minutes on the bacterial suspension of E. faecalis with high virulence origin of the streptococcosis case in Bangladesh which were infected tilapia and catfish at a concentration of 2 × 105–4 × 105 CFU ml−1 causes death 72 hours of post-infection and causes first symptoms clinically 24 hours of post-infection.
Fig. 2. The pathological examination of red tilapia’s internal organs (Oreochromis hybrid) is artificially infected by E. faecalis strain 7INB, Bogor, Indonesia. (A) Fluid in the stomach (red arrow). (B) Gas accumulation in the intestines (red arrows). (C) Liver becomes pale with a brittle texture (asterisk). (D) Non-infectious red tilapia liver as normal liver control (asterisk).
Fig. 3. Molecular identification and re-isolation of E. faecalis strain 7INB, Bogor, Indonesia, from the LD50 study in artificially infected red tilapia (Oreochromis hybrid). M. Marker (100 bp). (1) E. faecalis strain 7INB. (2) E. faecalis strain 7INB re-isolation from the blood (PO 2.1 × 108 CFU ml−1). (3) E. faecalis strain 7INB re-isolation from the liver (PO 2.1 × 108 CFU ml−1). (4) E. faecalis strain 7INB re-isolation from the blood (IP 2.1 × 108 CFU ml−1). (5) E. faecalis strain 7INB re-isolation from the liver (IP 2.1 × 108 CFU ml−1). NTC. Non-template control. K(–). Negative control. The concentration of the E. faecalis strain 7INB could cause clinical symptoms of up to 80% of mortality at 2.1 × 108 CFU ml−1. The LD50 of E. faecalis strain 7INB for red tilapia was obtained at 1.99 × 108 CFU ml−1 for the PO route, and 0.79 × 108 CFU ml−1 for the IP route. These findings may be difficult to find in nature. Nevertheless, it needs to be paid attention to the stress factors in fish due to intensive maintenance in aquaculture ponds that reduces the fish’s immune system. The immunosuppressive condition can cause an increase in the concentration of E. faecalis, which initially enters the host’s body in small amounts and finds the quorum sensing points in the digestive tract of tilapia. The digestive tract environment does not become an obstacle to colonization for this bacterium, which is resistant to extreme environments (Arias and Murray, 2012). The findings of clinical symptoms in the field on red tilapia prove that the E. faecalis can still cause the symptoms of streptococcosis; even with low pathogenicity. The different routes of infection are thought to affect the resulting mortality in treated fish. Bacterial infection on the PO route showed more mortality causes (80%) than the IP route (40%). Report study of artificially streptococcal bacterial infection using isolated S. agalactiae (concentration of 7 × 107 CFU per 0.1 ml or 7 × 108 CFU ml−1) conducted by Iregui et al. (2016) on red tilapia showed that the main route of infection with these bacteria is through the gastrointestinal tract if compared to the route of infection via immersion. Observation through a transmission electron microscope showed that S. agalactiae can perform adhesions on the intestinal epithelial cells of fish and perform binary fission. After that, the bacterium begins to enter the intestinal tissue through blood vessels, which causes the bacterium to enter the bloodstream and consequently cause septicemia. Based on the mortality rates recorded for both routes of infection in the current study, it is now presumed that the main port of E. faecalis bacteria’s entry is also via the gastrointestinal tract. This route causes death and clinical symptoms relatively more severe than the IP route of infection at the same bacterial concentration, which is the same as studies of S. agalactiae reported. This can make it possible to remember E. faecalis is also a bacterium that is tolerant of acidic conditions with pH 2.9–4.2 at 37°C or pH 5.0 at 25°C (Morandi et al., 2005; Mubarak and Soraya, 2018); alkaline conditions with a pH of 9.6; as well as existence bile salts (Stuart et al., 2006). As for tilapia’s digestive tract condition, generally, a pH range of 2.5–8 is suggested to allow E. faecalis to survive (El-Beltagy et al., 2004; Zhao et al., 2011; Zhou et al., 2013). With its physiological properties that can survive in these extreme conditions, E. faecalis cause streptococcosis in the tilapia industry, which must be taken seriously if its existence is found in a pond. However, it still needs the serial studies of the E. faecalis observations of ranges based upon specific time in each route of infection. It should be possible at a later date to know the real-time conditions of this bacterium in the tilapia’s digestive tract per hour.
Fig. 4. An overview of red tilapia (Oreochromis hybrid) blood smear artificially infected with the E. faecalis. (A) Monocyte (red arrow). (B) Lymphocyte (red arrow). (C) Neutrophil (red arrow). (D) Heterophil (red arrow). (E) Basophil (red arrow). (F) Eosinophil (red arrow). (G) Gram-positive coccus bacteria were found in blood smears examination (red arrows). (H) Gram staining of E. faecalis. Profile of clinical symptoms caused by artificial infection of E. faecalis in this study also shares some common clinical signs of septicemia caused by streptococcosis in tilapia reported by Osman et al. (2017). These are hemorrhage in the pectoral fin base, erosion on caudal (tail) and pectoral fins, hemorrhage uni- and bilaterally in the eye, and cloudy eye (opacity in the eye), which is uni- and bilateral. Clinical symptoms are also in line with streptococcosis symptoms that have been widely reported in clinical terms, both natural and artificial infection (Siti-Zahrah et al., 2008; Khafagy et al., 2009; Amal and Zamri-Saad, 2011; Anshary et al., 2014; Rahman et al., 2017; Rizkiantino et al., 2020). Clinical symptoms in the form of hemorrhage at the base of the dorsal fin and black spots on the skin of the ventral area of mouth and gill (Fig. 1c–e) were consistent with the findings reported by Fawzy et al. (2014) in S. dysgalactiae and Enterococcus gallinarum strain infection. This finding is a novelty report where it turns out that E. faecalis is also capable of causing clinical symptoms such as hemorrhage at the base of the dorsal fin and black spots on the skin of the ventral area of the mouth and gill. An examination of the internal organs also found symptoms similar to several studies of artificial infection of E. faecalis before; namely, the liver becomes pale with a brittle texture. Notable pathological changes in the form of fluid in the stomach and gas accumulation in the intestines of PO-infected fish with a concentration of 2.1 × 108 CFU ml−1 can also be found in a small proportion of individuals who were diagnosed. This anomaly can not be determined as the cause of these clinical symptoms because, physiologically, E. faecalis produces lactic acid from glucose fermentation but is not accompanied by gas production (Klein, 2003). However, this suspicion can arise because it is related to changes in the digestive tract microbiome, including the intestine, due to the abundant presence of E. faecalis bacterium, affecting physiological properties of other normal microflora in the intestine, which are individual in the treated fish so only found in one or two fish. It is suspected that the erosion of the caudal (tail) and pectoral fins can be worsened by the cannibalism efforts against individual fish who are sick and weak by healthy fish. Naumowicz et al. (2017) states that a disease or health problem in cultured fish that causes stunted growth may also increase the incidence of cannibalism. The clinical symptoms generated during the study of suspected artificial infections can not be separated from the presence of virulence factors possessed by E. faecalis. Enterococcus faecalis has the virulence gene of gelE, which encodes for the gelatinase enzyme, closely related to infection and cloudy eye (Engelbert et al., 2004). The bacteria circulates in the bloodstream and moves towards the eyes, colonizes, and produces gelatinase in this organ. This enzyme can break down protein present in the eye’s cornea so that the protein becomes degraded which is characterized by opacity or cloudiness in the eye. The virulence gene of gelE also has a role as toxin, metalloendopeptidase, and hydrolyzes other bioactive compounds, such as collagen and hemoglobin (Su et al., 1991). Hemorrhage occurred in several parts of the body, such as the base of the pectoral fin, dorsal fin, and eyes, which are thought to result from systemic infections or septicemia. Septicemia is a clinical condition characterized by the emergence of a systemic inflammatory response syndrome or an organ failure. Gram-positive bacteria are reported to cause many sepsis events in humans and animals (American College of Chest Physicians/Society of Critical Care Medicine, 1992; Angus et al., 2001). Lipoteichoic acid present in the matrix peptidoglycan and the diversity of Gram-positive bacteria’s cell wall structure act as a precursor to the resulting exaggerated inflammatory response eliciting the inflammatory response that varies significantly between individuals infected (Yipp et al., 2002). Septicemia or bacteremia can be correlated with the presence of an E. faecalis virulence factor in the form of a surface protein encoded by esp virulent genes (Creti et al., 2004). Surface protein plays a role in the process of bacterial contact with a layer in the host tissue. Therefore, this virulence factor also correlates with E. faecalis adhering, besides the virulence factor of aggregation substance, in the abiotic environment to form a biofilm (Toledo-Arana et al., 2001). The condition of septicemia in this study was characterized by a change in the liver that becomes paler and has a brittle texture, positive culture results from fish blood that have died or moribund, and the presence of Gram-positive bacteria in the form of coccus on blood smear examination. To the authors’ knowledge, the positive culture results from fish blood that has died or moribund and the presence of Gram-positive bacteria in the form of coccus on blood smear examination caused by E. faecalis infection is the recent report which can prove the existence of bacteria circulating in the blood of fish that are artificially infected by E. faecalis. This laboratory examination is expected to assist in diagnosing clinical symptoms of septicemia due to E. faecalis when there are streptococcosis cases in the field. Septicemia can also be characterized by a change in the hematological profile system, such as anemia, leukocytosis, and thrombocytopenia (Goyette et al., 2004). Based on the results of the counting of the average total leukocytes was performed in each treatment group obtained an increase of twofold on the mean total leukocytes in the group with a bacterial concentration of 2.1 × 108 CFU ml−1, both PO and IP, when compared with the non-infectious group. Changes in the total leukocyte profile and the differential leukocytes obtained in this study are in line with the results of a study conducted by Martins et al. (2008), which is in artificial infection with the Enterococcus sp. with the bacterial concentration of 1 × 106 CFU ml−1 can increase the total leukocytes nearly twofold when compared with the uninfected tilapia group. In that study, lymphocytes were also reported as leukocytes with the highest number compared to neutrophils and macrophages. The lymphocytes could be triggered to produce because of antigen exposure. This white blood cell will be conducted fission and produces more immature lymphocytes and causes lymphocytes to increase and higher than monocytes and neutrophils. Based on the results obtained, it can be concluded that E. faecalis used during the study is one causative agent of streptococcosis with low pathogenicity levels. It requires a higher enough PO dose of infection that can cause death in individuals than other E. faecalis strains in a previous study. The route of infection can also affect the severity of clinical symptoms. However, this bacterium causes variation in clinical symptoms in each infected fish. It is also capable of causing septicemia when analyzed based on changes in hematology profile and the discovery of bacteria upon observation of blood smear during examination. This information is expected to increase knowledge of clinical symptoms of streptococcosis caused by E. faecalis in the fisheries community in Indonesia. AcknowledgmentsThe authors would like to thank the Deputy of Research Affirmation and Development, Ministry of Research and Technology-National Agency for Research and Innovation, the Republic of Indonesia for Masters to Doctoral Program for Superior Bachelor (PMDSU) Scholarship Batch IV, which fully funded in this study. Conflict of interestThe authors declare that there is no conflict of interest. Author contributionsRR conducted a microbiological examination toward the specimen of E. faecalis, the LD50 study in treated fish, and wrote the manuscript. IWTW, FHP, and RDS as advisors in microbiological and immunological examination in this study. SP conducted the molecular examination using PCR procedures. DBW conducted water quality examination, aquaria maintenance, fed the treated fish, and helped in the pathological anatomy examination. All authors read and approved the final version of the manuscript. ReferencesAmal, M.N.A. and Zamri-Saad, M. 2011. Streptococcosis in tilapia (Oreochromis niloticus): a review. Pertanika J. Trop. Agric. Sci. 34, 195–206. American College of Chest Physicians/Society of Critical Care Medicine. 1992. Consensus conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit. Care Med. 20, 864–874. Angus, D.C., Linde-Zwirble, W.T., Lidicker, J., Clermont, G., Carcillo, J. and Pinsky, M.R. 2001. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29, 1303–1310. Anshary, H., Kurniawan, R.A., Sriwulan, S., Ramli, R. and Baxa, D.V. 2014. Isolation and molecular identification of the etiological agents of streptococcosis in Nile tilapia (Oreochromis nicotilus) cultured in net cages in Lake Sentani, Papua, Indonesia. Springerplus. 3, 627. Arias, C.A. and Murray, B.E. 2012. The rise of the Enterococcus: beyond vancomycin resistance. Nat. Rev. Microbiol. 10, 266–278. Austin, B. and Austin, D.A. 2012. Bacterial fish pathogens: disease of farmed and wild fish. Springer Science+Business Media Dordrecht, Berlin, Germany. Creti, R., Imperi, M., Bertuccini, L., Fabretti, F., Orefici, G., Di Rosa, R. and Baldassarri, L. 2004. Survey for virulence determinants among Enterococcus faecalis isolated from different sources. J. Med. Microbiol. 53, 13–20. Dias, M.K.R., Sampaio, L.S., Proitetti-Junior, A.A., Yoshioka, E.T.O., Rodrigues, D.P., Rodriguez, A.F.R., Ribeiro, R.A., Faria, F.S.E.D.V., Ozório, R.O.A. and Tavares-Dias, M. 2016. Lethal dose and clinical signs of Aeromonas hydrophila in Arapaima gigas (Arapaimidae), the giant fish from Amazon. Vet. Microbiol. 188, 12–15. El-Beltagy, A.E., El-Adawy, T.A., Rahma, E.H. and El-Bedawey, A.A. 2004. Purification and characterization of an acidic protease from the viscera of bolti fish (Tilapia nicotila). Food Chem. 86, 33–39. El-Sayed, N. and El-Gheit, A. 2005. Streptococcus infection among farmed and wild tilapia fish in Egypt. Egypt. J. Exp. Biol. (Zool.). 1, 1–7. Engelbert, M., Mylonakis, E., Ausubel, F.M., Calderwood, S.B. and Gilmore, M.S. 2004. Contribution of gelatinase, serine protease, and fsr to the pathogenesis of Enterococcus faecalis endophthalmitis. Infect. Immun. 72, 3628–3633. Evans, J.J., Pasnik, D.J., Klesius, P.H. and Al-Ablani, S. 2006. First report of Streptococcus agalactiae and Lactococcus garvieae from a wild bottlenose dolphin Tursiops truncatus. J. Wildl. Dis. 42, 561–569. Fawzy, N.M., Osman, K.M, Ibrahim, M.E.E., Ali, M.N.M. and Abd-Elrahman, S.S. 2014. Streptococcosis in tilapia: clinico-pathological picture of experimentally infected tilapia. Life Sci. J. 11, 1005–1012. Ferguson, H.W., Morales, J.A. and Ostland, V.E. 1994. Streptococcosis in aquarium fish. Dis. Aquat. Org. 19, 1–6. Goyette, R.E., Key, N.S. and Ely, E.W. 2004. Hematologic changes in sepsis and their therapeutic implications. Semin. Respir. Crit. Care Med. 25, 645–659. Hamilton, M.A., Russo, R.C. and Thurston, R.V. 1977. Trimmed Spearman-karber method for estimating median lethal concentrations in toxicity bioassays. Environ. Sci. Technol. 11, 714–719. Haslam, D.B. and Geme III, J.W.S. 2018. Classification of streptococci. In Principles and practice of pediatric infectious diseases, 5th ed.., Eds., Long, S., Prober, C. and Ficher M. Amsterdam, NL: Elsevier. Iregui, C.A., Comas, J., Vásquez, G.M. and Verján, N. 2016. Experimental early pathogenesis of Streptococcus agalactiae infection in red tilapia Oreochromis spp. J. Fish Dis. 39, 205–215. Khafagy, A.A.R., Eid, H.M.I., Abou El-Atta, M.E.I. and Abd. El-Fattah, L. 2009. Isolation of Enterococcus faecalis from tilapia in Lake Temsah in Ismailia governorate. Suez Canal Vet. Med. J. 14, 45–54. Klein, G. 2003. Taxonomy, ecology, and antibiotic resistance of enterococci from food and the gastro-intestinal tract. Int. J. Food Microbiol. 88, 123–131. Kwon, A.S., Kang, B.J., Jun, S.Y., Yoon, S.J., Lee, J.H. and Kang, S.H. 2017. Evaluating the effectiveness of Streptococcus parauberis bacteriophage Str-PAP-1 as an environmentally friendly alternative to antibiotics for aquaculture. Aquaculture 468, 464–470. Linden, P. 2003. Can enterococcal infections initiate sepsis syndrome? Curr. Infect. Dis. Rep. 5, 372–378. Martins, M.L., Mouriño, J.L.P., Amaral, G.V., Vieira, F.N., Dotta, G., Jatobá, A.M.B, Pedrotti, F.S., Jerônimo, G.T., Buglione-Neto, C.C. and Pereira-Jr, G. 2008. Haematological changes in Nile tilapia experimentally infected with Enterococcus sp. Braz. J. Biol. 68, 657–661. Martins, M.L., Tavares-Dias, M., Fujimoto, R.Y., Onaka, E.M. and Nomura, D.T. 2004. Haematological alterations of Leporinus macrocephalus (Osteichthyes: Anostomidae) naturally infected by Goezia leporini (Nematoda: Anisakidae) in fish pond. Arq. Bras. Med. Vet. Zootec. 56, 640–646. Morandi, S., Brasca, M., Alfieri, P, Lodi, R. and Tamburini, A. 2005. Influence of pH and temperature on the growth of Enterococcus faecium and Enterococcus faecalis. Le Lait, INRA Editions. 85, 181–192. Mubarak, Z. and Soraya, C. 2018. The acid tolerance response and pH adaptation of Enterococcus faecalis in extract of lime Citrus aurantifolia from Aceh Indonesia. F1000Research. 7, 287. Naumowicz, K., Pajdak, J., Terech-Majewska, E. and Szarek, J. 2017. Intracohort cannibalism and methods for its mitigation in cultured freshwater fish. Rev. Fish Biol. Fisheries. 27, 193–208. Netto, L.N., Leal, C.A.G. and Figueiredo, H.C.P. 2011. Streptococcus dysgalactiae as an agent of septicaemia in Nile tilapia, Oreochromis niloticus (L.). J. Fish Dis. 34, 251–254. Osman, K.M., Al-Maary, K.S., Mubarak, A.S., Dawoud, T.M., Moussa, I.M.I., Ibrahim, M.D.S., Hessain, A.M., Orabi, A. and Fawzy, N.M. 2017. Characterization and susceptibility of streptococci and enterococci isolated from Nile tilapia (Oreochromis niloticus) showing septicaemia in aquaculture and wild sites in Egypt. BMC Vet. Res. 13, 357. Rahman, M., Rahman, M.M., Deb, S.C., Alam, M.S., Alam, M.J. and Islam, M.T. 2017. Molecular identification of multiple antibiotic resistant fish pathogenic Enterococcus faecalis and their control by medicinal herbs. Sci. Rep. 7, 3747. Rizkiantino, R., Wibawan, I.W.T., Pasaribu, F.H., Soejoedono, R.D., Arnafia, W., Ulyama, V. and Wibowo, D.B. 2020. Isolation and characterization of the Enterococcus faecalis strain isolated from red tilapia (Oreochromis hybrid) in Indonesia: a preliminary report. J. Surv. Fish. Sci. 7, 27–42. Rosenfeld, G. 1947. Corante pancrômico para hematologia e citologia clínica. Nova combinação dos componentes do May-Grunwald e do Giemsa num só corante de emprego rápido. Mem. Inst. Butantan. 20, 329–334. Ryan, K.J. and Ray, C.G. 2004. Sherris medical microbiology: an introduction to infectious diseases. McGraw Hill Companies Inc., New York, NY, pp: 294–295. Siti-Zahrah, A., Padilah, B., Azila, A., Rimatulhana, R. and Shahidan, R. 2008. Multiple streptococcal species infection in cage-cultured red tilapia but showing similar clinical signs. In Diseases in Asian aquaculture VI. Eds., M. Bondad-Reantaso, M.G. Mohan, C. V. Crumlish, and M. Subasinghe, R.P. Manila, Manila, Philippines: Fish Health Section Asian Fisheries Society, pp: 313–320. Stoskopf, MK. 2010. Fish medicine Volume I. 2nd ed. Baltimore, MD: ART Sciences LLC. Stuart, C.H., Schwartz, S.A., Beeson, T.J. and Owatz, C.B. 2006. Enterococcus faecalis: its role in root canal treatment failure and current concepts in retreatment. J. Endod. 32, 93–98. Su, Y.A., Sulavik, M.C., He, P., Makinen, K.K., Makinen P.L., Fiedler, S., Wirth, R. and Clewell, D.B. 1991. Nucleotide sequence of the gelatinase gene (gelE) from Enterococcus faecalis subsp. liquefaciens. Infect. Immun. 59, 415–420. Taufek, N.M., Harith, H.H., Abd Rahim, M.H., Ilham, Z., Rowan, N. and Wan-Mohtar, W.A.A.Q.I. 2020. Performance of mycelial biomass and exopolysaccharide from Malaysian Ganoderma lucidum for the fungivore red hybrid Tilapia (Oreochromis sp.) in Zebrafish embryo. Aquacult. Rep. 17, 100322. Taukhid, T. and Purwaningsih, U. 2011. Screening of Streptococcus spp. isolates as an antigen candidate in vaccine development, and its efficacy to prevent streptococcosis on tilapia, Oreochromis niloticus. J. Ris. Akuakultur. 6, 103–118. Toledo-Arana, A., Valle, J., Solano, C., Arrizubieta, M.J., Cucarella, C., Lamata, M., Amorena, B., Leiva, J., Penadés, J.R. and Lasa, I. 2001. The enterococcal surface protein, Esp, is involved in Enterococcus faecalis biofilm formation. Appl. Environ. Microbiol. 67, 4538–4545. Toranzo, A.E., Magariños, B. and Romalde, J.L. 2005. A review of the main bacterial fish diseases in mariculture systems. Aquaculture 246, 37–61. Wan-Mohtar, W.A.A.Q.I., Taufek, N.M., Yerima, G., Rahman J., Thiran, J.P., Subramaniam K. and Sabaratnam, V. 2021. Effect of bioreactor-grown biomass from Ganoderma lucidum mycelium on growth performance and physiological response of red hybrid tilapia (Oreochromis sp.) for sustainable aquaculture. Org. Agr. 11, 327–335. Yipp, B.G., Andonegui, G., Howlett, C.J., Robbins, S.M., Hartung, T., Ho, M. and Kubes, P. 2002. Profound differences in leukocyte-endothelial cell responses to lipopolysaccharide versus lipoteichoic acid. J. Immunol. 168, 4650–4658. Zhao, L., Budge, S.M., Ghaly, A.E., Brooks, M.S. and Dave, D. 2011. Extraction, purification and characterization of fish pepsin: a critical review. J. Food Process. Technol. 2, 126. Zhou, A., Yin, F., Zhao, L., Gong, C., Benjakul, S., Liu, X. and Cao, Y. 2013. Purification and characterization of trypsin from the intestine of genetically improved nile tilapia (Oreochromis nicotilus). J. Aquat. Food Prod. Technol. 22, 421–433. | ||
| How to Cite this Article |
| Pubmed Style Rizkiantino R, Pasaribu FH, Soejoedono RD, Purnama S, Wibowo DB, IWTW, . Experimental Infection of Enterococcus faecalis in Red Tilapia (Oreochromis hybrid) Revealed Low Pathogenicity to Cause Streptococcosis. Open Vet J. 2021; 11(2): 309-318. doi:10.5455/OVJ.2021.v11.i2.16 Web Style Rizkiantino R, Pasaribu FH, Soejoedono RD, Purnama S, Wibowo DB, IWTW, . Experimental Infection of Enterococcus faecalis in Red Tilapia (Oreochromis hybrid) Revealed Low Pathogenicity to Cause Streptococcosis. https://www.openveterinaryjournal.com/?mno=60365 [Access: November 09, 2024]. doi:10.5455/OVJ.2021.v11.i2.16 AMA (American Medical Association) Style Rizkiantino R, Pasaribu FH, Soejoedono RD, Purnama S, Wibowo DB, IWTW, . Experimental Infection of Enterococcus faecalis in Red Tilapia (Oreochromis hybrid) Revealed Low Pathogenicity to Cause Streptococcosis. Open Vet J. 2021; 11(2): 309-318. doi:10.5455/OVJ.2021.v11.i2.16 Vancouver/ICMJE Style Rizkiantino R, Pasaribu FH, Soejoedono RD, Purnama S, Wibowo DB, IWTW, . Experimental Infection of Enterococcus faecalis in Red Tilapia (Oreochromis hybrid) Revealed Low Pathogenicity to Cause Streptococcosis. Open Vet J. (2021), [cited November 09, 2024]; 11(2): 309-318. doi:10.5455/OVJ.2021.v11.i2.16 Harvard Style Rizkiantino, R., Pasaribu, F. H., Soejoedono, R. D., Purnama, S., Wibowo, D. B., , I. W. T. W. & (2021) Experimental Infection of Enterococcus faecalis in Red Tilapia (Oreochromis hybrid) Revealed Low Pathogenicity to Cause Streptococcosis. Open Vet J, 11 (2), 309-318. doi:10.5455/OVJ.2021.v11.i2.16 Turabian Style Rizkiantino, Rifky, Fachriyan Hasmi Pasaribu, Retno Damajanti Soejoedono, Sucitya Purnama, Danny Bagus Wibowo, I Wayan Teguh Wibawan, and . 2021. Experimental Infection of Enterococcus faecalis in Red Tilapia (Oreochromis hybrid) Revealed Low Pathogenicity to Cause Streptococcosis. Open Veterinary Journal, 11 (2), 309-318. doi:10.5455/OVJ.2021.v11.i2.16 Chicago Style Rizkiantino, Rifky, Fachriyan Hasmi Pasaribu, Retno Damajanti Soejoedono, Sucitya Purnama, Danny Bagus Wibowo, I Wayan Teguh Wibawan, and . "Experimental Infection of Enterococcus faecalis in Red Tilapia (Oreochromis hybrid) Revealed Low Pathogenicity to Cause Streptococcosis." Open Veterinary Journal 11 (2021), 309-318. doi:10.5455/OVJ.2021.v11.i2.16 MLA (The Modern Language Association) Style Rizkiantino, Rifky, Fachriyan Hasmi Pasaribu, Retno Damajanti Soejoedono, Sucitya Purnama, Danny Bagus Wibowo, I Wayan Teguh Wibawan, and . "Experimental Infection of Enterococcus faecalis in Red Tilapia (Oreochromis hybrid) Revealed Low Pathogenicity to Cause Streptococcosis." Open Veterinary Journal 11.2 (2021), 309-318. Print. doi:10.5455/OVJ.2021.v11.i2.16 APA (American Psychological Association) Style Rizkiantino, R., Pasaribu, F. H., Soejoedono, R. D., Purnama, S., Wibowo, D. B., , I. W. T. W. & (2021) Experimental Infection of Enterococcus faecalis in Red Tilapia (Oreochromis hybrid) Revealed Low Pathogenicity to Cause Streptococcosis. Open Veterinary Journal, 11 (2), 309-318. doi:10.5455/OVJ.2021.v11.i2.16 |