| Original Article | ||
Open Vet J. 2021; 11(2): 301-308 Open Veterinary Journal, (2021), Vol. 11(2): 301–308 Original Research First seroprevalence investigation of epizootic haemorrhagic disease virus in LibyaAbdusalam Mahmoud1*, Maria Luisa Danzetta2, Daria di Sabatino2, Massimo Spedicato2, Zakaria Alkhatal3, Abdunaser Dayhum1, Franceseco Tolari4, Mario Forzan4, Maurizio Mazzei4 and Giovanni Savini21Department of Preventive Medicine, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya 2Istituto Zooprofilattico Sperimentale dell'Abruzzo e del Molise “G. Caporale”, (IZSAM), Teramo, Italy 3National Center of Animal Health, Tripoli, Libya 4Department of Veterinary Science, University of Pisa, Pisa, Italy *Corresponding Author: Abdusalam Sharef Mahmoud. Department of Preventive Medicine, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya. Email: Abd.Mahmoud [at] uot.edu.ly Submitted: 13/03/2021 Accepted: 21/05/2021 Published: 21/06/2021 © 2021 Open Veterinary Journal
AbstractBackground: Epizootic haemorrhagic disease (EHD) is a vector-borne viral disease of domestic and wild ruminants. Epizootic haemorrhagic disease virus (EHDV) is transmitted by Culicoides spp. EHDV is a member of the Orbivirus genus within the Reoviridae family. It shares many morphological and structural characteristics with other members of the genus, such as the bluetongue virus, African horse sickness virus, and equine encephalosis virus. Aims: The purpose of our study was to investigate the epidemiological situation of EHDV in Libya in order to gain some knowledge about the presence of this virus in the country. Methods: In this study, we investigated the seroprevalence of EHDV in Libya, testing 855 blood samples collected during 2015. The samples were collected from domestic ruminants (cattle, sheep, and goats) originating from 11 provinces of Libya. Sera were tested by competitive enzyme-linked immunosorbent assays and positive samples confirmed by serum neutralization test. Results: The overall seroprevalence of EHDV was estimated to be 4% (95% confidence intervals=2.8%–5.4%). Small ruminant seroprevalence was significantly (p=0.016) higher than that found in cattle. Neutralizing antibodies against EHDV-6 were detected in a sheep from the western region of Libya. Conclusion: This study suggests that EHDV has circulated or is circulating in Libya, and sheep could play an important role in the epidemiology of EHDV, and the virus may still be circulating in North Africa. Keywords: EHD, EHDV-6, Seroprevalence, Libya. IntroductionEpizootic haemorrhagic disease (EHD) is an infectious, non-contagious viral disease transmitted by insects of the Culicoides genus affecting wild and domestic ruminants. The Epizootic haemorrhagic disease virus (EHDV) is a member of the Orbivirus genus within the Reoviridae family. It shares many morphological and structural characteristics with other members of the genus, such as the bluetongue virus, African horse sickness virus, and equine encephalosis virus (Maclachlan et al., 2004). The viral genome of EHDV is composed of 10 segments of double-stranded RNA that encode seven structural (VP1–VP7) and three or possibly four nonstructural proteins (NS1–NS3 and NS3a) (Mertens et al., 2005). EHDV was first described and isolated from white tailed deer during 1955 in New Jersey (Shope et al., 1960). The first EHDV strain that was demonstrated to be pathogenic for cattle was the Ibaraki strain (IBAV) isolated in Japan in 1959 (Omori et al., 1969; Inaba, 1975). Based on the recent genetic and phylogenetic analysis of the capsid proteins responsible for the EHDV serotype specificity (VP2 and VP5) and according to the International Committee on Taxonomy of Viruses, currently seven serotypes of the virus are officially recognized. Furthermore, EHDV-3, EHDV-318, and IBAV strains are now considered as EHDV-1, EHDV-6, and EHDV-2, respectively (Anthony et al., 2009; Maan et al., 2010). In recent years, however, novel EHDV strains from South Africa (Wright, 2013), Japan (Shirafuji et al., 2017) (ON-4/B/98 strain), and China have been proposed as new possible serotypes. During September and October of 2006 in Indiana and Illinois, EHDV-6 was reported as a novel reassortant strain between the Australian prototype of EHDV-6 (CSIRO 753) and the North American prototype of EHDV-2 (Alberta) (Allison et al., 2012). In the Mediterranean Basin, four serotypes of EHDV have been reported, EHDV-1, EHDV-2, and EHDV-6 in Turkey, EHDV-6 in Algeria, Morocco, and Tunisia (Ben Dhaou et al., 2016), and EHDV-7 in Israel and Jordan (Yadin et al., 2008). In Israel, the outbreaks sustained by EHDV-7 in cattle during 2006 caused relevant economic losses affecting 83 dairy and 22 beef herds with morbidity rates ranging from 5% to 80% and mortality rates below 1% (Yadin et al., 2008; Kedmi et al., 2010). However, no morbidity was reported in other domestic and wild ruminants despite their proximity to cattle herds (Kedmi et al., 2010). In July 2007, an outbreak of EHDV-6 in cattle was recorded in Turkey (Temizel et al., 2009). Even if the pathways by which these serotypes were introduced into these countries remain unclear, it is important to note an alarming similarity between the current EHD scenario and that of bluetongue (BT), which took place at the end of 1990. During that period, some BTV serotypes that were initially circulating in Algeria, Tunisia, Turkey, and Israel, were able to cross the Mediterranean Sea, causing outbreaks in several European countries (Savini et al., 2011). The purpose of this study was to investigate the epidemiological situation of EHDV in Libya in order to gain some knowledge about the presence of this virus in the country. Materials and MethodsDuring 2015, 855 serum samples were collected from domestic ruminants, including 165 cattle, 555 sheep, and 135 goats. In each farm, a maximum of 10 serum samples was gathered regardless of the herd size. Information on sex and age (expressed in months) was recorded. The samples were collected from 96 farms representing the 11 provinces distributed in four regions of Libya (eastern, western, northern, and southern region) (Fig. 1). Samples were collected, and submitted to the Istituto Zooprofilattico Sperimentale dell’Abruzzo e del Molise “G. Caporale” (IZSAM). The samples were tested by competitive enzyme-linked immunosorbent assays (c-ELISA), by the commercial kit LSIVetTM Ruminant EHDV-serum (Life Technology, Waltham, MA) which can detect specific antibodies against EHDV serogroups without cross reactions to BTV serotypes. c-ELISA positive samples were further analyzed by serum neutralization test (SNT) with little modification (Sailleau et al., 2020). The SNT was performed to determine the neutralizing titer and the serotype (EHDV-1, -2, -3, -4, -5, -6, or -7) responsible for the infection. The antibody titre was defined as the highest dilution able to inhibit at least 75% of the virus cytopathic effect. To the authors’ knowledge, all the samples were collected from animals which neither experienced EHDV vaccination nor showed clinical signs of EHD at the time of sampling. Statistical analysisStatistical analysis was performed using XLSTAT. The seroprevalence and 95% confidence intervals (CI) were calculated using the Bayesian approach of the Beta distribution. Seroprevalence was calculated by order of species, sex, and age for all the animals tested, considering both small ruminants and cattle. Animals were grouped into three age classes: kids (less than 6 months), young (between 6 and 24 months), and adults (more than 24 months). Association between the outcome variables (status of EHDV infection in small ruminants and cattle) and its potential risk factors (sex and a farmed species) were screened in a univariate analysis using Fisher’s exact test. Chi-square test was used for species, age classes, and regions. A p-value < 0.05 was considered to be significant for EHDV infection. Odds-ratio and 95% CI were calculated for each risk factor considered.
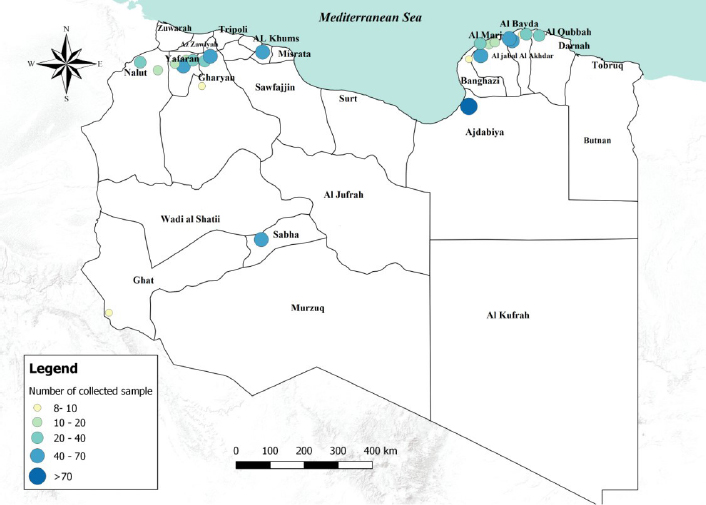
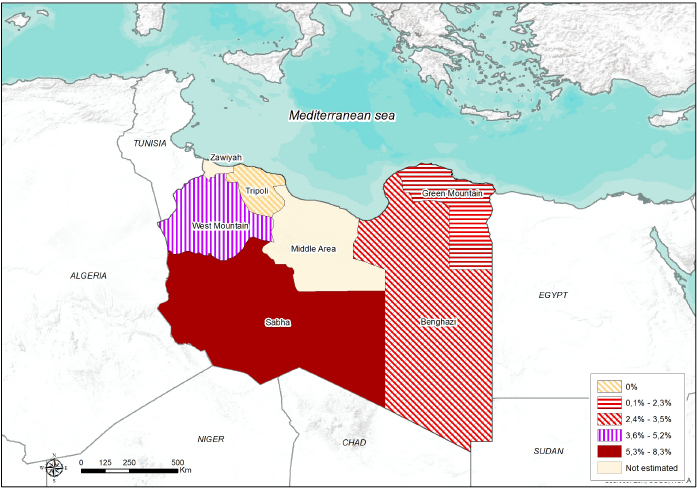
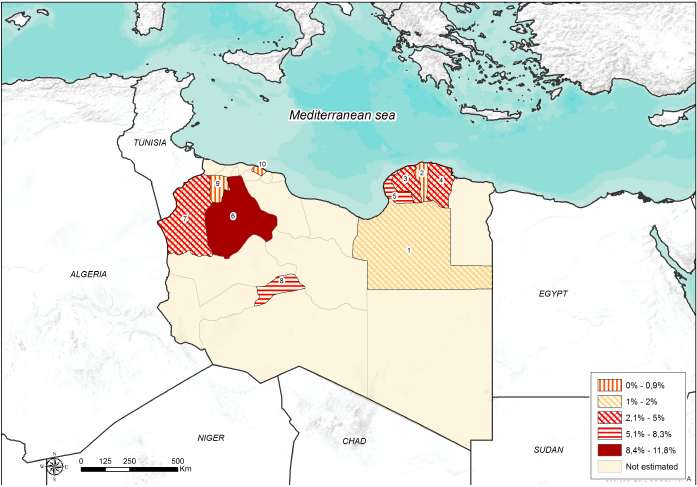
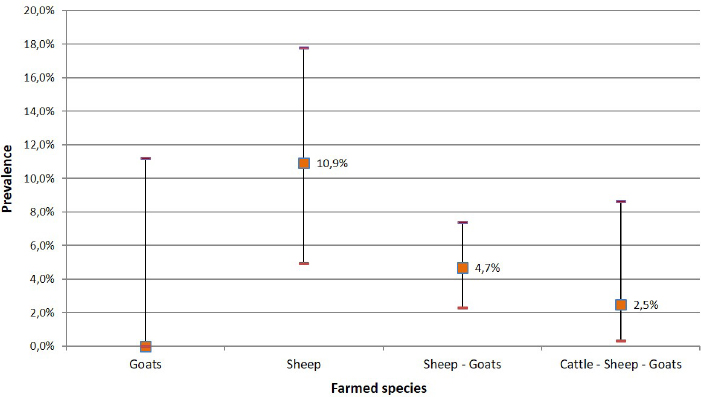
Fig. 1. Number of collected samples (sheep, goats and cattle) represented by sampled areas and by sample size classes. Ethical approvalNo consent of Ethics required for the research. ResultsThe overall seroprevalence rate (small ruminants and cattle) of EHDV was estimated to be 4% (95% CI=2.8%–5.4%). Of the 555 sheep tested, 32 (5.76%; 95% CI=3.83%–7.71%) were found positive, none of the 135 goats tested were positive and only 1 out of 165.cattle (0.61%; 95% CI=0.04%–3.31%) resulted positive. The highest seroprevalence of EHDV (8.3%; 95% CI=1.9%–19.6%) was reported in the Southern region (Sabha) (Fig. 2). The highest (11.8%; 95% CI=7.1%–19.2%) and lowest (1.2%; 95% CI=2.8%–6.4%) seroprevalences were recorded in small ruminants of Gharyan and Al Jabal al Akhdar provinces, respectively. In Yafran-Jadu province, none of the small ruminants (130) have tested positive (Fig. 3 and Table 1). The sole cattle (0.61%; 95% CI=0.000%–1.79%) tested c-ELISA positive was from Al-Marj in the Eastern Region. In Gharyan province, the small ruminants sampled were only adults, and they represented 24.3% of all the small adult ruminants sampled in the study. The highest seroprevalence was recorded in adult animals (5.3%; 95% CI=3.6%–7.5%). The highest seroprevalence among females and males was recorded in females (4.3%; 95% CI=2.9%–6.2%). The results of the univariate analysis showed that only independent variables of animal age group and farm-type species (Fig. 4 and Table 2) were statistically significant. Of the 32 c-ELISA positive sheep, only one was confirmed by the SN assay against EHDV-6 serotype. Due to the exiguous number of SN-positive animals, statistical analysis was performed only on ELISA data.
Fig. 2. Seroprevalence and spatial distribution of EHD virus in five Libyan regions.
Fig. 3. Seroprevalence and spatial distribution of EHD virus according to Libyan province. Table 1. Seroprevalence of EHD virus in Libyan small ruminants according to Provinces.
DiscussionIn recent years, we have assisted in bringing significant disruption in the global distribution of vector-borne diseases (VBD), which in most cases led to a northward range expansion. Since the end of the last century, BT, more than any other VBD, has spread northwards into Europe beyond its traditional range. EHDV shares a similar biological niche in terms of vectors and hosts as BTV, and therefore, global EHDV distribution likely mirrors that of the BTV (Savini et al., 2011). Consequently, the presence of EHDV in Northern Africa countries could pose a risk of introducing the virus to Europe, as happened for BTV. Despite the occurrence of BTV in Europe, no cases of EHDV infection have been reported so far (Savini et al., 2011).
Fig. 4. Seroprevalence of EHD virus according to farmed species.
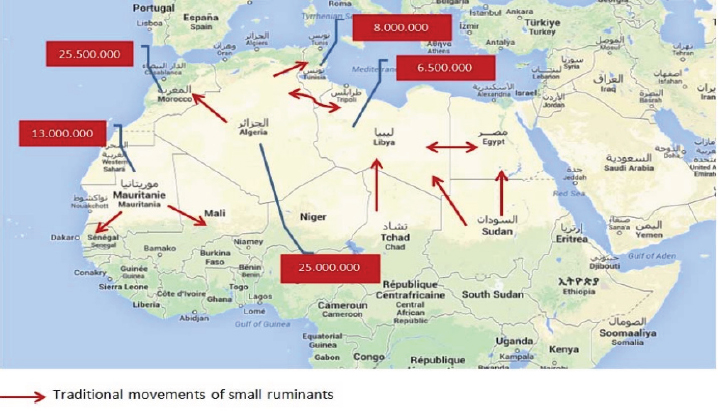
Fig. 5. Major pathways of the traditional movements of small ruminants in the Maghreb region (OIE, 2015). This study represents the first investigation on EHDV seroprevalence and risk factors in domestic ruminants in Libya. The overall seroprevalence of EHDV was estimated as 4% (CI 95%=2.8%–5.4%). Unexpectedly, seroprevalence of small ruminants was significantly (p=0.016) higher than in cattle. Our findings revealed higher seroprevalence of the EHDV in sheep (32 out of 555) than other species, indicating their susceptibility for infection by EHDV in agreement with epidemiological data reported in Western Turkey (Yavru et al., 2014). On the other hand, it should be mentioned that, during a serological study conducted in Israel to investigate the involvement of sheep during the outbreaks of EHD that occurred in 2006, no serological evidence of infection was found in sheep (Kedmi et al., 2010). Differences in seropositivity in sheep and goats in the same herd location were unexpected but maybe explained with vector activity oranimal dynamics and breeding systems. Neutralizing antibodies were detected only in one sheep sample. Although not much is known on how long serological tests can detect EHDV antibodies in infected animals, it is believed that they behave like those of BTV. In other words, c-ELISA is likely to be more sensitive than SNT. It is capable of detecting antibodies earlier and for a longer time compared to SNT. The sheep sample showed neutralizing antibodies against serotype EHDV-6. Still, no clinical signs were observed in the herd in accordance with the observations that sheep are susceptible to EHDV infection but rarely develop clinical symptoms while their involvement in the epidemiology of EHDV is still not well understood (Kedmi et al., 2010). The EHDV-6 positive animal was a nine years old ewe, sampled in the Province of Gharyan (West Region). The animal was farmed in a flock of 550 sheep and 50 goats. Table 2. Epizootic hemorrhagic disease in Libya: results of univariate analysis by species, age group, regions, farmed species, and provinces.
Transitional movement of small ruminants from all the neighboring countries to Libya occurs regularly, particularly during the holy months. Therefore, the presence of EHDV-6 in the West region of Libya may be due to animal trades (Fig. 5). Alternatively, the circulation of this serotype in the Northwest of Libya may be a consequence of the emergence of EHDV-6 during 2006 in North Africa (Tunisia, Algeria, and Morocco) [European Food and Safety Authority (EFSA), 2009; Ben Dhaou et al., 2016]. Because of political instability in Libya, the sample collection was opportunistic, and some areas were investigated through the collection of samples from small ruminants but not from cattle (South Region) or vice versa (North Region). No difference (p=0.903) was highlighted between the seroprevalences recorded in the East and the West Regions. Comparatively, a single sample site was investigated in the South Region, and that revealed the highest seroprevalence (8.3%) of EHDV; therefore, it cannot be considered as representative of the whole South Region. To assess the risk for EHDV spreading, it should be necessary to monitor animal movements from neighboring countries and inside Libya. In addition, it would be essential to improve diagnostic surveillance on trade animals. This approach could indeed be useful not only for EHDV but also for other animal diseases. ConclusionSumming up our results, we suggest, sheep may play an important role in the epidemiology of EHDV, and the virus may still be circulating in North Africa. Therefore, for a better understanding of the epidemiological patterns of EHDV and vectors biting midges, further studies and investigation should be planned. AcknowledgementsThe Authors would like to express their appreciations to the staff of the Libyan National Center for Animal Health (NCAH) for their collaboration, especially to those who contributed in collecting samples and filling questionnaires. The authors would like to express their gratitude to the staff of the National Reference of IZSAM, Teramo, Italy, for providing fund and samples testing. Conflict of interestAll authors declare that there is no conflict of interest. ReferencesAllison A.B., Holmes E.C., Potgieter A.C., Wright I.M., Sailleau C., Breard, E., Ruder, M.G. and Stallknecht, D.E. 2012. Segmental configuration and putative origin of the reassortantorbivirus, epizootic hemorrhagic disease virus serotype 6, strain Indiana. Virology. 424, 67–75. Anthony, S.J., Maan, S., Maan, N., Kgosana, L., Bachanek-Bankowska, K., Batten, C., Darpel, K.E., Sutton, G., Attoui, H. and Mertens, P.P.C. 2009. Genetic and phylogenetic analysis of the outer-coat proteins VP2 and VP5 of epizootic haemorrhagic disease virus (EHDV): comparison of genetic and serological data to characterise the EHDV serogroup. Virus Res. 145(2), 200–210. Ben Dhaou, S., Sailleau, C., Babay, B., Viarouge, C., Sghaier, S., Zientara, S., Hammami, S. and Breard, E. 2016. Molecular characterisation of epizootic haemorrhagic disease virus associated with a Tunisian 312 outbreak among cattle in 2006. Acta Vet. Hung. 64, 250–262. European Food and Safety Authority (EFSA). 2009. Epizootic hemorrhagic disease (EHD). EFSA J. 7(12), 1418. Inaba, U. 1975. Ibaraki disease and its relationship to bluetongue. Aust. Vet. J. 51, 178–185. Kedmi, M., Van Straten, M., Ezra, E., Galon, N. and Klement, E. 2010. Assessment of the productivity effects associated with epizootic hemorrhagic disease in dairy herds. J. Dairy Sci. 93, 2486–2495. Maan, N.S., Maan, S., Nomikou, K., Johnson, D.J., El Harrak, M., Madani, H., Yadin, H., Incoglu, S., Yesilbag, K., Allison, A.B., Stallknecht, D.E., Batten, C., Anthony, S.J. and Mertens, P.P. 2010. RT-PCR assays for seven serotypes of epizootic haemorrhagic disease virus & their use to type strains from the Mediterranean region and North America. PLoS One. 5(9), e12782; doi:10.1371/journal.pone.0012782 Maclachlan, N.J. and Osburn, B.I. 2004. Epizootic hemorrhagic disease. In Infectious diseases of livestock, 2nd ed. Eds., Coetzer J.A.W. and Tustin, R.C., New York, NY: Oxford University Press, pp: 1227–1230. Mertens, P.P.C., Attoui, H., Duncan, R. and Dermody, T.S. 2005. Reoviridae. In Virus taxonomy. Eighth report of the international committee on taxonomy of viruses. Eds., Fauquet, C.M., Mayo, M.A., Maniloff, J., Desselberger, U. and Ball, L.A., Elsevier/Academic Press, London, UK, pp: 447–454. OIE. 2015. Report on the activities and working programmes (2014–2015). OIE regional and sub regional representations for Africa. 21st Conference of the OIE Regional Commission for Africa Rabat, 2015 Feb 17–20, Rabat, Morocco. Omori, T., Inaba, Y., Morimoto, T., Tanaka, Y. and Ishitani, R. 1969. Ibaraki virus, an agent of epizootic disease of cattle resembling bluetongue. I. Epidemiologic, clinical and pathologic observations and experimental transmission to calves. Jap. J. Microbiol. 2, 139–157. Sailleau, C., Breard, E., Viarouge, C., Belbis, G., Lilin, T., Vitour, D. and Zientara, S. 2020. Experimental infection of calves with seven serotypes of epizootic hemorrhagic disease virus: production and characterization of reference sera. Vet. Ital. 55(4), 339–346. Savini, G., Afonso, A., Mellor, P., Aradaib, I., Yadin, H., Sanaa, M., Wilson, W., Monaco, F. and Domingo, M. 2011. Epizootic hemorrhagic disease. Res. Vet. Sci. 91, 1–17. Shirafuji, H., Kato, T., Yamakawa, M., Tanaka, T., Minemori, Y. and Yanase, T. 2017. Characterization of genome segments 2, 3 and 6 of epizootic hemorrhagic disease virus strains isolated in Japan in 1985–2013: identification of their serotypes and geographical genetic types. Infect. Genet. Evol. 53, 38–46. Shope, R.E., MacNamara, L.G. and Mangold, R. 1960. A virus-induced epizootic hemorrhagic disease of the Virginia white-tailed deer (Odocoileus virginianus). J. Exp. Med. 111, 155–170. Temizel, E.M., Yesilbag, K., Batten, C., Senturk, S., Maan, N.S., Clement-Mertens, P.P. and Batmaz, H. 2009. Epizootic hemorrhagic disease in cattle, Western Turkey. Emerg. Infect. Dis. 15, 317–319. Wright, I.M. 2013. Serological and genetic characterisation of putative new serotypes of bluetongue virus and epizootic haemorrhagic disease virus isolated from an alpaca. NorthWest University, Potchefstroom, South Africa. Yadin, H., Brenner, J., Bumbrov, V., Oved, Z., Stram, Y., Klement, E., Perl, S., Anthony, S., Maan, S., Batten, C. and Mertens, P.P. 2008. Epizootic haemorrhagic disease virus type 7 infection in cattle in Israel. Vet. Rec. 162, 53–56. Yavru, S., Erol, N., Avci, O., Esin, E. and Pasa, S. 2014. Isolation of epizootic hemorrhagic disease virus from sheep in western Turkey. Rev. Méd. Vét. 165, 20–24. | ||
| How to Cite this Article |
| Pubmed Style Mahmoud ASA, Zanetta ML, Sabatino DD, Spedicato M, Alkatal Z, Dayhum A, Tolari F, Forzan M, Mazzei M, Savini G. First seroprevalence investigation of epizootic haemorrhagic disease virus in Libya. Open Vet J. 2021; 11(2): 301-308. doi:10.5455/OVJ.2021.v11.i2.15 Web Style Mahmoud ASA, Zanetta ML, Sabatino DD, Spedicato M, Alkatal Z, Dayhum A, Tolari F, Forzan M, Mazzei M, Savini G. First seroprevalence investigation of epizootic haemorrhagic disease virus in Libya. https://www.openveterinaryjournal.com/?mno=62057 [Access: November 08, 2024]. doi:10.5455/OVJ.2021.v11.i2.15 AMA (American Medical Association) Style Mahmoud ASA, Zanetta ML, Sabatino DD, Spedicato M, Alkatal Z, Dayhum A, Tolari F, Forzan M, Mazzei M, Savini G. First seroprevalence investigation of epizootic haemorrhagic disease virus in Libya. Open Vet J. 2021; 11(2): 301-308. doi:10.5455/OVJ.2021.v11.i2.15 Vancouver/ICMJE Style Mahmoud ASA, Zanetta ML, Sabatino DD, Spedicato M, Alkatal Z, Dayhum A, Tolari F, Forzan M, Mazzei M, Savini G. First seroprevalence investigation of epizootic haemorrhagic disease virus in Libya. Open Vet J. (2021), [cited November 08, 2024]; 11(2): 301-308. doi:10.5455/OVJ.2021.v11.i2.15 Harvard Style Mahmoud, A. S. A., Zanetta, . M. L., Sabatino, . D. D., Spedicato, . M., Alkatal, . Z., Dayhum, . A., Tolari, . F., Forzan, . M., Mazzei, . M. & Savini, . G. (2021) First seroprevalence investigation of epizootic haemorrhagic disease virus in Libya. Open Vet J, 11 (2), 301-308. doi:10.5455/OVJ.2021.v11.i2.15 Turabian Style Mahmoud, Abdusalam Sharef A., Maria Lusia Zanetta, Daria Di Sabatino, Massimo Spedicato, Zakaria Alkatal, Abdunser Dayhum, Francesco Tolari, Mario Forzan, Maurizio Mazzei, and Giovanni Savini. 2021. First seroprevalence investigation of epizootic haemorrhagic disease virus in Libya. Open Veterinary Journal, 11 (2), 301-308. doi:10.5455/OVJ.2021.v11.i2.15 Chicago Style Mahmoud, Abdusalam Sharef A., Maria Lusia Zanetta, Daria Di Sabatino, Massimo Spedicato, Zakaria Alkatal, Abdunser Dayhum, Francesco Tolari, Mario Forzan, Maurizio Mazzei, and Giovanni Savini. "First seroprevalence investigation of epizootic haemorrhagic disease virus in Libya." Open Veterinary Journal 11 (2021), 301-308. doi:10.5455/OVJ.2021.v11.i2.15 MLA (The Modern Language Association) Style Mahmoud, Abdusalam Sharef A., Maria Lusia Zanetta, Daria Di Sabatino, Massimo Spedicato, Zakaria Alkatal, Abdunser Dayhum, Francesco Tolari, Mario Forzan, Maurizio Mazzei, and Giovanni Savini. "First seroprevalence investigation of epizootic haemorrhagic disease virus in Libya." Open Veterinary Journal 11.2 (2021), 301-308. Print. doi:10.5455/OVJ.2021.v11.i2.15 APA (American Psychological Association) Style Mahmoud, A. S. A., Zanetta, . M. L., Sabatino, . D. D., Spedicato, . M., Alkatal, . Z., Dayhum, . A., Tolari, . F., Forzan, . M., Mazzei, . M. & Savini, . G. (2021) First seroprevalence investigation of epizootic haemorrhagic disease virus in Libya. Open Veterinary Journal, 11 (2), 301-308. doi:10.5455/OVJ.2021.v11.i2.15 |