| Case Report | ||
Open Vet J. 2021; 11(4): 672-679 Open Veterinary Journal, (2021), Vol. 11(4): 672–679 Case Report Retinal detachment secondary to vitreoretinopathy in two closely related warmblood horsesEline Vercruysse1*, Carolina Naranjo2, Aurélie Sauvage1, Maxime Vandersmissen3, Magda Grauwels1 and Sébastien Monclin11Department of Ophthalmology, Veterinary Teaching Hospital, University of Liege, Liege, Belgium 2IDEXX Laboratories, Barcelona, Spain 3Department of Medical Imaging, Veterinary Teaching Hospital, University of Liege, Liege, Belgium *Corresponding Author: Eline Vercruysse. Department of Ophthalmology, Veterinary Teaching Hospital, University of Liege, Liege, Belgium. Email: evercruysse [at] uliege.be Submitted: 09/03/2021 Accepted: 03/11/2021 Published: 19/11/2021 © 2021 Open Veterinary Journal
AbstractBackground: To describe the clinical, diagnostic imaging, and histopathological findings of two visually impaired closely related horses and to identify a possible cause. Case Description: Two warmblood horses, with a common dam and sire, were presented to the ophthalmology department of Liège for investigation of impaired vision. Information collected included physical and ophthalmic examination findings, results of ocular ultrasound, electroretinogram, magnetic resonance imaging (MRI), and histopathology. Ophthalmic examination, ocular ultrasound and MRI revealed a complete retinal detachment (RD) in the left eye and vitreous synaeresis in both eyes of both horses. Electroretinograms showed a normal response in both right eyes but a total loss of the retinal response in their left eyes. Histopathologic examination revealed multifocal retinal dysplasia in both left eyes. Conclusion: In these two horses, RD has likely been caused by the congenital posterior segment abnormalities of the vitreous and the retina. A vitreoretinopathy is highly suspected and is possibly hereditary in these closely related siblings. Keywords: Horse, Retinal detachment, Retinal dysplasia, Synaeresis, Vitreoretinopathy. IntroductionRetinal detachment (RD) is a focal or total separation of the neurosensory retina (NSR) from the underlying retinal pigment epithelium (RPE). This implies a disruption between the photoreceptors outer segments and RPE with a loss of function and secondary retinal degeneration in the affected area. Depending on the degree of detachment, the patient will be visually impaired or completely blind. The diagnosis of RD is mainly based on the ophthalmic examination and ultrasonography (US) (Allbaugh et al., 2017). RD in horses has seldom been reported in the literature. Unilateral or bilateral RD can be caused by congenital or acquired disorders. Congenital RD is suggested to be secondary to incomplete invagination of the optic vesicle, causing nonattachment of the NSR to the RPE (Cutler et al., 2000; Cook, 2013). It is also described to result from unequal growth of the developing outer and inner sheet of the retina or from congenital chorioretinitis (Nell and Walde, 2010). It can be an isolated finding but is more frequently associated with other congenital defects such as multiple congenital ocular anomalies (MCOA) (Andersson et al., 2011). Severe retinal dysplasia and multiple congenital defects such as coloboma, microphakia, microphthalmos, and lens luxation can be associated with congenital RD but these abnormalities are uncommon in horses (Wilkie, 2005). Acquired RD is seen in association with inflammatory, traumatic and neoplastic disorders as well as by increased and decreased size of the globe (Nell and Walde, 2010). The most common causes of acquired RD are equine recurrent uveitis (ERU) and trauma (Matz-Rensing, 1996; Strobel et al., 2007). The objective of this report is to document two cases of RD in two closely related horses and to identify a possible cause. Case DetailsHistoryTwo warmblood horses, a 2-year-old, bay colored, Standardbred mare and a 3-year-old, bay colored, Standardbred gelding, were presented to the ophthalmology service of the equine clinic of the University of Liège, Belgium for investigation of decreased vision. Both horses were born and bred in the same stable and shared a common dam and sire. Both gestation and foaling were uneventful and no previous systemic illnesses were recollected. There was no history of trauma or previous ocular problems reported but poor vision was suspected due to their abnormal behavior. The mare had always shown a fearful behavior and would bump into objects on her left side. The gelding had abnormal bilateral scleral show since birth. No significant differences in vision between bright- and dim environment were noticed. Ophthalmic examinationA complete ophthalmic examination was performed by an European College of Veterinary Ophthalmologists (ECVO) board-certified veterinary ophthalmologist (Sébastien Monclin), before and after pupil dilation. This included slit-lamp biomicroscopy (SL-17 Portable Slit Lamp, Kowa), binocular indirect ophthalmoscopy (Keeler Vantage Plus), applanation tonometry (Tono-Pen VETTM, Reichert), and fluorescein staining. Case 1The mare was easily startled, extremely nervous in a new environment and could easily be led into stationary objects on her left side. The menace response was normal in the right eye (oculus dexter (OD)) and absent in the left eye (oculus sinister (OS)). The dazzle and palpebral reflexes were normal in both eyes (oculi uterque (OU)). The direct pupillary light reflex OS and consensual pupillary light reflex OD were diminished and slow. The remaining pupillary light reflexes were within normal limits (Fig. 1). A mild anisocoria was present with minimal mydriasis OS. Slit-lamp biomicroscopic examination revealed no adnexal, ocular surface, or anterior segment abnormalities. The vitreous was liquefied OU with presence of vitreous floaters. The optic nerve heads (ONH) were subjectively thought to be in an abnormal position, being positioned too ventrally. The fundus OD seemed otherwise within normal limits. A giant retinal tear and partial disinsertion of the dorsal retina were observed OS. The retina was completely detached and radiated from the rim of the optic disk in transparent folds extending towards the posterior lens surface. The torn dorsal end of the retina had a typical scrolled and wrinkled appearance. These features were consistent with a rhegmatogenous retinal detachment (RRD). Intraocular pressure measured 24 mmHg OD and 27 mmHg OS. The fluorescein stain test was negative OU. Case 2The ophthalmic examination of the gelding was identical to that of the mare except for a lateral divergent strabismus with a slight rotation of the globe within the orbit OU regardless of head positioning. The ventronasal sclera were continuously exposed (Fig. 2A and B). The equator of the lens was visible OU after pupil dilation, and an incipient posterior cortical cataract was present OS (Fig. 2C and D). Intraocular pressure measured 27 mmHg OD and 26 mmHg OS.
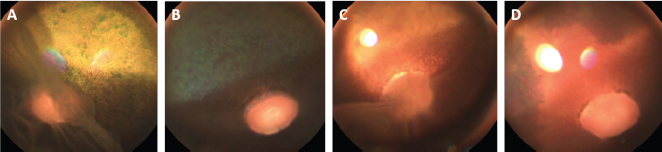
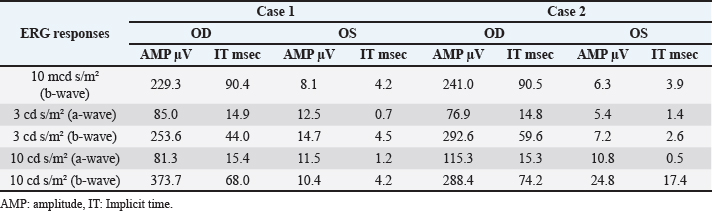
Fig . 1. Photographs of the fundus of the left eye of case 1 with a retinal detachment, giant retinal tear and partial disinsertion of the dorsal retina (A); normal fundus of the right eye of case 1 (B); subalbinotic fundus of the left eye of case 2 with a retinal detachment, giant retinal tear and partial disinsertion of the dorsal retina (C); normal sulabinotic fundus of the right eye of case 2 (D). Due to poor prognosis, the owner elected euthanasia of both horses and consented for additional pre- and postmortem examinations. ElectroretinographyThe ERG protocols were performed under scotopic conditions 24 hours after the initial ophthalmic examination. Pupil dilation was achieved in both eyes with Tropicamide 0.5% (Tropicol®, Théa). The horses were sedated with detomidine (10 µg/kg IV) in combination with morphine (0.1 mg/kg IV). Boluses of detomidine (6 µg/kg IV) were given, when needed, throughout the ERG protocol. Auriculopalpebral nerve blocks were performed bilaterally using 2 ml of 2% lidocaine (Xylocaine®, Astrazeneca). After desensitization of the cornea with a topical anesthetic (Minims® tetracaine hydrochloride 1% w/v ophthalmic solution, Bausch & Lomb Inc.), the electrodes were placed as previously described by Ben-Shlomo et al. (2012). The electrodes were connected to the Handheld Multispecies ERG (HMsERG) Model 1000 (RetVetCorp, Inc., Columbia, MO) with mini-Ganzfeld bowl. Artificial tears (Thilotears® ophthalmic gel, Falcon) were administered on both eyes during and immediately after the procedure to prevent drying of the cornea. The ERG QuickRetCheck protocol was initiated after a 20-minute dark adaptation to evaluate the function of both rod and cone photoreceptors. Three flash intensities were used: the rod photoreceptors were recorded in response to a low light intensity stimulus (four averaged flashes of 10 mcd s/m2) and a mixed rod-cone response was evaluated by a high light intensity stimulus (one single flash of 3,000 and 10,000 mcd s/m2). Once the protocol OD was finished, the electrodes were set around OS, in a maintained scoptopic condition. The results were comparable for both horses and all ERG responses were attenuated for both OS (Table 1, Fig. 3). US and magnetic resonance imaging (MRI)Ultrasonography was performed under sedation with detomidine (10 µg/kg IV), after application of tetracaine 1% on both eyes. A standard transpalpebral approach of the globe and retrobulbar area was performed using a linear transducer set at 8 MHz.
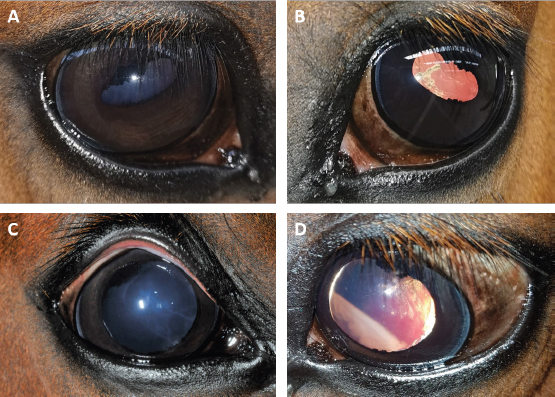
Fig. 2. Case 2 with a permanent globe deviation and scleral show, before (A, B) and after pupil dilatation (C, D). Right eye with lens equator visible at 8 o’clock and vitreous floaters evident after pupil dilatation (C). Left eye with a posterior cortical cataract. The lens equator is visible in the nasal quadrant and the detached retina is evident nasally (D). Table 1. Results of bilateral electroretinographic recordings in both horses obtained at the three flash intensities used (10, 300 and 10,000 mcd s/m2) from the right and the left eye of case 1 and 2.
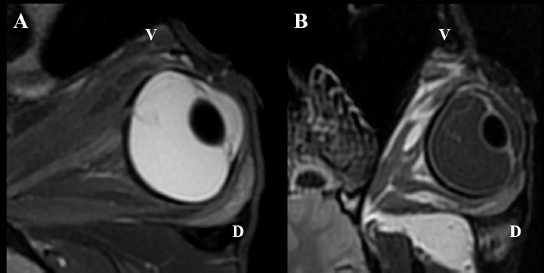
Following euthanasia, MRI of the heads was performed, using a high field (1.5T) system (GE, Signa Explorer, GE Healthcare, Wauwatosa, WI). T1, fluid-attenuated inversion recovery (FLAIR), and T2 sequences in the sagittal, frontal and transverse planes were acquired. T2 sequences were also acquired in an oblique plane, in the axes of the ONH of each eye. Intraocular lesions found on US and MRI of both horses were consistent with the ophthalmic examination. Complete RD OS and hyperechoic vitreous floaters OU were noted on US (Fig. 4). RD was also evident OS on MRI, more conspicuously on T2W and FLAIR sequences. In T2 sequences, the detached retina was identified as a V-shaped membrane of intermediate signal inside the hyperintense vitreous and reaching the optic disk (Fig. 5A), while it was slightly hyperintense in comparison to the hypointense vitreous chamber on FLAIR acquisitions (Fig. 5B). There was a subjectively abnormal positioned optic disk OU on US and MRI, suggesting ectopic ONH. OS was flattened at the level of the ONH in both horses. Except for a subarachnoid emphysema due to postmortem changes, the brain looked otherwise normal on MRI. Based on US, the axial globe length measured 39.2 mm OS and 41.5 mm OD of case 1 and 45 mm OU in case 2.
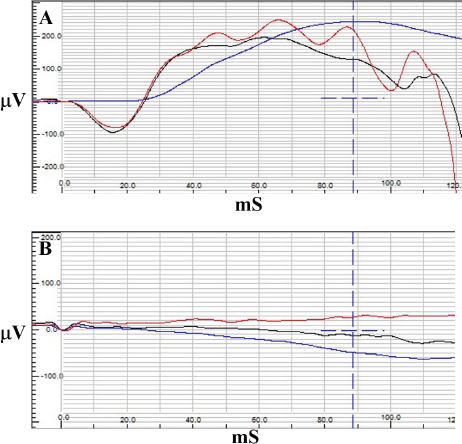
Fig. 3. Electroretinography results of case 1 with normal traces of rod (blue) and combined rod-cone response (red and black) OD (A) and a flat tracing OS (B).
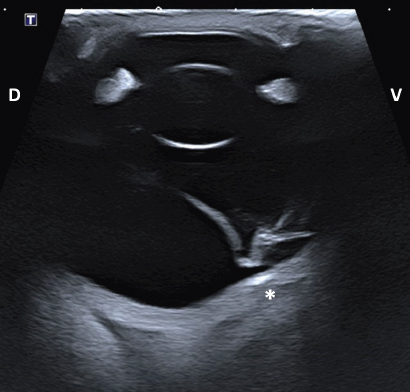
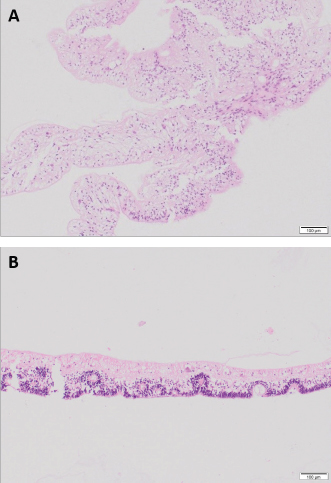
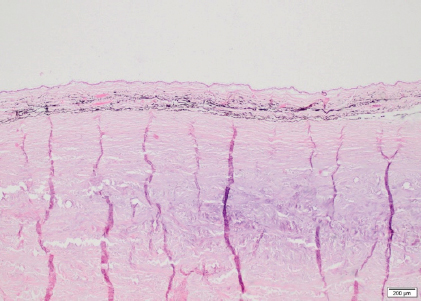
Fig. 4. Transpalpebral ultrasonographic image OS of case 1 in a vertical longitudinal axis. A “seagull sign” is visible in the vitreous, consistent with RD. * Note the depression at the caudoventral aspect of the globe, in the area of the optic disk (not visible on this isolated image). D: dorsal; V: ventral. HistopathologyThe enucleated globes were fixed in 10% neutral buffered formalin. They were routinely processed for histopathology and stained with hematoxylin and eosin. On the cut surface, the consistency of the vitreous was markedly decreased OU and the retina completely detached OS in both horses. The axial globe lengths measured 4 cm OD and 4.2 cm OS for case 1 and 4.5 cm OU for case 2. Case 1Both eyes showed no significant microscopic abnormalities of the anterior segment. All the retinal layers were preserved OD, including abundant ganglion cells. The retina OS was detached, with rounded edges at the periphery, consistent with a retinal tear. The central area of the retina OS was mainly composed of glial tissue and showed marked loss of all its layers (Fig. 6A). The peripheral retina OS preserved all its layers more frequently, but with occasional rosettes (Fig. 6B). Infrequent rosettes were also noted in the retina OS adjacent to the ONH. There was moderate gliosis of the ONH OS. Small mineral deposits were present peripapillary within the outer layers of the choroid OD. The posterior sclera immediately adjacent to the ONH showed chondroid metaplasia of the inner and mid layers OS (Fig. 7).
Fig. 5. MRI OS, case 2. T2W oblique plane image highlighting the hypointense-detached retina reaching the optic disk and in direct continuity with the optic nerve. Note the hyperintense periphery of the optic nerve, corresponding to the normal thin layer of cerebrospinal fluid surrounding it (A). FLAIR frontal plane image showing the difference of signal between the slightly hyperintense retina within the hypointense vitreous chamber (B). D: dorsal; V: ventral. Case 2The posterior lens capsule OS was lined with epithelial cells consistent with migration and few morgagnian globules were visible within the posterior cortex adjacent to this area. The remaining anterior segment was otherwise unremarkable. All the retinal layers were preserved OD, whereas the retina was completely detached OS with loss of its normal layering in the peripapillary region. The peripheral retina OS preserved some of the layers although ganglion cells were sparse and there were segmental areas of fusion of the inner and outer nuclear layers. At the superior aspect of the ora ciliaris retinae OS there was a small remnant of peripheral retina that showed rounded edges consistent with a tear. The vitreous showed mild deposit of fibrillar eosinophilic material within its anterior portions OD and surrounding detached retina OS. The optic nerve OD was within normal limits whereas the intraocular portions of the nerve OS were atrophied. The sclera OS presented chondroid metaplasia of the scleral collagen immediately adjacent to the optic nerve. DiscussionA complete RD OS and vitreous degeneration OU were identified in these two closely related horses. The ERG results were comparable for both horses and were within normal limits OD compared to previously described values (Ben-Shlomo et al., 2012; Church and Norman, 2012). The ERG response OS was absent, shown as a flat ERG tracing, believed to be a consequence of the depletion of chromophores in the photoreceptor outer segment following RD (Hoffman et al., 2018). Axial globe lengths measured on US corresponded to measurements taken on histopathology. The axial globe lengths of case 1 were considered within normal limits OU when compared to published values (40.52 ± 2.67 mm) (Grinninger et al., 2010; Don, 2013). However, the axial globe lengths of case 2 were slightly increased OU while intraocular pressures were within normal limits (Miller et al., 1991; Michau, 2017). Furthermore, no signs consistent with glaucoma were detected on ophthalmic and histopathological examination, and thus the globe sizes of case 2 were considered as an anatomical variation. The continuously exposed sclera in case 2 was most likely the consequence of the globe size. In our patients’ eyes, histopathological examination revealed changes, more obvious OS than OD in both horses, and more severe in case 2 compared to case 1. The most pronounced abnormality was the detached retina OS associated with a retinal tear. The central detached retina did not show obvious neuronal components and was composed basically of glial tissue. The peripheral detached retina still contained some of the neuronal layers but also occasional small rosettes, although very sparse and embedded within the glial areas. These features are more consistent with retinal dysplasia with extensive areas of malformation or lack of development of the retinal layers rather than atrophy. These areas with absent layering are therefore probably part of the same congenital abnormalities. This is likely secondary to the retinal lesions previously described, or part of a more generalized vitreoretinopathy, in which an abnormal relationship between the retina and the vitreous may facilitate the detachment and tear. There was cartilaginous metaplasia of the central posterior sclera OS. This has been noted anecdotally as a degenerative change in aged horses but its significance in a young animal is difficult to determine. It has been described in Suffolk sheep but its clinical significance remains unknown (Smith et al., 2011). The presence of an incipient cataract OS was confirmed in case 2. No obvious features of a congenital cataract were noted, including duplication, wrinkling or irregular and frayed capsule, lysis of the nucleus, or vascular structures adhered to the lens capsule (Dubielzig et al., 2010). Therefore, it was interpreted as an acquired cataract, secondary to the RD.
Fig. 6. Detached retina OS, case 1. Central area of the retina mainly composed of glial tissue and marked loss of all its layers (A). Peripheral retina displays all its layers but marked disorganization is noted, with some rosettes formed by the outer nuclear layer extending into the inner nuclear layer consistent with retinal dysplasia (B) (H&E coloration, 100 µm).
Fig. 7. Sclera OS, case 1. Cartilaginous metaplasia in the inner and mid layers of the posterior sclera immediately adjacent to the optic nerve (H&E coloration, 200 µm). There is a paucity of reports on RD in horses published in the literature. RD can be associated with developmental or congenital defects or can be due to acquired infectious or non-infectious causes. In a retrospective study of 40 horses (46 eyes) with RD, an etiological diagnosis consistent with ERU and trauma was made in 67.5% and 25% of the cases, respectively. In the remaining 7.5% of the cases, the cause for RD included congenital cataracts, optic neuritis, or congenital ocular abnormalities (Strobel et al., 2007). In the two horses in this case report, there were no signs of active or chronic intraocular inflammation, excluding ERU and trauma as possible causes. Congenital RD is rarely reported in horses and is mostly associated with MCOA (Andersson et al., 2011). MCOA syndrome is an inherited condition that is strongly related to coat color, called Silver coat. It is associated with a dominant missense mutation in PMEL17 gene and causes a dilution of black and brown pigment into gray or white. These two horses are bay colored without a coat color dilution. Besides the RD, both horses did not show any other abnormalities consistent with MCOA (Ramsey et al., 1999; Grahn et al., 2008; Andersson et al., 2011; Ségard et al., 2013) and thus MCOA was excluded as a cause of RD. In normal eyes, both the vitreous body and RPE are involved in maintaining a normal retinal attachment. The vitreous body exerts a gentle and widely distributed force on the inner retina while the RPE actively maintains an osmotic gradient that keeps the NSR in apposition with the RPE. Other factors that are believed to maintain a normal retinal attachment are the tight junctions of the outer limiting membrane and RPE (Dubielzig et al., 2010). If the vitreous and/or RPE are malfunctioning, the NSR is not properly supported and can more easily separate from the RPE. The term “synaeresis” is used to indicate a degenerative breakdown of the vitreous gel, resulting in liquefaction and development of fluid-filled cavities within the vitreous. Liquefaction and opacities (vitreous “floaters”) of the vitreous are signs of vitreal degeneration, which may occur after inflammation or due to age or unknown causes (Papaioannou and Dubielzig, 2013). In humans, different types of inherited congenital abnormalities of the vitreous and retina (vitreoretinopathy) are described: Stickler syndrome types 1 and 2, autosomal dominant vitreoretinochoroidopathy, Wagner syndrome, and snowflake vitreoretinal degeneration. They are all characterized by the presence of severe degeneration or maldevelopment of the vitreous, early onset progressive cataract, and an increased predisposition to (RRD) (Edwards, 2008). RRD is characterized by a retinal tear and migration of fluid from the vitreous into the subretinal space. This can be primary or secondary. Primary RRD occur spontaneously and are preceded by degeneration or changes of the vitreous which predisposes to RD. They are not the result of inflammation, trauma, surgery, or other eye disorder(s) (Vainisi et al., 2013). Certain canine breeds (e.g., Brussels Griffon, Chihuahua, Chinese Crested, Havanese, Italian Greyhound, Shih Tzu, Whippet) are predisposed to RRD due to a primary breakdown of the vitreous hydrogel. Further research is needed in order to determine a hereditary etiology (Boeve and Stades, 2013; Papaioannou and Dubielzig, 2013). In the Shih Tzu, primary RRD is presumed to be preceded by alteration of the vitreous and is usually presented in middle-aged dogs, rather than young dogs. The vitreous is usually liquefied but condensed at the inner retinal surface (Dubielzig et al., 2010). They develop giant retinal tear likely induced by intense head shaking. An eosinophilic matrix material in the vitreous is seen in ocular vitreoretinopathy of the Shih Tzu (Papaioannou and Dubielzig, 2013). Based on our ophthalmic and ultrasonographic examination, we suspected a congenital vitreoretinopathy in the two horses due to the presence of bilateral liquid vitreous body with secondary unilateral RRD and giant retinal tear. Other causes of vitreal liquefaction were ruled out including chronic intraocular inflammation, aging of the vitreous, lens luxation, cataract, glaucoma, or trauma. Shih Tzu with RRD have been reported to have dense fibrillary eosinophilic material in the vitreous. In our horses, the little vitreous present on the histopathological slides showed mild deposit of fibrillar eosinophilic material that was less dense compared to Shih Tzus. In contrast to our horses, the retina of the Shih Tzu does not display the rosettes and severe areas of gliosis seen on histopathological examination in our horses. To our knowledge, vitreoretinopathy has not yet been described in horses. Due to the congenital posterior segment abnormalities seen in our horses comparable to the vitreoretinopathy described in humans and Shih Tzu dogs, we suspect that RD can be the consequence of a primary vitreoretinopathy. Because of the clinical and histopathological similarities between these two closely related horses, a hereditary etiology was highly suspected. Conflict of interestThe authors declare that there is no conflict of interest Authors’ contributionsEline Vercruysse performed all the examinations/tests, drafted the manuscript, and revised the manuscript. Aurélie Sauvage performed the ERG. Maxime Vandersmissen performed the medical imaging. Carolina Naranjo interpreted the histopathology, generated relevant figures, helped to draft the manuscript, and provided critical review. Magda Grauwels helped to draft, revise the manuscript, and provided critical review. Sébastien Monclin was the clinician responsible for the entire supervision. ReferencesAllbaugh, R.A., Townsend, W.M. and Wilkie, D.A. 2017. Disease of the equine vitreous and retina. In Equine ophthalmology, 3rd ed. Ed., Gilger, B.C. Ames, IA: Wiley Blackwell, pp: 469–507. Andersson, L.S., Axelsson, J., Dubielzig, R.R., Lindgren, G. and Ekesten, B. 2011. Multiple congenital ocular anomalies in Iceland horses. BMC Vet. Res. 7, 21. Ben-Shlomo, G., Plummer, C., Barrie, K. and Brooks, D. 2012. Characterization of the normal dark adaptation curve of the horse. Vet. Ophthalmol. 15, 42–45. Boeve, M.H. and Stades, F.C. 2013. Diseases and surgery of the canine vitreous. In Veterinary ophthalmology, 5th ed. Eds., Gelatt, K.N., Gilger, B.C. and Kern, T.J. Ames, IA: Wiley Blackwell, pp: 1287–1302. Church, M.L. and Norman, J.C. 2012. Electroretinogram responses of the normal thoroughbred horse sedated with detomidine hydrochloride. Vet. Ophthalmol. 15, 77–83. Cook, C.S. 2013. Ocular embryology and congenital malformations. In Veterinary ophthalmology, 5th ed. Eds., Gelatt, K.N., Gilger, B.C. and Kern, T.J. Ames, IA: Wiley Blackwell, pp: 3–38. Cutler, T.J., Brooks, D.E., Andrew, S.E., Denis, H.M., Biros, D.J., Gelatt, K.N., Komaromy, A.M. and Kallberg, M. 2000. Disease of the equine posterior segment. Vet. Ophthalmol. 3, 73–82. Don, A.S. 2013. Ophthalmic Anatomy. In Veterinary ophthalmology, 5th ed. Eds., Gelatt, K.N., Gilger, B.C. and Kern, T.J. Ames, IA: Wiley Blackwell, pp: 61. Dubielzig, R.R., Ketring, K.L., McLellan, G.J. and Albert, D.M. 2010. The retina. In Veterinary ocular pathology: a comparative review, 1st ed. Eds., Dubielzig, R.R., Ketring, K.L., McLellan, G.J. and Albert, D.M. Philadelphia, PA: Saunders Elsevier, pp: 349–398. Edwards, A.O. 2008. Clinical features of the congenital vitreoretinopathy. Eye 22, 1233–1242. Grahn, B.H., Pinard, C., Archer, S., Bellone, R., Forsyth, G. and Sandmeyer, L.S. 2008. Congenital ocular anomalies in purebred and crossbred Rocky and Kentucky Mountain horses in Canada. Can. Vet. J. 49, 675–681. Grinninger, P., Skalicky, M. and Nell, B. 2010. Evaluation of healthy equine eyes by use of retinoscopy, keratometry, and ultrasonographic biometry. Am. J. Vet. Res. 71, 677–681. Hoffman, A., Sisler, S., Pappania, M., Hsu, K., Ross, M. and Ofri, R. 2018. Electroretinography is a prognostic indicator for postoperative vision in dogs undergoing retinal reattachment surgery. Vet. Ophthalmol. 21(3), 273–280. Matz-Rensing, K. 1996. Retinal detachment in horses. Equine. Vet. J. 28, 111–116. Michau, T.M. 2017. Equine glaucoma. Vet. Clin. North Am. Equine. Pract. 33(3), 519–540. Miller, P.E., Pickett, J.P. and Majors, L.J. 1991. Evaluation of two applanation tonometers in horses. Am. J. Vet. Res. 51(6), 935–937. Nell, B. and Walde, I. 2010. Posterior segment diseases. Equine. Vet. J. 42(37S), 69–79. Papaioannou, N.G. and Dubielzig, R.R. 2013. Histopathological and immunohistochemical features of vitreoretinopathy in Shih Tzu Dogs. J. Comp. Path. 148, 230–235. Ramsey, D.T., Ewart, S.L., Render, J.A., Cook, C.S. and Latimer, C.A. 1999. Congenital ocular abnormalities of Rocky Mountain Horses. Vet. Ophthalmol. 2, 47–59. Ségard, E.M., Depecker, M.C., Lang, J., Gemperli, A. and Cadoré, J.L. 2013. Ultrasonographic features of PMEL17 (Silver) mutant gene-associated multiple congenital ocular anomalies (MCOA) in Comtois and Rocky Mountain horses. Vet. Ophthalmol. 16(6), 429–435. Smith, J.D., Hamir, A.N. and Greenlee, J.J. 2011. Cartilaginous metaplasia in the sclera of Suffolk sheep. Vet. Pathol. 48(4), 827–829. Strobel, B.W., Wilkie, D.A. and Gilger, B.C. 2007. Retinal detachment in horses: 40 cases (1998–2005). Vet. Ophthalmol. 10, 380–385. Vainisi, S.J., Wolfer, J.C. and Hoffman, A.R. 2013. Surgery of the canine posterior segment. In Veterinary ophthalmology, 5th ed. Eds., Gelatt, K.N., Gilger, B.C. and Kern, T.J. Ames, IA: Wiley Blackwell, pp: 3–38. Wilkie, D.A. 2005. Retinal dysplasia. Unpublished data cited by Gilger, B.C. In Equine ophthalmology, 1st ed. Ed., Gilger, B.C. St. Louis, MO: Elsevier Inc., pp: 350. | ||
| How to Cite this Article |
| Pubmed Style Vercruysse E, Sauvage A, Vandersmissen M, Naranjo C, Grauwels M, Monclin S. Retinal detachment secondary to vitreoretinopathy in two closely related warmblood horses. Open Vet J. 2021; 11(4): 672-679. doi:10.5455/OVJ.2021.v11.i4.18 Web Style Vercruysse E, Sauvage A, Vandersmissen M, Naranjo C, Grauwels M, Monclin S. Retinal detachment secondary to vitreoretinopathy in two closely related warmblood horses. https://www.openveterinaryjournal.com/?mno=62445 [Access: October 06, 2024]. doi:10.5455/OVJ.2021.v11.i4.18 AMA (American Medical Association) Style Vercruysse E, Sauvage A, Vandersmissen M, Naranjo C, Grauwels M, Monclin S. Retinal detachment secondary to vitreoretinopathy in two closely related warmblood horses. Open Vet J. 2021; 11(4): 672-679. doi:10.5455/OVJ.2021.v11.i4.18 Vancouver/ICMJE Style Vercruysse E, Sauvage A, Vandersmissen M, Naranjo C, Grauwels M, Monclin S. Retinal detachment secondary to vitreoretinopathy in two closely related warmblood horses. Open Vet J. (2021), [cited October 06, 2024]; 11(4): 672-679. doi:10.5455/OVJ.2021.v11.i4.18 Harvard Style Vercruysse, E., Sauvage, . A., Vandersmissen, . M., Naranjo, . C., Grauwels, . M. & Monclin, . S. (2021) Retinal detachment secondary to vitreoretinopathy in two closely related warmblood horses. Open Vet J, 11 (4), 672-679. doi:10.5455/OVJ.2021.v11.i4.18 Turabian Style Vercruysse, Eline, Aurlie Sauvage, Maxime Vandersmissen, Carolina Naranjo, Magda Grauwels, and Sbastien Monclin. 2021. Retinal detachment secondary to vitreoretinopathy in two closely related warmblood horses. Open Veterinary Journal, 11 (4), 672-679. doi:10.5455/OVJ.2021.v11.i4.18 Chicago Style Vercruysse, Eline, Aurlie Sauvage, Maxime Vandersmissen, Carolina Naranjo, Magda Grauwels, and Sbastien Monclin. "Retinal detachment secondary to vitreoretinopathy in two closely related warmblood horses." Open Veterinary Journal 11 (2021), 672-679. doi:10.5455/OVJ.2021.v11.i4.18 MLA (The Modern Language Association) Style Vercruysse, Eline, Aurlie Sauvage, Maxime Vandersmissen, Carolina Naranjo, Magda Grauwels, and Sbastien Monclin. "Retinal detachment secondary to vitreoretinopathy in two closely related warmblood horses." Open Veterinary Journal 11.4 (2021), 672-679. Print. doi:10.5455/OVJ.2021.v11.i4.18 APA (American Psychological Association) Style Vercruysse, E., Sauvage, . A., Vandersmissen, . M., Naranjo, . C., Grauwels, . M. & Monclin, . S. (2021) Retinal detachment secondary to vitreoretinopathy in two closely related warmblood horses. Open Veterinary Journal, 11 (4), 672-679. doi:10.5455/OVJ.2021.v11.i4.18 |