| Original Article | ||
Open Vet J. 2021; 11(2): 330-336 Open Veterinary Journal, (2021), Vol. 11(2): 330–336 Original Research Morphology and histology of paryphasmata and hemibaculum of Varanus salvator based on sexual maturityAlif Yahya Al-ma’ruf1, Regita Permata Sari1, Imam Mustofa2*, Suzanita Utama2, Chairul Anwar3, Maslichah Mafruchati3, Eka Pramyrtha Hestianah3, Lita Rakhma Yustinasari3, Benjamin Christoffel Tehupuring3, Djoko Legowo4 and Boedi Setiawan51Pet and Wild Animals Interest Group, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 2Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 3Division of Veterinary Anatomy, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 4Division of Veterinary Pathology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 5Division of Veterinary Clinic, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia *Corresponding Author: Imam Mustofa. Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: imam.mustofa [at] fkh.unair.ac.id Submitted: 10/03/2021 Accepted: 06/06/2021 Published: 30/06/2021 © 2021 Open Veterinary Journal
AbstractBackground: Varanus salvator is one of the reptiles being hunted by human beings for several purposes, including traditional medicine. The studies about reproductive biology aspects were limited. Aim: This study aimed to determine the morphology, histology, and histometry of V. salvator paryphasmata and hemibaculum based on Snout-Vent Length (SVL) as an indicator of sexual maturity. Methods: This study examined 18 pairs of hemipenis of V. salvator with SVL more and less than 40 cm in equal number. Paryphasmata and hemibaculum parts were observed visually and micro-sliced, then stained with Hematoxylin-Eosin (HE). The histological observation was conducted under a 40×, 100×, and 400× magnification of a light microscope. The histometry of the paryphasmata was examined using 13 Megapixels Coolpad and OptiLab Plus for microscopic pictures. The chondrocyte cell area was measured using the Optilab Plus and Image Raster three applications. Results: The sizes of glans of hemipenis, paryphasmata, and hemibaculum increased according to the increasing of SVL. The average paryphasmata row number, epidermis, and loose connective tissue thickness were not significantly different (p > 0.05). However, dense connective tissue was thicker (p < 0.05), which corresponds to SVL. Hemibaculum was composed of fibrous and hyaline cartilage characterized by chondrocyte cells. The SVL also affects (p < 0.05) the ossification of hyaline in hemipenis, while the chondrocyte cell area followed the equation −1.87E7 + 7.09E5* SVL. Conclusion: The SVL size of V. salvator affects the paryphasmata, hemibaculum, thickness of dense connective tissue of paryphasmata, and the area of chondrocyte cells. Keywords: Chondrocytes, Glans hemipenis, Sexual maturity, Snout-Vent Length. IntroductionVaranus salvator, commonly known as water monitor, can be found from South Asia to Southeast Asia. This species is the most widespread among all the Varanidae. It is one of the reptiles that is being hunted by human beings for various purposes (Bhattacharya and Koch, 2018). The baby lizards for pets, their skin as a raw material of crafting, and meat for consumption. The other body parts of this lizard are traditionally believed to be an antidote to some disease (Dadang, 2012). Lizard oil for skin diseases (Uyeda et al., 2014), the meat can maintain stamina, treat asthma, and even increase male vitality (Nijman, 2016). Samples subjected to liquid chromatography-mass spectrometry were detected 123 potential anticancer peptides identified from V. salvator (Jeyamogan et al., 2020). Although V. salvator is considered to have a low-risk extinction [(CITES (Convention on International Trade in Endangered Species) of Wild Fauna and Flora, 2013], it is crucial to prevent excessive exploitation. So far, this species’ study was still limited to ecological research, body morphology, and commercial harvesting. However, studies about reproductive biology aspects, especially in male ones, were less reported. It is also known that the male V. salvator has a reproductive organ called hemipenis, which is developed along with the development of the body. Hemipenis was measured using Snout-Vent Length (SVL), i.e., length from the snout (head tip) to the cloaca. The hemipenis is undeveloped if the SVL ≤ 40 cm (sexually immature), while the size of SVL > 40 cm (sexually mature) will have a more developed and apparent perfect form of hemipenis. There was a cartilaginous formation at the hemipenis tip like a cone-shaped (Kusuma et al., 2017) called hemibaculum (Vitt and Caldwell, 2014).Although morphologies and sizes of the hemipenis in several species of varanids have been known (Rowe and Arnqvist, 2011), i.e., there was fibrous cartilage as an internal skeleton of hemipenis. Several characteristics remain unknown (Hosken et al., 2018). Therefore, histological studies of the paryphasmata and bony structures in the hemipenis tissue will add the basic knowledge. This study was conducted to comprehensively describe the morphology, histology, and histometry of paryphasmata and hemibaculum of water monitor (V. salvator) of different size of SVL. Materials and MethodsAnimalsNine hemipenes of V. salvator with SVL ≤ 40 cm and nine of those with SVL > 40 cm representing both sexual maturity status. Samples were collected from a water monitor slaughtering place in Gang Nyambek, Pagerwojo, Buduran, Sidoarjo, Indonesia. Procedures of studyThe SVL measurement of the V. salvator, i.e., length of the straight line from the straight ventral side from the snout to the cloaca, is done using a tape meter. Hemipenis samples were obtained from dissecting cloaca using a sharp knife and stored samples in plastic pots prefilled with 10% formaldehyde. Paryphasmata and hemibaculum parts were observed visually, and micro-sliced with the paraffin method on the transversal cut at the lateral side, then stained with HE. The evaluation of the stained micro-slides was conducted under a 40×, 100×, and 400× magnification microscope for the paryphasmata and hemibaculum. The histometry of the paryphasmata was examined using 13 Megapixels Coolpad and OptiLab Plus for microscopic pictures, while Wacom Intuos Manga CTH-480S software for drawing the illustration of hemipenis morphology. The chondrocyte cell area was measured using the Optilab Plus and Image Raster 3 applications. Data analysisThe morphology, histological data of paryphasmata, and hemibaculum of hemipenis are descriptively presented. Meanwhile, parameters of the thickness of the paryphasmata histological structure and area of chondrocyte cells were conducted randomly on five microscopic views and averaged; All the parameters were analyzed for the normality using the Kolmogorov–Smirnov test, then statistically analyzed using Student t-test. The SVL and chondrocyte cell area analyzed by the Pearson’s correlation, followed by the simple linear regression to estimate the chondrocyte cell area based on SVL. All statistical analysis was conducted to a 95% significance level using Statistical Product and Service Solutions Version 23. Ethical approvalThis study’s proposal was approved by the Animal Care and Use Committee of Universitas Airlangga no. 525/HRECCFODM/VII/2019. ResultsThe ventral part of the tail could distinguish the male V. salvator from the females. The male ones have a pair of hemipenes located at the ventral part of the tail base, bulging from under the skin. There was a long tendon on the caudal part of each hemipenis that function to push to make the hemipenis everted through the cloaca (Fig. 1). Morphologically hemipenis consists of radix hemipenis (the base of the penis that was located near the retractor muscle), corpus hemipenis (body section of the penis), and glans hemipenis (the tip of the penis that consist of paryphasmata and hemibaculum). Homogeneity examination using a Kolmogorov–Smirnov test of all the parameters showed that the data were normally distributed (p > 0.05). Morphology of hemipenisThe V. salvator has two same-sized hemipenes located in the caudal part of the cloaca at the tail’s inner side. There was a flower-like jagged structure on the base of glans of the hemipenis known as paryphasmata and two different sized hemipenis bones on the tip of each hemipenis that was known as hemibaculum. The paryphasmata started being visible on the nearly mature V. salvator, but it was fully developed on the mature ones. The stomach side of the paryphasmata is divided by a sulcus spermaticus until it approaches the smaller hemibaculum. The development of hemipenis included glans hemipenis, wherein the paryphasmata and hemibaculum exist, was follow the size of SVL, as seen in Figure 2: (5)–(8). When the SVL was ≤ 40 cm, the hemipenis has not fully developed. The paryphasmata and hemibaculum on the hemipenis glans were also still very small. Therefore, it was difficult to dissect the hemipenis in a shorter SVL. In V. salvator with SVL 40 cm, the hemipenes began to develop, in conjunction with the paryphasmata and hemibaculum that began to show a definite shape. Meanwhile, the animals with SVL > 40 cm hemipenis had developed entirely and grown more significantly. The paryphasmata showed a definite form, and the hemibaculum was visible and protruding as the tip of the hemipenes. Sometimes, the number of rows of the paryphasmata was not equal between left and right hemipenis, but it was not significantly different (p > 0.05).
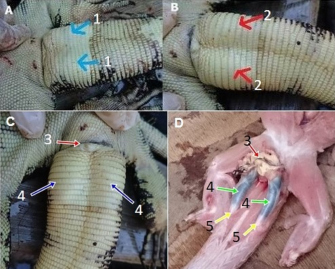
Fig. 1. The difference between male and female V. salvator based on the form of the ventral tail near the cloaca (A=female, B=male, 1=female no bulge, 2=male, there are bulges of hemipenis). Covered hemipenis (C): 3=cloaca, 4=hemipenis inside the tail’s base part. uncovered hemipenis (D): 3=cloaca, 4=hemipenis; 5=tendon.
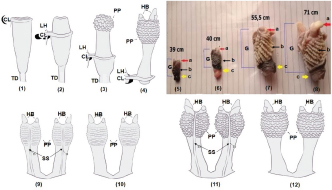
Fig. 2. Illustration of hemipenis eversion of mature V. salvator (1–4). CL: cloaca; HB=hemibaculum; LH=left hemipenis; PP=paryphasmata; TD=tendon. The macroscopic view of V. salvator hemipenis at each SVL: (5), (6), (7), and (8) were 39, 40, 55.5, 71 cm, respectively. a=hemibaculum, b=paryphasmata, c=corpus hemipenis, G=glans hemipenis. Illustration of hemipenis of immature (9–10) and mature (11–12) V. salvator. HB=hemibaculum; PP=paryphasmata; SS=sulcus spermaticus; 9 and 11=ventral side; 10 and 12=dorsal side. Histology of paryphasmataThe paryphasmata surface of V. salvator with SVL ≤ 40 cm already had a winding shape (Fig. 2) but was not thoroughly visible compared to SVL > 40 cm. The epidermis layer had more basophilic color than the dermis, consisting of stratified squamous epithelium with many melanocytes. However, there was no stratum corneum found. Under the epidermis layer, there was a layer of loose connective tissue consisted of many capillary blood vessels. The loose connective tissue in the paryphasmata of V. salvator with SVL > 40 cm was thicker, and it contained more blood vessels than those of SVL ≤ 40 cm. The dense connective tissue layer consisted of thick irregular bundles of collagen fibers running in various directions, but no gland was found. Some parts of the dense connective tissue on hemipenis of V. salvator with SVL > 40 cm had more solid structures with slightly heterogenic colored collagen fibers (Fig. 3). Histology of hemibaculumThe hemibaculum was composed of dense connective and cartilage tissues. The cartilage was a mixture of hyaline and fibrous cartilage. The hyaline cartilage was characterized by the presence of chondrocyte cells inside the lacunae. Moreover, there were tendons of collagen fibers spread evenly contained chondrocyte cells; hence, they were easily identified as fibrous cartilage. Hyaline cartilage was mostly located in the apical region leading to the medial part of the hemibaculum; it flanked fibrous cartilage and dense connective tissue with collagen fibers. Dense irregular connective tissue and skeletal muscle as a retractor muscle of the hemipenis were found at the distal part of the hemibaculum. Fibrous cartilage was scattered randomly. It could be in the middle of hyaline cartilage or attached to dense connective tissue, while hyaline cartilage was located at the apical and medial part of the hemibaculum (Fig. 4).
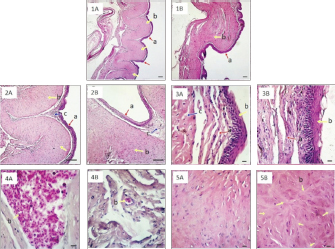
Fig. 3. Histology of paryphasmata of V. salvator with HE staining. Observation under 40× magnification (1A: SVL ≤ 40 cm; 1B: SVL > 40 cm): a=epidermis; b=dense connective tissue; bar: 100 μm. Observation under 100× magnification (2A: SVL ≤ 40 cm; 2B: SVL > 40 cm): a=epidermis; b=dense connective tissue; c=loose connective tissue; bar: 100 μm; Observation under 400× magnification (3A: SVL ≤ 40 cm; 3B: SVL > 40 cm): a=epidermis; b=loose connective tissue becomes thicker on SVL > 40 cm; c=dense connective tissue; bar: 10 μm. Loose connective tissue under 400× magnification (4A: SVL ≤ 40 cm; 4B: SVL > 40 cm): b=erythrocyte; bar: 10 μm. Dense connective tissue under 400× magnification (5A: SVL ≤ 40 cm; 5B: SVL > 40 cm): b=collagen fibers; bar: 10 μm. The development of glans hemipenis followed the development of V. salvator SVL, wherein there was a hemibaculum. Histologically, the hemibaculum of V. salvator with SVL ≤ 40 cm had fields of microscopic view 17–32, while SVL > 40 had 31–56. In the hemibaculum of shorter SVL, ossification of hyaline cartilage was not found; in contrast, hardened cartilage was found in longer SVL. At the apical part of the hemibaculum of V. salvator with SVL 35 cm (Fig. 4. section 2A), loose connective tissue wraps the blood vessels and skeletal muscle, there was no cartilage seen yet. Figure 4 section 2B showed that histology of hemibaculum SVL 68 cm showed perichondrium, which contained chondroblasts cells inside, hyaline cartilage contained chondrocyte cells, collagen also filled with chondrocyte cells, and it could be considered as fibrous cartilage. The hemibaculum was covered with stratified squamous epithelium with loose connective tissue and blood vessels under the epithelium, which lines hyaline cartilage and fibrous cartilage. Under the blood vessels, the dense irregular connective tissue was thick and looks very dense, and also, there was much hyaline cartilage almost the whole of the microscopic view.
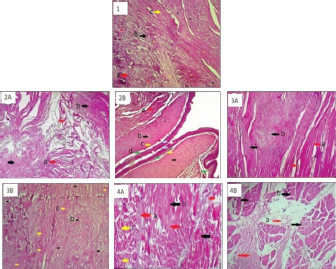
Fig. 4. Histology of V. salvator hemibaculum with HE staining (1). a=collagen; b=hyaline cartilage; c=fibrous cartilage. Apical part of hemibaculum SVL ≤ 40 cm (2A): a=skeletal muscle; b=loose connective tissue, and SVL >40 cm (2B): a=fibrous cartilage, b=hyaline cartilage, c=perichondrium, d=blood vessel. Medial part of hemibaculum SVL ≤ 40 cm (3A): a=fibrous cartilage, b=hyaline cartilage, and SVL > 40 cm (3B): b=hyaline cartilage, c=fibrous cartilage. Distal part of hemibaculum SVL ≤ 40 cm (4A): a=hyaline cartilage, b=collagen tissue, c=skeletal muscle, and SVL > 40 cm (4B): a=dense irregular connective tissue, b=skeletal muscle. Magnification 100×. The medial part of the hemibaculum (Fig. 4, section 3A) of the V. salvator with SVL 35 cm showed fibrous cartilage and hyaline cartilage. Meanwhile, the hemibaculum of V. salvator with SVL 68 cm (Fig. 4 section 3B) showed the dominance of hyaline cartilage over fibrous cartilage. The medial part of the hemibaculum was composed of dense connective tissue that flanked hyaline cartilage and fibrous cartilage. The collagen tissue looked thicker compared to the cartilage tissue, and the cartilage was dominated by hyaline. The distal part of the hemibaculum of V. salvator with SVL 35 cm (Fig. 4 section 4A) showed hyaline cartilage, collagen tissue, and skeletal muscles, while on hemibaculum of V. salvator with SVL 68 cm (Fig. 4 section 4B) showed that hyaline cartilage was dominating compared to fibrous cartilage. The dense connective tissue flanks hyaline cartilage and fibrous cartilage. The collagen tissue looks thicker than cartilage tissue, and the medial part of the hemibaculum was dominated by hyaline cartilage. There were alternately dark and bright lines seen under a microscope with a magnification of 400×.
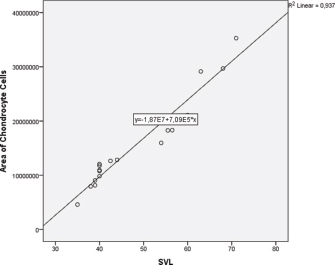
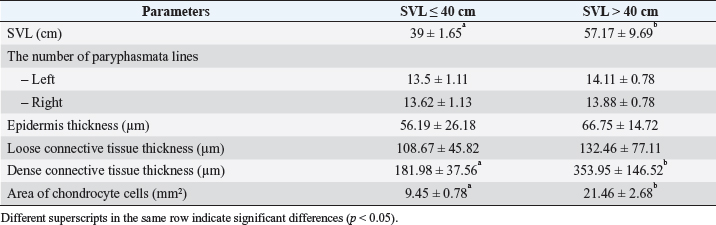
Fig. 5. Linear regression of chondrocyte cells area based on SVL of V. salvator. There was no significant difference (p=0.47) between the average number of paryphasmata rows left and right, the thickness of the epidermis, and loose connective tissue; whereas, the dense connective tissue was thicker (p =0.002) correspond to longer SVL (Table 1). The SVL linearly affects the chondrocyte cells’ area ( p =0.00016) following the equation: the area of chondrocyte cells=−1.87E7 + 7.09E5*SVL (Fig. 5). The determination coefficient of this linear regression was 93.7%, which means that the chondrocyte cell change area was 93.7% caused by SVL change. Table 1. The histological metric of paryphasmata and area of chondrocyte cells of hemibaculum of V. salvator based on SVL.
DiscussionMale V. salvator are sexually mature when they are at the end of their second year with over a metre in total length and 50 (Andrews, 1995) or around 40 cm (Shine et al., 1996) SVL. The SVL was used to determine the sexual maturity of V. salvator to describe hemipenis development. This method was generally used for reptiles (Kusuma et al., 2017) rather than tail length because male and female reptiles with the same SVL will have different tail lengths. Male reptile tails will be thicker and longer than female tails, so tail length measurement to determine sexual maturity will be less accurate (Sreekar et al., 2011). Morphology of hemipenisHemipenis was a cylindrical (truncus form) organ supported by dense connective tissue at the corpus cavernous with many blood vessels for erection function (Mahfud et al., 2017). In the erect state, the blood pressure in the corpus cavernous increased, and blood filled the hemipenis, and at the same time, propulsar muscles pushed the hemipenis out for mating (Porto et al., 2013). These blood vessels also have a drainage function to reduce the hemipenis’ size after mating is complete (Mahfud et al., 2017). Macroscopically, the hemibaculum was white with quite a hard consistency. In addition, the size of the SVL affected the size of hemipenis, i.e., when the SVL was 36 cm, the cartilage was undeveloped, when the SVL was 39 cm, the cartilage size was tiny, and for an SVL of 58 cm, the cartilage of the hemipenis was like the adult human’s pinky color fingernails. Both hemibaculum structures were visible when the hemipenis had a full erection, but they were folded beneath the tail skin when there was no erection (Mahfud et al., 2015). A cylindrical retractor muscle, commonly known as the propulsar muscle extending from the distal hemipenis tip to the base area of the hemipenis. During the erection, the propulsar muscle contraction caused the hemipenis to come out through the cloaca (evagination), but during relaxation, the hemipenis returned to its place of origin (invagination). In an erection state, the hemipenis wall’s outer side was found with the curvature of the sulcus spermaticus from the caput to the radix of hemipenis, which functions to dispense sperm when mating. When the hemipenis was fully erect, a tongue-shaped structure appeared at the mesiodistal part; it was white with a quite hard texture like cartilage tissue. In a non-erection state, this form was in the truncus hemipenis, and it was located right in the caudal region (Mahfud et al., 2015). Histology of paryphasmataThe paryphasmata of the hemipenis was formed by an epidermis tissue and a winding-shaped dense connective tissue. This epidermis consisted of stratified squamous epithelium with melanocytes on the basal layer. Many fibers could recognize dense connective tissue stuck together with less fibroblast and extracellular matrix (Hestianah et al., 2013). The epidermis and the loose connective tissue of the paryphasmata are not adequately attached to the dense connective tissue but rather coat its outer part. The size of the paryphasmata corresponded to the size of the hemipenis and SVL. The paryphasmata of the longer SVL had a lighter color that was caused by the melanocytes on the epidermis becoming rarer as the hemipenis became larger [see Fig. 2 (5)–(8)]. There were many blood vessels in the loose connective tissue layer in the paryphasmata. Blood vessels could be recognized by epithelium that formed capillaries, sometimes with erythrocytes inside the lumen (Hestianah et al., 2013). The paryphasmata became larger during the erect state as much blood-filled this structure. The tissue structure of the paryphasmata was hard but quite bendy because it had dense connective tissue that consisted of collagen fibers. The edges of the paryphasmata were not sharp as they are only used to attach inside the vagina. Histology of the hemibaculumThe hemibaculum was composed of cartilaginous tissue and dense connective tissue. The cartilage on the hemibaculum consisted of both fibrous and hyaline cartilage. There was perichondrium lining the hyaline cartilage, the collagen cells looked smooth and widely spread, and the chondrocyte cells were inside the lacunae. On the fibrous cartilage, chondrocyte cells blend into the surrounding connective with the surrounding connective tissue. The chondrocyte cells usually appeared parallel to the direction of the fiber of collagen tissue (Hestianah et al., 2013), but in the current study, the collagen fiber’s direction was not observable. Hyaline cartilage had intercellular material consisting of smooth collagen fibers, which had the same refractive as amorphous cells so that the matrix appears homogeneous. Fibrous cartilage never stands alone but gradually merges with hyaline cartilage or adjacent dense connective tissue, and the intercellular material was formed from enormous amounts of coarse collagen fibers. It was suspected that cartilage in the hemibaculum functioned as support and made it easier for the hemipenis entrance to the female cloaca. The cartilage did not undergo hardening because of its function, which requires flexibility, so it does not easily fracture during mating. In mammals, there was also bone formation in the penis (os penis) called the baculum. The baculum was an extraskeletal bone located in the penis of a few species in several orders of mammals such as carnivores, insectivores, rodents, bats, and primates. On the dog, an os penis with lower mineral density was suspected to decrease the stiffness and reduce the risk of fracture during mating (Sharir et al., 2011). The hemibaculum and baculum may have the same function: strengthening and supporting the penis for mating success. The difference between those was in its composition; the hemibaculum of V. salvator consists of hyaline cartilage and fibrous cartilage, whereas the baculum of the dog consists of a spongious bone (Saadon, 2016). Based on the explanation in this study, there was nothing special of V. salvator penile based on histologic analysis to confirm an aphrodisiac’s efficacy. However, this lizard’s meat is consumed as a protein source and for several other purposes as traditional medicine (Uyeda et al., 2014). Therefore, it is important to carry out further studies to confirm the medicinal properties of V. salvator. At the same time, people should be made aware of the importance for preserving this species. It could be concluded that the SVL size of the V. salvator linearly affects the size of the glans hemipenis, paryphasmata, and hemibaculum but does not affect the number of rows of paryphasmata. Increases also occurred in the thickness of dense connective tissue of paryphasmata, and the area of chondrocyte cells followed the equation=−1.87E7 + 7.09E5*SVL. Further study to observe spermatogenic and supporting cells on testis histology of the V. salvator based on SVL is suggested. AcknowledgmentsThe authors would like to thank A. Arimbi, Head of Division of Veterinary Pathology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya. Special thanks to J. Jumawan, Veterinary Pathology laboratory assistant, for technical support and help. Conflict of interestThe authors declare that there is no conflict of interest. Authors contributionAlif Yahya Al-ma’ruf and Regita Permata Sari conceived and designed the study, collected, and drafted the manuscript. Imam Mustofa, Suzanita Utama, and Eka Paramytha Hestianah critical review/revision, Chairul Anwar and Lita Rakhma Yustinasari evaluated histological slide, Maslichah Mafruchati summarized the entire data, Benjamin Christoffel Tehupuring interpreted anatomical finding, Djoko Legowo and Boedi Setiawan analyzed and interpreted of data. ReferencesAndrews, H.V. 1995. Sexual maturation in Varanus salvator (Laurenti, 1 768),with notes on growth and reproductive effort. Herpetol. J. 5, 189–194. Bhattacharya, S. and Koch, A. 2018. Effects of traditional beliefs leading to conservation of water monitor lizards (Varanus salvator) and threatened Marshlands in West Bengal, India. Herpetol. Conserv. Biol. 13(2), 408–414. CITES (Convention on International Trade in Endangered Species) of Wild Fauna and Flora. 2013. Available via http://www.cites.org/eng/app/appendices.php (Accessed 19 September 2020). Dadang, R.S. 2012. Types of reptiles traded in Banten. Fau. Indones. 11, 4–9. Hestianah, E.P., Anwar C., Kuncorojakti, S. and Yustinasari, L.R. 2013. Veterinary histology teaching book. PT Revka Petra Media, Indonesia. Hosken, D.J., Archer C.R., House C.M., and Wedell, N. 2018. Penis evolution across species: divergence and diversity. Nat. Rev. Urol. 16, 98–106. Jeyamogan, S., Khan, N.A, Sagathevan, K. and Siddiqui, R. 2020. Anticancer properties of Asian water monitor lizard (Varanus salvator), python (Malayopython reticulatus) and Tortoise (Cuora kamaroma amboinensis). Anticancer Agents Med Chem, 20, 1558. https://doi.org/10.2174/1871520620666200504103056. Kusuma, I.A., Alfiyanto, D.B., Srianto, P., Triakoso, N. and Legowo, D. 2017. Morfometry study of hemipenis Varanus salvator on length measurement of Snouth Vent Length (SVL) and body weight. KnE Life Sci. 3(6), 742–752. Mahfud, M., Winarto, A. and Nisa, C. 2015. Anatomy of the reproductive organ of male Varanus salvator (Reptil: Varanidae). Acta. Vet. Indones. 3, 1–7. Mahfud, M., Winarto, A. and Nisa, C. 2017. Morphology of the hemipenes of water monitor lizard (Varanus salvator bivittatus). J. Sain Vet. 35, 111–117. Nijman, V. 2015. Water monitor lizards for sale as novelty food in Java, Indonesia. Biawak 9(1), 28–32. Nijman, V. 2016. Perceptions of sundanese men towards the consumption of water monitor lizard meat in West Java, Indonesia. Biawak 10(1), 22–25. Porto, M., de Oliveira, M.A., Pissinatti, L., Rodrigues, R.L. and Rojas-Moscoso, J.A. 2013. The evolutionary implications of hemipenial morphology of Rattlesnake Crotalus durissus terrificus. PLoS One 8, 1–8. Rowe, L. and Arnqvist, G. 2011. Sexual selection and the evolution of genital shape and complexity in water striders. Evolution 66, 40–54. Saadon, A.H. 2016. Anatomical and histological study of local dog penis. Mirror Res. Vet. Sci. Anim. 5, 8–14. Sharir, A., Israeli, D., Milgram, J., Currey, J.D., Monsonego-Ornan, E. and Shahar, R. 2011. The canin baculum: the structure and mechanical properties of an unusual bone. J. Struct. Biol. 175, 451–456. Shine, R., Harlow, P.S., Keogh, J.S. and Boeadi, B. 1996. Commercial harvesting of giant lizards: the biology of water monitors Varanus salvator in southern Sumatra. Biol. Conserv. 77(2–3), 125–134. Sreekar, R., Katya, S., Shyam, N.R. and Chetana, B.P. 2011. Predicting lizard gender: sexual dimorphism in Calotes rouxe (Reptilia: Agamidae) from Agumbe, Karnataka, India. Herpetological Conserv. Biol. 6, 75–80. Uyeda, L., Iskandar, E., Purbatrapsila, A., Pamungkas, J., Wirsing, A. and Kyes, K. 2014. Water monitor lizard (Varanus salvator) satay: a treatment for skin ailments in Muarabinuangeun and Cisiih, Indonesia. Biawak 8(1), 35–38. Vitt, L.J. and Caldwell, J.P. 2014. Squamates part I: Lizard. Herpetol. 4, 581–582. | ||
| How to Cite this Article |
| Pubmed Style AYA, RPS, Mustofa I, Utama S, Anwar C, Mafruchati M-, Hestianah EP, Yustinasari LR, Tehupuring BC, Legowo DR, Setiawan B. Morphology and histology of paryphasmata and hemibaculum of Varanus salvator based on sexual maturity. Open Vet J. 2021; 11(2): 330-336. doi:10.5455/OVJ.2021.v11.i2.18 Web Style AYA, RPS, Mustofa I, Utama S, Anwar C, Mafruchati M-, Hestianah EP, Yustinasari LR, Tehupuring BC, Legowo DR, Setiawan B. Morphology and histology of paryphasmata and hemibaculum of Varanus salvator based on sexual maturity. https://www.openveterinaryjournal.com/?mno=62826 [Access: July 27, 2024]. doi:10.5455/OVJ.2021.v11.i2.18 AMA (American Medical Association) Style AYA, RPS, Mustofa I, Utama S, Anwar C, Mafruchati M-, Hestianah EP, Yustinasari LR, Tehupuring BC, Legowo DR, Setiawan B. Morphology and histology of paryphasmata and hemibaculum of Varanus salvator based on sexual maturity. Open Vet J. 2021; 11(2): 330-336. doi:10.5455/OVJ.2021.v11.i2.18 Vancouver/ICMJE Style AYA, RPS, Mustofa I, Utama S, Anwar C, Mafruchati M-, Hestianah EP, Yustinasari LR, Tehupuring BC, Legowo DR, Setiawan B. Morphology and histology of paryphasmata and hemibaculum of Varanus salvator based on sexual maturity. Open Vet J. (2021), [cited July 27, 2024]; 11(2): 330-336. doi:10.5455/OVJ.2021.v11.i2.18 Harvard Style , A. Y. A., , . R. P. S., Mustofa, . I., Utama, . S., Anwar, . C., Mafruchati, . M. -., Hestianah, . E. P., Yustinasari, . L. R., Tehupuring, . B. C., Legowo, . D. R. & Setiawan, . B. (2021) Morphology and histology of paryphasmata and hemibaculum of Varanus salvator based on sexual maturity. Open Vet J, 11 (2), 330-336. doi:10.5455/OVJ.2021.v11.i2.18 Turabian Style , Alif Yahya Al-maruf, Regita Permata Sari, Imam Mustofa, Suzanita Utama, Chairul Anwar, Maslichah - Mafruchati, Eka Paramytha Hestianah, Lita Rakhma Yustinasari, Benjamin Christoffel Tehupuring, Djoko R Legowo, and Boedi Setiawan. 2021. Morphology and histology of paryphasmata and hemibaculum of Varanus salvator based on sexual maturity. Open Veterinary Journal, 11 (2), 330-336. doi:10.5455/OVJ.2021.v11.i2.18 Chicago Style , Alif Yahya Al-maruf, Regita Permata Sari, Imam Mustofa, Suzanita Utama, Chairul Anwar, Maslichah - Mafruchati, Eka Paramytha Hestianah, Lita Rakhma Yustinasari, Benjamin Christoffel Tehupuring, Djoko R Legowo, and Boedi Setiawan. "Morphology and histology of paryphasmata and hemibaculum of Varanus salvator based on sexual maturity." Open Veterinary Journal 11 (2021), 330-336. doi:10.5455/OVJ.2021.v11.i2.18 MLA (The Modern Language Association) Style , Alif Yahya Al-maruf, Regita Permata Sari, Imam Mustofa, Suzanita Utama, Chairul Anwar, Maslichah - Mafruchati, Eka Paramytha Hestianah, Lita Rakhma Yustinasari, Benjamin Christoffel Tehupuring, Djoko R Legowo, and Boedi Setiawan. "Morphology and histology of paryphasmata and hemibaculum of Varanus salvator based on sexual maturity." Open Veterinary Journal 11.2 (2021), 330-336. Print. doi:10.5455/OVJ.2021.v11.i2.18 APA (American Psychological Association) Style , A. Y. A., , . R. P. S., Mustofa, . I., Utama, . S., Anwar, . C., Mafruchati, . M. -., Hestianah, . E. P., Yustinasari, . L. R., Tehupuring, . B. C., Legowo, . D. R. & Setiawan, . B. (2021) Morphology and histology of paryphasmata and hemibaculum of Varanus salvator based on sexual maturity. Open Veterinary Journal, 11 (2), 330-336. doi:10.5455/OVJ.2021.v11.i2.18 |