| Review Article | ||
Open Vet J. 2023; 13(2): 131-142 Open Veterinary Journal, (2023), Vol. 13(2): 131–142 Review Article Main mites associated with dermatopathies present in dogs and other members of the Canidae familyPamela Thomson1*, Nicole Carreño1, and Andrea Núñez2,31Laboratorio Microbiología Clínica y Microbioma, Escuela de Medicina Veterinaria, Facultad de Ciencias de la Vida, Universidad Andrés Bello, Santiago, Chile 2Escuela de Medicina Veterinaria, Facultad de Recursos Naturales y Medicina Veterinaria, Universidad Santo Tomás, Talca, Chile 3Facultad de Medicina Veterinaria y Agronomía, Universidad de las Américas, Santiago, Chile *Corresponding Author: Pamela Thomson. Laboratorio Microbiología Clínica y Microbioma, Escuela de Medicina Veterinaria, Facultad de Ciencias de la Vida, Universidad Andrés Bello, Santiago, Chile. Email: pamela.thomson [at] unab.cl Submitted: 01/07/2022 Accepted: 05/01/2023 Published: 02/02/2023 © 2023 Open Veterinary Journal
AbstractDermatological diseases of parasitic origin are one of the most frequent in the clinical practice of dogs and cats. Mites such as Sarcoptes scabiei, Otodectes cynotis, Demodex canis, and Cheyletiella spp., commonly affect domestic dogs. However, the impact generated by these mites on populations of wildlife animals and the mechanisms involved in their epidemiological dynamics are still not clear. In recent decades, the migration of populations and their interaction with domestic environments and vice versa have generated a worrying threat due to the transmission of some of these ectoparasites. Some reports have suggested that sarcoptic mange represents an emerging threat to wildlife. Given the outbreaks of greater magnitude and geographical extension. The objective of this review is to contribute to the state of the art of the main mites that cause dermatopathies in members of the Canis lupus familiaris family and other members of the Canidae family. For this, a systematic search was carried out in the Embase and PubMed databases. Infections caused by mites, mainly scabies, continue to be diseases with a worldwide distribution, affecting mammals and humans. Although they are long-standing diseases, the effects that are generated in wild canids are still unknown. A comprehensive evaluation is required to generate guidelines in favor of the conservation of some species of foxes and wolves present in different regions of the world. Keywords: Sarcoptic mange, Otoacariasis, Demodicosis, Cheyletiellosis, Canids. IntroductionMites are distributed throughout the world and have an affinity for a varied group of mammalian hosts, including man. They belong to the phylum Arthropoda, class Arachnida, and subclass Acari. Unlike ticks, they are smaller and do not have a leathery covering, and some species have spiracles on the cephalothorax (Pulido et al., 2016). With more than 30,000 species described, the main mites that cause dermatopathies detected in the Canidae family are: Sarcoptes scabiei, Otodectes cynotis Demodex canis, and Cheyletiella spp. (Rodríguez et al., 2003; Souza et al., 2008; Craig, 2016). Dermatopathies of parasitic origin in small animals are the most common, presenting with a high casuistry in veterinary consultation (Beugnet et al., 2014). Since the skin is the most exposed organ, the clinical manifestation of these attacks can be mild or marked and be associated with clinical signs such as: inflammation, erythema, intense itching, and presentation of scabs, among others (Birchard and Sherding, 2005; Pulido et al., 2016). Except for Demodex, transmission after birth occurs by direct contact from a carrier or infested animal, or less frequently by indirect contact through the environment or fomites (Campbell, 2007). Today, constant updating in dermatology is essential, both for the clinic of small animals and for the conservation of wild species. It is essential to have sensitive and specific diagnostic methods to be able to investigate this type of mites. To achieve a better understanding of the dynamics of diseases that affect the skin in areas where a great variety of species converge, it is necessary to know the factors that can favor the transmission of parasitic agents, as well as the implications that these can have in wildlife conservation (Escobar et al., 2022). Due to their prevalence, importance, and distribution, we will describe the state of the art of S. scabei, O. cynotis D. canis, and Cheyletiella spp. in Canis lupus familiaris and other members of the Canidae family. Sarcoptes scabieiThe mite S. scabiei var canis is the etiological agent of sarcoptic mange or scabies, a transmissible, very pruritic, and non-seasonal skin infestation (Miller et al., 2012), which affects humans and animals. The parasite burrows deep into the epidermis, causing intense itching, inflammation, and in some cases, disruption of the skin barrier (Skerratt et al., 1999; Simpson et al., 2016). In highly susceptible wild populations, the mite can spread rapidly, reduce reproduction, causing mass mortality events (Hartley and English, 2005), especially when other threats such as habitat destruction are added (Kalema-Zikusoka et al., 2002; Vogelnest and Woods, 2008). Sarcoptic mange has been reported in at least 12 orders, 39 families, and 148 species of domestic and wild mammals, making it one of the most important ectoparasites in recent decades (Escobar et al., 2022). LifecycleThe mite life cycle is completed by an incubation period of 1 to 2 weeks in he dog (Domínguez-Santas et al., 2022). Larvae, nymphs, and immature adults represent the stages of contagion. Adults are small (200–400 µm), oval, and white with two pairs of short legs. The females have an average life of 30 days, digging galleries in the epidermis at a rate of 2–3 ml per day. Eggs are then laid in these tunnels, from which larvae hatch and migrate to the skin surface to feed (Campbell, 2005). Sarcoptes scabiei is a parasite that is very sensitive to desiccation since its survival time outside the host depends on the temperature and relative humidity of the environment (Campbell, 2005). TransmissionSarcoptic mange is a cosmopolitan, non-seasonal disease, without predisposition by race, sex, or age. The incubation period for S. scabiei in dogs ranges from 10 days to 8 weeks, generally being longer the first time, an animal is infected. It produces a highly contagious dermatitis among dogs, and its transmission occurs mainly by direct contact with an infected animal. Indirect transmission through hair or fomites has also been reported (Curtis, 2004; Arlian and Morgan, 2017) since some variants of S. scabiei manage to survive outside the host for 14 to 21 days under optimal conditions. Other estimates for the survival period range from hours to 5 days. Since males die after mating, sarcoptic mange is primarily spread by fertilized females (Curtis, 2004; Beugnet et al., 2014). There is currently evidence that the clinical picture is due to a hypersensitivity reaction. Many canines develop a type IV hypersensitivity reaction to S. scabiei antigens, so the presence of 10 to 15 mites is enough to cause severe clinical signs in a hypersensitive individual (Ihrke, 2006; Bhat et al., 2017). The immunological response against S. scabiei has shown that antigens from the parasite's saliva or buccal appendages induce an immune response. Cases have been described in which despite the presence of mites on the skin, the papules and itching did not appear until a month or more after inoculation (Saari et al., 2019). Clinical presentationThe distribution pattern of S. scabiei primarily involves the head, mainly the ear area, the elbows, and tarsi. The dorsal area of the nose and periorbital region, chest, and base of the tail are also affected (Fig. 1), eventually spreading rapidly to the rest of the body (Scott et al., 2001; Nwufoh et al., 2021). Due to the intense itching, the main lesion is a papule that is usually associated with scaling, crusting, and secondary superficial pyoderma. In general, the distal parts of the limbs and the tail are usually left free (Bandi and Saikumar, 2013; Pulido et al., 2016). In more prolonged cases, it is common to observe hyperpigmentation of the affected skin and generalized lymphadenopathy (Jofré et al., 2009). The mite incubation period has not been defined, but typically intense pruritus manifests itself 21 to 30 days after exposure (Jofré et al., 2009). Some dogs never develop the classic mange lesions. They scratch incessantly and have few or no lesions apart from mild erythema and occasional excoriations (Scott et al., 2001; Jofré et al., 2009). DiagnosisThe diagnosis of sarcoptic mange begins with the clinical history of non-seasonal intense itching, in normally affected areas such as: the ear edge, elbows, and ankles; associated or not, to contact with other affected animals (Jofré et al., 2009). The definitive diagnosis of S. scabiei is the parasitological examination by skin scraping and microscopic visualization, without delving into the dermis. The presence of mites, eggs, or their feces confirms the diagnosis (Mueller et al., 2020). A minimum of five areas should be scraped, always including margins of the ears, elbows, tarsi, and non-excoriated papular lesions. The test has a low sensitivity for Sarcoptes, reported to be positive in about 30% to 50% of affected dogs (Scott, 2013). Other authors also point out the suboptimal sensitivity and specificity of parasitological diagnosis based on microscopic examination of skin scrapings; added to that the collection of samples with a scalpel blade often causes discomfort to patients. For this reason, one of the main objectives of the World Molecular Network for Sarcoptes has been to successfully design and develop molecular diagnostic methods, such as polymerase chain reaction (PCR) or quantitative PCR (qPCR), techniques that have been used to confirm the diagnosis of different clinical grades of sarcoptic mange in animals (Angelone-Alasaad et al., 2015).
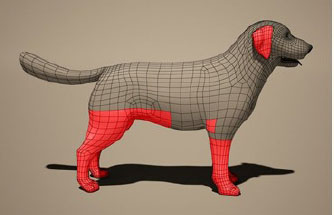
Fig. 1. Dermogram of sarcoptic mange caused by Sarcoptes scabiei in Canis lupus familiaris. Fraser et al. (2018) indicate that diagnosis by PCR from skin scrapings is an advance compared to traditional microscopy, which generates more false negatives. The results have been shown to be replicable to the diagnosis of S. scabiei in other host species, including humans. Likewise, Nwufoh et al. (2021) indicate that the diagnostic errors and false negatives that accompany the microscopy diagnostic technique can be limited with the use of PCR from scrapings and skin biopsies. This tool is highly sensitive for detecting sarcoptic mange in dogs. Diagnosis using q PCR has now been developed, combined with the use of an alternative non-invasive swab sampling technique, which offers the possibility of improving the diagnosis of scabies; however, further studies will be needed to better define the diagnostic performance of this test (Chng et al., 2021). To this, we can add dermatoscopy, a rapid and non-invasive detection method in dogs with sarcoptic mange. The degree of the infestation, the chronicity of the disease, and the number of parasites on the skin surface can influence the sensitivity of dermoscopy as a diagnostic technique, so traditional diagnostic methods should also be used (Legnani and Buckley, 2022). Otodectes cynotisOtodectes cynotis is the mite that causes otoacariasis, one of the most frequent causes of external otitis in dogs. It is estimated that up to 50% of cases of external otitis in dogs are caused by this mite (Arther et al., 2015). LifecycleThe life cycle of O. cynotis lasts 3 weeks, with survival outside the host depending on temperature and relative humidity, ranging from 5 to 17 days (Jofré et al., 2009). Under normal conditions, a maximum development of 12 days can be expected. The egg is deposited with a cement that adheres to the substrate, and after 4 days of incubation, it hatches to produce the hexapoda larva. The larva feeds actively for 3–10 days to give rise to the octapoda protonymph, which moults into a deutonymph. This form is absorbed by the adult male, both being united by the posterior dorsal suckers of the nymph's body. Sexual dimorphism occurs only in the adult form. Adult females live about 60 days and have a terminal anus and four pairs of legs. All male feet have terminal cups, while the last pair of female feet is rudimentary and does not extend beyond the body margin. Otodectes cynotis is highly mobile, but it is not a burrowing mite of the host's integument, rather it lives on the surface of the skin (Scott et al., 2001). TransmissionThe species O. cynotis is a mite with worldwide distribution, highly contagious among dogs, being particularly prevalent in juveniles since older animals can acquire immunity to this parasite. The cat is the natural reservoir of the mite and functions as a source of infection for the dog and other animals (Medleau and Hnilica, 2006). This parasite feeds on the epithelial residues and tissue fluids on the surface of the external auditory canal and the adjacent skin, causing intense irritation and, consequently, external otitis. In this way, the host is exposed to the mite antigen and immunized against it (Scott et al., 2001). Delayed host hypersensitivity has not been observed, but there is evidence that the host develops antibodies early and in the late stages of the disease (Scott et al., 2001; Medleau and Hnilica, 2006). Clinical signsThe clinical signs associated with O. cynotis are variable. In general, they tend to present ear irritation, inflammation of the external ear, intense ear itching with minimal secretion, and thick and dry brown earwax (Pulido et al., 2016). The lesions can be restricted to the external auditory canal, but it is also common to find mites in other regions of the body such as the neck and legs (Fig. 2). Ear mites are highly contagious and are more prevalent in young animals and without host specificity (Machicote, 2011). DiagnosisThe diagnosis of ear mange is generally made by bilateral otoscopy or video otoscopy using a veterinary speculum and cotton dissection forceps, inserted into the external auditory canal, to investigate the presence of O. cynotis (Six et al., 2016; Trajano, et al., 2020). The mites found should be placed on a slide for identification. However, diagnosis of Otodectes can also be made with material collected by surface scraping or on sticky tape (Scott et al., 2001). Combarros et al. (2019) compared different diagnostic techniques, obtaining that the diagnostic sensitivity of otoscopy alone was 67%, while obtaining the sample with a curette was 93%, and 57% for the smear. Interestingly, by associating otoscopy and curette, they obtained a sensitivity of 100% compared to 86% when otoscopy and smear were combined.
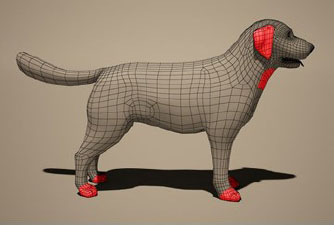
Fig. 2. Dermogram of otocariasis due to Otodectes cynotis in Canis lupus familiaris. Demodex spp.Since 1841, when Simon first described the Demodex in a dog's ear and later Demodex folliculorum var. hominis (Spickett, 1961), at least 140 species have been identified in 11 different orders of domestic and wild mammals (Lacey et al., 2011). Demodex is a genus of highly specialized mites that inhabit hair follicles and sebaceous glands, forming part of the normal microbiota in canines, which is why it is present in most healthy dogs (Birchard and Sherding, 2005; Foley et al., 2021). Demodex canis is the most frequently isolated species in dogs, to which is added: a short form called Demodex cornei (Gross et al., 2005; Izdebska and Rolbiecki, 2018) and a long form called Demodex injai (Hillier and Desch, 2002). The short form has distinct morphological characteristics and preferentially inhabits the stratum corneum of the epidermis in association with D. canis (Scott et al., 2001; Sivajothi et al., 2015). Whereas D. injai mites seem to preferentially inhabit hair follicles and sebaceous glands (Scott et al., 2001). LifecycleThe life cycle of D. canis lasts between 18 to 35 days and includes 4 evolutionary stages: fusiform egg, hexapod larva, nymph, and adult; a cycle that can develop at a temperature between 16°C and 41°C (Scott et al., 2001). Adult females produce slightly elongated eggs, which measure 80 μm in length and 30 μm in width, once hatched, the elongated hexapod larvae measure between 90 and 100 μm. Once the octopod nymph stage is reached, they reach a length of 130 to 200 μm. In its adult stage, this mite can reach sizes of 150 to 300 μm, with females being the largest and longest (Izdebska and Rolbiecki, 2018). This mite inhabits hair follicles, sebaceous glands, and less frequently apocrine sweat glands, feeding on cells, secretions, and epidermal debris (Freitas, 2012). Demodex canis do not survive outside hair follicles, on the body surface, or outside the host, however, under laboratory conditions, they can live up to 37 days (Scott et al., 2001). TransmissionTransmission of D. canis occurs through direct contact between the mother and her litter, or between pups in the same litter, during the first 48 to 72 hours postpartum (Scott et al., 2001). Demodicosis is not considered contagious between healthy animals after the neonatal period, this has been proven, with the coexistence between dogs with generalized demodicosis and healthy dogs in a confined environment; or with the inoculation of solutions containing mites in the skin of healthy animals; in both cases, the infection cannot be generated (Rhodes, 2004; Tilley and Smith, 2004; Mueller et al., 2020). Demodex canis is present in small numbers as a commensal on the skin and in the ear canal in 30% to 80% of healthy dogs, but only a few develop the disease (Scott et al., 2001; Hill, 2002). Therefore, demodectic, follicular, or red mange is the result of the excessive proliferation of Demodex spp. mites in the skin of dogs (Beco et al., 2007). Hereditary predisposition has been supported by a higher prevalence of the disease in purebred dogs, some more affected than others, by the association of D. canis with hereditary diseases (Mueller et al., 2020). The literature indicates that the breeds with the greatest predisposition to demodicosis are Boston Terrier, Jack Russel Terrier, Scottish Terrier, West Highland White Terrier (Bowden et al., 2018), Boxer, Bulldog, Chihuahua, Dalmatian, Dachshund, Doberman pinscher, Great Dane, Afghan, Alaskan Malamut, German Shepherd, Shar-pei, Weimaraner (Salo, 2011). This genetic predisposition in dogs is due to a defect in the T cells in response to the parasite, in addition to polymorphisms in the human and canine leukocyte antigen (Tilley and Smith, 2004; Medleau and Hnilica, 2006; Huisinga et al., 2007). Clinical presentationDemodicosis is classified according to the location and extent of the lesions. This classification is made because there is a different prognosis and treatment for each presentation (Perdomo, 2010; Paterson et al., 2014). Localized demodicosisLocalized demodicosis is considered when there are four or fewer lesions on the dog's body with a diameter of up to 2.5 cm (Mueller et al., 2009; Mueller et al., 2012; Dash et al., 2017). In the localized presentation, a generally circumscribed region of the skin develops mild erythema and partial alopecia, and may or may not have pruritus and seborrhea, scaling, and hyperpigmentation. The most common site is the face, especially the periocular area and the corners of the mouth. The largest number of cases occurs in animals between 3 and 6 months of life. The disease can develop some level of secondary infection, where it usually heals spontaneously (Charach, 2018). Generalized demodicosisGeneralized demodicosis is one of the most serious canine dermatoses (Pulido et al., 2016). It is characterized by the presentation of five or more affected areas, with multiple lesions that cover an entire region of the body, and/or pododermatitis in two or more limbs (Fig. 3) (Neuber, 2004; Miller et al., 2012). It presents with erythema, comedones, alopecia, follicular papules, pustules, and scales (Pulido et al., 2016). Lymphadenopathy is commonly associated with the disease and secondary bacterial infections are very common (Fourie et al., 2013) and can lead to suppurative folliculitis-furunculosis, which in the worst scenarios ends in deep pyoderma (Ferrer et al., 2014).
Fig. 3. Dermogram of generalized demodicosis due to Demodex canis. Diagnostic methodsThe standard method for the diagnosis of demodicosis continues to be the microscopic identification of the parasite in the material obtained by the deep scraping of skin and scales, which is easy to perform, low cost, and highly sensitive (Mueller et al., 2020). It is recommended to carry out multiple scrapings in the direction of hair growth, of approximately 1 cm2 of the affected skin, together with this the skin should be scraped, until capillary bleeding is observed, and it is advisable to pinch the skin during scraping to increase the number of mites collected from hair follicles (Mueller et al., 2020). Curettage should be done especially in the transitional areas between the skin and lesions, which cover at least three to six different locations (Scott et al., 2001). The material is transferred to a slide and mixed with mineral oil or paraffin for examination under the microscope at low magnification and low light intensity. The evaluation of the sample must be immediate, since the deterioration of the mites could occur (Mueller et al., 2020). Another alternative diagnostic technique is the trichogram, which is often used in dogs with difficult restraint and/or to examine sensitive areas such as the periocular, perioral, and interdigital areas, which are the most affected areas in dogs with demodicosis (Fondati et al., 2010). A hemostat is usually used to remove hair as close to the surface and in the direction of hair growth for subsequent microscopic observation (Beco et al., 2007). This technique is considered a reliable diagnostic method despite being considered less sensitive than skin scraping (Beco et al., 2007). The duct tape test is also validated for the diagnosis of canine demodicosis, although its sensitivity is not as high as skin scraping (Lousada et al., 2016; Vogelnest and Garibotto, 2016; Mueller et al., 2020). The sample is collected by pressing a strip of transparent acetate tape against the surface of the hair and skin and is subsequently observed under a microscope (Scott et al., 2001). Interestingly, Barillas et al. (2019) point out that there are no significant differences in the total number of mites between the samples obtained by deep skin scrapings and the impressions of the adhesive tape plus skin pinching, recommending the latter for therapeutic monitoring of dogs with generalized demodicosis. Faced with negative results of skin scrapings and trichograms, demodicosis cannot be ruled out; if there is clinical suspicion, a skin biopsy is recommended (Mueller et al., 2012). Some studies even point to the use of biopsy to detect the expression of major histocompatibility complex molecules, concluding that the expression of these molecules is elevated in the skin of dogs suffering from demodicosis, presuming that this is related to the development and maintenance of follicular inflammation, in the interdigital region in purebred dogs such as the Shar-pei (Huisinga et al., 2007). Cheyletiella spp.This mite is the cause of Cheyletiellosis, a zoonotic disease that affects dogs, cats, and rabbits. It is an obligatory and permanent parasite (Fonseca et al., 2021). The main species described are C. parasitivorax, which affects rabbits, C. blakei, cats, and C. yasguri, dogs (Angarano and Parish, 1994). The eggs contain the pre-larva, and the larval stage develops into the egg of the nymphs. The infestation can spread through direct contact and through fomites (Fonseca et al., 2021). Cheyletiella mites do not burrow galleries and are characterized by hook-shaped anterior palps. Dermatitis in humans occurs by contact with an affected animal and can present as papular lesions. The mites live in the outer layer of the host epidermis and feed on surface debris and tissue fluids (Angarano and Parish, 1994). In humans, lesions range from mild dermatitis to more severe disease with systemic symptoms, so diagnosis can be challenging (Reynolds and Elston, 2017). LifecycleThis obligate parasite develops its complete evolutionary cycle in the host, in approximately 3 weeks to 35 days (Miller et al., 2012; Monteiro, 2017). The evolutionary stages of the parasites are egg, larva, two nymphal stages, and adults (Acha and Syfres, 2001). The larva, nymph, and adult male die within 48 hours of separation from a host. On the other hand, eggs and females can live 10 days outside the host, so environmental control is necessary to avoid reinfestation (Acha and Syfres, 2001; Miller et al., 2012). They are large mites, between 400 μm long and 300 μm wide, with a saddle-shaped body and, due to their whitish color and rapid locomotion (Elsheika et al., 2018), they are commonly called “dandruff walker” (Jacob and Lorber, 2015). They are easily recognized by their well-developed palps, which resemble a pair of legs and have claws at their ends, facing the mouthparts. The legs end in two rows of hair and the tarsal appendages combined, instead of suckers or claws (Acha and Syfres, 2001). TransmissionCheyletiella mites are highly contagious ectoparasites, and it is common for the entire litter and dogs in a household to be infested. Direct contact is the most important route of infestation. The infestation can spread between dogs and through fomites such as combs and brushes. They have already been observed in larger ectoparasites, such as lice and fleas, and it is assumed that they can help spread their dissemination and environmental contamination (Näreaho et al., 2018). As in sarcoptic mange, there is no racial or sexual predilection, it can occur in adult dogs, but it is more frequent in young individuals, weakened animals, and in overcrowded and unsanitary conditions, which makes them more susceptible to infestation (Jacob and Lorber, 2015; Tait, 2020). Because Cheyletiella moves easily between different hosts, any history of contact with dogs, cats, or rabbits is important. Owners should be asked if the pet visits kennels, parks, or any other place where it lives or shares with other animals. Because there are asymptomatic carriers, the disease can become apparent after the introduction of a new pet into the home (Tait, 2020). Clinical signsPruritus may be absent or vary from mild to severe. In dogs, it can present with scaling in the dorsal area, and papulopustular dermatitis (White, 2016). Erythema, scaling, alopecia, and crusting typically affect the dorsum of infested rabbits, but the face and ventral abdomen may also be involved (Mellgren and Bergvall, 2008). In humans, the lesions caused by the mite appear pruritic, papular, and diffuse on the arms, chest, and abdomen. The disease is usually transient and can last between 1 and 2 weeks (Tan, 1997). Being a zoonotic disorder, the recent appearance of pruritic skin lesions in one or more of the owners should increase the suspicion of this condition. Erythematous macules on the arms, trunk, and buttocks may develop into papules, vesicles, and pustules. The severity is variable. Once animals and their environment have been successfully treated, human infestations typically resolve without treatment within 3 weeks (Craig, 2016). DiagnosisDiagnosis is made from history and clinical signs and confirmed by observation of mites or eggs in skin abrasions, fur brushing, or tape strips. They can sometimes be difficult to find, and the lack of demonstration of mites should not be taken as evidence of their absence on the animal. Skin scraping techniques are described, and the sample is deposited on a slide with coverslips and examined under the low-power objective of a good microscope. Brushing the coat, by rubbing or combing, collects scales and debris on a large sheet of white paper. The samples can be examined under a magnifying glass or under a microscope. Through hair removal since the eggs adhere to the hairs (Craig, 2016). Adhesive tape strips are useful for revealing Cheyletiella spp. mites (Halliwell, 2016). Mites in wild canidsS. scabiei in wild canidsThe "itch mite" or "scabies mite" S. scabiei causes scabies in humans and sarcoptic mange in domestic and wild animals. This mite has a wide host range due to its ability to adapt and has spread throughout the world presumably through human expansion. Although the disease caused by S. scabiei has been well-studied in humans and domestic animals, there is still little information on this pathogen in wild animals (Pulido et al., 2016; Escobar et al., 2022). The course of the disease is identical to that of Canis familiaris, with alopecia and intense itching as the main clinical sign. So much so, that in 2020, it was documented that intense itching is a useful clinical sign to obtain an approximation to the diagnosis of the disease in wildlife, which has subsequently been corroborated with molecular analyses (Montecino-Latorrea et al., 2020). Some authors state that sarcoptic mange infection alters the skin microbiome of infected canids, facilitating a secondary bacterial infection as observed in humans and other mammals infected with S. scabiei, so they suggest studying this pathology from the perspective of health (Rowe et al., 2019; Escobar et al., 2022). In Latin America, data on the disease is very scarce, resulting in unknown impacts on wild populations. It has been observed that most wild canids in Latin America are solitary animals, or tend to live in small families, unlike what has been reported in North America. Despite this, some outbreaks in native canids have been reported (Deem et al., 2002; Diaz-Luque et al., 2014; Verdugo et al., 2016), with potential transmission from domestic dogs or infected prey (Verdugo et al., 2016). In Chile, it has been detected non-recurringly since 2004, including its detection within protected wild areas, a fact that has generated a worrying scenario due to a projection that considers the increase in the mortality rate that could be associated with this parasite (Montecino-Latorre et al., 2020). A review carried out in North America mentions records of the detection of these species of mites in wildlife dating back to the 1900s, being associated with clinical manifestations such as itching, alopecia, and erythema in animals such as the desert fox or northern fox (Vulpes macrotis mutica), the gray wolf (C. lupus), the red wolf (Canis rufus), the Mexican wolf (Canis lupus baileyi), the red fox (Vulpes vulpes), the grey fox (Urocyon cinereoargenteus), and the coyote (Canis latrans) (DeCandia et al., 2019; Niedringhaus et al., 2019). Sarcoptic mange has been closely studied in Yellowstone National Park, where S. scabiei invaded a reintroduced wolf population in 2007 (Almberg et al., 2012; Almberg et al., 2015; Cross et al., 2016), in these infected wolf’s changes such as decreased movement, selection of warmer habitats, increased food consumption. and greater social dependence among pack members were observed. On the other hand, in Switzerland, sarcoptic mange has been reported in red foxes since 1835; however, the first case diagnosed in the framework of health surveillance was in 1959 and since then it has spread throughout the country, reporting an annual prevalence of mange in foxes between 0.1% and 12% estimated by camera traps, which has confirmed the endemism of this agent (Pisano et al., 2019). In Iberia, sarcoptic mange is also endemic in the red fox (Vulpes vulpes) and the first confirmed cases of mange in wolves have recently been reported. However, knowledge about S. scabiei in wolves is scarce due to the sampling difficulties inherent to these species. In endangered species, mange causes conservation problems because it can decimate isolated populations and contribute to extinction. Despite this, the Iberian Peninsula still maintains one of the largest populations of wolves (C. lupus) in Europe (Oleaga et al., 2011). On the other hand, in Estonia, sarcoptic mange has been detected in red foxes (Vulpes vulpes); curiously these foxes have begun to populate urban areas of the city because their natural habitat has been decimated (Plumer et al., 2014). O. cynotis in wild canidsOtodectes cynotis is a nonburrowing, obligate parasitic mite, and all life stages typically occur in the host's ear, near the eardrum. In cases of high infestation, they can also be found on the tail, back, and head (Wall and Shearer, 2001). The presence of O. cynotis has been recorded in different domestic species throughout the literature, but in recent years it has also been detected in wildlife. causing "ear mange" or "ear mange." For example, in Brazil, it has been reported in two species of foxes: crab-eating fox (Cerdocyon thous) and Pampas fox (Lycaloppex gymnocercus) (Huang-Bastos et al., 2020). A study carried out in Chile detected and identified O. cynotis by molecular techniques in the Chilote fox or Darwin's fox (Lycalopex fulvipes) and in the Culpeo fox (Lycalopex culpaeus) in 76% and 73% respectively (Briceño et al., 2020). In central Italy, not only has the presence of O. cynotis been detected in red foxes (Vulpes vulpes), but in that same study, the presence of S. scabiei was also reported in animals with skin lesions; the severity of the lesions and the poor body conditions observed in most of the animals indicate that attention should be paid to this infestation in the wild fox population (Perrucci et al., 2016). On the other hand, some authors found that the presence of O. cynotis in the ear canal of Santa Catalina Island foxes (Urocyon littoralis catalinae) was associated with reduced microbial diversity in the ear canal and the presence of a community dominated by Staphylococcus pseudintermedius in the ear canal (Moriarty et al., 2015; DeCandia et al., 2020; Trevelline et al., 2020). The main complication of infestation by this parasite is related to the accumulation of cerumen and brown exudate in the pinna, which has the potential to obstruct and generate hyperplasia of the ceruminous glands (Briceño et al., 2020). Demodex sp. in wild canidsThere is currently no precise information on the presence of Demodex sp. in wild canids in Latin America, despite this, there are international studies that document those traces of Demodex sp. in wild wolf feces with a prevalence of 0.2% in the Riding Mountain National Park in Manitoba, Canada (Stronen et al., 2011). Given the increase in the feral canine population in the world, we must consider generating studies to determine the status of infection by this ectoparasite. Cheyletiella in wild canidsThe disease is rare in wild canids; however, its true incidence may be underestimated since mites can be difficult to find, and asymptomatic carriers are also reported (Tait, 2020). Lledó et al. (2015), examined 400 red foxes for endo and ectoparasites in the province of Soria, and C. yasguri was identified in two of them. Interestingly, the authors conclude that foxes could act as sentinel species for diseases transmitted by both ectoparasites and endoparasites. Since foxes can move through various habitats, feed on many food sources, and carry a variety of parasites that can cause emerging diseases in humans, this situation poses a risk of contact with domestic animals and humans (Lledó et al., 2015). ConclusionUnlike sarcoptic mange, there are few records of cases produced by Demodex in wildlife; probably because it is characterized by only affecting individuals in immunocompromised states and/or with some degree of genetic predisposition. Despite this, it is still relevant to evaluate the presence of this parasite in wild animals. Sarcoptic mange is a re-emerging zoonotic disease that is very common in the clinic of domestic dogs, which also affects the welfare and conservation of wild species, a situation that has been reported in several countries around the world. Due to its global distribution facilitated by transmission routes and a large number of host species, it constitutes a major threat to fauna conservation. The presence of domestic canines ever closer to ecosystems with susceptible species generates significant concern and calls for action to be taken soon. The effects caused by O. cynotis in wildlife are still a subject of study, although its presence in wild canids from different countries is known, the severity of the clinical picture that it can cause is unknown, so it is necessary to continue studying its characteristics in non-domestic species. Cheyletiellosis is an important differential diagnosis in patients with itching. This disease poses a diagnostic challenge as there are asymptomatic carriers. By remaining viable for a few days in the environment, this situation can favor the spread of other animal species, including humans. The impact that this environmental contamination can have on other species of wild animals, such as foxes, where it can present as an emerging disease, should also be considered. The treatment, control, and prevention of pathologies caused by mites in dogs must be covered from a "one health" perspective, since they are not only a daily clinical problem, but also a threat to the conservation of different species and ecosystems. AcknowledgmentThe authors would like to thank the ANID PAI Project # 77190079. Conflict of interestThe authors declare that they have no conflicts of interest. Authors’ contributionPamela Thomson: Conception and design of the study, wrote the first draft of the manuscript, critically revised the manuscript, funding acquisition. Nicole Carreño: writing the first draft of the manuscript and design figures. Andrea Núñez: Writing one of the topics and critically revising the manuscript. ReferencesAcha, P.N. and Syfres, B. 2001. Zoonosis y enfermedades transmisibles comunes al hombre y a los animales, 3rd ed. Washington, DC, pp: 413. Available via http://iris.paho.org/xmlui/bitstream/handle/123456789/711/9275119936.pdf?sequence=2&isAllowed=y/ 08/03/2022. Almberg, E.S., Cross, P.C., Dobson, A.P., Smith, D.W. and Hudson, P.J. 2012. Parasite invasion following host reintroduction: a case study of Yellowstone’s wolves. Philos. Trans. R. Soc. B. Biol. Sci. 367, 2840–2851. Almberg, E.S., Cross, P.C., Dobson, A., Smith, D.W., Metz, M.C., Stahler D.R. and Hudson, P.J. 2015. Social living mitigates the costs of a chronic illness in a cooperative carnivore. Ecol. Lett.18, 660–667. Angarano, D.W. and Parish, L.C. 1994. Comparative dermatology: parasitic disorders. Clin. Dermatol. 12, 543–550. Angelone-Alasaad, S., Molinar Min, A., Pasquetti, M., Alagaili, A.N., D’Amelio, S., Berrilli, F., Obanda, V., Gebely, M., Soriguer, R. and Rossi, L. 2015. Universal conventional and real-time PCR diagnosis tools for Sarcoptes scabiei. Parasit. Vectors. 14(8), 587. Arlian, L.G. and Morgan, M.S. 2017. A review of Sarcoptes scabiei: past, present, and future. Parasit. Vectors. 10(1), 297. Arther, R., Davis, W., Jacobsen, J. and Lewis, V. 2015. Clinical evaluation of the safety and efficacy of 10% imidacloprid + 2.5% moxidectin topical solution for the treatment of ear mite (Otodectes cynotis) infestations in dogs. Vet. Parasitol. 10, 64–68. Bandi, K.M. and Saikumar, C. 2013. Sarcoptic mange: a zoonotic ectoparasitic skin disease. J. Clin. Diagn. Res. 7(1), 156–157. Barillas, O.F., Bajwa, J., Guillot, J. and Arcique, A. 2019. Comparison of acetate tape impression, deep skin scraping, and microscopic examination of hair for therapeutic monitoring of dogs with juvenile generalized demodicosis: a pilot study. Can. Vet. J. 60(6), 596–600. Beco, L., Fontaine, F. and Bergvall, K. 2007. Comparison of skin scrapes and hair plucks for detecting Demodex mites in canine demodicosis, a multicenter, prospective study. In Annual Conference of the European Society of Veterinary Dermatology. European College of Vet Dermatol., vol. 18, p 381. Beugnet, F., Bourdeau, P., Chalvet-Monfray, K., Cozma, V., Farkas, R., Guillot, J., Halos, L., Joachim, A., Losson, B., Miró, G., Otranto, D., Renaud, M. and Rinaldi, L. 2014. Parasites of domestic owned cats in Europe: co-infestations and risk factors. Parasit. Vectors. 7, 291. Bhat, S.A., Mounsey, K.E., Liu, X. and Walton, S.F. 2017. Host immune responses to the itch mite, Sarcoptes scabiei, in humans. Parasit. Vectors. 10(1), 385. Birchard, S.J. and Sherding, R.G. 2005. Saunders manual of small animal practice-e-book. Amsterdam, The Netherlands: Elsevier Health Sciences. Bowden, D., Outerbridge, C., Kissel, M., Baron, J. and White, S. 2018. Canine demodicosis: a retrospective study of a veterinary hospital population in California, USA (2000-2016). Vet. Dermatol. 29(1), 19–e10. Briceño, C., González-Acuña, D., Jiménez, J.E., Bornscheuer, M.L., Funk, S.M. and Knapp, L.A. 2020. Ear mites, Otodectes cynotis, on wild foxes (Pseudalopex spp.) in Chile. J. Wildl. Dis. 56(1), 105–112. Campbell, K. 2005. Other external parasites. In Textbook of veterinary internal medicine: diseases of the dog and cat, 6th ed. Eds., Ettinger, S.J. and Feldman, E.C. St. Louis, MO: W.B. Saunders Company, pp: 66–70. Campbell, K. 2007. Other external parasites. Veterinary internal medicine, diseases of the dog and cat, 6th ed. Madrid, Spain: Elsevier, p. 67. Charach, M.G. 2018. Demodicosis: new treatment, common misdiagnosis. Can. Vet. J. 59(5), 545–547. Chng, L., Holt, D.C., Field, M., Francis, J.R., Tilakaratne, D., Dekkers, M.H., Robinson, G., Mounsey, K., Pavlos, R., Bowen, A.C., Fischer, K., Papenfuss, A.T., Gasser, R.B., Korhonen, P.K., Currie, B.J., McCarthy, J.S. and Pasay, C. 2021. Molecular diagnosis of scabies using a novel probe-based polymerase chain reaction assay targeting high-copy number repetitive sequences in the Sarcoptes scabiei genome. PLoS One 15(2), e 0009149. Combarros, D., Boncea, A.M., Brément, T., Bourdeau, P. and Bruet, V. 2019. Comparison of three methods for the diagnosis of otoacariasis due to Otodectes cynotis in dogs and cats. Vet. Dermatol. 30(4), 334–e96. Craig, M. 2016. Surface mites in dogs, cats, and rabbits. Companion. Anim. 21(12); https://doi.org/10.12968/coan.2016.21.12.678. Cross, P.C., Almberg, E.S., Haase, C.G., Hudson, P.J., Maloney, S.K., Metz, M.C., Munn, A.J., Nugent, P., Putzeys, O., Stahler, D.R., Stewart, A.C. and Smith, D.W. 2016. Energetic costs of mange in wolves estimated from infrared thermography. Ecology 97, 1938–1948. Curtis, C.F. 2004. Current trends in the treatment of Sarcoptes, Cheyletiella and Otodectes mite infestations in dogs and cats. Vet. Dermatol. 15, 108–111. Dash, S., Jyotiranjan, T., Priya Das, L., Sahoo, R., Mohapatra, S. and Das, M. 2017. Management of demodicosis (Demodex canis) associated with secondary bacterial infections in Dog. J. Pharm. Innov. 6(9), 372–375. DeCandia, A., Brenner, L., King, J. and VonHoldt, B. 2020. Ear mite infection is associated with altered microbial communities in genetically depauperate Santa Catalina Island foxes (Urocyon littoralis catalinae). Mol. Ecol. 29(8), 1463–1475. DeCandia, A., Leverett, K. and VonHoldt, B. 2019. Of microbes and mange: consistent changes in the skin microbiome of three canid species infected with Sarcoptes scabiei mites. Parasit. Vectors. 12(1), 488. Deem, S.L., Noss, A.J., Cuéllar, R.L., Villarroel, R., Linn, M.J. and Forrester, D.J. 2002. Sarcoptic mange in free-ranging pampas foxes in the Gran Chaco, Bolivia. J. Wildl. Dis. 38, 625–628. Diaz-Luque, J.A.D., Müller, H., González, L. and Berkunsky, I. 2014. Clinical signs suggestive of mange infestation in a free-ranging maned wolf (Chrysocyon brachyurus) in the Moxos Savannahs of Beni, Bolivia. Mastozool. Neotrop. 21, 135–138. Dominguez-Santas, M., Roustan-Gullon, G. and Alfageme-Roldan, F. 2022. Sonographic findings in scabies. J. Ultrasound. doi: 10.1007/s40477-022-00700-4. Epub ahead of print. PMID: 36028790. Elsheika, H.M., Wright, I. and Mcgarry, J. 2018. Parasites and pets: a veterinary nursing guide. CABI, publishing,Wallingford, Oxforshire, UK, pp: 158. Escobar, L.E., Carver, S., Cross, P., Rossi, L., Almberg, E.S., Yabsley, M.J., Niedringhaus, K.D., Van Wick, P., Dominguez-Villegas, E., Gakuya, F., Xie, Y., Angelone, S., Gortázar, C. and Astorga, F. 2021. Sarcoptic mange: an emerging panzootic in wildlife. Transbound. Emerg. Dis. 69(3), 927–942. Ferrer, L., Ravera, I. and Silbermayr, K. 2014. Immunology and pathogenesis of canine demodicosis. Vet. Dermatol. 25, 427–432. Foley, R., Kelly, P., Gatault, S. and Powell, F. 2021. Demodex: a skin resident in man and his best friend. J. Eur. Acad. Dermatol. Venereol. 35(1), 62–72. Fondati, A., De Lucia, M., Furiani, N., Monaco, M., Ordeix, L. and Scarampella, F. 2010. Prevalence of Demodex canis-positive healthy dogs at trichoscopic examination. Vet. Dermatol. 21(2), 146–151. Fonseca, V.D.M., Pereira, M.F., Ribeiro, S.G. and Vieira, V.P. 2021. Taxonomia, morfologia e ciclo do ácaro Cheyletiella, uma revisa revisão de literatura. Re. C. M. V. 26, 47–60. Fourie, J., Dumont, P., Halos, L., Beugnet, F. and Pollmeier, M. 2013. Efficacy of a topical application of Certifect® (fipronil 6.26% w/v, amitraz 7.48% w/v, (S)- methoprene 5.63% w/v) for the treatment of canine generalized demodicosis. Parasite 20, 46. Fraser, T.A., Martin, A., Polkinghorne, A. and Carver, S. 2018. Comparative diagnostics reveals PCR assays on skin scrapings is the most reliable method to detect Sarcoptes scabiei infestation. Vet. Parasitol. 251, 119–124. Gross, T.L., Ihrke, P.J., Walder, E.J. and Affolter, V.K. 2005. Pustular and nodular diseases with adnexal destruction. Canine demodicosis. In Skin diseases of the dog and cat – clinical and histopathologic diagnosis, 2nd ed. Eds., Gross, T.L., Ihrke, P.J., Walder, E.J. and Affolter, V.K. Hoboken, NJ: Blackwell Publishing, pp: 442–447. Halliwell, R. 2016. The diagnostic approach to pruritus. In the Proceedings of the 2016 Continuing Education Programme of the World Congress of Veterinary Dermatology, 31 May–4 June, Bordeaux, France, pp. 5–13. Hartley, M. and English, A. 2005. Sarcoptes scabei var. wombati infection in the common wombat (Vombatus ursinus). Eur. J. Wildl. Res. 51, 117–121. Hill, P.B. 2002. Small animal dermatology – a practical guide to the diagnosis and management of skin diseases in dogs and cats. Oxford, UK:Butterworth-Heinemann, pp: 6–9, e270–274. Hillier, A. and Desch, C.E. 2002. Large-bodied Demodex mite infestation in 4 dogs. J. Am. Vet. Med. Assoc. 220, 623–627. Huang-Bastos, M., Bassini-Silva, R., Rolim, L.S., O´Connor, B., Ochoa, R., Barros-Battesti, D.M. and Jacinavicius, F.C. 2020. Otodectes cynotis (Sarcoptiformes: Psoroptidae): new records on wild carnivores in Brazil with a case report. J. Med. Entomol. 57(4), 1090–1095. Huisinga, M., Failing, K. and Reinacher, M. 2007. MHC class II expression by follicular keratinocytes in canine demodicosis--an immunohistochemical study. Vet. Immunol. Immunopathol. 118(3–4), 210–220. Ihrke, P.J. 2006. New approaches to common canine ectoparasites. In Proceedings of the 31st World Small Animal Veterinary Association (WSAVA) Congress: Prague, Czech Republic, p. 11–14. Izdebska, J.N. and Rolbiecki, L. 2018. The status of Demodex cornei: description of the species and developmental stages, and data on demodex mites in the domestic dog Canis Lupus familiaris. Med. Vet. Entomol. 32(3), 346–357. Jacob, J. and Lorber, B. 2015. Diseases transmitted by man’s best friend: the dog. Microbiol. Spectr. 3(4), IOL5-0002-2015. Jofré, M.L., Noemí, H.I., Neira, O.P., Saavedra, U.T. and Díaz, L.C. 2009. Animal mites transmissible to humans and associated zoonosis. Rev. Chilena. Infectol. 26(3), 248–257. Kalema-Zikusoka, G., Kock, R.A. and Macfe, E.J. 2002. Scabies in free-ranging mountain gorillas (Gorilla beringei) in Bwindi Impenetrable National Park, Uganda. Vet. Rec. 150, 12–15. Lacey, N., Raghallaigh, S. and Powell, F. 2011. Demodex mites–commensals, parasites or mutualistic organisms? Vet. Dermatol. 222, 128–130. Legnani, S. and Buckley, L. 2022 The use of dermoscopy to support the diagnosis of sarcoptic mange in two dogs. Vet. Dermatol. 33(3), 255–e67. Lledó, L., Giménez-Pardo, C., Saz, J.V. and Serrano, J.L. 2015. Wild red foxes (Vulpes vulpes) as sentinels of parasitic diseases in the Province of Soria, Northern Spain. Vector. Borne. Zoonotic. Dis. 15(12), 743–749. Lousada, R., Fonseca, I. and Nunes, T. 2016. New atraumatic technique for Demodex canis diagnosis. Vet. Dermatol. 27(Suppl. 1), 114. Machicote, G. 2011. Dermatología canina y felina. Zaragoza, España: Servet editorial, pp. 115–135. Medleau, L. and Hnilica, A. 2006. Parasitic skin disorders. In Small animal dermatology – a color atlas and therapeutic guide, 2nd ed. Saunders Elsevier, St. Louis, Missouri, E.E.U.U., pp: 102–108.. Mellgren, M. and Bergvall, K. 2008. Treatment of rabbit cheyletiellosis with selamectin or ivermectin: a retrospective study. Acta. Vet. Scand. 50, 1. Miller, W.H. Jr., Griffin, C.E. and Campbell, K.L. 2012. Parasitic skin disease. In Muller and Kirk's small animal dermatology. Eds., Mosby, E. St Louis, MO: Saunders, pp: 284–342. Montecino-Latorre, D., Napolitano, C., Briceño, C. and Uhart, M. 2020. Sarcoptic mange: an emerging threat to Chilean wild mammals? Perspect. Ecol. Conserv. 18, 267–276. Monteiro, S.G. 2017. Parasitologia na medicina veterinária / Silvia Gonzalez Monteiro, 2nd ed. Rio de Janeiro, Brazil: Roca, p. 370. Moriarty, M.E., Vickers, T.W., Clifford, D.L., Garcelon, D.K., Gaffney, P.M., Lee, K.W., King, J.L., Duncan, C.L. and Boyce, W.M. 2015. Ear mite removal in the Santa Catalina Island fox (Urocyon littoralis catalinae): controlling risk factors for cancer development. PLoS One 10(12), e0144271. Mueller, R., Bensignor, E., Ferrer, L., Holm, B. and Lemarie, S. 2012. Treatment of demodicosis in dogs: 2011 clinical practice guidelines. Vet. Dermatol. 23(2), 86–96. Mueller, R.S., Meyer, D., Bensignor, E. and Sauter-Louis, C. 2009. Treatment of canine generalized demodicosis with a ‘spot-on’ formulation containing 10% moxidectin and 2.5% imidacloprid (Advocate®, Bayer Healthcare). Vet. Dermatol. 20(5–6), 441–446. Mueller, R.S., Rosenkrantz, W., Bensignor, E., Karaś-Tęcza, J., Paterson, T. and Shipstone, M.A. 2020. Diagnosis and treatment of demodicosis in dogs and cats: clinical consensus guidelines of the World association for veterinary dermatology. Vet. Dermatol. 31(1), 4–e2. Näreaho, A., Nikander, S. and Saari, S. 2018. Arachnida in: canine parasites and parasitic diseases, 1st ed. Amsterdam, The Netherlands: Elsevier, pp. 187–228. Neuber, A.E. 2004. What is your diagnosis? Juvenile-onset generalized demodicosis. J. Small. Anim. Pract. 45(11), 575–576. Niedringhaus, K.D., Brown, J.D., Sweeley, K.M. and Yabsley, M.J. 2019. A review of sarcoptic mange in North American wildlife. Int. J. Parasitol. Parasites. Wildl. 9, 285–297. Nwufoh, O.C., Sadiq, N.A., Fagbohun, O., Adebiyi, A., Adeshina, R., Emmanuel, E. and Emikpe, B.O. 2021. Molecular detection and characterization of Sarcoptes scabiei var canis using skin scrapings and skin biopsies. J. Parasit. Dis. 45(1), 258–262. Oleaga, A., Casais, R., Balseiro, A., Espí, A., Llaneza, L., Hartasánchez, A. and Gortázar, C. 2011. New techniques for an old disease: sarcoptic mange in the Iberian wolf. Vet. Parasitol. 181(2–4), 255–266. Paterson, T., Halliwell, R., Fields, P., Louw, M. and Ball, G. 2014. Canine generalized demodicosis treated with varying doses of a 2.5% moxidectin + 10% imidacloprid spot-on and oral ivermectin: parasiticidal effects and long-term treatment out comes. Vet. Parasitol. 205(3–4), 687–696. Perdomo, F. 2010. Sarna demodécica en perros: un estudio actual sobre su importancia en la clínica de pequeñas especies. Veracruz, Mexico: Universidad de Veracruzana, pp. 30–31. Perrucci, S., Verin, R., Mancianti, F. and Poli, A. 2016. Sarcoptic mange and other ectoparasitic infections in a red fox (Vulpes vulpes) population from central Italy. Parasite. Epidemiol. Control. 1(2), 66–71. Pisano, S.R.R., Zimmermann, F., Rossi, L., Capt, S., Akdesir, E., Bürki, R., Kunz, F., Origgi, F.C. and Ryser-Degiorgis, M.P. 2019. Spatiotemporal spread of sarcoptic mange in the red fox (Vulpes vulpes) in Switzerland over more than 60 years: lessons learnt from comparative analysis of multiple surveillance tools. Parasit. Vectors. 12(1), 521. Plumer, L., Davison, J. and Saarma, U. 2014. Rapid urbanization of red foxes in Estonia: distribution, behaviour, attacks on domestic animals, and health-risks related to zoonotic diseases. PLoS One 9(12), e115124. Pulido, A., Castañeda, R., Ibarra, H., Gómez, L. and Barbosa, A. 2016. Microscopía y principales características morfológicas de algunos ectoparásitos de interés veterinario. RIVEP 27(1), 91–113. Reynolds, H.H. and Elston, D.M. 2017. What’s eating you? Cheyletiella mites. Cutis 99(5), 335–336. Rhodes, K.H. 2004. Demodicosis. The 5-minute veterinary consult clinical companion – small animal dermatology, 1st ed. Philadelphia, PA: Lippincott Williams & Wilkins, pp. 203–209. Rodríguez, R., Ortega, A., Rosado, J. and Bolio, G. 2003. Factors affecting the prevalence of mange-mite infestations in stray dogs of Yucatán, Mexico. Vet. Parasitol. 115, 61–65. Rowe, M.L., Whiteley, P.M. and Carver, S. 2019. The treatment of sarcoptic mange in wildlife: a systematic review. Parasit. Vectors. 12, 99. Saari, S., Näreaho, A. and Nikander, S. 2019. Arachnida. Canine parasites and parasitic diseases, 1st ed. Amsterdam, The Netherlands: Elsevier, pp. 187–228. Salo, E. 2011. Clinical forms of canine demodicosis. There is more than just alopecia. Clin. Vet. Peq. Anim. 31(2), 67–75. Scott, D. 2013. Canine scabies. In 10th Annual Dermatology Chapter Meeting of the Australian New Zealand College of Veterinary Scientists, 2013 July 12–13, pp: 33–34. Scott, D.W., Miller, W.H. and Griffin, C.E. 2001. Parasitic skin diseases. Canine demodicosis. In Muller and Kirk’s—small animal dermatology, 6th ed. Eds., Miller, W.H., Griffin, C.E. and Campbell K.L. Philadelphia, PA: W.B. Saunders Company (Philadelphia), pp: 457–474. Simpson, K., Johnson, C.N. and Carver, S. 2016. Sarcoptes scabiei: the mange mite with mighty efects on the common wombat (Vombatus ursinus). PloS One 11, e0149749. Sivajothi, S., Sudhakara Reddy, B. and Rayulu, V.C. 2015. Demodicosis caused by Demodex canis and Demodex cornei in dogs. J. Parasit. Dis. 39(4), 673–676. Six, R., Becskei, C., Mazaleski, M., Fouriec, J., Mahabir, S., Myers, M. and Slootmans, N. 2016. Efficacy of sarolaner, a novel oral isoxazoline, against two common mite infestations in dogs: Demodex spp. and Otodectes cynotis. Vet. Parasitol. 222, 62–66. Skerratt, L.F., Middleton, D. and Beveridge, I. 1999. Distribution of life cycle stages of Sarcoptes scabiei var wombati and efects of severe mange on common wombats in Victoria. J. Wildl. Dis. 35, 633–646. Souza, C., Ramadinha, R., Scott, F. and Pereira, M. 2008. Factors associated with the prevalence of Otodectes cynotis in an ambulatory population of dogs. Pesq. Vet. Bras. 28(8), 1–7. Spickett, S.G. 1961. Studies on Demodex folliculorum Simon (1842). I life history. Parasitol 51(1–2), 181–192. Stronen, A.V., Sallows, T., Forbes, G.J., Wagner, B. and Paquet, P.C. 2011. Diseases and parasites in wolves of the riding mountain national park region, Manitoba, Canada. J. Wildl. Dis. 47(1), 222–227. Tait, J. 2020. Cheyletiellosis small animal dermatology for technicians and nurses section IV parasitic skin diseases, Wiley Online Library, pp: 139–146. Tan, J.S. 1997. Human zoonotic infections transmitted by dogs and cats. Arch. Intern. Med. 157(17), 1933–1943. Tilley, L.P. and Smith, F.W. 2004. Demodicosis. The 5-minute veterinary consult canine and feline, 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins (Philadelphia), pp. 322–323. Trajano, J., Claudino, L., Mangueira, M., do Nascimento, L., Ferreira, T., Ribeiro, F., William, A. and Ribeiro, V. 2020. Prevalence and clinical aspects of Otodectes cynotis infestation in dogs and cats in the semi-arid region of Paraíba, Brazil. Acta. Sci. Vet. 48, 1725. Trevelline, B.K., Stephenson, J.F. and Kohl, K.D. 2020. Two's company, three's a crowd: exploring how host-parasite-microbiota interactions may influence disease susceptibility and conservation of wildlife. Mol. Ecol. 29(8), 1402–1405. Verdugo, C., Espinoza, A., Moroni, M., Valderrama, R. and Hernandez, C. 2016. Sarcoptic mange in a South American gray fox (Chilla Fox; Lycalopex griseus), Chile. J. Wildl. Dis. 52, 738–741. Vogelnest, L. and Garibotto, V. 2016. Evaluation of the squeeze tape impression for the diagnosis of canine demodicosis. Vet. Dermatol. 27(Suppl. 1), 38. Vogelnest, L. and Woods, R. 2008. Medicine of Australian mammals, 1st ed. Melbourne, Australia: CSIRO Publishing. Wall, R. and Shearer, D. 2001. Veterinary ectoparasites: biology, pathology, and control, 2nd ed. Oxford, UK: Blackwell Science, p. 288. White, S. 2016. Scaling and nodular diseases of rabbits and rodents. In Proceedings of the Continuing Education Programme of the World Congress of Veterinary Dermatology. Available via http://www.wavd.org/images/pdf/WCVD8CEProceedings.pdf: 410. | ||
| How to Cite this Article |
| Pubmed Style Thomson P, Carreño N, Nuñez A. Main mites associated with dermatopathies present in dogs and other members of the Canidae family. Open Vet J. 2023; 13(2): 131-142. doi:10.5455/OVJ.2023.v13.i2.1 Web Style Thomson P, Carreño N, Nuñez A. Main mites associated with dermatopathies present in dogs and other members of the Canidae family. https://www.openveterinaryjournal.com/?mno=65213 [Access: November 09, 2024]. doi:10.5455/OVJ.2023.v13.i2.1 AMA (American Medical Association) Style Thomson P, Carreño N, Nuñez A. Main mites associated with dermatopathies present in dogs and other members of the Canidae family. Open Vet J. 2023; 13(2): 131-142. doi:10.5455/OVJ.2023.v13.i2.1 Vancouver/ICMJE Style Thomson P, Carreño N, Nuñez A. Main mites associated with dermatopathies present in dogs and other members of the Canidae family. Open Vet J. (2023), [cited November 09, 2024]; 13(2): 131-142. doi:10.5455/OVJ.2023.v13.i2.1 Harvard Style Thomson, P., Carreño, . N. & Nuñez, . A. (2023) Main mites associated with dermatopathies present in dogs and other members of the Canidae family. Open Vet J, 13 (2), 131-142. doi:10.5455/OVJ.2023.v13.i2.1 Turabian Style Thomson, Pamela, Nicole Carreño, and Andrea Nuñez. 2023. Main mites associated with dermatopathies present in dogs and other members of the Canidae family. Open Veterinary Journal, 13 (2), 131-142. doi:10.5455/OVJ.2023.v13.i2.1 Chicago Style Thomson, Pamela, Nicole Carreño, and Andrea Nuñez. "Main mites associated with dermatopathies present in dogs and other members of the Canidae family." Open Veterinary Journal 13 (2023), 131-142. doi:10.5455/OVJ.2023.v13.i2.1 MLA (The Modern Language Association) Style Thomson, Pamela, Nicole Carreño, and Andrea Nuñez. "Main mites associated with dermatopathies present in dogs and other members of the Canidae family." Open Veterinary Journal 13.2 (2023), 131-142. Print. doi:10.5455/OVJ.2023.v13.i2.1 APA (American Psychological Association) Style Thomson, P., Carreño, . N. & Nuñez, . A. (2023) Main mites associated with dermatopathies present in dogs and other members of the Canidae family. Open Veterinary Journal, 13 (2), 131-142. doi:10.5455/OVJ.2023.v13.i2.1 |