| Original Article | ||
Open Vet J. 2021; 11(4): 619-634 Open Veterinary Journal, (2021), Vol. 11(4): 619–634 Original Research Analysis of risk factors for canine mast cell tumors based on the Kiupel and Patnaik grading system among dogs with skin tumorsAna Luísa Martins1,2*, Fátima Faria Carvalho1, João R. Mesquita1,3,4, Fátima Gärtner1,5,6 and Irina Amorim1,51Institute of Biomedical Sciences Abel Salazar (ICBAS), University of Porto, Porto, Portugal 2Faculty of Sciences of the University of Porto (FCUP), Porto, Portugal 3Faculty of Pharmacy of the University of Porto (FFUP), Porto, Portugal 4Epidemiology Research Unit (EPIUnit), Instituto de Saúde Pública da Universidade do Porto, Porto, Portugal 5Instituto de Investigação e Inovação em Saúde da Universidade do Porto (i3S), Porto, Portugal 6Institute of Molecular Pathology and Immunology of the University of Porto (IPATIMUP), Porto, Portugal *Corresponding Author: Ana Luísa Martins. Institute of Biomedical Sciences Abel Salazar (ICBAS), University of Porto, Porto, Portugal. Email: analuisam11 [at] gmail.com Submitted: 30/03/2021 Accepted: 20/10/2021 Published: 11/11/2021 © 2021 Open Veterinary Journal
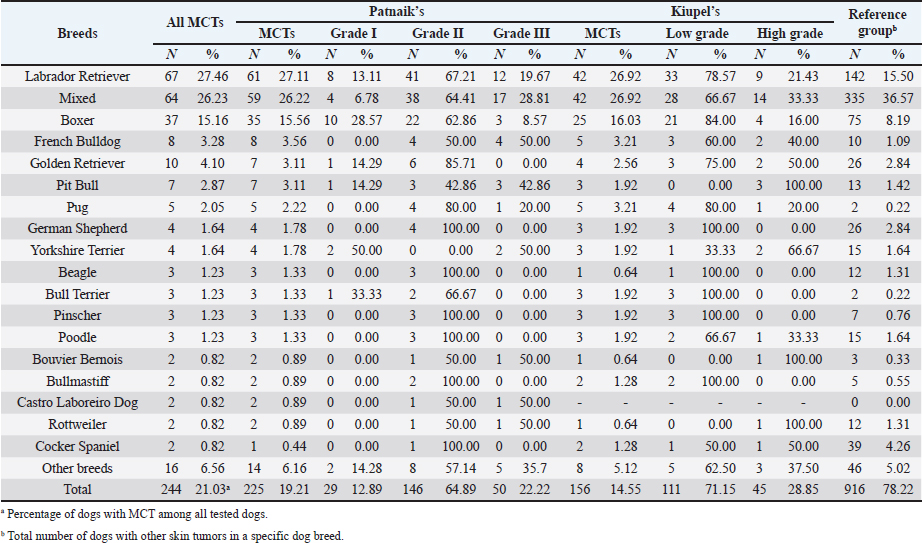
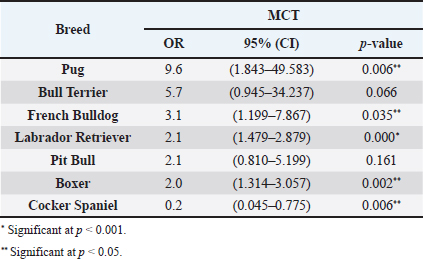
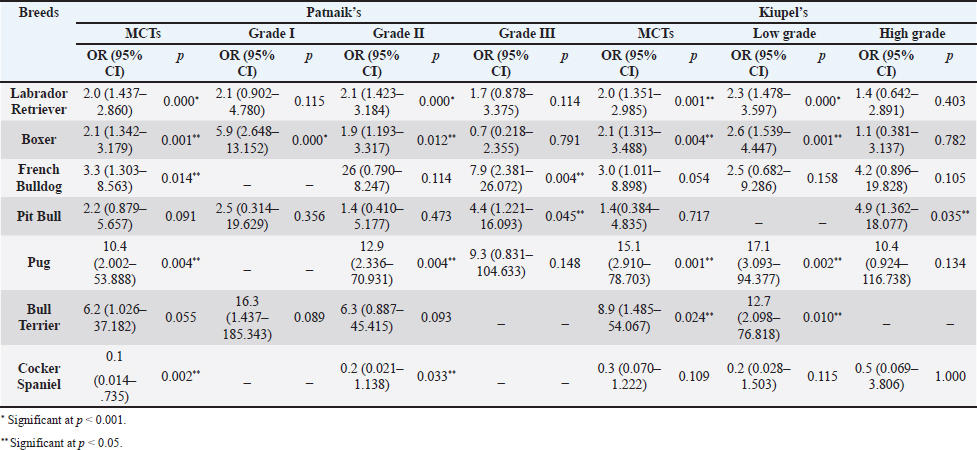
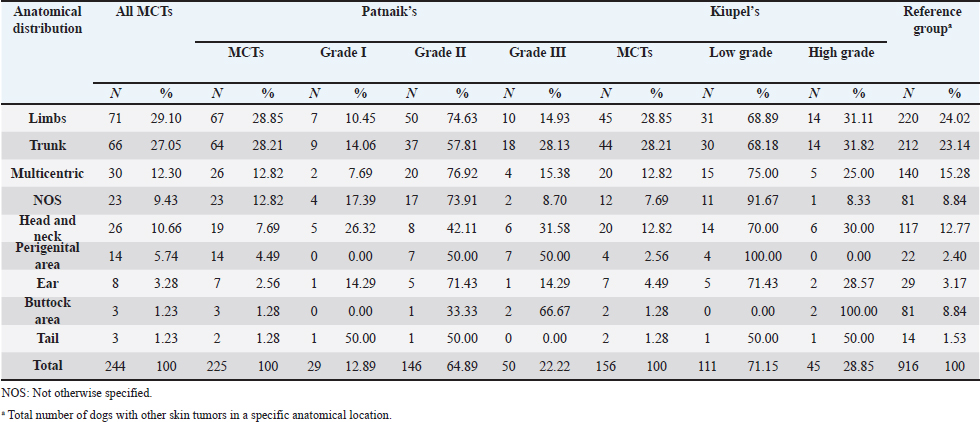
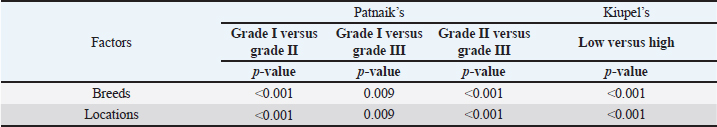
AbstractBackground: Skin tumors are the most frequently diagnosed lesions, of which 7%–21% are mast cell tumors (MCTs). There is a great effort to identify factors that can influence the prospective course of MCTs. Although, the histological grade is considering an important predictor helping to determine the malignancy and metastatic potential of MCTs. Aim: In this study, an epidemiological analysis of risk factors (breed, age, sex, and anatomical site) for dogs having MCTs was evaluated considering the respective MCTs histological grade in comparison to other skin tumors. Methods: The study included 244 dogs affected by cutaneous MCTs from a universe of 1,185 dogs diagnosed with skin tumors. A univariable analysis with Fisher exact test was performed to determine the odds ratios (ORs) with 95% confidence intervals (CIs). Results: Boxers had a higher predisposition to Patnaik’s grade I (OR=5.9, 95% CI 2.648–13.152) and to Kiupel’s low-grade MCTs (OR=2.6, 95% CI 1.539–4.447). Labrador retrievers (OR=2.1, 95% CI 1.423–3.184), and pugs (OR=12.9, 95% CI 2.336–70.931) had a predisposition for Patnaik’s grade II MCTs and Kiupel’s low-grade lesions (OR=2.3, 95% CI 1.478–3.597; OR=17.1, 95% CI 3.093–94.377, respectively). French bulldogs had a higher risk to grade III MCTs (OR=7.9, 95% CI 2.381–26.072). Pit bulls had a predisposition to grade III MCTs and Kiupel’s high-grade tumors (OR=4.4, 95% CI 1.221–16.1 and OR=4.962, 95% CI 1.362–18.077, respectively). Bull terriers (OR=12.7, 95% CI 2.098–76.818) presented higher risk for having low-grade MCTs. The perigenital area and trunk exhibit a greater risk for high grading lesion (OR=6.6, 95% CI 2.679–16.334; OR=1.9, 95% CI 1.028–3.395, respectively) and the limbs had a predisposition to grade II tumor (OR=1.6, 95% CI 1.134–2.395). A decreased risk of having MCT was seen in older dogs (from 7–10 years to 11–18 years) compared to that in the reference group (4–6 years). Conclusion: When comparing to canine skin tumors, this study showed a relationship between MCT histological grading and the risk factors, age, breed, and topography of canine MCTs. The variations noted in the clinical presentation of MCTs amongst predisposed dog breeds reinforces the relevance of the genetic background in MCTs carcinogenesis. Keywords: Dog, Kiupel, Mast cell tumor, Patnaik, Risk. IntroductionOne of the leading causes of mortality in dogs is cancer, accounting from 14% to 27% of all deaths in previous studies (Adams et al., 2010; Dobson, 2013). The quantity of diagnosed tumors is continuously rising since improved health care for pets currently broadens their life expectancy, allowing for the diagnosis of late-in-life diseases, such as cancer (Adams et al., 2010; Villamil et al., 2011; Dobson, 2013; Grüntzig et al., 2015; Śmiech et al., 2017, 2019). One of the most frequently diagnosed tumors are skin tumors, and approximately 7%–21% are mast cell tumors (MCTs) (Welle et al., 2008; Grüntzig et al., 2015). MCTs can be presented as isolated, small, and single case or have a multicentric growth (Blackwood et al., 2012). Plus they can infiltrate the neighboring tissues and metastasize to lymphatic system and internal organs, displaying a wide-ranging clinical course of action (Misdorp, 2004; Murphy et al., 2004; Welle et al., 2008; Blackwood et al., 2012; Śmiech et al., 2018). There is a great effort to identify factors that can influence the prospective course of this disease (Warland and Dobson, 2013; Śmiech et al., 2017, 2018, 2019). One of the most important tumor predictors that can determine its morphological characteristics, metastatic potential, response to treatment as well as prognosis is the histological grade (Zemke et al., 2002; Murphy et al., 2004; Wu et al., 2006; Dobson and Scase, 2007; Romansik et al., 2007; Fröberg et al., 2009; Brønden et al., 2010; Berlato et al., 2012; Costa Poggiani et al., 2012). Prior to 2011, the most commonly used MCTs histological grading system was the three-grade Patnaik system which recognizes three different categories: grade I, II, and III (Patnaik et al., 1984; Śmiech et al., 2017). Grade I are well-differentiated tumors and mostly develop in the dermis and less often in subcutaneous tissue (Śmiech et al., 2017; Tamlin et al., 2020). These tumors do not invade nearby tissues, hardly ever metastasize and generally, after surgical removal with clean margins, they do not recur (Tamlin et al., 2020). They have a predictably good long-term prognosis and a 12-month survival probability up to 100% (Tamlin et al., 2020). Grade II, or intermediate grade tumors, present higher tendency to invade neighboring, extend into the deeper layers of the skin and disseminate to other parts of the body, in comparison to grade I (Tamlin et al., 2020). When these tumors are incompletely surgical removed or with narrow margins, they have more chances to recur. About 87%–92% of these tumors have a 12-month survival probability (Tamlin et al., 2020). Grade III or high grade tumors are poorly differentiated, invade deep into the skin and underlying tissue and are extremely aggressive, presenting a metastasis rate of 55%–95% (Tamlin et al., 2020). Require aggressive therapeutic management since patients detain a poor long-term prognosis and about 16%–46% probability of 12-month survival (Tamlin et al., 2020). Animal outcome with grade II MCTs can be difficult to predict (Sabattini et al., 2015). In some cases, these lesions can behave either as grade I tumors, or more aggressively, as grade III (Garrett, 2014; Sabattini et al., 2015). An additional challenge is the trustworthiness of the grade evaluated and attributed by the pathologist since there is some degree of subjectivity within this system (Sabattini et al., 2015) . As a result of these challenges related with behavior variability and unpredictable clinical course, Kiupel et al. (2011) proposed a new 2-grade classification system divided only in low-grade and high grade, based on the morphology of the cell’s nucleus and the number of mitotic figures. Low-grade tumors have a higher frequency, usually between 59% and 89%, and high-grade tumors a smaller frequency of 11%–41% (Tamlin et al., 2020). High grade tumors have a more aggressive behavior, a tendency to recur and metastasize, and a reduced survival time. In the case of high-grade MCTs, the median survival time is about 4 months (12-month survival probability of 24%) and for low-grade MCTs is more than 2 years (12-month survival probability is about 95%) (Kiupel et al., 2011; Tamlin et al., 2020). The relationship of the dog’s age, sex, and body weight, in castrated or sterilized dogs with MCTs has been already demonstrated (Dobson et al., 2002; Villamil et al., 2011; White et al., 2011; Warland and Dobson, 2013; Artuković et al., 2014; Leidinger et al., 2014; Grüntzig et al., 2015; Shoop et al., 2015; Grüntzig et al., 2016). Even though MCTs can be found in any segment of the body, several research studies hypothesized a potential prognostic significance for several sites, i.e. digit, scrotal, inguinal, or perianal and mucocutaneous junctions (Misdorp, 2004; Dobson and Scase, 2007; Welle et al., 2008; Blackwood et al., 2012). A particular study showed that tumor anatomical location could be correlated to a better or worse prognosis (Pizzoni et al., 2018). Nevertheless, MCTs can be frequently found on the trunk (50%–60%), limbs (25%–40%), and head and neck (10%) (Welle et al., 2008). MCTs development in dogs can happen at all ages; however, the majority of cases are diagnosed amongst 7.5 and 9 years of age (Misdorp, 2004; Dobson and Scase, 2007; Welle et al., 2008; O’Connell and Thomson, 2013). Numerous studies have shown a breed-related predisposition to certain cutaneous tumors (Fröberg et al., 2009). Nevertheless, the relationship between breed and clinical aspects of disease is not sufficiently explored. A comparison of MCTs grading within each breed, as well as the impact of the other factors will offer more information about the complex biology of these neoplasms and simultaneously, may provide based evidence for the implementation of genetic studies focusing on the etiology. Hence, a retrospective analysis of the risk of having MCTs, and the respective histological grades presented could be extremely helpful for prognosis. There are no epidemiological studies in the veterinary literature based on both the Kiupel and the Patnaik classification system, which results remain the most common used prognostic factors for determining the course of the disease. The aim of this study was to conduct an epidemiological analysis of the risk of dogs having MCT graded by the Patnaik’s and Kiupel’s classification in comparison to other skin tumors, taking into account the dog’s breed, age, sex, and tumor’s anatomical location. Materials and MethodsThe study consisted in the analysis of 1,185 canine cutaneous tumors from the archives of the Laboratory of Veterinary Pathology from Institute of Biomedical Sciences Abel Salazar (LPV-ICBAS), University of Porto, sent for histological evaluation and diagnosed during 2014–2020. Within this group, cases previously diagnosed as cutaneous MCTs were selected and clinicopathological data (breed, age, sex, anatomical location) from each dog were collected. All the cases were revised and subclassified according with at least one of the following classifications systems: the three-grade malignancy scale of Patnaik et al. (1984) and the two-grade malignancy scale of Kiupel et al. (2011). With the clinicopathological data collected, four age groups were distinguished: dogs under 3 year of age, 4–6 years, 7–10 years, and 11–18 years. Additionally, nine anatomical locations were established: (1) buttock area, (2) ear, (3) head and neck, (4) limbs, (5) multicentric, (6) perigenital area, (7) tail, (8) trunk, and (9) Skin not otherwise specified (Skin, NOS). A reference group was defined comprising dogs diagnosed with cutaneous tumors others than MCTs, with the restriction that subcutaneous MCTs were excluded from the analysis. When applicable, z-test and t-test were performed. All the data recorded were submitted to statistical analysis using the IBM® Statistical Package for the Social Sciences® (SPSS®) Statistics version 26 (IBM SPSS, Armonk, NY). Values of p < 0.05 were considered to be significant. The risk of having MCT according to breed, sex, location, and age was determined based on the odds ratio (OR). Univariable analysis with Fisher exact test was executed for each variable to determine the ORs with 95% confidence intervals (CIs). For each specific breed, ORs were calculated by comparing the MCT incidence in the analyzed breed with that in the other breeds diagnosed with other cutaneous tumors (reference group). Analogous calculations were conducted for tumor location. For the calculations of ORs relative to age, the group of dogs with ages between 4 and 6 years were regarded as the basal group. Males were the basal group in the determination of ORs for sex. Ethical approvalAll the examined samples were collected for diagnostic purposes as a part of routine and standard care and based on the best clinical judgement of their attending practitioners. The investigators had no influence on the execution of any clinical procedure and only used the data resulting from the histopathological diagnosis. ResultsIn this study, 244 MCTs were retrieved from LPV-ICBAS database, accounting for 21.03% of all cutaneous tumors diagnosed. All 244 MCTs were classified with at least one of this classification systems, when possible both classifications were used. The analysis involved 244 cases of cutaneous MCTs from 222 dogs, representing a total of 34 breeds (33 purebreds and mixed breed). Some breeds were represented in the reference group but not in the MCTs group due to the low frequency reflected in the later and for that reason, 44 breeds of the reference group were replaced into a subgroup, named “other breeds.” Patnaik and Kiupel classification systems137 MCT cases were classified with both systems, 88 and 19 cases were classified only with Patnaik’s or Kiupel’s system, respectively. Out of the total, we had 225 cases classified with the Patnaik classification system and 156 with Kiupel classification system. Breed’s evaluationThe greatest number of MCTs in purebreds were diagnosed in Labrador Retrievers (n=67; 27.46%) followed by Boxers (n=37; 15.16%), Golden retrievers (n=10; 4.10%), French bulldogs (n=8; 3.28%), Pit bulls (n=7; 2.87%), and Pugs (n=5; 2.05%) (Table 1). Mixed breed dogs were the second most common, when considering all breeds (n=64, 26.23%). Data on the frequencies of breeds according to Patnaik and Kiupel classification system were similar to the data from all MCTs and are presented in Table 1. Differences in the proportion of breeds amongst the grades of each classification system were found (Table S1). Regarding Patnaik’s scheme, Z-test was calculated between grade I versus grade II (p-value=<0.001), grade I versus grade III (p-value=0.009), and grade II versus grade III (p-value=<0.001). In Kiupel’s classification system between low grade versus high grade (p-value=<0.001). The highest predisposition for MCT (Table 2), in comparison to other cutaneous tumors, was detected in Pugs (OR=9.6; 95% CI 1.843–49.583), French bulldogs (OR=3.1; 95% CI 1.199–7.867), Labrador retrievers (OR=2.1; 95% CI 1.479–2.879), and Boxers (OR=2.0; 95% CI 1.314–3.057). Cocker spaniels (OR=0.2; 95% CI 0.045–0.775) also had a significant value thought for a decreased predisposition to having MCTs. Concerning breed predisposition, in cases classified with Patnaik’s or Kiupel’s histological grade (Table 3), some breeds presented increased risk for having MCT, though in the general analysis of MCTs (i.e., without classification discrimination–—Table 2) they do not show increased risk. In the Patnaik’s classification system (Table 3), Boxers had a higher predisposition to grade I and grade II (OR=5.9; 95% CI 2.648–13.152; and OR=1.9; 95% CI 1.193–3.317, respectively). Two other breeds had an increased risk for grade II MCTs, namely Labrador retrievers (OR=2.1; 95% CI 1.423–3.184) and Pugs (OR=12.9; 95% CI 2.336–70.931). French bulldogs and Pit bulls have a noted predisposition to grade III MCTs (OR=7.9; 95% CI 2.381–26.072 and OR=4.4; 95% CI 1.221–16.093, respectively). For Kiupel’s (Table 3), in terms of low-grade MCTs, Labrador retrievers (OR=2.3; 95% CI 1.478–3.597), Boxers (OR=2.6; 95% CI 1.539–4.447), Pugs (OR=17.1; 95% CI 3.093–94.377), and Bull terriers (OR=12.7; 95% CI 2.098–76.818) presented higher risk. Furthermore, a predisposition to high-grade tumors was noted in pit bulls (OR=4.9; 95% CI 1.362–18.077). Anatomical distribution evaluationIn terms of anatomical distribution, the greatest numbers of MCTs were noted in the limbs (n=71, 29.10%) and they were dominated by Patnaik’s grade II and Kiupel’s low-grade tumors (n=50, 74.63% and n=31, 68.89%, respectively), followed by trunk (n=66, 27.05%), multicentric growth (n=30, 12.30%), and head and neck (n=26, 10.66%). All these three locations had the highest frequency on grade II (n=37, 57.91%; n=20, 76.92% and n=8, 42.11%, respectively) and low-grade tumors (n=30, 68.18%; n=15, 75.00% and n=14, 70.00%, respectively) (Table 4). The highest frequency for the grade III and high-grade tumors were for buttock area (n=2, 66.67% and n=2, 100.00%, respectively), perigenital area (n=7, 50.00% in grade III), and tail (n=1, 50.00% in high grade) (Table 4). Z-test was calculated for anatomical location in the two classification systems and a difference in proportions was seen (Table S1). Regarding Patnaik’s classification system, z-test was calculated between grade I versus grade II (p-value=<0.001), grade I versus grade III (p-value=0.009), and grade II versus grade III (p-value=<0.001). In Kiupel’s classification system between low grade versus high grade (p-value=<0.001). Table 1. Frequency of MCTs in various breeds of dogs, in general and according to the Patnaik grading system and the Kiupel grading system.
Table 2. ORs and 95% CIs for MCT in various dog breeds. 2-sided Fisher exact test.
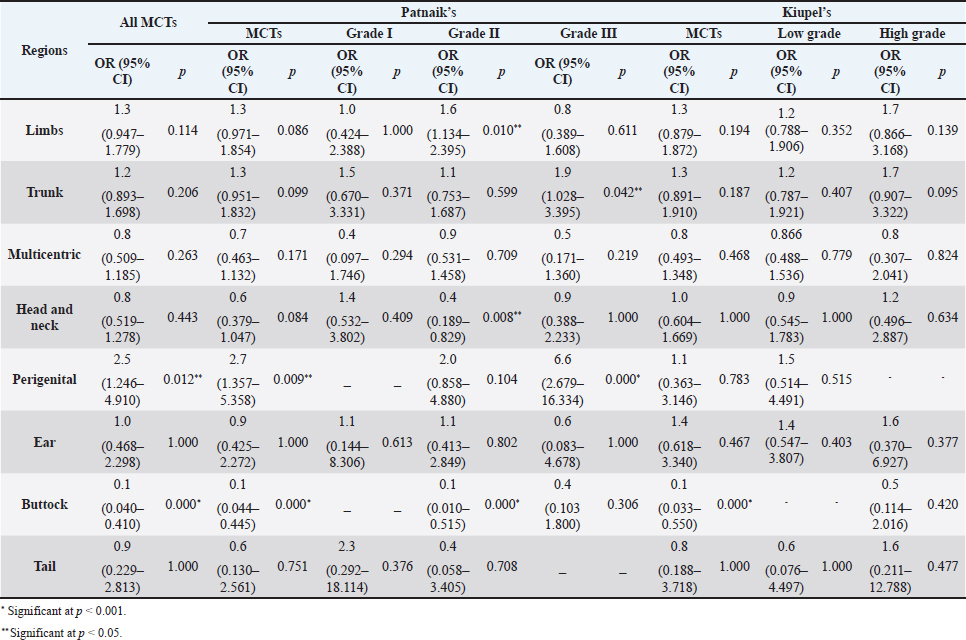
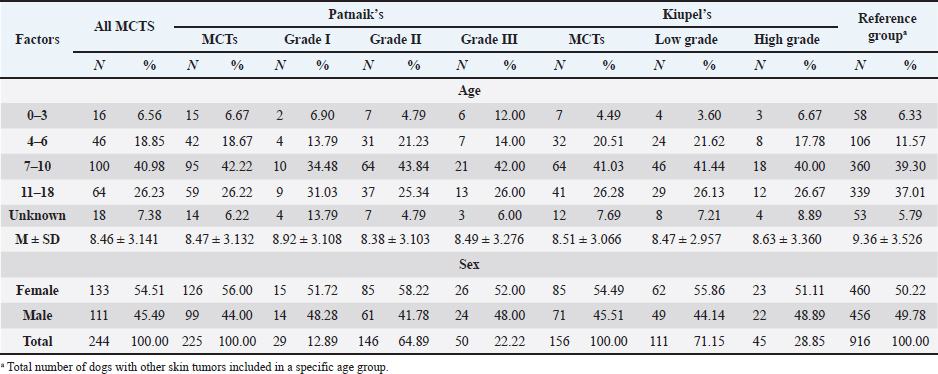
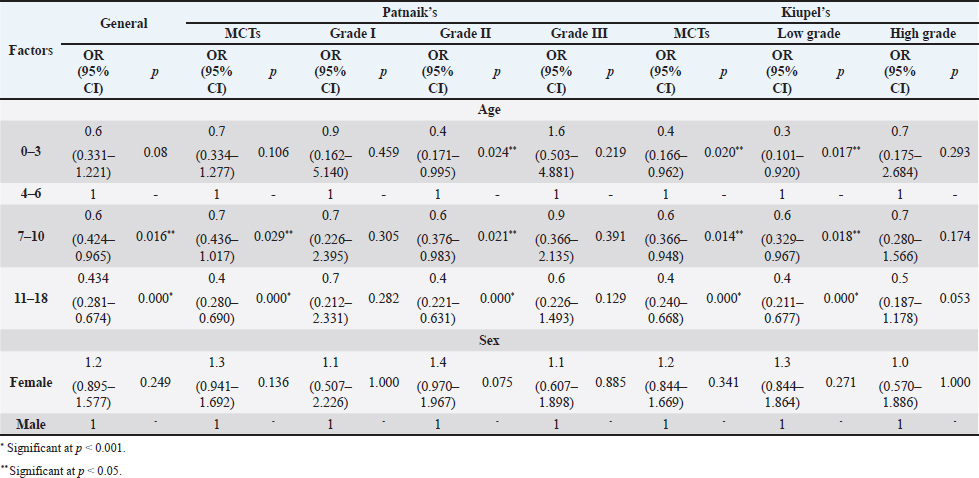
The perigenital area was considered of significant highest risk for having cutaneous MCT (OR=2.5; 95% CI 1.246–4.910) (Table 5). In contrast, buttock area presented a decreased risk for having these neoplasms (OR=0.1; 95% CI 0.040–0.410). In the analysis of the lesions distribution when classified according with Patnaik system, the perigenital area exhibits a substantially greater risk of being affected with a high grading (grade III) lesion (OR=6.6; 95% CI 2.679–16.334) (Table 5). Another location with high OR and high risk for grade III was the trunk (OR=1.9; 95% CI 1.028–3.395). In turn, the limbs were found to be the region with the highest risk for having grade II tumor (OR=1.6; 95% CI 1.134–2.395). Buttock area, head, and neck presented a decreased risk for grade II tumors (OR=0.1; 95% CI 0.010–0.515 and OR=0.4; 95% CI 0.189–0.829, respectively). Based on Kiupel classification, MCTs distribution amongst the different anatomical regions did not display significant values (Table 5). Sex and age-group evaluationData representing the frequency of MCTs in the analyzed canine population considering dog’s sex and the four established age groups are shown in Table 6. The frequency of MCTs is higher in the older age groups (7–0 and 11–18 years, accounting for 40.98% and 26.23%, respectively). In terms of the frequency distribution in the classification systems (Table 6), for Patnaik’s system in grade I, II, and III majority of the frequencies were in the two oldest groups (7–10 and 11–18 years) and in Kiupel’s system, for low grade and high grade, the same distribution occurs. All age groups had majority of cases classified as grade II or lower (Table 6). Female dogs (54.51%) were most affected than males (45.49%). As shown in Table 6, even when analyzing female and male dogs’ distribution within the histological grading’s systems, the same tendency was seen. A difference in mean age was seen between the total MCT group and the reference group; between MCTs classified with the Patnaik classification system and the reference group as well as between MCTs classified with the Kiupel classification system and the reference group (Table S2). For the age group risk evaluation, an analysis comparing the other groups with the youngest dog group (0–3 years) was performed; however, no significant outcome was noted. However, since, the younger group had the lower frequency in comparison with the other groups, we performed another analysis with a different reference group. A risk analysis was performed comparing the others with the group of 4–6 years. In this analysis, we encounter a decreased risk (OR=0.6; 95% CI 0.424–0.965 and OR=0.4; 95% CI 0.281–0.674) for having MCT in the older dogs (from 7 to 10 years and 11 to 18 years, respectively) compared to that in the reference group (4–6 years) (Table 7). There was no significant value for the youngest group of less than 3 years old. In the Patnaik distribution for grade II tumors, all of the groups (0–3, 4–6, and 7–10 years) had a decreased risk in relation to the group of 4–6 year-old dogs (OR=0.4; 95% CI 0.171–0.995; OR=0.6; 95% CI 0.376–0.983 and OR=0.4; 95% CI 0.221–0.631, respectively) (Table 7) For the Kiupel distribution in the low-grade tumors, there was also a decreased risk for all the groups (0–3, 4–6, and 7–10 years) (OR=0.3; 95% CI 0.101–0.920; OR=0.6; 95% CI 0.329–0.967 and OR=0.4; 95% CI 0.211–0.677, respectively) (Table 7). There was not noted risk for gender. Table 3. ORs and 95% CIs for MCT in various dog breeds according to the Patnaik’s and Kiupel’s grading system. (p) p-value. 2-sided Fisher exact test.
Table 4. Frequency of MCTs in general and according to Patnaik’s classification systems and Kiupel’s classification system by tumor location.
Table 5. ORs and 95% CIs for MCT in various anatomical locations, in all general MCTs and according to Patnaik’s and Kiupel’ classification systems. (p) p-value. 2-sided fisher exact test.
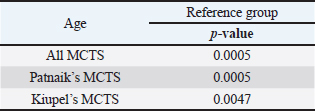
Table 6. Frequency of MCT in general and according to Patnaik and Kiupel’s classification by age and sex. (M) Mean and (SD) Standard deviation.
Table 7. ORs and 95% CIs for MCT for age and sex according to Patnaik and Kiupel’s classification system. (p) p-value. 2-sided fisher exact test.
DiscussionMCTs accounted for 21.03% of all examined skin tumors which corresponds to the frequency found in other studies (7%–21%) (Finnie and Bostock, 1979; Welle et al., 2008; Villamil et al., 2011). The results of this study indicated an increased risk for having MCT in four breeds: Labrador retriever, Boxer, French bulldog, and Pugs (Table 2). The results were similar to those found by other authors, namely, in the UK (where the highest risk was predicted for Boxers, Labrador retrievers, golden retrievers, and Staffordshire bull terriers) and in Poland (Shar-pei, American Staffordshire terrier, Labrador retriever, French bulldog, and Boxer) (Warland and Dobson, 2013; Śmiech et al., 2018). Variances in MCTs incidence amongst breeds can be linked to the geographical region where the study was performed, breed preferences or popularity in that geographical area and also to the choice of the reference group, since in some studies it comprised insured populations (Dobson et al., 2002; Warland and Dobson, 2013), registration in kennel associations (Warland and Dobson, 2013; Leidinger et al., 2014), or hospitalized dogs (Villamil et al., 2011; White et al., 2011; Warland and Dobson, 2013). In this study, the reference group comprised dogs with skin tumors, free of cutaneous MCTs. In contrast to previous results (Warland and Dobson, 2013; Śmiech et al., 2018, 2019), herein Golden retrievers were not found to have a significant association with MCT occurrence, highlighting possible differences amongst the study populations. However, regardless of all the putative variations factors previously mentioned, an overall increase risk of having MCTs in Boxers has been consistently noted (Villamil et al., 2011; White et al., 2011; Warland and Dobson, 2013; Leidinger et al., 2014; Shoop et al., 2015), and it was also corroborated in this study. Additionally, the statistical analysis also revealed an increased risk in French bulldogs. In the literature, there is a hypothesis that Boxers and French bulldogs may be genetically related, sharing a common ancestor in their phylogenetic evolution which can possibly explain this specific breed susceptibility to this particular tumor histotype (Peters, 1969; Dobson, 2013). Nevertheless, further studies are needed to unveil this particular association. Predisposition for having MCTs in Labrador retrievers has been shown in previously studies (White et al., 2011; Warland and Dobson, 2013). These observations were also confirmed in this study with this breed presenting increased risk for Kiupel low grade tumors and for Patnaik grade II tumors, as already suggested by others (Śmiech et al., 2018). However, some studies indicate a higher risk for more aggressive tumors, which was not shown here. Nevertheless, grade II tumors may display variations in behavior and can act either more like grade I tumors or more aggressively, like grade III tumors. Boxers and Pugs are characterized by higher susceptibility for having low grade MCTs as seen in multiple available published data (Bostock, 1986; McNiel et al., 2006; London and Thamm, 2012; Dobson, 2013; Mochizuki et al., 2017). Our analysis confirmed these findings and demonstrated an increased risk for lower grade tumors, in both classification systems. Boxers depicted an increased risk for Kiupel low-grade tumors and for Patnaik grade I and II lesions. In Pugs, there was an increased risk to Kiupel’s low-grade lesions and an increased risk for Patnaik grade II lesions; however, there was no data available in our series for grade I analysis in this specific breed. French bulldogs had an increased risk for having this specific tumor which goes toward previous observations (Śmiech et al., 2018, 2019). However, only in Patnaik’s classification system, an increased risk for overall tumor development and for having grade III tumors was achieved. These findings clearly contrast with those publicized that claimed that this breed presents an increased risk for low-grade MCTs (Śmiech et al., 2017, 2018, 2019). Moreover, Bull terrier and Pit bull, although not revealing an increased risk for overall MCT development, both presented significant results in the analysis performed for each classification system. Bull terriers, within Kiupel’s classification system displayed an increases risk for low-grade MCT; while, within Patnaik system, this breed also had an increased risk, but did not reach statistical significance. For Pit bulls, in both classification systems, even though with no increased risk noted in overall MCT development, an increased risk for high grade and Grade III tumors was encountered, which is in concordance with results from other studies (Trappier et al., 2014). Both Pit bulls and Bull terriers are a result of a cross between Bulldogs and Terriers (Definitive and Guide, 2013). Therefore, underlying genetic factors can probably play a role in increasing the risk of these breeds to this type of lesions, as well as other related breeds such as Staffordshire bull terrier. As indicated in the literature, MCTs with a different presentation occur in dog breeds that are phylogenetically related with Bulldogs; for instance, Boxers have a predisposition to lower grade tumors as seen in several studies and American Staffordshire terrier may be at increased risk for high-grade tumors (Śmiech et al., 2019) and again, both breeds are hypothesized to descend from bulldogs and terriers. Thus, further studies are needed for a better comprehension of the possible common genetic background of some breeds and the development of some diseases (Peters, 1969; Dobson, 2013; Mochizuki et al., 2017). The current analysis also demonstrated a decreased risk of having MCT in one breed, Cocker spaniel (Table 2), which is consistent with the literature (Villamil et al., 2011; Warland and Dobson, 2013; Shoop et al., 2015). Differences between the risk according with both classification systems, can be partially explained by the frequencies variations of these specific breeds in each system. Other processes adding up to the genetic influence compound may be relevant for MCTs carcinogenesis and can be responsible for the biological behavior of tumors in a specific breed. Investigations of mitochondrial DNA conducted in recent years have demonstrated somatic mutations in the mitochondrial DNA D-loop in MCTs, which may also be associated with neoplastic transformation (Śmiech et al., 2016). Since both classification systems had different frequencies (n=225 for Patnaik’s and n=156 for Kiupel’s), this could be responsible for some discrepancies in the analysis. The current investigation demonstrated correlations between the anatomical distribution and having MCTs (Śmiech et al., 2018, 2019). Of all the locations evaluated, the statistical analysis demonstrated that the perigenital area presented the highest risk of having MCTs. In the perigenital area, concerning the risk associated with the histological grading, based on the Kiupel’s system, all the cases were classified as low grade and no increasing risk was detected. However, with Patnaik’s classification, this same location revealed higher risk for having grade III lesions. The analysis of all MCTs demonstrated a decreased risk for the buttock area although this location presented low number of representative cases. Herein, a high risk for higher grade lesions for the perigenital area was found which goes toward the available literature when report the inguinal, scrotal, and perianal areas, as well as the mucocutaneous junctions, as anatomical sites associated with a worse prognosis (Misdorp, 2004; Sfiligoi et al., 2005; Dobson and Scase, 2007; Welle et al., 2008; Blackwood et al., 2012). Nonetheless, a less favorable prognosis is also related to the surgical procedure approach which can lead to an incomplete tumor resection (Murphy et al., 2004; Blackwood et al., 2012). With the Patnaik’s system, an increased risk for grade III tumors in the trunk was noted. As described in the literature, there is a tendency for the development of high-grade MCTs in the inguinal and axillary regions, which in our analysis were included in the trunk area. These results are in accordance with some authors that described a predisposition for tumors of higher grade in this location (Śmiech et al., 2017, 2018). However, putative causative factors for this occurrence should be investigated. For Patnaik grade II tumors, an increased risk was detected in the limbs and a decreased risk was found in the buttock area, head, and neck. With this regard, the published data remains controversial. In some studies, the thoracic limb presented a higher risk of having high grade tumors (Śmiech et al., 2018), while in others, this region presented a higher probability of grade I tumors occurrence (Śmiech et al., 2017). A completely different distribution was shown in a research performed in dogs from Croatia (Grabarević et al., 2009): grade III tumors were found primarily on the pelvic limb and neck. Other findings showed a decreased risk for Kiupel low grade and high grade tumors in the head, and a higher risk for either high grade and low grade tumor in the anus (Śmiech et al., 2018). On the other hand, the head and neck were reported to display a higher probability of being affected by Patnaik grade I tumors (Śmiech et al., 2017). According to the literature, results can be very variable and our findings concerning risk for Patnaik grade II tumors do not fit in those published studies. However, this specific histotype can be very unpredictable in terms of behavior thus, being able to exhibit either more aggressive or less aggressive features. These differences in several studies can be explained by the methodological approaches established, like the different anatomical division of the body regions, simplifying the designation of the body site or merging some areas of the body into a single region (Śmiech et al., 2017). For a more thorough and viable analysis of the MCTs anatomical distribution prediction, a universal division of the animal body should be used. Currently, we observed a decrease risk of having MCT in adult dogs aged 7–10 and 11–18 years old, acting as a protective factor, in comparison to the reference group previously defined and comprised of younger dogs (4–6 years). Shoop et al. (2015) observed a 41-fold increase risk of MCT development in 10-year-old dogs, although the reference group used was composed of 2-years old dogs. Additionally, Villamil et al. (2011) noted a higher incidence of MCTs in dogs older than 7 years. In the present investigation, the statistical analyses revealed a few correlations amongst dog’s age and the malignancy grade of MCTs. In the Patnaik distribution for grade II tumors and in the Kiupel distribution for low-grade tumors, all of the groups (0–3, 4–6 and 7–10 years) had a decreased risk, acting as a protective factor, in relation to the reference group. These results, contradict other researches, however the chosen methodology and the reference group can influence the results and ours is different from other studies. Also, the proportions of the different age groups differ between studies. In most epidemiological studies, no correlations were exhibited between the age and the risk of developing different MCT histological grades (Villamil et al., 2011; Shoop et al., 2015). Regarding the risk of having MCTs in females and males, there are many discrepancies in the veterinary literature although the great majority claim that there is no association (Miller, 1995; Dobson and Scase, 2007; Welle et al., 2008; Shoop et al., 2015; Pizzoni et al., 2018). These current results confirmed the absence of this association. However, some published statistics suggest that castration and sterilization increase risk of having MCT, (Kiupel et al., 2005; White et al., 2011; Zink et al., 2014; Mochizuki et al., 2017) underlining a possible role of sex hormones in their oncogenesis. As the reproductive status of the target canine population under study was not identified, the conclusions herein obtained cannot be properly interpreted. Overall, both classifications presented similar results with regard to the risk analysis of the variables in question (breed, location of MCTs lesions, age, and sex). However, there are some disparities that might be caused by the sampling method and the reduced number of cases. We emphasize, as limitations, that the data presented are obtained exclusively from a national laboratory which limits the sample size and may influence the results. Additionally, results regarding global risk factors for MCTs development could also be influenced by the reference group selected, since this was calculated based on the group of skin tumors and not a general reference group. Notwithstanding the advances in MCT treatment procedures and prognostic aspects, the etiology of this disease has not been yet entirely clarified (Blackwood et al., 2012; Kandefer-Gola et al., 2015a, 2015b, 2015c, 2015d; Sledge et al., 2016). This study demonstrated a relationship between histological grading system and clinicopathological characteristics, such as age, and location of canine MCT. Retrospective studies conducted in large animal populations present a valuable contribution to the clinical nature of MCTs knowledge. Information obtained in the present study can be used for the prediction or to determine the impact of several risk factors in breeds that are predisposed to having MCTs. The variations noted in the clinical presentation of MCTs amongst predisposed dog breeds reinforces the relevance of the genetic background in MCTs carcinogenesis. Must be highlighted that modern-day dog breeds were designed via selection of particular phenotypical characteristics and inbreeding decreases genetic variation and ends in the development of numerous hereditary disorders, which may include neoplasia. AcknowledgmentsThe authors would like to thank the support received in the Biochemistry Master of the ICBAS and Faculty of Sciences (FCUP), University of Porto. This article reports the research’s Master work of Ana Luísa Martins under the supervision of Irina Amorim and Fátima Gärtner. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionsALM wrote the manuscript. ALM collected and analyzed the data. FF carried out the HE and IHC staining. JRM performed the statistical analysis. IA and FG critically revised the manuscript for important intellectual content. FG and IA design of the study. All authors read and approved the final manuscript. ReferencesAdams, V.J., Evans, K.M., Sampson, J. and Wood, J.L.N. 2010. Methods and mortality results of a health survey of purebred dogs in the UK. J. Small Anim. Pract. 51(10), 512–524. Artuković, B., Medven, L., Hohšteter, M., Šoštarić-Zuckermann, I.C., Kurilj, A.G., Beck, A., Huber, D., Grabarević, D., Severin, K. and Grabarević, Ž. 2014. Prevalence of cutaneous mast cell sarcoma in dogs in Croatia. Vet. Arh. 84(6), 601–614. Berlato, D., Stewart, J., Newton, R., Maglennon, G.A., Monti, P., Flindall, A. and Murphy, S. 2012. Evaluation of minichromosome maintenance protein 7 as a prognostic marker in canine cutaneous mast cell tumours. Vet. Comp. Oncol. 10(2), 135–142. Blackwood, L., Murphy, S., Buracco, P., De Vos, J.P., De Fornel-Thibaud, P., Hirschberger, J., Kessler, M., Pastor, J., Ponce, F., Savary-Bataille, K. and Argyle, D.J. 2012. European consensus document on mast cell tumours in dogs and cats. Vet. Comp. Oncol. 10(3), e1–e29. Bostock, D.E. 1986. Neoplasms of the skin and subcutaneous tissues in dogs and cats. Br. Vet. J. 142(1), 1–19. Brønden, L.B., Eriksen, T. and Kristensen, A.T. 2010. Mast cell tumours and other skin neoplasia in Danish dogs–data from the Danish Veterinary Cancer Registry. Acta Vet. Scand. 52(1), 6. Costa Poggiani, S., Maria Terra, E., Torres Neto, R., Tinucci Costa, M. and Laufer Amorim, R. 2012. Canine cutaneous mast cell tumor: biologic behavior and its correlation with prognostic indicators. Open J. Vet. Med. 2(4), 255–261. Definitive, T.H.E. and Guide, V. 2013. The dog encyclopedia: the definitive visual guide. London, UK: Dorling Kindersley. Dobson, J.M., Samuel, S., Milstein, H., Rogers, K. and Wood, J.L.N. 2002. Canine neoplasia in the UK: estimates of incidence rates from a population of insured dogs. J. Small Anim. Pract. 43, 240–246. Dobson, J.M. and Scase, T.J. 2007. Advances in the diagnosis and management of cutaneous mast cell tumours in dogs. J. Small Anim. Pract. 48(8), 424–431. Dobson, J.M. 2013. Breed-predispositions to cancer in pedigree dogs. ISRN Vet. Sci. 2013, 94127. Finnie, J.W. and Bostock, D.E. 1979. Skin neoplasia in dogs. Aust. Vet. J. 55(12), 602–604. Fröberg, G.K., Lindberg, R., Ritter, M. and Nordlind, K. 2009. Expression of serotonin and its 5-HT1A receptor in canine cutaneous mast cell tumours. J. Comp. Pathol. 141(2–3), 89–97. Garrett, L. 2014. Canine mast cell tumors: diagnosis, treatment, and prognosis. Vet. Med. Res. Rep. 5, 49–58. Grabarević, Ž., Špoljar, J.B., Kurilj, A.G., Šoštarić-Zuckermann, I.C., Artuković, B., Hohšteter, M., Beck, A., Džaja, P. and Strmečki, N.M. 2009. Mast cell tumor in dogs–incidence and histopathological characterization. Coll. Antropol. 33(1), 253–258. Grüntzig, K., Graf, R., Hässig, M., Welle, M., Meier, D., Lott, G., Erni, D., Schenker, N.S., Guscetti, F., Boo, G., Axhausen, K., Fabrikant, S., Folkers, G. and Pospischil, A. 2015. The Swiss Canine Cancer Registry: a retrospective study on the occurrence of tumours in dogs in Switzerland from 1955 to 2008. J. Comp. Pathol. 152, 161–171. Grüntzig, K., Graf, R., Boo, G., Guscetti, F., Hässig, M., Axhausen, K.W., Fabrikant, S., Welle, M., Meier, D., Folkers, G. and Pospischil, A. 2016. Swiss Canine Cancer Registry 1955–2008: occurrence of the most common tumour diagnoses and influence of age, breed, body size, sex and neutering status on tumour development. J. Comp. Pathol. 155(2–3), 156–170. Kandefer-Gola, M., Nowak, M., Madej, J.A., Dzimira, S., Ciaputa, R. and Janus, I. 2015a. Useful immunohistochemical indicators in canine mast cell tumours. Acta Vet. Hung. 63(1), 49–59. Kandefer-Gola, M., Nowak, M., Dzimira, S., Janus, I. and Ciaputa, R. 2015b. COX-2 and mPGES-1 expression in canine mast cell tumours. Med. Weter 71, 1–5. Kandefer-Gola, M., Madej, J.A., Dzimira, S., Nowak, M., Janus, I. and Ciaputa, R. 2015c. Comparative analysis of markers of cell proliferation in canine mast cell tumours according to current classifications. Pol. J. Vet. Sci. 18(2), 241–247. Kandefer-Gola, M., Madej, J.A., Dzimira, S., Janus, I., Nowak, M. and Ciaputa, R. 2015d. Immunohistochemical evaluation of neoangiogenesis in canine mast cell tumours. Bull. Vet. Inst. Pulawy. 37(12), 1642–1646. Kiupel, M., Webster, J.D., Miller, R.A. and Kaneene, J.B. 2005. Impact of tumour depth, tumour location and multiple synchronous masses on the prognosis of canine cutaneous mast cell tumours. J. Vet. Med. Ser. A Physiol. Pathol. Clin. Med. 52(6), 280–286. Kiupel, M., Webster, J.D., Bailey, K.L., Best, S., DeLay, J., Detrisac, C.J., Fitzgerald, S.D., Gamble, D., Ginn, P.E., Goldschmidt, M.H., Hendrick, M.J., Howerth, E.W., Janovitz, E.B., Langohr, I., Lenz, S.D., Lipscomb, T.P., Miller, M.A., Misdorp, W., Moroff, S., Mullaney, T.P., Neyens, I., O'Toole, D., Ramos-Vara, J., Scase, T.J., Schulman, F.Y., Sledge, D., Smedley, R.C., Smith, K.W., Snyder, P., Southorn, E., Stedman, N.L., Steficek, B.A., Stromberg, P.C., Valli, V.E., Weisbrode, S.E., Yager, J., Heller, J. and Miller, R. 2011. Proposal of a 2-tier histologic grading system for canine cutaneous mast cell tumors to more accurately predict biological behavior. Vet. Pathol. 48(1), 147–155. Leidinger, E.F., Freeman, K., Kirtz, G., Hooijberg, E.H. and Sick, K. 2014. Breed related odds ratio and anatomic distribution of canine mast cell tumours in Austria: retrospective study of cases in the years 2000–2010. Tierarztl. Prax. Ausgabe K Kleintiere–Heimtiere 42(6), 367–373. London, C.A. and Thamm, D.H. 2012. Mast cell tumors. Withrow MacEwen’s Small Animal Clinical Oncology, 5th ed. St. Louis, MO: Saunders Elsevier, pp: 126–130. McNiel, E.A., Prink, A.L. and O’Brien, T.D. 2006. Evaluation of risk and clinical outcome of mast cell tumours in pug dogs. Vet. Comp. Oncol. 4(1), 2–8. Miller, D.M. 1995. The occurrence of mast cell tumors in young Shar-Peis. J. Vet. Diagn Invest. 7(3), 360–363. Misdorp, W. 2004. Mast cells and canine mast cell tumours. A review. Vet. Q. 26(4), 156–169. Mochizuki, H., Motsinger-Reif, A., Bettini, C., Moroff, S. and Breen, M. 2017. Association of breed and histopathological grade in canine mast cell tumours. Vet. Comp. Oncol. 15, 829–839. Murphy, S., Sparkes, A.H., Smith, K.C., Blunden, A.S. and Brearley, M.J. 2004. Relationships between the histological grade of cutaneous mast cell tumours in dogs, their survival and the efficacy of surgical resection. Vet. Rec. 154(24), 743–746. O’Connell, K. and Thomson, M. 2013. Evaluation of prognostic indicators in dogs with multiple, simultaneously occurring cutaneous mast cell tumours: 63 cases. Vet. Comp. Oncol. 11(1), 51–62. Patnaik, A.K., Ehler, W.J. and MacEwen, E.G. 1984. Canine cutaneous mast cell tumor: morphologic grading and survival time in 83 dogs. Vet. Pathol. 21(5), 469–474. Peters, J.A. 1969. Canine mastocytoma: excess risk as related to ancestry. J. Natl. Cancer Inst. 42(3), 435–443. Pizzoni, S., Sabattini, S., Stefanello, D., Dentini, A., Ferrari, R., Dacasto, M., Giantin, M., Laganga, P., Amati, M., Tortorella, G. and Marconato, L. 2018. Features and prognostic impact of distant metastases in 45 dogs with de novo stage IV cutaneous mast cell tumours: a prospective study. Vet. Comp. Oncol. 16(1), 28–36. Romansik, E.M., Reilly, C.M., Kass, P.H., Moore, P.F. and London, C.A. 2007. Mitotic index is predictive for survival for canine cutaneous mast cell tumors. Vet. Pathol. 44(3), 335–341. Sabattini, S., Scarpa, F., Berlato, D. and Bettini, G. 2015. Histologic grading of canine mast cell tumor: is 2 better than 3? Vet. Pathol. 52, 70–73. Sfiligoi, G., Rassnick, K.M., Scarlett, J.M., Northrup, N.C. and Gieger, T.L. 2005. Outcome of dogs with mast cell tumors in the inguinal or perineal region versus other cutaneous locations: 124 cases (1990–2001). J. Am. Vet. Med. Assoc. 226(8), 1368–1374. Shoop, S.J., Marlow, S., Church, D.B., English, K., McGreevy, P.D., Stell, A.J., Thomson, P.C., O’Neill, D.G. and Brodbelt, D.C. 2015. Prevalence and risk factors for mast cell tumours in dogs in England. Canine Genet. Epidemiol. 2(1), 1; doi:10.1186/2052-6687-2-1. Sledge, D.G., Webster, J. and Kiupel, M. 2016. Canine cutaneous mast cell tumors: a combined clinical and pathologic approach to diagnosis, prognosis, and treatment selection. Vet. J. 215, 43–54. Śmiech, A., Ślaska, B., Surdyka, M., Grzybowska-Szatkowska, L., Łopuszyński, W. and Różańska, D. 2016. Identification of additional mitochondrial DNA mutations in canine mast cell tumours. Acta Vet. Scand. 58(1), 28; doi:10.1186/s13028-016-0210-y. Śmiech, A., Ślaska, B., Łopuszyński, W., Jasik, A., Szczepanik, M. and Wilkołek, P. 2017. Epidemiological study of canine mast cell tumours according to the histological malignancy grade. Pol. J. Vet. Sci. 20, 455–465. Śmiech, A., Lsopuszyński, W., Ślaska, B., Bulak, K. and Jasik, A. 2019. Occurrence and distribution of canine cutaneous mast cell tumour characteristics among predisposed breeds. J. Vet. Res. 63(1), 141–148. Śmiech, A., Şlaska, B., Łopuszyński, W., Jasik, A., Bochyńska, D. and Da¸browski, R. 2018. Epidemiological assessment of the risk of canine mast cell tumours based on the Kiupel two-grade malignancy classification. Acta Vet. Scand. 60, 1–9. Tamlin, V.S., Bottema, C.D.K. and Peaston, A.E. 2020. Comparative aspects of mast cell neoplasia in animals and the role of KIT in prognosis and treatment. Vet. Med. Sci. 6, 3–18. Trappier, M.C., Popovitch, C.A., Goldschmidt, M.H., Goldschmidt, K.H. and Risbon, R.E. 2014. Scrotal tumors in dogs: a retrospective study of 676 cases (1986–2010). Can. Vet. J. 55(1), 1229–1233. Villamil, J.A., Henry, C.J., Bryan, J.N., Ellersieck, M., Schultz, L., Tyler, J.W. and Hahn, A.W. 2011. Identification of the most common cutaneous neoplasms in dogs and evaluation of breed and age distributions for selected neoplasms. J. Am. Vet. Med. Assoc. 239(7), 960–965. Warland, J. and Dobson, J. 2013. Breed predispositions in canine mast cell tumour: a single centre experience in the United Kingdom. Vet. J. 197, 496–498. Welle, M.M., Bley, C.R., Howard, J. and Rüfenacht, S. 2008. Canine mast cell tumours: a review of the pathogenesis, clinical features, pathology and treatment. Vet. Dermatol. 19(6), 321–339. White, C.R., Hohenhaus, A.E., Kelsey, J. and Procter-Gray, E. 2011. Cutaneous MCTs: associations with spay/neuter status, breed, body size, and phylogenetic cluster. J. Am. Anim. Hosp. Assoc. 47, 210–216. Wu, H., Hayashi, T. and Inoue, M. 2006. Immunohistochemical expression of Mdm2 and p53 in canine cutaneous mast cell tumours. J. Vet. Med. Ser. A Physiol. Pathol. Clin. Med. 53(2), 65–68. Zemke, D., Yamini, B. and Yuzbasiyan-Gurkan, V. 2002. Mutations in the juxtamembrane domain of c-KIT are associated with higher grade MCTs in dogs. Vet. Pathol. 2002, 529–535. Zink, M.C., Farhoody, P., Elser, S.E., Ruffini, L.D., Gibbons, T.A. and Rieger, R.H. 2014. Evaluation of the risk and age of onset of cancer and behavioral disorders in gonadectomized Vizslas. J. Am. Vet. Med. Assoc. 244(3), 309–319. Supplementary MaterialTable S1. Z-test evaluation for proportions within grades for breeds and anatomical locations.
Table S2. T-test evaluation for the factor age, for general group of all MCTs, and according to Patnaik and Kiupel’s classification system.
| ||
| How to Cite this Article |
| Pubmed Style ALM, FF, JM, FG, Amorim I. Analysis of risk factors for canine mast cell tumors based on the Kiupel and Patnaik grading system among dogs with skin tumors. Open Vet J. 2021; 11(4): 619-634. doi:10.5455/OVJ.2021.v11.i4.12 Web Style ALM, FF, JM, FG, Amorim I. Analysis of risk factors for canine mast cell tumors based on the Kiupel and Patnaik grading system among dogs with skin tumors. https://www.openveterinaryjournal.com/?mno=68760 [Access: October 06, 2024]. doi:10.5455/OVJ.2021.v11.i4.12 AMA (American Medical Association) Style ALM, FF, JM, FG, Amorim I. Analysis of risk factors for canine mast cell tumors based on the Kiupel and Patnaik grading system among dogs with skin tumors. Open Vet J. 2021; 11(4): 619-634. doi:10.5455/OVJ.2021.v11.i4.12 Vancouver/ICMJE Style ALM, FF, JM, FG, Amorim I. Analysis of risk factors for canine mast cell tumors based on the Kiupel and Patnaik grading system among dogs with skin tumors. Open Vet J. (2021), [cited October 06, 2024]; 11(4): 619-634. doi:10.5455/OVJ.2021.v11.i4.12 Harvard Style , A. L. M., , . F. F., , . J. M., , . F. G. & Amorim, . I. (2021) Analysis of risk factors for canine mast cell tumors based on the Kiupel and Patnaik grading system among dogs with skin tumors. Open Vet J, 11 (4), 619-634. doi:10.5455/OVJ.2021.v11.i4.12 Turabian Style , Ana Luísa Martins, Fátima Faria, João Mesquita, Fátima Gärtner, and Irina Amorim. 2021. Analysis of risk factors for canine mast cell tumors based on the Kiupel and Patnaik grading system among dogs with skin tumors. Open Veterinary Journal, 11 (4), 619-634. doi:10.5455/OVJ.2021.v11.i4.12 Chicago Style , Ana Luísa Martins, Fátima Faria, João Mesquita, Fátima Gärtner, and Irina Amorim. "Analysis of risk factors for canine mast cell tumors based on the Kiupel and Patnaik grading system among dogs with skin tumors." Open Veterinary Journal 11 (2021), 619-634. doi:10.5455/OVJ.2021.v11.i4.12 MLA (The Modern Language Association) Style , Ana Luísa Martins, Fátima Faria, João Mesquita, Fátima Gärtner, and Irina Amorim. "Analysis of risk factors for canine mast cell tumors based on the Kiupel and Patnaik grading system among dogs with skin tumors." Open Veterinary Journal 11.4 (2021), 619-634. Print. doi:10.5455/OVJ.2021.v11.i4.12 APA (American Psychological Association) Style , A. L. M., , . F. F., , . J. M., , . F. G. & Amorim, . I. (2021) Analysis of risk factors for canine mast cell tumors based on the Kiupel and Patnaik grading system among dogs with skin tumors. Open Veterinary Journal, 11 (4), 619-634. doi:10.5455/OVJ.2021.v11.i4.12 |