| Original Article | ||
Open Vet J. 2022; 12(6): 980-984 Open Veterinary Journal, (2022), Vol. 12(6): 980–984 Original Research Serum concentrations of osteoprotegerin, brain-derived nerve factor, angiotensin II, and endothelin-1 in aging dogsMitsuhiro IsakaLaboratory of Companion Animal Surgery, School of Veterinary Medicine, Rakuno Gakuen University, Hokkaido, Japan Submitted: 14/07/2022 Accepted: 13/11/2022 Published: 15/12/2022 *Corresponding Author: Mitsuhiro Isaka. Laboratory of Companion Animal Surgery, School of Veterinary Medicine, Rakuno Gakuen University, Hokkaido, Japan. Email: m-isaka [at] rakuno.ac.jp © 2022 Open Veterinary Journal
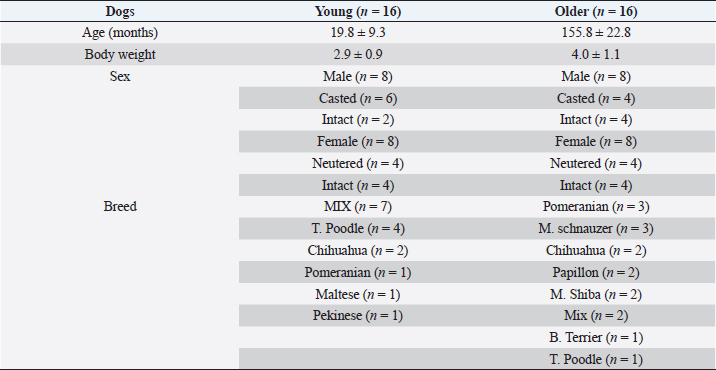
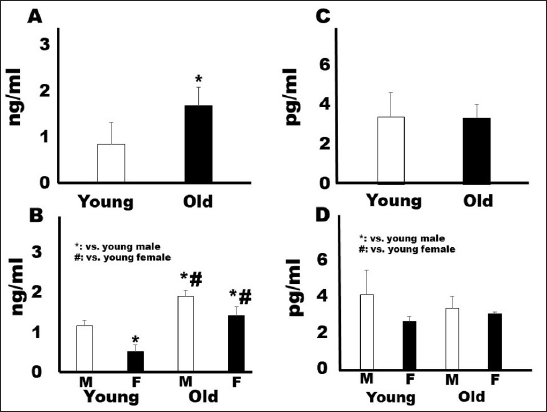
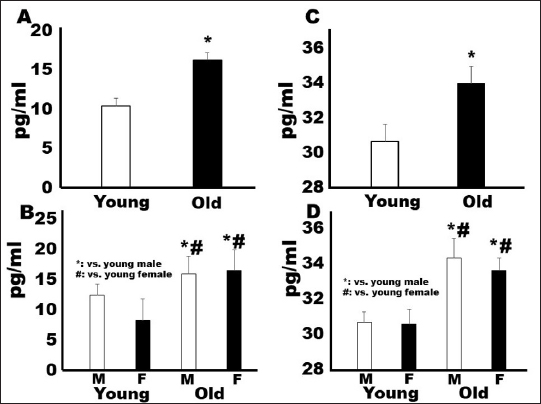
AbstractBackground: Gerontology is a major research topic in veterinary medicine; however, there are few reports on changes in biomarker levels in aged dogs. Aim: The purpose of this preliminary study was to evaluate the differences in serum biomarker levels between young (less than 36 months) and old (over 108 months) companion dogs. Methods: We measured the serum concentrations of brain-derived neurotrophic factor (BDNF), osteoprotegerin (OPG), angiotensin II (ANGII), and endothelin-1 (ET-1) in both groups (young: n=16, 19.8 ± 9.3 months old; old: n=16, 155.8 ± 22.8 months old). Results: Although the concentrations of BDNF did not differ between the two groups, the OPG, ANGII, and ET-1 levels were significantly higher in the old companion dogs than in the young dogs (p < 0.05). Conclusion: OPG, ANGII, and ET-1 concentrations may increase in dogs during aging. Keywords: Angiotensin II, Brain-derived growth factor, Dogs, Endothelin-1, Osteoprotegerin. IntroductionGerontology is a major research topic in human and veterinary medicine (Lee et al., 2020). In recent years, comparisons between dog and human methylomes have revealed a nonlinear relationship that translates dog years to human years. These studies also showed that the timing of major physiological milestones was similar between the two species (Hoffman et al., 2018; Sándor and Kubinyi, 2019). In a study of dogs, it was reported that several inflammatory and immunological substances, such as immunoglobulin M and 8-hydroxy-2- deoxyguanosine, increased with age, whereas immunoglobulin G and C-reactive protein levels did not change (Alexander et al., 2018). In addition, growth hormone and insulin growth factor-1 were reported as potential biomarkers for aging in dogs (Lee et al., 2020). Unlike mice and rats, companion dogs are affected by diverse spontaneously occurring diseases similar to those in humans, such as age-related neurological, cardiovascular, and musculoskeletal diseases (Inoue et al., 2015). For example, osteoprotegerin (OPG) is steadily released from vascular endothelial cells in response to inflammatory stimuli, which can be an important factor in osteoporosis (Coulson et al., 2017). However, brain-derived neurotrophic factor (BDNF) has been reported to rescue synaptic and muscle function and selective molecular mechanisms in muscles that might underlie improved cellular functions, especially those associated with cognition (Miniksar et al., 2021). In addition, major problems are observed in the cardiovascular system in geriatric medicine. Angiotensin II (ANGII) is a well-known peptide hormone that causes vasoconstriction and elevates blood pressure, primarily through the ANGII-converting enzymes in the lungs, endothelial cells, kidney epithelial cells, and brain (Forrester et al., 2018). It is also associated with sarcopenia (Marzetti et al., 2013). Endothelin-1 (ET-1), a potent endogenous vasoconstrictor produced mainly by the vascular endothelium, is another essential biomarker (Yanagisawa et al., 1988). However, the levels of these biomarkers have not been measured in young and old companion dogs. It has been reported that the secretion of some substances differs depending on aging, body weight, and sex in dogs (Piccione et al., 2004). Therefore, the purpose of this preliminary study was to investigate the difference in each parameter between young and elderly companion dogs, including sex differences. Materials and MethodsAnimalsThe dogs in this study were referred to Rakuno Gakuen University between March 2020 and February 2021 due to medial patellar luxation without heart murmur and hypertension. The dogs were fed with commercial pet food. The definitions of young and old were as follows: dogs aged less than 36 months were considered young, while dogs aged over 108 months were considered old. All dogs weighed less than 5 kg. This study was approved by the Rakuno Gakuen University, School of Veterinary Medicine Institutional Animal Care and Use Committee (approval No. VH19A10). Measurements of biomarkersSerum samples (1 ml) were collected from the saphenous vein in a serum-separating tube (2 ml, Fujifilm. Co. Ltd. Tokyo, Japan). The collected blood was left still for 15 minutes and centrifugated at 1,500 rpm for 15 minutes. The collected sera were stored at −80°C until the time of measurement, i.e., approximately 4 weeks. All measurements were performed commercially using canine enzyme-linked immunosorbent assay (ELISA) kits in duplicates. Canine ET-1 levels were measured using a canine ET-1 ELISA kit (Cat No, MBS2606443, CA), with a sensitivity of 5 pg/ml. BDNF levels were measured using a canine BDNF ELISA kit (Cat No, RK00516, Tokyo, Japan), with a sensitivity of 0.056 ng/ml. Canine ANGII levels were measured using an ANGII ELISA kit (Cat No, MBS705510, CA), with a sensitivity of 4.68 pg/ml. Lastly, canine OPG levels were measured using a canine OPG ELISA kit (Cat No, MBS011402, CA), with a sensitivity of 0.1 pg/ml. Statistical analysisThe Shapiro–Wilk test was used to assess the normality of all variables. Data are presented as mean ± standard error. Unpaired Student’s t-tests were used to compare differences between the young and old dogs. Analysis of variance (ANOVA) with a post-hoc Tukey–Kramer test was used to determine sex differences in all young and old dogs. Differences were considered significant at p < 0.05. ResultsAs shown in Table 1, the mean age of the young group was of 19.8 ± 9.3 months, while that of the old group was 155.8 ± 22.8 months. The body weight (kg) of the young group (2.9 ± 0.9) was lower than that of the old group (4.0 ± 1.1); however, the difference was not statistically significant. There were eight males (six neutered, two intact) and eight females (four spayed, four intact) in the young group and eight males (four neutered, four intact), and eight females (four spayed, four intact) in the old group. As for the breeds, the young group included mixed (n=7), Toy Poodle (n=4), Chihuahua (n=2), Pomeranian (n=1), Maltese (n=1), and Pekingese (n=1), while the old group included Pomeranian (n=3), Miniature Schnauzer (n=3), Chihuahua (n=2), Papillon (n=2), Shiba Inu (n=2), Mixed (n=2), Boston Terrier (n=1), and Toy Poodle (n=1). The serum concentration of the musculoskeletal biomarker OPG was significantly higher in the old group (1.7 ± 0.39 ng/ml) than in the young group (0.86 ± 0.47 ng/ml; p < 0.05) (Fig. 1A). In addition, ANOVA revealed significant differences between young males (1.19 ± 0.14 ng/ml) and young females (0.54 ± 0.17 ng/ml), young males and old males (1.92 ± 0.14 pg/ml), and young males and old females (1.43 ± 0.23 ng/ml), as seen in Figure 1B. In contrast, the serum concentration of BDNF did not significantly differ among the four groups (Fig. 1C). The results were as follows: young, 3.39 ± 1.23 ng/ml; old, 3.35 ± 0.69 ng/ml; young males, 4.1 ± 1.36 ng/ml; young females, 2.6 ± 0.28 ng/ml; old males, 3.39± 0.67 pg/ml; and old females, 3.06 ± 0.11 ng/ml (Fig. 1D). The serum concentration of the cardiovascular biomarker ANG II was significantly higher in the old group (16.3 ± 3.0 pg/ml) than in the young group (10.5 ± 3.5 pg/ml; p < 0.05) (Fig. 2A). Specifically, the ANGII levels were significantly elevated in old males (16.0 ± 3.3 pg/ml) and females (16.7 ± 3.3 ng/ml) than in young males (12.6 ± 1.8 pg/ml) and females (8.4 ± 3.6 pg/ml) (Fig. 2B). Similarly, the serum concentration of ET-1 in the old group (34.0 ± 0.96 pg/ml) was significantly higher than that in the young group (30.68 ± 0.69 pg/ml; p < 0.05, Fig. 2C); specifically, the levels were higher in old males (34.4 ± 1.1 pg/ml) and females (33.65 ± 0.69 ng/ml) than in young males (30.7 ± 0.58 pg/ml) and females (30.63 ± 0.83 pg/ml) (Fig. 2D). Table 1. Characteristics of dogs.
Fig. 1. Comparison of musculoskeletal system biomarkers by sex and age. (A): OPG comparison between young and old. (B): OPG comparison between the sexes in each age group. (C): BDNF comparison between young and old. (D): BDNF comparison between the sexes in each age group. OPG, osteoprotegerin; BDNF, brain-derived neurotrophic factor. DiscussionIn this preliminary study, serum OPG, ANGII, and ET-1 concentrations were significantly higher in older companion dogs than in younger companion dogs. The BDNF levels did not differ between the two groups in our study. However, a decrease in serum BDNF levels is negatively correlated with cognitive decline and memory in dogs (Sechi et al., 2015). In our preliminary study, none of the elderly dogs showed symptoms of cognitive dysfunction. Collectively, it suggested necessitating an understanding of the relationship between BDNF levels and cognitive dysfunction syndrome in elderly dogs in the future. In our study, serum OPG concentrations were higher in older dogs than in younger dogs, a trend that has also been observed in humans (Coulson et al., 2017). Although there have been a few reports of osteoporosis in veterinary medicine (Weigel and Alexander, 1981), circulating levels of OPG are higher in older adult humans than in young humans and positively associated with whole-body bone mineral density, suggesting an association with osteoporosis (Coulson et al., 2017). Despite the small sample sizes, our study showed sex-based differences in young and old dogs, which was also observed in a previous study (Hoffman et al., 2018), indicating that there may be sex-based differences in bone metabolism during aging. Our findings for the cardiovascular biomarkers, ANGII and ET-1 may be similar to previous findings in humans (Yu et al., 2015; Forrester et al., 2018). The ANG-converting enzyme 2 activity in plasma and tissue is elevated in dogs with heart disease (Larouche-Lebel et al., 2019). In addition, ET-1 is known to be a cardiovascular biomarker in dogs (Prosek et al., 2004). Thus, although we did not evaluate the blood pressure or perform echocardiography or other general blood tests, we believe ANG II and ET-1 might associate with aging in dogs. In the sex-based comparisons between young and old dogs, there were significant differences in all the parameters in our study, except for BDNF. In a previous study, limited sex-based effects were noted in either longevity or causes of death in companion dogs, suggesting that the most significant sex-based differences in most canine populations may be due to the effects of neutering (Hoffman et al., 2018). In our study, we included both intact and neutered dogs. Thus, future studies should focus on evaluating the effects of neutering in dogs. The limitations of this study include the limited number of dogs in each group and the inclusion of only two broad categories (young and elderly companion dogs) for testing the concentration of each biomarker. We plan to conduct further studies on biomarkers in aging dogs. In conclusion, this study shows that OPG, ANGII, and ET-1 change between young and elderly dogs, which could lead to an improved understanding of canine gerontology.
Fig. 2. Comparison of musculoskeletal system biomarkers by sex and age. (A): ANGII comparison between young and old. (B): ANGII comparison between the sexes in each age group. (C): ET-1 comparison between young and old. (D): ET-1 comparison between the sexes in each age group. ANGII, angiotensin II; ET-1, endothelin-1. AcknowledgmentsWe would like to thank Editage (www.editage.com) for English language editing. Conflict of interestThe author does not have any financial or personal relationships that could inappropriately influence or bias the content of this article. ReferencesAlexander, J.E., Colyer, A., Haydock, R.M., Hayek, M.G. and Park, J. 2018. Understanding how dogs age: longitudinal analysis of markers of inflammation, immune function, and oxidative stress. J. Gerontol. A. Biol. Sci. Med. Sci. 73, 720–728. Coulson, J., Bagley, L., Barnouin, Y., Bradburn, S., Butler-Browne, G., Gapeyeva, H., Hogrel, J.Y., Maden- Wilkinson, T., Maier, A.B., Meskers, C., Murgatroyd, C., Narici, M., Pääsuke, M., Sassano, L., Sipilä, S., Al-Shanti, N., Stenroth, L., Jones, D.A. and McPhee, J.S. 2017. Circulating levels of dickkopf-1, osteoprotegerin and sclerostin are higher in old compared with young men and women and positively associated with whole-body bone mineral density in older adults. Osteoporos. Int. 28, 2683–2689. Forrester, S.J., Booz, G.W., Sigmund, C.D., Coffman, T.M., Kawai, T., Rizzo, V., Scalia, R. and Eguchi, S. 2018. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol. Rev. 98, 1627–1738. Hoffman, J.M., O’Neill, D.G., Creevy, K.E. and Austad, S.N. 2018. Do female dogs age differently than male dogs? J. Gerontol. A. Biol. Sci. Med. Sci. 73, 150–156. Inoue, M., Hasegawa, A., Hosoi, Y. and Sugiura, K. 2015. Breed, gender and age pattern of diagnosis for veterinary care in insured dogs in Japan during fiscal year 2010. Prev. Vet. Med. 119, 54–60. Larouche-Lebel, É., Loughran, K.A., Oyama, M.A., Solter, P.F., Laughlin, D.S., Sánchez, M.D., Assenmacher, C. A., Fox, P.R. and Fries, R.C. 2019. Plasma and tissue angiotensin- converting enzyme 2 activity and plasma equilibrium concentrations of angiotensin peptides in dogs with heart disease. J. Vet. Intern. Med. 33, 1571–1584. Lee, S.H., Kim, J.W., Lee, B.C. and Oh, H.J. 2020. Age-specific variations in hematological and biochemical parameters in middle- and large-sized of dogs. J. Vet. Sci. 21, e7. Marzetti, E., Calvani, R., Cesari, M., Buford, T.W., Lorenzi, M., Behnke, B. J. and Leeuwenburgh, C. 2013. Mitochondrial dysfunction and sarcopenia of aging: from signaling pathways to clinical trials. Int. J. Biochem. Cell. Biol. 45, 2288– 2301. Miniksar, Ö.H., Çiçekçioğlu, F., Kılıç, M., Honca, M., Miniksar, D.Y., Gocman, A.Y., Kaçmaz, O. and Öz, H. 2021. Decreased brain derived neurotrophic factor levels may predict early perioperative neurocognitive disorder in patients undergoing coronary artery bypass surgery: a prospective observational pilot study. J. Clin. Anesth. 71, 110235. Piccione, G., Fazio, F., Giudice, E., Grasso, F., Caola, G. 2004. Blood lipids, fecal fat and chymotrypsin excretion in the dog: influence of age, body weight and sex. J. Vet. Med. Sci. 66, 59–66. Prosek, R., Sisson, D.D., Oyama, M.A., Biondo, A.W. and Solter, P.F. 2004. Plasma endothelin-1 immunoreactivity in normal dogs and dogs with acquired heart disease. J. Vet. Intern. Med. 18, 840–844. Sándor, S. and Kubinyi, E. 2019. Genetic pathways of aging and their relevance in the dog as a natural model of human aging. Front. Genet. 10, 948. Sechi, S., Chiavolelli, F., Spissu, N., Cerbo, A.D., Canello, S., Guidetti, G., Fiore, F. and Cocco, R. 2015. An antioxidant dietary supplement improves brain-derived neurotrophic factor levels in serum of aged dogs: preliminary results. J. Vet. Med. 2015, 412501. Yanagisawa, M., Kurihara, H., Kimura, S., Tomobe, Y., Kobayashi, M., Mitsui, Y., Yazaki, Y., Goto, K. and Masaki, T. 1988. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 332, 411–415. Yu, A.P., Tam, B.T., Yau, W.Y., Chan, K.S., Yu, S.S., Chung, T.L. and Siu, P.M. 2015. Association of endothelin-1 and matrix metallopeptidase-9 with metabolic syndrome in middle- aged and older adults. Diabetol. Metab. Syndr. 7, 111. Weigel, J. and Alexander, J.W. 1981. Aging and the musculoskeletal system. Vet. Clin. North. Am. Small. Anim. Pract. 11, 749–764. | ||
| How to Cite this Article |
| Pubmed Style Isaka M, . Serum Concentrations of Osteoprotegerin, Brain-Derived Nerve Factor, Angiotensin II, and Endothelin-1 in aging dogs. Open Vet J. 2022; 12(6): 980-984. doi:10.5455/OVJ.2022.v12.i6.26 Web Style Isaka M, . Serum Concentrations of Osteoprotegerin, Brain-Derived Nerve Factor, Angiotensin II, and Endothelin-1 in aging dogs. https://www.openveterinaryjournal.com/?mno=68824 [Access: April 26, 2024]. doi:10.5455/OVJ.2022.v12.i6.26 AMA (American Medical Association) Style Isaka M, . Serum Concentrations of Osteoprotegerin, Brain-Derived Nerve Factor, Angiotensin II, and Endothelin-1 in aging dogs. Open Vet J. 2022; 12(6): 980-984. doi:10.5455/OVJ.2022.v12.i6.26 Vancouver/ICMJE Style Isaka M, . Serum Concentrations of Osteoprotegerin, Brain-Derived Nerve Factor, Angiotensin II, and Endothelin-1 in aging dogs. Open Vet J. (2022), [cited April 26, 2024]; 12(6): 980-984. doi:10.5455/OVJ.2022.v12.i6.26 Harvard Style Isaka, M. & (2022) Serum Concentrations of Osteoprotegerin, Brain-Derived Nerve Factor, Angiotensin II, and Endothelin-1 in aging dogs. Open Vet J, 12 (6), 980-984. doi:10.5455/OVJ.2022.v12.i6.26 Turabian Style Isaka, Mitsuhiro, and . 2022. Serum Concentrations of Osteoprotegerin, Brain-Derived Nerve Factor, Angiotensin II, and Endothelin-1 in aging dogs. Open Veterinary Journal, 12 (6), 980-984. doi:10.5455/OVJ.2022.v12.i6.26 Chicago Style Isaka, Mitsuhiro, and . "Serum Concentrations of Osteoprotegerin, Brain-Derived Nerve Factor, Angiotensin II, and Endothelin-1 in aging dogs." Open Veterinary Journal 12 (2022), 980-984. doi:10.5455/OVJ.2022.v12.i6.26 MLA (The Modern Language Association) Style Isaka, Mitsuhiro, and . "Serum Concentrations of Osteoprotegerin, Brain-Derived Nerve Factor, Angiotensin II, and Endothelin-1 in aging dogs." Open Veterinary Journal 12.6 (2022), 980-984. Print. doi:10.5455/OVJ.2022.v12.i6.26 APA (American Psychological Association) Style Isaka, M. & (2022) Serum Concentrations of Osteoprotegerin, Brain-Derived Nerve Factor, Angiotensin II, and Endothelin-1 in aging dogs. Open Veterinary Journal, 12 (6), 980-984. doi:10.5455/OVJ.2022.v12.i6.26 |