| Original Article | ||
Open Vet J. 2021; 11(3): 364-369 Open Veterinary Journal, (2021), Vol. 11(3): 364–369 Original Research Methicillin-resistant Staphylococcus schleiferi subspecies coagulans associated with otitis externa and pyoderma in dogsJoel André Palomino-Farfán*, Luis Guillermo Alvarez Vega, Sonia Yenny Calle Espinoza, Sofia Gonzales Magallanes and Juan José Siuce MorenoLaboratory of Veterinary Bacteriology and Mycology, Faculty of Veterinary Medicine, National University of San Marcos, Lima, Perú *Corresponding Author: Joel André Palomino-Farfán. Laboratory of Veterinary Bacteriology and Mycology, Faculty of Veterinary Medicine, National University of San Marcos, Lima, Perú. Email: jpalominof [at] unmsm.edu.pe Submitted: 29/04/2021 Accepted: 27/06/2021 Published: 20/07/2021 © 2021 Open Veterinary Journal
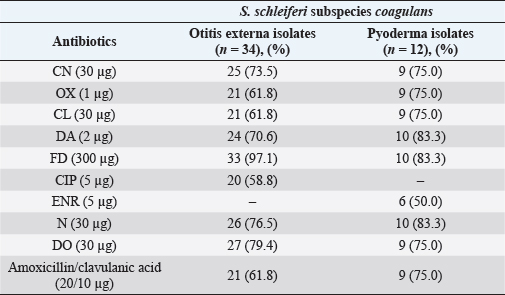
AbstractBackground: Dermatological infections are the most common cases in the daily pet clinic. Since its discovery in 1990, Staphylococcus schleiferi subspecies coagulans have been reported more frequently in canine otitis externa and pyoderma and even in cases of zoonoses. Aim: Detect the presence of S. schleiferi subsp. coagulans of canine otitis externa and pyoderma, its antimicrobial resistance, and the presence of mecAgen. Methods: Three-hundred-thirty-one swabs from dogs with otitis externa and pyoderma were cultured on bacteriological agar for bacterial isolation and subsequent biochemical and molecular identification. The identified S. schleiferi subsp. coagulans were evaluated for their antimicrobial susceptibility using the Kirby–Bauer technique, including an oxacillin disk, and subsequently, a PCR was run to identify which ones had the mecA gene. Results: Thirty-four (22.97%) and twelve (6.56%) isolates were identified as S. schleiferi subspecies coagulans from otitis externa and pyoderma, respectively. Fluoroquinolones, the most widely used group of antibiotics in Peru, showed a susceptibility of 58.82% (20/34) in cases of otitis externa and 50% (6/12) in cases of canine pyoderma. Meanwhile, nitrofurantoin was the antibiotic with the best efficacy in both cases, with 97% (33/34) in otitis externa and 83% (10/12) in pyoderma. Furthermore, 40% (13/34) of S. schleiferi subsp. coagulans isolated from otitis externa were resistant to methicillin, and 85.29% (29/34) had the mecA gene. On the other hand, the only methicillin-resistant isolate from pyoderma was also the only one with a mecA gene. Conclusion: This study is the first report of S. schleiferi subsp. coagulans in Peru, finding a higher percentage than reported in other South American countries. Keywords: Staphylococcus schleiferi, CoPS, Methicillin-resistant, Oxacillin, mecA gene. IntroductionThe cutaneous and ear microbiota of dogs is composed of bacteria, fungi, and parasites, which protect the ear surface from other environmental microorganisms. Lack of knowledge of this function and using antiseptic solutions as cleaners allows the entry and colonization of pathogenic microorganisms (Nuttall and Cole, 2004; Ngo et al., 2018). Carrying out treatments without really knowing the primary cause or the related microorganism generates a delay in the recovery of the patient in addition to producing multidrug bacterial resistance or causing severe damage in the dog, such as a ruptured eardrum (Newman et al., 2015). Staphylococci are the predominant bacteria in otitis external and pyoderma in dogs. Coagulase-positive Staphylococcus (CoPS) have been more clinically aggressive and share antimicrobial-resistant genes (Devriese et al., 2005; Griffeth et al., 2008; Sasaki et al., 2010). Staphylococcus schleiferi subsp. coagulans are commonly confused with other CoPS due to their similar morphological and biochemical characteristics (Devriese et al., 2005). In this way, its identification and epidemiological importance are biased. Likewise, the differentiation between the subspecies of S. schleiferi is complex because they have polymorphisms of equal length and only performing a PCR is not enough; this must be complemented with other biochemical tests (Yamashita et al., 2005). Additionally, human cases of S. schleiferi subsp. coagulans have been reported (Kumar et al., 2007; Thibodeau et al., 2012; Swe et al., 2016), and in one of the cases where infection by a dog with recurrent otitis and pyoderma was suspected (Kumar et al., 2007). Strains of S. schleiferi subsp. Methicillin-resistant coagulans (Kania et al., 2004; Bemis et al., 2006; Kumar et al., 2007; Griffeth et al., 2008; Kawakami et al., 2010; Davis et al., 2013; Swe et al., 2016) and fluoroquinolones (Muñoz et al., 2012; Davis et al., 2013) have been reported. The aim of the current study was to detect the presence of S. schleiferi subsp. coagulans of canine otitis externa and pyoderma, its antimicrobial resistance, and the presence of mecAgen. Materials and MethodsSampling and isolationA collection of 331 samples were collected by ear (148) and skin (183) swabs from dogs with otitis externa and pyodermas. All dogs were examined by the San Marcos National University’s Veterinarian Clinic from 2017 to 2018. The samples were cultured on tryptic soy agar (TSA; Merck, Germany) at 37°C for 24 hours. Identification of Staphylococcus speciesGram-positive cocci, catalase-positive, and typical colonial morphology (whitish round colonies) were identified as Staphylococci. Tube coagulase test with rabbit plasma (Bactident coagulase; Merck, Germany) was performed, and only CoPS strains were selected for further investigation. Other biochemical tests were acetoin production, acid production from trehalose and mannitol, and clotting factor. These strains were stored in 15% glycerol at −20°C until use. Antimicrobial susceptibility testsAntimicrobial susceptibility tests were performed with a commercially paper disk diffusion method using Muller–Hinton Agar. The most popular antimicrobial agents used for the treatment of otitis externa and pyoderma were tested: gentamicin (CN), oxacillin (OX), cephalexin (CL), clindamycin (DA), nitrofurantoin (FD), ciprofloxacin (CIP) for otitis externa and enrofloxacin (ENR) for pyoderma, neomycin (N), doxycycline (DO), and amoxicillin with clavulanic acid (AMC). 16S rDNA-specific PCR for identification of S. schleiferi subsp. coagulansAll CoPS strains were subcultured on TSA with 5% sheep blood at 37°C overnight. Bacterial DNA was extracted following the instructions from the kit (GeneJET Genomic DNA purification; Thermo Fisher Scientific, USA). A pair of primers was designed to amplify the 1,369 bp region of the 16S rDNA of S. schleiferi subsp. coagulans. Primer-1 (5′–GAACGGACAAGGAGCTTGCTCCTTTGAA-3′) and primer-2 (5′–TTACAAACTCTCGTGGTGTGAA-3′) correspond to the nucleotide residues 61–88 and 1,407–1,429, respectively. The PCR was performed in a 20 μl reaction volume. Each reaction mixture contained 1 μl (17–240 ng) of the sample DNA solution, 10 μl of 1X MasterMix (2XPCR MasterMix; abm, Canada), 1 μl (0.5 μM) of each primer, and completed with 7 μl of ultra-pure water. The amplification reaction was carried out in a standard thermal cycler (GenAmp Biometra; Gmbb Analytik Jena AG, Germany), using the following program: 1 minute at 94°C, followed by 30 cycles of 1 minute at 94°C, 1 minute at 57°C and 2 minutes at 72°C. The program was completed with an additional 2 minutes at 72°C. After the amplification, 5 μl of the reaction mixture was analyzed by electrophoresis on a 2% agarose gel in Tris-borate-EDTA buffer at 90 V for 90 minutes. A charge buffer Safe-Green was used with all the samples and a 100 bp ladder of 1.5 kb. Detection of the mecA genThe identification of this gen was carried out using a similar PCR method as described previously. A pair of primers was designed to amplify the 533 bp region. Primer-1 (5′-AAAATCGATGGTAAAGGTTGGC-3′) and primer-2 (5′-AGTTCTGCAGTACCGGATTTGC-3′). The PCR was performed in a 20 μl reaction volume. Each reaction mixture contained 3 μl of the sample DNA solution, 10 μl of 1X MasterMix, 0.4 μl of each primer, and completed with 6.2 μl of ultra-pure water. The PCR program was 45 seconds at 94°C, followed by 35 cycles of 45 seconds at 94°C, 45 seconds at 50°C, and 1 minute at 72°C. The program was completed with an additional 2 minutes at 72°C. After the amplification, 5 μl of the reaction mixture was analyzed by electrophoresis on a 1.5% agarose gel in Tris-borate-EDTA buffer at 70V for 100 minutes. The same charge buffer was used with all the samples and a 50 bp ladder of 1 kb. ResultsStaphylococci were isolated from external auditory meatus in 112 (76.87%) of 148 samples of otitis externa and from skin lesions in 141 (77.05%) of 183 samples of pyoderma. The staphylococcal isolates from otitis externa (n=112) and pyoderma (n=141) analyzed by PCR for the amplification of a 1,369 bp product of the 16S rDNA showed that 30.34% (34/112) and 8.5% (12/141) were identified as S. schleiferi subsp. coagulans, respectively (Fig. 1). Two DNA samples of isolates S027 and S058, positive for S. schleiferisubsp. coagulans were sent to the Macrogen Laboratory (Korea) for purification and sequencing. The sequences of isolates S027 and S058 are deposited in GenBank with the codes MN497847 and MN497848, respectively. The results of the antimicrobial susceptibility of S. schleiferi subsp. coagulans isolated from otitis externa (n=34) and pyoderma (n=12) were favorable with an efficacy level greater than 70% for CN (25/34 and 9/12), DA (24/34 and 10/12), FD (33/34 and 10/12), N (26/34 and 10/12) and DO (27/34 and 9/12). Beta-lactams (CL, OX, and AMC) obtained a moderately effective response in cases of otitis externa (21/34) but favorable in cases of pyoderma (11/12). Fluoroquinolones showed the lowest value in terms of efficacy with 58.8% (20/34) and 50% (6/12) of the otitis externa and pyoderma isolates, respectively (Table 1). Regarding multidrug resistance, it was found that 17/34 and 3/12 of the S. schleiferi subsp. coagulans isolated from otitis externa and pyoderma, respectively, were resistant to 3 or more antibiotic groups. Furthermore, mecA gene was detected in 85.29% (n=34) and 8.3% (n=1) of S. schleiferi subsp. coagulans from samples of otitis externa and pyoderma, respectively (Fig. 2). DiscussionExcept for the study carried out by Bugden (2013) in Australia, where 35% (1,256/3,541) of the bacteria isolated from otitis externa was Pseudomonas aeruginosa, several studies have shown that Staphylococcus sp. is the primary bacterium found in canine otitis externa, in a range of 50%–60% (Yamashita et al., 2005; Penna et al., 2010; Muñoz et al., 2012; Zur et al., 2016). The present study found that 75.68% (112/148) of the otitis externa samples and 77.05% (141/183) of the pyoderma samples were positive for the genus Staphylococcus.
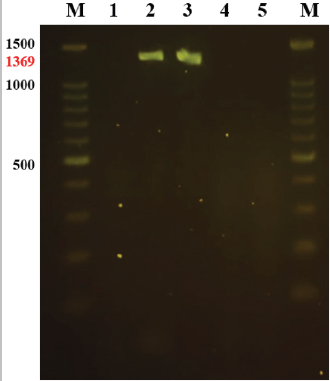
Fig. 1. Agarose gel electrophoresis 2% for the detection of S. schleiferi subsp. coagulans. Lanes: M, 1.5 kb marker; 1, blank; 2, isolate S027 (GenBank: MN497847)—1,369 bp; 3, isolate S058 (GenBank: MN497848)—1,369 bp; 4, strain of S. aureus (ATCC 4330); 5, strain of S. pseudintermedius. The frequency of isolation of S. schleiferi subsp. coagulans from canine pyoderma in the present study was 6.56% (12/183), a value very similar to the 5.93% (8/135) reported Muñoz et al. (2012) in Chile; but with respect to the otitis externa samples, there is a significant difference of little more than double, wherein this study they were 22.97% (34/148) compared to that reported by Muñoz et al. (2012) where it was 10.78% (11/102). This may be because Muñoz et al. (2012) used the BBL ™ Crystal GP diagnostic kit (Becton Dickinson®) instead of the molecular diagnostic method used in this study.
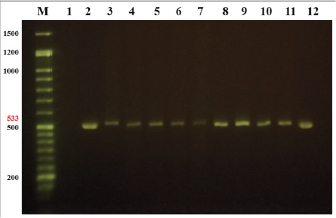
Fig. 2. Agarose gel electrophoresis 1.5% for the detection of mecA gen in S. schleiferi subsp. coagulans. Lanes: M, 1.5 kb marker; 1, blank; 2, strain of Methicillin-resistant S. aureus (ATCC 4330)—533 bp; 3, isolate S012; 4, isolate S017; 5, isolate S019; 6–10, isolates S027-31; 11, isolate S039; 12, isolate S040. Table 1. Antibiotics susceptibility profile of S. schleiferi subspecies coagulans isolated from otitis externa and pyoderma in dogs.
Staphylococcus pseudintermedius is the most reported CoPS in canine otitis externa and pyoderma, with frequencies around 87.9% (Zur et al., 2016; Alvarez et al., 2020), so many veterinarians assume that it is this bacterium. In this study, it has been observed that at least for the cases of external otitis, 30.34% of the Staphylococcus species were S. schleiferi subsp. coagulans, a value to take into account for the cases reported in Peru. Yamashita et al. (2005) and Swe et al. (2016) mention the coagulase and urease tests as beneficial for the differentiation of S. schleiferi subspecies, but the urease test was not considered in this study because there is a percentage of variability in terms of interpretation, as earlier reported by Zdovc et al. (2004). This study shows a relatively low susceptibility of 58.8% (20/34) for CIP and 50% (6/12) for ENR, antibiotics belonging to the group of fluoroquinolones used empirically as the first option for treatment of otitis externa and pyoderma. These results are similar to those found by Kunder et al. (2015) and Palomino-Farfán et al. (2020), where the susceptibility to CIP and ENR was 47.7% and 51.3%, respectively. Staphylococcus species have been reported to possess genes that are resistant to fluoroquinolones; this could explain why the susceptibility is so low (Muñoz et al., 2012; Schwarz et al., 2018). Another group of antibiotics used as first-line treatment for skin and ear diseases is CL and amoxicillin. In the present study, a susceptibility of 61.8% (21/34) of the ear isolates and 91.67% (11/12) of the skin was found, being higher than those found by Kunder et al. (2015) of 38.5%, but lower than those reported by Penna et al. (2010) of 77.5% and Muñoz et al. (2012) where they found 100% sensitivity to this group of β-lactams. This could be due to the empirical CL treatment that many veterinarians perform when observing coccoid forms on cytology of ear or skin discharge without really knowing their antibiotic response (Wiebe, 2015). Strains of Staphylococcus that phenotypically show resistance to OX are known as Methicillin-Resistant Staphylococcus, the best-known species being Staphylococcus aureus (MRSA). MRSAs are of great importance in public health because they cause most nosocomial infections in human medicine (Sakoulas et al., 2001). This study demonstrated that there is a resistance to OX in 38.2% (13/34) of the otitis externa isolates, being almost identical to that shown by Cain et al. (2011), where they found the resistance of 39% (20/51) of isolates of S. schleiferi coagulase positive. These percentages are lower than those found by Kunder et al. (2015), where they evaluated the resistance of S. schleiferi isolated from dogs in two regions of the United States, finding values of 62% (72/116) and 73% (74/101) of strains resistant to methicillin. Kunder et al. (2015) reported that exposure to recurrent infections and constant antibiotic treatments favor the colonization of resistant Staphylococcus methicillin. The consequences of this are reflected in not only their resistance to penicillin (penicillin, amoxicillin, and ampicillin) and first and second-generation cephalosporins (CL, cephalothin, and cefazolin), but it may also be related to resistance to macrolides, aminoglycosides, tetracyclines, and fluoroquinolones (Morris et al., 2006; Kawakami et al., 2010). Considering the relationship between owners and pets, many of these resistant bacteria could pose a risk of zoonosis or reverse zoonosis (Frank et al., 2009). In conclusion, the detection of S. schleiferi subsp. coagulans are higher in cases of otitis externa than in canine pyoderma. Antimicrobial resistance is increasing, as shown in this study, antibiotics commonly used to treat otitis externa and pyodermas such as fluoroquinolones are not effective, so it is crucial to perform a bacteriological culture with its respective antibiogram to know exactly its resistance profiles. Likewise, the presence of methicillin-resistant S. schleiferi subsp. coagulans have been detected, 38.24% (13/34) in cases of external otitis and 8.3% (1/12) in cases of pyoderma, which in some cases have been shown to be multidrug-resistant and possess the mecA gene. AcknowledgmentsA special thanks to the Vice-Rector for Research and Postgraduate Studies of the National University of San Marcos for having financed this research, as well as to all the staff of the Bacteriology laboratory who supported us. Conflict of interestThe authors declare that there is no conflict of interest. ReferencesAlvarez, V.L., Siuce Moreno, J., Palomino-Farfán, J., Gonzales Magallanes, S., Sedano, A. and Calle Espinoza, S. 2020. Molecular detection of Staphylococcus pseudintermedius in canine pyoderma. Rev. Investig. Vet. del Peru. 31(3). Bemis, D.A., Jones, R.D., Hiatt, L.E., Ofori, E.D., Rohrbach, B.W., Frank, L.A. and Kania, S.A. 2006. Comparison of tests to detect oxacillin resistance in Staphylococcus intermedius, Staphylococcus schleiferi, and Staphylococcus aureus isolates from canine hosts. J. Clin. Microbiol. 44(9), 3374–3376. Bugden, D.L. 2013. Identification and antibiotic susceptibility of bacterial isolates from dogs with otitis externa in Australia. Aust. Vet. J. 91(1–2), 43–46. Cain, C.L., Morris, D.O., O’Shea, K. and Rankin, S.C. 2011. Genotypic relatedness and phenotypic characterization of Staphylococcus schleiferi subspecies in clinical samples from dogs. Am. J. Vet. Res. 72(1), 96–102. Davis, M.F., Cain, C.L., Brazil, A.M. and Rankin, S.C. 2013. Two coagulase-negative Staphylococci emerging as potential zoonotic pathogens: wolves in sheep’s clothing? Front. Microbiol. 4(123), 1–4. Devriese, L.A., Vancanneyt, M., Baele, M., Vaneechoutte, M., De Graef, E., Snauwaert, C., Cleenwerck, I., Dawyndt, P., Swings, J., Decostere, A. and Haesebrouck, F. 2005. Staphylococcus pseudintermedius sp. nov., a coagulase-positive species from animals. Int. J. Syst. Evol. Microbiol. 55(4), 1569–1573. Frank, L.A., Kania, S.A., Kirzeder, E.M., Eberlein, L.C. and Bemis, D.A. 2009. Risk of colonization or gene transfer to owners of dogs with meticillin-resistant Staphylococcus pseudintermedius. Vet. Dermatol. 20(5–6), 496–501. Griffeth, G.C., Morris, D.O., Abraham, J.L., Shofer, F.S. and Rankin, S.C. 2008. Screening for skin carriage of methicillin-resistant coagulase-positive Staphylococci and Staphylococcus schleiferi in dogs with healthy and inflamed skin. Vet. Dermatol. 19(3), 142–149. Kania, S.A., Williamson, N.L., Frank, L.A., Wilkes, R.P., Jones, R.D. and Bemis, D.A. 2004. Methicillin resistance of Staphylococci isolated from the skin of dogs with pyoderma. Am. J. Vet. Res. 65(9), 1265–1268. Kawakami, T., Shibata, S., Murayama, N., Nagata, M. and Nishifuji, K. 2010. Antimicrobial susceptibility and methicillin resistance in Staphylococcus pseudintermedius and Staphylococcus schleiferi subsp. coagulans isolated from dogs with pyoderma in Japan. J. Vet. Med. Sci. 72(12), 1615–1619. Kumar, D., Cawley, J.J., Alvarez, A. and Alvarez, S. 2007. Case of Staphylococcus schleiferi subspecies coagulans endocarditis and metastatic infection in an immune compromised host. Transpl. Infect. Dis. 9, 336–338. Kunder, D.A., Cain, C.L., O’Shea, K., Cole, S.D. and Rankin, S.C. 2015. Genotypic relatedness and antimicrobial resistance of Staphylococcus schleiferi in clinical samples from dogs in different geographic regions of the United States. Vet. Dermatol. 26(6), 406–411. Morris, D.O., Rookt, K.A., Shofer, F.S. and Rankin, S.C. 2006. Screening of Staphylococcus aureus, Staphylococcus intermedius, and Staphylococcus schleiferi isolates obtained from small companion animals for antimicrobial resistance: a retrospective review of 749 isolates (2003–04). Eur. Soc. Vet. Dermatol. 17(5), 332–337. Muñoz, L., Molina, M., Heresmann, M., Abusleme, F., Ulloa, M., Borie, C., San Martin, B., Silva, V. and Anticevic, S. 2012. Primer reporte de aislamiento de Staphylococcus schleiferi subspecies coagulans en perros con pioderma y otitis externa en Chile. Arch. Med. Vet. 44(3), 261–265. Newman, A.W., Estey, C.M., Mcdonough, S., Cerda-Gonzalez, S., Larsen, M. and Stokol, T. 2015. Cholesteatoma and meningoencephalitis in a dog with chronic otitis externa. Vet. Clin. Pathol. 44(1), 157–163. Ngo, J., Taminiau, B., Fall, P.A., Daube, G. and Fontaine, J. 2018. Ear canal microbiota – a comparison between healthy dogs and atopic dogs without clinical signs of otitis externa. Vet. Dermatol. 29(5), 425-e140. Nuttall, T. and Cole, L.K. 2004. Ear cleaning: the UK and US perspective. Vet. Dermatol. 15(2), 127–136. Palomino-Farfán, J.A., Alvarez Vega, L., Siuce Moreno, J. and Calle Espinoza, S. 2020. Antimicrobial resistance in coagulase-positive Staphylococci (CoPS) isolated from dogs with external otitis. Rev. Investig. Vet. del Peru 31(1). Penna, B., Varges, R., Medeiros, L., Martins, G.M., Martins, R.R. and Lilenbaum, W. 2010. Species distribution and antimicrobial susceptibility of Staphylococci isolated from canine otitis externa. Vet. Dermatol. 21(3), 292–296. Sakoulas, G., Gold, H.S., Venkataraman, L., Degirolami, P.C., Eliopoulos, G.M. and Qian, Q. 2001. Methicillin-resistant Staphylococcus aureus: comparison of susceptibility testing methods and analysis of mecA-positive susceptible strains. J. Clin. Microbiol. 39(11), 3946–3951. Sasaki, T., Tsubakishita, S., Tanaka, Y., Sakusabe, A., Ohtsuka, M., Hirotaki, S., Kawakami, T., Fukata, T. and Hiramatsu, K. 2010. Multiplex-PCR method for species identification of coagulase-positive Staphylococci. J. Clin. Microbiol. 48(3), 765–769. Schwarz, S., Feßler, A.T., Loncaric, I., Wu, C., Kadlec, K., Wang, Y. and Shen, J. 2018. Antimicrobial resistance among Staphylococci of animal origin. Microbiol. Spectr. 6(4), 1–29. Swe, T., Naing, A.T., Baqui, A. and Khillan, R. 2016. Methicillin-resistant Staphylococcus schleiferi subspecies coagulans infection in a patient with hepatocellular carcinoma. J. Investig. Med. High Impact Case Rep. 4(3), 1–3. Thibodeau, E., Boucher, H., DeNofrio, D., Pham, D.T. and Snydman, D. 2012. First report of a left ventricular assist device infection caused by Staphylococcus schleiferi subspecies coagulans: a coagulase-positive organism. Diagn. Microbiol. Infect. Dis. 74(1), 68–69. Wiebe, V.J. 2015. Drug therapy for infectious diseases of the dog and cat. Ames, Iowa: John Wiley & Sons, Inc. Yamashita, K., Shimizu, A., Kawano, J., Uchida, E., Haruna, A. and Igimi, S. 2005. Isolation and characterization of Staphylococci from external auditory meatus of dogs with or without otitis externa with special reference to Staphylococcus schleiferi subsp. coagulans isolates. J. Vet. Med. Sci. 67(3), 263–268. Zdovc, I., Ocepek, M., Pirš, T., Krt, B. and Pinter, L. 2004. Microbiological features of Staphylococcus schleiferi subsp. coagulans, isolated from dogs and possible misidentification with other canine coagulase-positive Staphylococci. J. Vet. Med. Ser B Infect. Dis. Vet. Public Health 51(10), 449–454. Zur, G., Gurevich, B. and Elad, D. 2016. Prior antimicrobial use as a risk factor for resistance in selected Staphylococcus pseudintermedius isolates from the skin and ears of dogs. Vet. Dermatol. 27(6), 468-e125. | ||
| How to Cite this Article |
| Pubmed Style Farfán JAP, Vega LGA, Espinoza SYC, Magallanes SG, Moreno JJS, . Methicillin-resistant Staphylococcus schleiferi subspecies coagulans isolated from dogs with otitis externa and pyoderma in Peru. Open Vet J. 2021; 11(3): 364-369. doi:10.5455/OVJ.2021.v11.i3.5 Web Style Farfán JAP, Vega LGA, Espinoza SYC, Magallanes SG, Moreno JJS, . Methicillin-resistant Staphylococcus schleiferi subspecies coagulans isolated from dogs with otitis externa and pyoderma in Peru. https://www.openveterinaryjournal.com/?mno=77444 [Access: September 01, 2024]. doi:10.5455/OVJ.2021.v11.i3.5 AMA (American Medical Association) Style Farfán JAP, Vega LGA, Espinoza SYC, Magallanes SG, Moreno JJS, . Methicillin-resistant Staphylococcus schleiferi subspecies coagulans isolated from dogs with otitis externa and pyoderma in Peru. Open Vet J. 2021; 11(3): 364-369. doi:10.5455/OVJ.2021.v11.i3.5 Vancouver/ICMJE Style Farfán JAP, Vega LGA, Espinoza SYC, Magallanes SG, Moreno JJS, . Methicillin-resistant Staphylococcus schleiferi subspecies coagulans isolated from dogs with otitis externa and pyoderma in Peru. Open Vet J. (2021), [cited September 01, 2024]; 11(3): 364-369. doi:10.5455/OVJ.2021.v11.i3.5 Harvard Style Farfán, J. A. P., Vega, L. G. A., Espinoza, S. Y. C., Magallanes, S. G., Moreno, J. J. S. & (2021) Methicillin-resistant Staphylococcus schleiferi subspecies coagulans isolated from dogs with otitis externa and pyoderma in Peru. Open Vet J, 11 (3), 364-369. doi:10.5455/OVJ.2021.v11.i3.5 Turabian Style Farfán, Joel André Palomino, Luis Guillermo Alvarez Vega, Sonia Yenny Calle Espinoza, Sofia Gonzales Magallanes, Juan José Siuce Moreno, and . 2021. Methicillin-resistant Staphylococcus schleiferi subspecies coagulans isolated from dogs with otitis externa and pyoderma in Peru. Open Veterinary Journal, 11 (3), 364-369. doi:10.5455/OVJ.2021.v11.i3.5 Chicago Style Farfán, Joel André Palomino, Luis Guillermo Alvarez Vega, Sonia Yenny Calle Espinoza, Sofia Gonzales Magallanes, Juan José Siuce Moreno, and . "Methicillin-resistant Staphylococcus schleiferi subspecies coagulans isolated from dogs with otitis externa and pyoderma in Peru." Open Veterinary Journal 11 (2021), 364-369. doi:10.5455/OVJ.2021.v11.i3.5 MLA (The Modern Language Association) Style Farfán, Joel André Palomino, Luis Guillermo Alvarez Vega, Sonia Yenny Calle Espinoza, Sofia Gonzales Magallanes, Juan José Siuce Moreno, and . "Methicillin-resistant Staphylococcus schleiferi subspecies coagulans isolated from dogs with otitis externa and pyoderma in Peru." Open Veterinary Journal 11.3 (2021), 364-369. Print. doi:10.5455/OVJ.2021.v11.i3.5 APA (American Psychological Association) Style Farfán, J. A. P., Vega, L. G. A., Espinoza, S. Y. C., Magallanes, S. G., Moreno, J. J. S. & (2021) Methicillin-resistant Staphylococcus schleiferi subspecies coagulans isolated from dogs with otitis externa and pyoderma in Peru. Open Veterinary Journal, 11 (3), 364-369. doi:10.5455/OVJ.2021.v11.i3.5 |