| Short Communication | ||
Open Vet J. 2021; 11(3): 346-355 Open Veterinary Journal, (2021), Vol. 11(3): 346–355 Short Communication The potential anti-African swine fever virus effects of medium chain fatty acids on in vitro feed model: An evaluation study using epidemic ASFV strain circulating in VietnamHa Thi Thanh Tran1,†, Anh Duc Truong1,†, Duc Viet Ly1, Tuan Van Hoang1, Nhu Thi Chu1, Huyen Thi Nguyen1, Anh Thi Kieu Dang1, Maartje De Vos2, Kobe Lannoo2, Geert Bruggeman2 and Hoang Vu Dang1*1Department of Biochemistry and Immunology, National Institute of Veterinary Research (NIVR), Hanoi, Vietnam 2Royal Agrifirm Group, Apeldoorn, The Netherlands †These authors contributed equally to this work *Corresponding Author: Hoang Vu Dang. Department of Biochemistry and Immunology, National Institute of Veterinary Research, Hanoi, Vietnam. Email: dangvuhoang [at] nivr.gov.vn Submitted: 11/05/2021 Accepted: 22/06/2021 Published: 15/07/2021 © 2021 Open Veterinary Journal
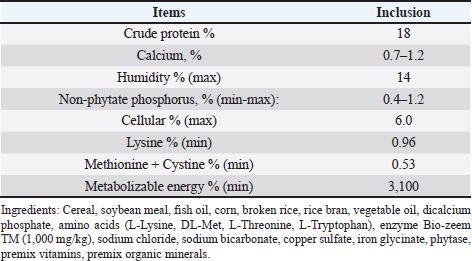
AbstractBackground: African swine fever (ASF) is an important disease affecting swine and has a significant economic loss in both the developed and developing world. Aim: In this study, we evaluated the potential effects of medium-chain fatty acids (MCFAs) in individual and synergistic forms to prevent and/or reduce ASF virus (ASFV) infection using in vitro feed model. Methods: The cytotoxicity of MCFAs on porcine alveolar macrophages cells was evaluated by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. The potential effects of MCFAs, including C8 (caprylic acid), C8-C6-C10 (caprylic acid-caproic acid-capric acid; 1:1:1 ratio) and C8-C10-C12 (caprylic acid-capric acid-lauric acid; 1:1:1 ratio) against a field ASFV strain isolated in the capital Hanoi of Vietnam, were further examined by real-time PCR and haemadsorption assays in in vitro feed model. Results: Our results indicated that all tested products do not induce cytotoxicity at the dose of 100 μg/ml and are suitable for further in vitro examination. These products have shown a strong antiviral effect against ASFV infectivity at doses of 0.375% and 0.5%. Interestingly, the synergistic MCFAs have shown clearly their potential activities against ASFV in which at a lower dose of 0.25%, pre-treatment with product two and three induced significant increases at the level of Cq value when compared to positive control and/or product 1 (p < 0.05). However, the viral titre was not changed after 24 hours post-inoculation when compared to positive control. Our findings suggested that all tested products, both individual and synergistic forms of MCFAs, have possessed a strong anti-ASFV effect, and this effect is dose-dependence in in vitro feed model. Additionally, synergistic effects of MCFAs are more effective against ASFV when compared to individual forms. Conclusion: Together, the findings in this study indicate that MCFAs, both individual and synergistic forms, inhibit against a field ASFV strain in the feed model, which may support minimizing the risk of ASF transmission in the pig population. Further studies focusing on in vivo anti-ASFV effects of MCFAs are important to bring new insight into the mode of ASFV-reduced action by these compounds in swine feed. Keywords: African swine fever virus, Virus isolation, MCFA, Pig, Vietnam. IntroductionAfrican swine fever (ASF) was first observed in Kenya in 1909 following the introduction of the European (exotic) domestic pig and was described retrospectively in 1921 as a highly contagious hemorrhagic disease in both wild boar and domestic pigs (Eustace Montgomery, 1921). ASF virus (ASFV) has extremely high morbidity, mortality spreads rapidly, and causes severe illnesses up to 100%; thus, it is notifiable as an important disease with the World Organization for Animal Health (OIE) (OIE, 2012). In August 2018, ASFV made its biggest leap into China and then outbreaks in Asiatic countries that have highlighted the urgent need for effective antiviral agents against ASFV, which can provide an alternative tool for combating this disease (Zhou et al., 2018; Kim et al., 2019; Tran et al., 2019; Kim et al., 2020). Due to the lack of vaccines, drugs, and effective control measures, ASF remains a serious threat to global pork production (Gaudreault and Richt, 2019; Sanchez et al., 2019). Currently, one of the most effective measures to control and prevent ASFV-spread is biosecurity (Revilla et al., 2018; Dixon et al., 2019b), including biosecurity at the farm, local and national levels. The recent results demonstrated that ASFV could be easily transmitted orally through natural consumption of both liquid and feed, indicating the important role of feed in the emergence of ASFV outbreaks in new pig populations throughout the world (Mazur-Panasiuk et al., 2019; Niederwerder et al., 2019). Disinfection and the proper use of disinfectants of pathogenic in feed and water are a basic and the most important role in the aspect of biosecurity and based on decreasing the percentage of pathogenic micro-organisms in the feed (Davies et al., 2017; Revilla et al., 2018; Eble et al., 2019). The ideal of “feed and water biosecurity” should be characterized by non-toxicity, fast action, durability, resistance to influence from the environment, and more importantly, it should have the widest possible spectrum of biocidal activity including bacteria, viruses, and fungi. The incorrect definition of activity parameters such as concentration, contact time, and range may lead to the improper use of disinfectant products, whereby no effectiveness is achieved (Davies et al., 2017; Eble et al., 2019; Mazur-Panasiuk et al., 2019; Niederwerder et al., 2019). There are several substances or chemical compounds generally accepted as inactivating enveloped viruses, including the ASFV and the only studies on the use of disinfectants against the ASFV concerning tests on various surfaces, as revealed (Dee et al., 2014; Zhang et al., 2014; Cochrane et al., 2015; Hakobyan et al., 2019). To the best of authors’ knowledge, this is the first in vitro testing of medium-chain fatty acids (MCFAs) as chemicals/disinfectants against the ASFV strain circulating in Vietnam. Therefore, the aim of these experiments focused on (i) characterization of the ASFV strain isolated from the capital Hanoi outbreak in the Red River Delta region of Vietnam and (ii) evaluation of the potential effects of MCFAs, individual and synergistic forms, to prevent or reduce the amounts of viral particles of ASFV in in vitro feed model. Material and MethodsVirus isolation, DNA extraction, and PCR assayPreparation of porcine alveolar macrophages (PAM) cells was done as previously described (Tran et al., 2019). The PAM cells were cultured at 37°C in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS, 2 mM L-glutamine, 1,000 IU/ml penicillin, and 1 mg/ml streptomycin. The homogenate of the field pig spleen sample from ASFV confirmed outbreak in the capital Hanoi was virus-positive by conventional PCR using p72U/p72D specific primer (data not shown), and the haemadsorption (HAD) assay was used to inoculate PAM cells for virus isolation as previously described (Tran et al., 2019). The cell supernatants were collected after 4 days of inoculation, and the HAD assay for infectious virus particles was performed. The genomic DNA of ASFV was extracted by using the QIAamp DNA Mini Kit (QIAgen, Hilden, Germany) from cell supernatants. For molecular analysis, different PCRs were set up on ASF isolate: (i) the DNA of ASFV in the supernatant was detected by the real-time PCR for viral p72 gene-specific primer as recommended by OIE, and the real-time PCR was carried out on an Agilent AriaMx Real-Time PCR System (Agilent, Santa Clara, CA) according to the OIE-recommended procedure described in King et al. (2003); (ii) the C-terminal region of the p72 protein was amplified using primers p72U/D for genotype analysis (Gallardo et al., 2014; Quembo et al., 2018); (iii) a fragment of the EP402R gene encoding the CD2v protein using specific primer CD2-2F/2R according to the previously described for serotype analysis (Sanna et al., 2017); and (iv) a fragment of the tandem repeat sequences (TRS) in the intergenic area between the I73R and I329L genes using specific primer ECO1A/ECO1B according to the previously described (Gallardo et al., 2014; Sanna et al., 2017). Positive amplification control consisted of known DNA of ASFV positive, and negative amplification control consisted of nuclease-free sterile water. HAD assayThe titer of ASFV was performed by HAD assay and expressed as 50% HAD doses/ml (HAD50), as previously described (OIE, 2012; Tran et al., 2019). Briefly, the HAD assay was performed as previously described (Malmquist and Hay, 1960). Primary PAM cells were seeded in 96-well plates, and the samples were then added to the plates and titrated in triplicate using 10 × dilutions. The quantity of the ASFV was determined by the identification of characteristic rosette formation representing haemadsorption of erythrocytes around infected cells, according to the guideline of OIE (OIE, 2012). HAD was observed for 4 days, and the images were captured with Optika Vision Pro [OPTIKA S.r.l.ViaRigla, Ponteranica (BG) – Italy]. The HAD50 was calculated using the method of Reed and Muench (1938). Sequence analysis of the ASFV isolateThe correct size of the amplicons of the partial gene p72, EP402R, and TRS products was electrophoresed on a 1.5% agarose gel against a 100 bp DNA leader marker (Thermo Scientific) and visualized by UV irradiation and ethidium bromide staining (Sigma-Aldrich, St. Louis, MS). The correct size of the amplicons was isolated from the agarose gel and purified using the QIAQuick gel extraction kit (QIAgen) according to the manufacturer’s specifications for sequencing (1st BASE, Selangor, Malaysia). The chromatograms of amplicons and probe binding site sequences were analyzed using BioEdit and DNAstar program (DNASTAR Inc. Madison, WI). The nucleotide identity of the ASFV strain in the capital Hanoi outbreak of Vietnam compared with other sequences was performed using the Blast tool at the National Center for Biotechnology Information (NCBI) database and using the information of published sequences. The multiple sequence alignment was performed using the Lasergene software (DNASTAR Inc.). Phylogenetic analyses of nucleotides sequences of partial p72 and CD2v ASFV were constructed using the neighbor-joining method with a bootstrap value of 1,000 in the MEGA7 program (Kumar et al., 2016). Experimental design of feed assayThe 22.5 g of commercial swine feed (formula composition Table 1) samples were treated with 03 products of MCFAs, product 1 [RAG C8 (caprylic acid)], product 2 [RAG C6-C8-C10 (caprylic acid-caproic acid-capric acid); 1:1:1 ratio], and product 3 [RAG C8-C10-C12 (caprylic acid-capric acid-lauric acid); 1:1:1 ratio] (All products provided by Royal Agrifirm Group, Apeldoorn, The Netherlands; chemical structures as shown in Fig. 1A) at an application rate of 0.125%, 0.25%, 0.375%, 0.5% and then, spiked with 2.5 ml of DMEM (Gibco, Thermo Scientific) containing 1 × 106 HAD50/ml of ASFV. An additional 22.5 g of feed samples were inoculated with phosphate-buffered saline (PBS, Sigma-Aldrich) and used as negative controls, while an additional 22.5 g of feed samples were inoculated with 1 × 106 HAD50 of ASFV and used as positive controls. The final virus titration is 1 × 105 HAD50/g feed. Treatments of each group or control group were collected at 24-hour post-inoculation. The sample was collected for DNA extraction using the QIAamp DNA Mini Kit (QIAgen, Hilden, Germany) and subject to real-time PCR as described above. Cytotoxicity of MCFAs on PAM cellsPAM cells were harvested, counted by hemocytometer, and diluted with medium, yielding a concentration of 1 × 106 cells/ml. From this cell suspension, 100 μl was pipetted into 96-well microtiter plates (Nunc, Denmark) and incubated for 24 hours in a 5% CO2 incubator at 37°C. Cells were then treated with 25, 50, 100, 200, 400, and 1,000 μg/ml of MCFA products in culture medium and the final volume is 200 μl per well. Each concentration of MCFAs product was repeated 3-wells. The plate was then incubated in the 5% CO2 incubator at 37°C for 24 hours for determining the cytotoxicity and cell proliferation by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT kit, Abcam), according to the manufacturer's protocols. Table 1. Calculated energy and nutrient content of the commercial feed.
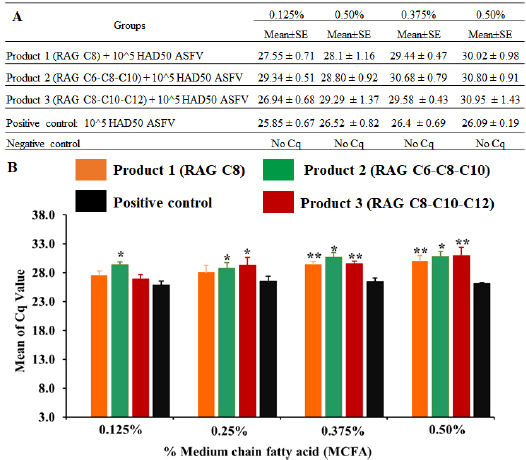
Fig. 1. (A) Chemical structures of MCFA derivatives. (B) The effects of MCFAs on PAMs cell at the cell viability by MTT assay. Data are presented as the mean ± SEM of three independent experiments indicate the significant differences between the control and treatment groups was determined by one-way analysis of variance (a: p < 0.05). Statistical analysisStatistical analysis was performed using IBM SPSS software (SPSS 23.0 for Windows; IBM, Chicago, IL). A p-value < 0.05 was considered to be statistically significant. Differences among the groups were tested by Duncan’s multiple comparison methods. Ethical approvalThe study was conducted in compliance with the institutional rules for the care and use of laboratory animals and using a protocol approved by the Ministry of Agriculture and Rural Development Vietnam (TCVN 8402:2010). Results and DiscussionASF is one of the most important viral diseases of wild boar and domestic pigs, causing significant socio-economic losses in affected countries (Dixon et al., 2019a). Depending on host characteristics and the circulating viral isolate, clinical signs may vary from the highly lethal form with 100% mortality to sub-clinical and a clinical form (Chenais et al., 2019; Cwynar et al., 2019; Dixon et al., 2019a, 2019b). Since the developed vaccines against ASFV have failed to induce effective protection, it is reasonable to evaluate antiviral agents against this virus (Revilla et al., 2018; Gaudreault and Richt, 2019; Sanchez et al., 2019). The aim of this study focused on (i) characterization of ASFV strain isolated from the capital Hanoi outbreak in the Red River Delta region of Vietnam and (ii) investigation of the antiviral activities of MCFAs at different composting materials, including C8 (caprylic acid), caprylic acid-caproic acid-capric acid (C8-C6-C10) and C8-C10-C12 (caprylic acid-capric acid-lauric acid), against ASFV infection in vitro. We first screened the cytotoxicity of MCFAs on PAM cells, the permissive cells for ASFV infection in vitro, by using the MTT assay according to the manufacturer’s protocols. As shown in Figure 1B, at the dose of 200 μg/ml, products 1 and 3 have shown a less cytotoxic effect when compared to product 2 on PAM cells, suggesting the anti-proliferative effects of the C6 component in product 2. All products do not induce cytotoxicity at the dose of 100 μg/ml (Fig. 1B), suggesting that all products are suitable for further in vitro examination. It has been reported previously that C6 and capric acid (C10) compounds at low concentrations may cause an alteration in cell membrane structures. However, the cytotoxic effects on mammalian cells may not reflect the adverse effects in humans and animals because of many physiological factors, including mucins and serum (Zentek et al., 2011). To examine antiviral effects of chemical/disinfectants, information of viral field strain used in whole experiments is very important, including genetic characterization. An ASFV isolated from an outbreak in the Capital Hanoi of Vietnam was employed. Some virus assays, including virus isolation in combination with HAD assay and conventional PCR for genotyping and serotyping, have been performed to confirm this field strain. A strong positive HAD was observed in culture, indicating the success of viral isolation (Fig. 2A). This isolate was named Pig/Hanoi/2019/01. Further conventional PCR was conducted to verify HAD positive data as recommended by OIE and genetic characterization of Pig/Hanoi/2019/01 based on the sequencing analysis of the p72, CD2v, and TRS region gene. As expected, positive 478-bp, 816-bp, and 356-bp bands of PCR products were appeared on the gel, respectively (Fig. 2B). Nucleotide sequence comparisons using the Basic Local Alignment Search Tool (http://blast.ncbi. nlm.nih.gov/Blast.cgi) revealed that the p72 and CD2v sequences of Pig/Hanoi/2019/01were 100% identical to those of China isolates of ASFV SY18, Pig/HLJ/2018, AnhuiXCGQ, and CN201801 (Ge et al., 2018; Li et al., 2018; Zhou et al., 2018). Phylogenetic analysis of p72 and CD2v of epidemic ASFV strain, Pig/Hanoi/2019/01, demonstrated that this isolate belongs to genotype II and serotype 8 and closely related with ASFVs isolated from China in 2018 (Zhou et al., 2018), Russia in 2012 and 2017 (Kolbasov et al., 2018) and Estonia 2014 (Zani et al., 2018) (Fig. 3A and B). On the other hand, the TRS regions in the intergenic region (IGR) between I73R and I329L has been extremely useful for resolving epidemiologic complexities at the genotype, region, and country levels (Gallardo et al., 2014; Sanna et al., 2017; Quembo et al., 2018), such that additional genome markers are required to evaluate the origin and to map the outbreak of closely related ASF isolates circulating in Viet Nam. Our results showed that the Pig/Hanoi/2019/01strain had an intergenic region II variant with an additional 10 nucleotide (5′-GGAATATATA-3′) into the TRS region between the I73R and I329L genes (Fig. 3C). The intergenic region II variant of the Pig/Hanoi/2019/01strain was identified to those described in isolates Ukr12/Zapo, Belgium 2018/1 (Sanna et al., 2017). The same TRS insertion was also found in China isolates of ASFV SY18, Pig/HLJ/2018, AnhuiXCGQ, and CN201801 (Ge et al., 2018; Li et al., 2018; Zhou et al., 2018) and differ from ASFV isolated in Georgia in 2007 (Chapman et al., 2011). The sequences of p72, CD2v, and TRS genes of Pig/Hanoi/2019/01 strain have been deposited in GenBank as the accession numbers of MT332151-3, respectively. Up until now, at least two different variants of IGR located between the I73R and I329L genes of ASF virus strains in Vietnam have been reported, and most ASFV strains circulating in this country belong to the p72 genotype II and IGR II variant (Tran et al., 2021). Therefore, the pig/Hanoi/2019/01 strain has been selected for this study as a representative strain of ASFV circulating in Vietnam. Recent research suggested that the susceptibility to chemicals/disinfectants depends on viral characteristics in which non-enveloped viruses are more resistant than enveloped viruses (Juszkiewicz et al., 2019). Moreover, some chemical synthesis or extract from a plant such as an acacetin, apigenin, genkwanin, rhoifolin, vitexin, and vitexin 2-O-rhamnoside are able to inhibit or reduce ASF virus-specific protein synthesis and viral factory formation in the Vero cell line system, in which apigenin showed potent inhibition of ASF virus-infected Vero cells with not display a cytopathic effect (Zhang et al., 2014; Hakobyan et al., 2016, 2019). However, there is no evidence of the survivability of ASFVs isolated from the field after pre-treatment with MCFAs. On the other hand, a recent report demonstrated that the activity of MCFAs could enhance the RNA degradation and mitigating of porcine epidemic diarrhea virus (PEDV) in swine feed and ingredients (Cochrane et al., 2015). To investigate the potential effects of MCFAs to reduce Pig/Hanoi/2019/01infectivity, we used in vitro feed model in which MCFA pre-treated feed was spiked to Pig/Hanoi/2019/01isolate at 105 HAD50/g feed, and the samples were collected after 24 hours post-inoculation. Real-time PCR amplification of the p72 gene was performed to detect the presence of viral DNA in the feed based on OIE recommended protocol. A recent in vivo report has indicated that the minimum infectious dose of ASFV in feed is 104TCID50, in which 40% of pigs orally exposed to ASFV have shown a positive result, while other studies suggested that the minimum dose of ASFV oral exposure should be 105 (Niederwerder et al., 2019). The results of MCFA pre-treated feed exposed to ASFV at the dose of 105 HAD50/g feed are summarized in Figure 4. No Cq value was obtained in the negative control group, while feed exposed to ASFV without MCFA treatment as a positive control group showed the Cq value ranged from 25.85 ± 0.67 to 26.52 ± 0.82 after 24 hours. It is indicated that the ASFV could survive in the feed and can be spread the ASFV in the new population of the pig industry (Food Safety Authority, 2014; Guinat et al., 2016; Niederwerder et al., 2019). Interestingly, a strong anti-ASFV effect was observed in all product treatments after 24h post-inoculation. Pre-treatment with product 1 significantly reduced ASFV replication at two highest doses of 0.375 and 0.5% (p < 0.01) (Fig. 4). Product 2 has shown the potential susceptibility to ASFV isolated in Vietnam in which all doses (0.125% and 0.25% with p < 0.05; 0.375%, and 0.5% with p < 0.01) induced a statistical increase in Cq value when compared to positive control. On the other hand, at the dose of 0.25%, product 3 caused significant enhancement in the levels of the Cq value when compared to a positive control (p < 0.05), and a significant increase in dose- dependence was recognized at doses 0.375 and 0.5% in product 3 group (p < 0.01) (Fig. 4). These results demonstrated that all tested products of MCFAs significantly increased in the Cq value when compared to the positive control (p < 0.01) at the highest doses of 0.375% and 0.5% at 24 hours after virus incubation (Fig. 4). Additionally, our findings also indicated that MCFAs in synergistic forms have shown promising candidates to reduce ASFV infectivity. Product 2 reduced ASFV infectivity at the lowest dose of 0.125%, while a significant increase in Cq value was noted in the product 3 group at a dose of 0.25% (p < 0.05), suggesting synergistic MCFA C6-C8-C10 is the most potential MCFAs against ASFV in feed model. It is clear that the antiviral effects of MCFAs on the reduction of ASFV infectivity in feed depend on the composting materials in the mixture and the dose. A recent study on the effects of MCFAs on PEDV infectivity has indicated that the C8 component has shown the strongest antiviral effect as an individual component and the MCFA blend (C8-C6-C10) is a promising product to reduce PEDV in feed. An agreement between our data on ASFV and a previous study on PEDV in feed was recognized in this study.
Fig. 2. HAD assay and PCR verification for virus isolation. (A) HAD assay of the spleen homogenate. The 10-times dilution of the supernatant of the homogenate was inoculated into PAM cells with 1% pig red blood cells. HAD was observed for 4 days. Scale bar: 200 μm. (B) PCR detection of ASFV isolated in PAM cells, including p72, CD2v, and TRS of ASFV genome.
Fig. 3. Phylogenetic analysis of Pig/Hanoi/2019/01 based on its partial p72 (A), EP402R gene encoding the CD2v protein (B) genes. The sequences of the p72 and EP402R gene encoding the CD2v protein genes of representative ASF virus were downloaded from the NCBI database. The neighbor-joining method was used to construct phylogenetic trees using MEGA 7 software. Numbers along branches indicate bootstrap values >70% (1,000 replicates). The black square indicates the ASF virus isolate from this study. Scale bars indicate nucleotide substitutions per site. (C) Alignment of the partial nucleotide sequence of the intergenic region between I73R and I329L of ASFV strain isolated from the capital Hanoi of Vietnam with reference ASFV strains. The mutation that results in the insertion of a single nucleotide internal repeat sequence (GGAATATATA) in the Pig/Hanoi/2019/01 is indicated by gray shading.
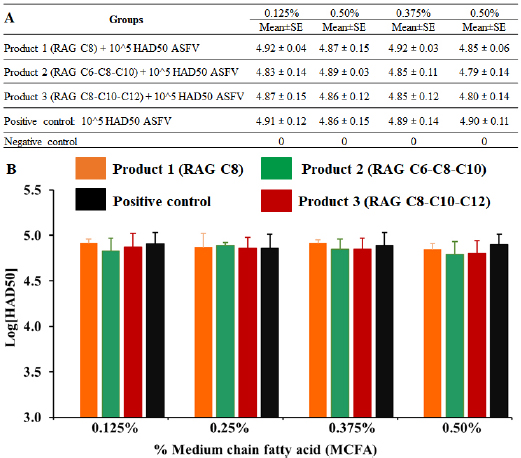
Fig. 4. MCFA pre-treated feed in exposure to 1 × 105 HAD50/g feed of Pig/Hanoi/2019/01 strain after 24-hour post-inoculation. The viral DNA of the ASF virus in supernatants collected from feed exposed and then, real-time PCR amplification of the p72 gene was performed to detect the presence of viral DNA in MCFAs treated feeds. Data are presented as the mean ± SEM of three independent experiments: (a) p < 0.05 and (b) p < 0.01. One of the most important steps when performing “feed and water biosecurity” is disinfection, and it may reduce or inhibit the risk of ASFV contamination in the environment or other pathogens (Juszkiewicz et al., 2019). A recent report demonstrated that the ASFV could be directly transmitted to contract pigs by contaminated fomites, drinking water, facilitate pig industry (Mazur-Panasiuk et al., 2019), excretions (Davies et al., 2017), and carriers (Eble et al., 2019). To the authors’ knowledge, real-time PCR is a wonderful tool to evaluate the susceptibility of chemicals/disinfectants to ASFV at “DNA level,” the HAD assay is important at “living material” for this purpose. As shown in Figure 5, the viral titre was not changed after 24 hours post-inoculation when compared to a positive control (p > 0.05). This finding suggests that all tested doses of MCFAs, both individual and synergistic forms, did not cause any change at viral titre after 24 hours post-inoculation. A high correlation between our current results and the data published by Jackman et al. (2020) was observed. Additionally, the difference between DNA level and virus titre of ASFV in the feed at 24 hours after ASFV exposure has been described previously by us in which treatment with Sal CURB RME liquid, a strong antiviral additive, caused a significant increase at Cq level. However, no statistical change was recognized at viral titre by HAD assay when compared to positive control (Tran et al., 2020).
Fig. 5. MCFA pre-treated feed in exposure to 1 × 105 HAD50/g feed of Pig/Hanoi/2019/01 strain after 24-hour post-inoculation. The ASF virus collected from feed exposed and then, HAD assay was performed for the viral titration in MCFAs treated feeds. Data are presented as the mean ± SEM of three independent experiments: (a) p < 0.05 and (b) p < 0.01. The antimicrobial activities of MCFA have been reported previously. Some studies have suggested the potential antiviral effects of caprylic acid (C8) and/or C10 on vesicular stomatitis virus or visa virus (Zentek et al., 2011). Additionally, the synergistic effects of MCFAs have shown more effective against pathogens when compared to their individual form in which combined effects of C8 and C10 caused a significant decline of bacterial flora, while no efficacy was found by individual C8 or C10 (Dierick et al., 2002). As the shortest member of the MCFA family, caproic acid C6 and its antimicrobial effects on bacterial growth have been reported in previous studies in which at the dose of 25 μg/ml, this acid has shown a complete inhibition of Actinobacillus actinomycetemcomitans growth, a gram-negative bacterium (Huang et al., 2011). The significant activities of C6 in combination with C8 and C10 against PEDV in previous study and our current ASFV experiment indicated that, this compound possessed both anti-bacterial and viral properties. Also, other studies have demonstrated that among all MCFAs, C12 componentis majority of antibacterial activities (Petschow et al., 1996; Vande Maele et al., 2016). The role of C12 component in synergistic MCFAs was markedly noted in our feed experiment, suggesting the susceptibility of lauric acid C12 to viral pathogens. Further studies are essential to elucidate the chemical properties and antiviral activities of this compound. The anti-bacterial effects on MCFAs have been reported in both in vitro and in vivo studies; however, the study of their effects on viral pathogens are limited. Although, it is difficult to be definitive regarding to molecular mechanism underlying MCFA, both individual and synergistic forms, activities against ASFV infectivity, it could be investigated in further studies, particularly in vivo model. ConclusionTo the authors’ knowledge, it is the first report on MCFA-activities against ASFV in in vitro feed model. Our findings suggested that the MCFAs possessed the potent activities as an antiviral feed mitigant on ASFV infectivity in vitro, and the synergistic forms of MCFAs (C6-C8-C10 and/or C8-C10-C12) have shown stronger anti-ASFV activities in vitro when compared to the individual form of MCFA(C8). Additionally, the effects of MCFAs on ASFV infectivity are dose- and component-dependent. Further studies focusing on in vivo anti- ASFV effects of MCFA are important to bring new insight into the mode of ASFV-reduced action of these compounds. AcknowledgementsThis work was supported by the Ministry of Science and Technology to Hoang Vu Dang (Project code: ĐTĐL.CN-75/19). The authors would like to thank the Royal Agrifirm Group for providing the funding and in-kind resources necessary to complete this project Authors’ contributionsHTTT, ADT, and HVD conceived and designed the experiments. HTTT, ADT, DVL, TVH, CTN, NTC, HTN, and ADT performed the experiments. HTTT, ADT, KL, GB, and HVD analyzed the data. HVD and KL contributed the reagents, materials, and analytical tools. HTTT, ADT, MDV, KL, GB, and HVD wrote the manuscript. All authors read and approved the final manuscript. Conflict of interestThe authors declare that they have no competing interests. ReferencesChapman, D.A., Darby, A.C., Da Silva, M., Upton, C., Radford, A.D. and Dixon, L.K. 2011. Genomic analysis of highly virulent Georgia 2007/1 isolate of African swine fever virus. Emerg. Infect. Dis. 17, 599–605. Chenais, E., Depner, K., Guberti, V., Dietze, K., Viltrop, A. and Stahl, K. 2019. Epidemiological considerations on African swine fever in Europe 2014-2018. Porcine Health Manag. 5, 6. Cochrane, R.A., Dritz, S.S., Woodworth, J.C., Huss, A.R., Stark, C.R., Hesse, R.A., Zhang, J., Tokach, M.D., Bai, J. and Jones, C.K. 2015. Evaluating chemical mitigation of porcine epidemic diarrhea virus (PEDV) in swine feed and ingredients. Kansas Agricultural Experiment Station, Research Reports No. 1. Cwynar, P., Stojkov, J. and Wlazlak, K. 2019. African swine fever status in Europe. Viruses 11(4), 310. Davies, K., Goatley, L.C., Guinat, C., Netherton, C.L., Gubbins, S., Dixon, L.K. and Reis, A.L. 2017. Survival of African swine fever virus in excretions from pigs experimentally infected with the georgia 2007/1 isolate. Transbound. Emerg. Dis. 64, 425–431. Dee, S., Neill, C., Clement, T., Christopher-Hennings, J. and Nelson, E. 2014. An evaluation of a liquid antimicrobial (Sal CURB®) for reducing the risk of porcine epidemic diarrhea virus infection of naive pigs during consumption of contaminated feed. BMC Vet. Res. 10, 220. Dierick, N.A., Decuypere, J.A., Molly, K., Van Beek, E. and Vanderbeke, E. 2002. The combined use of triacylglycerols (TAGs) containing medium chain fatty acids (MCFAs) and exogenous lipolytic enzymes as an alternative to nutritional antibiotics in piglet nutrition: II. In vivo release of MCFAs in gastric cannulated and slaughtered piglets by endogenous and exogenous lipases; effects on the luminal gut flora and growth performance. Livest. Prod. Sci. 76, 1–16. Dixon, L.K., Islam, M., Nash, R. and Reis, A.L. 2019a. African swine fever virus evasion of host defences. Virus Res. 266, 25–33. Dixon, L.K., Sun, H. and Roberts, H. 2019b. African swine fever. Antiviral Res. 165, 34–41. Eble, P.L., Hagenaars, T.J., Weesendorp, E., Quak, S., Moonen-Leusen, H.W. and Loeffen, W.L.A. 2019. Transmission of African swine fever virus via carrier (survivor) pigs does occur. Vet. Microbiol. 237, 108345. Eustace Montgomery, R. 1921. On a form of swine fever occurring in British East Africa (Kenya Colony). J. Comp. Pathol. Ther. 34, 159–191. Food Safety Authority, E. 2014. Evaluation of possible mitigation measures to prevent introduction and spread of African swine fever virus through wild boar. 12(3) 3616. Gallardo, C., Fernandez-Pinero, J., Pelayo, V., Gazaev, I., Markowska-Daniel, I., Pridotkas, G., Nieto, R., Fernandez-Pacheco, P., Bokhan, S., Nevolko, O., Drozhzhe, Z., Perez, C., Soler, A., Kolvasov, D. and Arias, M. 2014. Genetic variation among African swine fever genotype II viruses, eastern and central Europe. Emerg. Infect. Dis. 20, 1544–1547. Gaudreault, N.N. and Richt, J.A. 2019. Subunit vaccine approaches for African swine fever virus. Vaccines (Basel) 7(2), 56. Ge, S., Li, J., Fan, X., Liu, F., Li, L., Wang, Q., Ren, W., Bao, J., Liu, C., Wang, H., Liu, Y., Zhang, Y., Xu, T., Wu, X. and Wang, Z. 2018. Molecular characterization of African swine fever virus, China, 2018. Emerg. Infect. Dis. 24, 2131–2133. Guinat, C., Gogin, A., Blome, S., Keil, G., Pollin, R., Pfeiffer, D.U. and Dixon, L. 2016. Transmission routes of African swine fever virus to domestic pigs: current knowledge and future research directions. Vet. Rec. 178, 262–267. Hakobyan, A., Arabyan, E., Avetisyan, A., Abroyan, L., Hakobyan, L. and Zakaryan, H. 2016. Apigenin inhibits African swine fever virus infection in vitro. Arch. Virol. 161, 3445–3453. Hakobyan, A., Arabyan, E., Kotsinyan, A., Karalyan, Z., Sahakyan, H., Arakelov, V., Nazaryan, K., Ferreira, F. and Zakaryan, H. 2019. Inhibition of African swine fever virus infection by genkwanin. Antiviral Res. 167, 78–82. Huang, C.B., Alimova, Y., Myers, T.M. and Ebersole, J.L. 2011. Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch. Oral Biol. 56, 650–654. Jackman, J.A., Boyd, R.D. and Elrod, C.C. 2020. Medium-chain fatty acids and monoglycerides as feed additives for pig production: towards gut health improvement and feed pathogen mitigation. J. Anim. Sci. Biotechnol. 11, 44. Juszkiewicz, M., Walczak, M. and Wozniakowski, G. 2019. Characteristics of selected active substances used in disinfectants and their virucidal activity against ASFV. J. Vet. Res. 63, 17–25. Kim, H.J., Lee, M.J., Lee, S.K., Kim, D.Y., Seo, S.J., Kang, H.E. and Nam, H.M. 2019. African swine fever virus in pork brought into South Korea by travelers from China, August 2018. Emerg. Infect. Dis. 25, 1231–1233. Kim, S.H., Kim, J., Son, K., Choi, Y., Jeong, H.S., Kim, Y.K., Park, J.E., Hong, Y.J., Lee, S.I., Wang, S.J., Lee, H.S., Kim, W.M. and Jheong, W.H. 2020. Wild boar harbouring African swine fever virus in the demilitarized zone in South Korea, 2019. Emerg. Microbes Infect. 9, 628–630. King, D.P., Reid, S.M., Hutchings, G.H., Grierson, S.S., Wilkinson, P.J., Dixon, L.K., Bastos, A.D. and Drew, T.W. 2003. Development of a TaqMan PCR assay with internal amplification control for the detection of African swine fever virus. J. Virol. Methods 107, 53–61. Kolbasov, D., Titov, I., Tsybanov, S., Gogin, A. and Malogolovkin, A. 2018. African swine fever virus, Siberia, Russia, 2017. Emerg. Infect. Dis. 24, 796–798. Kumar, S., Stecher, G. and Tamura, K. 2016. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. Li, L., Wang, Q., Ge, S., Liu, Y., Liu, C., Liu, F., Hu, Y., Li, J., Bao, J., Ren, W., Zhang, Y., Xu, T., Sun, C., Li, L., Wang, S., Fan, X., Huang, B., Wu, X. and Wang, Z. 2018. Infection of African swine fever in wild boar, China, 2018. Transbound. Emerg. Dis. 66(3), 1395–1398. Malmquist, W.A. and Hay, D. 1960. Hemadsorption and cytopathic effect produced by African swine fever virus in swine bone marrow and buffy coat cultures. Am. J. Vet. Res. 21, 104–108. Mazur-Panasiuk, N., Zmudzki, J. and Wozniakowski, G. 2019. African swine fever virus – persistence in different environmental conditions and the possibility of its indirect transmission. J. Vet. Res. 63, 303–310. Niederwerder, M.C., Stoian, A.M.M., Rowland, R.R.R., Dritz, S.S., Petrovan, V., Constance, L.A., Gebhardt, J.T., Olcha, M., Jones, C.K., Woodworth, J.C., Fang, Y., Liang, J. and Hefley, T.J. 2019. Infectious dose of African swine fever virus when consumed naturally in liquid or feed. Emerg. Infect. Dis. 25, 891–897. OIE. African swine fever. In Manual of diagnostic tests and vaccines for terrestrial animals. Chapter 2.8.1. 2012. Petschow, B.W., Batema, R.P. and Ford, L.L. 1996. Susceptibility of helicobacter pylori to bactericidal properties of medium-chain monoglycerides and free fatty acids. Antimicrob. Agents Chemother. 40, 302–306. Quembo, C.J., Jori, F., Vosloo, W. and Heath, L. 2018. Genetic characterization of African swine fever virus isolates from soft ticks at the wildlife/domestic interface in Mozambique and identification of a novel genotype. Transbound. Emerg. Dis. 65, 420–431. Reed, L.J. and Muench, H. 1938. A simple method of estimaing fifty percent endpoints. Am. J. Epidemiol. 27, 493–497. Revilla, Y., Perez-Nunez, D. and Richt, J.A. 2018. African swine fever virus biology and vaccine approaches. Adv. Virus Res. 100, 41–74. Sanchez, E.G., Perez-Nunez, D. and Revilla, Y. 2019. Development of vaccines against African swine fever virus. Virus Res. 265, 150–155. Sanna, G., Dei Giudici, S., Bacciu, D., Angioi, P.P., Giammarioli, M., De Mia, G.M. and Oggiano, A. 2017. Improved strategy for molecular characterization of African swine fever viruses from Sardinia, based on analysis of p30, CD2V and I73R/I329L variable regions. Transbound. Emerg. Dis. 64, 1280–1286. Tran, H.T.T., Dang, A.K., Ly, D.V., Vu, H.T., Hoang, T.V., Nguyen, C.T., Chu, N.T., Nguye, V.T., Nguyen, H.T., Truong, A.D., Pham, N.T. and Dang, H.V. 2019. An improvement of real-time PCR system based on probe modification is required for accurate detection of African swine fever virus in clinical samples in Vietnam. Asian-Australas J. Anim. Sci. 33(10), 1683–1690. Tran, H.T.T., Truong, A.D., Dang, A.K., Ly, D.V., Nguyen, C.T., Chu, N.T., Hoang, T.V., Nguyen, H.T. and Dang, H.V. 2021. Circulation of two different variants of intergenic region (IGR) located between the I73R and I329L genes of African swine fever virus strains in Vietnam. Transbound Emerg Dis; doi:10.1111/tbed.13996. Tran, H.T.T., Truong, A.D., Ly, D.V., Vu, T.H., Hoang, V.T., Nguyen, T.C., Chu, T.N., Nguyen, T.H., Pham, N.T., Nguyen, T., Yersin, A.G. and Dang, H.V. 2020. Genetic characterisation of African swine fever virus in outbreaks in Ha Nam Province, Red River Delta Region of Vietnam, and activity of antimicrobial products against virus infection in contaminated feed. J. Vet. Res. 64, 207–213. Vande Maele, L., Heyndrickx, M., Maes, D., De Pauw, N., Mahu, M., Verlinden, M., Haesebrouck, F., Martel, A., Pasmans, F. and Boyen, F. 2016. In vitro susceptibility of Brachyspira hyodysenteriae to organic acids and essential oil components. J. Vet. Med. Sci. 78, 325–328. Zani, L., Forth, J.H., Forth, L., Nurmoja, I., Leidenberger, S., Henke, J., Carlson, J., Breidenstein, C., Viltrop, A., Hoper, D., Sauter-Louis, C., Beer, M. and Blome, S. 2018. Deletion at the 5’-end of estonian ASFV strains associated with an attenuated phenotype. Sci. Rep. 8, 6510. Zentek, J., Buchheit-Renko, S., Ferrara, F., Vahjen, W., Van Kessel, A.G. and Pieper, R. 2011. Nutritional and physiological role of medium-chain triglycerides and medium-chain fatty acids in piglets. Anim. Health Res. Rev. 12, 83–93. Zhang, W., Qiao, H., Lv, Y., Wang, J., Chen, X., Hou, Y., Tan, R. and Li, E. 2014. Apigenin inhibits enterovirus-71 infection by disrupting viral RNA association with trans-acting factors. PLoS One 9, e110429. Zhou, X., Li, N., Luo, Y., Liu, Y., Miao, F., Chen, T., Zhang, S., Cao, P., Li, X., Tian, K., Qiu, H.J. and Hu, R. 2018. Emergence of African swine fever in China, 2018. Transbound. Emerg. Dis. 65(6), 1482–1484. | ||
| How to Cite this Article |
| Pubmed Style Tran HTT, Truong DA, Ly VD, Hoang TV, Chu NT, Nguyen HT, Dang ATK, Vos MD, Lannoo K, Bruggeman G, Dang HV, . The potential anti- African swine fever virus effects of medium chain fatty acids on in vitro feed model: An evaluation study using a field ASFV strain isolated in Vietnam. Open Vet J. 2021; 11(3): 346-355. doi:10.5455/OVJ.2021.v11.i3.3 Web Style Tran HTT, Truong DA, Ly VD, Hoang TV, Chu NT, Nguyen HT, Dang ATK, Vos MD, Lannoo K, Bruggeman G, Dang HV, . The potential anti- African swine fever virus effects of medium chain fatty acids on in vitro feed model: An evaluation study using a field ASFV strain isolated in Vietnam. https://www.openveterinaryjournal.com/?mno=79280 [Access: July 27, 2024]. doi:10.5455/OVJ.2021.v11.i3.3 AMA (American Medical Association) Style Tran HTT, Truong DA, Ly VD, Hoang TV, Chu NT, Nguyen HT, Dang ATK, Vos MD, Lannoo K, Bruggeman G, Dang HV, . The potential anti- African swine fever virus effects of medium chain fatty acids on in vitro feed model: An evaluation study using a field ASFV strain isolated in Vietnam. Open Vet J. 2021; 11(3): 346-355. doi:10.5455/OVJ.2021.v11.i3.3 Vancouver/ICMJE Style Tran HTT, Truong DA, Ly VD, Hoang TV, Chu NT, Nguyen HT, Dang ATK, Vos MD, Lannoo K, Bruggeman G, Dang HV, . The potential anti- African swine fever virus effects of medium chain fatty acids on in vitro feed model: An evaluation study using a field ASFV strain isolated in Vietnam. Open Vet J. (2021), [cited July 27, 2024]; 11(3): 346-355. doi:10.5455/OVJ.2021.v11.i3.3 Harvard Style Tran, H. T. T., Truong, D. A., Ly, V. D., Hoang, T. V., Chu, N. T., Nguyen, H. T., Dang, A. T. K., Vos, M. D., Lannoo, K., Bruggeman, G., Dang, H. V. & (2021) The potential anti- African swine fever virus effects of medium chain fatty acids on in vitro feed model: An evaluation study using a field ASFV strain isolated in Vietnam. Open Vet J, 11 (3), 346-355. doi:10.5455/OVJ.2021.v11.i3.3 Turabian Style Tran, Ha Thi Thanh, Duc Anh Truong, Viet Duc Ly, Tuan Van Hoang, Nhu Thi Chu, Huyen Thi Nguyen, Anh Thi Kieu Dang, Maartje De Vos, Kobe Lannoo, Geert Bruggeman, Hoang Vu Dang, and . 2021. The potential anti- African swine fever virus effects of medium chain fatty acids on in vitro feed model: An evaluation study using a field ASFV strain isolated in Vietnam. Open Veterinary Journal, 11 (3), 346-355. doi:10.5455/OVJ.2021.v11.i3.3 Chicago Style Tran, Ha Thi Thanh, Duc Anh Truong, Viet Duc Ly, Tuan Van Hoang, Nhu Thi Chu, Huyen Thi Nguyen, Anh Thi Kieu Dang, Maartje De Vos, Kobe Lannoo, Geert Bruggeman, Hoang Vu Dang, and . "The potential anti- African swine fever virus effects of medium chain fatty acids on in vitro feed model: An evaluation study using a field ASFV strain isolated in Vietnam." Open Veterinary Journal 11 (2021), 346-355. doi:10.5455/OVJ.2021.v11.i3.3 MLA (The Modern Language Association) Style Tran, Ha Thi Thanh, Duc Anh Truong, Viet Duc Ly, Tuan Van Hoang, Nhu Thi Chu, Huyen Thi Nguyen, Anh Thi Kieu Dang, Maartje De Vos, Kobe Lannoo, Geert Bruggeman, Hoang Vu Dang, and . "The potential anti- African swine fever virus effects of medium chain fatty acids on in vitro feed model: An evaluation study using a field ASFV strain isolated in Vietnam." Open Veterinary Journal 11.3 (2021), 346-355. Print. doi:10.5455/OVJ.2021.v11.i3.3 APA (American Psychological Association) Style Tran, H. T. T., Truong, D. A., Ly, V. D., Hoang, T. V., Chu, N. T., Nguyen, H. T., Dang, A. T. K., Vos, M. D., Lannoo, K., Bruggeman, G., Dang, H. V. & (2021) The potential anti- African swine fever virus effects of medium chain fatty acids on in vitro feed model: An evaluation study using a field ASFV strain isolated in Vietnam. Open Veterinary Journal, 11 (3), 346-355. doi:10.5455/OVJ.2021.v11.i3.3 |