| Original Article | ||
Open Vet J. 2021; 11(4): 603-612 Open Veterinary Journal, (2021), Vol. 11(4): 603–612 Original Research Branham sign in dogs undergoing interventional patent ductus arteriosus occlusion or surgical ligation: A retrospective studyFilipe L. Madruga1*, Yolanda Martinez Pereira2, Ambra Panti3, Ian Handel2 and Geoff Culshaw21Small Animal Teaching Hospital, University of Liverpool, Leahurst Campus, Chester High Road, Neston, Wirral, CH64 7TE, UK 2The Royal (Dick) School of Veterinary Studies, University of Edinburgh, Easter Bush Campus, Roslin, EH25 9RG, UK 3Veterinary Specialists Scotland, Department of Veterinary Anaesthesia and Analgesia, 1 Deer Park Road, Livingston, EH54 8AG, UK *Corresponding Author: Filipe L. Madruga. Small Animal Teaching Hospital, University of Liverpool. Leahurst Campus, Chester High Road, Neston, Wirral, CH64 7TE, UK. Tel: +44 (0)151 795 6100. Email: f.lalanda-madruga [at] liverpool.ac.uk Submitted: 06/06/2021 Accepted: 18/10/2021 Published: 06/11/2021 © 2021 Open Veterinary Journal
AbstractBackground: The Branham sign is a baroreceptor response that follows patent ductus arteriosus (PDA) closure. Although described in dogs following both interventional and surgical ductal closure, a direct comparison of the Branham sign elicited by these two techniques has not been made. Aim: Since closure with an Amplatz canine ductal occluder (ACDO) occurs over 10 minutes and surgical ligation (SL) is more rapid, we hypothesized that the Branham sign following occlusion of a PDA with an ACDO would be less severe than following SL. Methods: Clinical records of dogs diagnosed with left-to-right shunting PDA between 2008 and 2018 were retrospectively reviewed. Of 139 dogs undergoing PDA occlusion, only 41 dogs (ACDO n=32, SL n=9) were included after applying exclusion criteria. Heart rate (HR) and blood pressure (BP) from occlusion time (T0) until 30 minutes post occlusion (T30) were recorded. Signalment and anesthetic protocol were also recorded. The influence of age and weight on the hemodynamic variations was assessed. Hemodynamic variables and calculations were compared between and within groups using a repeated measures general linear model, and post hoc tests were applied if significance was identified. Results: A mild Branham sign was present in both groups, and hemodynamic changes were not significantly different between groups. In both groups, there was a significant decrease in HR (11 bpm, 5.3–16.3; p < 0.001) (10.4%, 5.4–15.5; p < 0.001) and increase in diastolic BP (9.5 mmHg, 3–16; p=0.002) (23.5%, 7.1–39.9; p=0.002), but systolic BP did not change significantly (p=0.824). Age and weight did not influence Branham sign. Conclusion: The Branham sign in dogs is mild in both groups, lasts for at least 30 minutes, and is independent of the method of PDA closure. Keywords: ACDO, Branham sign, Dog, Ligation, PDA. IntroductionPatent ductus arteriosus (PDA) is a vascular connection between the ascending aorta and the pulmonary trunk that abnormally persists after birth. It is a common congenital heart abnormality in dogs and leads to left-sided congestive heart failure at a young age (Buchanan, 2001). Management options for PDA include surgical ligation (SL) via left fourth intercostal thoracotomy and transvascular occlusion. Surgical techniques include standard ligation, Jackson-Henderson technique, or hemostatic clips. Transvascular occlusion is by embolization coils, Amplatzer vascular plug 2 or, most frequently, Amplatz canine duct occluder (ACDO) (Ranganathan et al., 2018; Scansen, 2018; Wagner, 2019). The overall rate of successful occlusion is reported to be similar between methods, 95% for SL and 94% for ACDO (Blossom et al., 2010), but the less invasive nature of transvascular occlusion and the lower incidence of morbidity (7.1% vs. up to 28% with SL) (Ranganathan et al., 2018), mean that transvascular methods are frequently undertaken. Rapid closure of a PDA leads to profound and abrupt alterations to cardiac and vascular hemodynamics. These include an acute increase in mean blood pressure (BP) from volume overload in the presence of a closed ductus, which in turn stimulates the baroreceptors, leading to a reflex bradycardia (Wattanasirichaigoon and Pomposelli, 1997; Velez-Roa et al., 2004). This cardiovascular response is known as the Branham or Nicoladoni sign (Wattanasirichaigoon and Pomposelli, 1997). These events can be tracked closely by direct BP measurement and heart rate (HR) monitoring, and can be severe enough to require medical intervention (Pascoe, 2016). Recent publications have demonstrated the Branham sign in dogs following transvascular occlusion (De Monte et al., 2017; Parisi et al., 2020) but a direct comparison of BP and HR changes between surgical and non-surgical methods has not been made. Since closure of the ductus with a device takes several minutes longer than by SL (Stanley et al., 2003; Porciello et al., 2014), we hypothesized that the Branham sign following ACDO is less severe than following SL. This is of clinical relevance as a milder Branham sign would mean a reduced likelihood of clinical intervention following use of an ACDO, namely the use of muscarinic anticholinergics, though its use is usually unnecessary, unless bradycardia becomes hemodynamically significant (Robinson and Borgeat, 2016). It could also provide additional support for the use of newer generation transvascular devices in dogs for whom, thus far, surgery has been the only option, such as very small dogs. Material and MethodsComputerized records of dogs referred to our institution between January 2008 and January 2018 were reviewed. Dogs diagnosed with left-to-right shunting PDA were identified. Inclusion criteria were, closure of PDA with an ACDO (Infiniti Medical, Palo Alto, California) or by SL, complete general anesthesia record, recording of occlusion time, and HR/BP data available up to 30 minutes post occlusion. Exclusion criteria were absence of occlusion time identification (T0) and administration of an anticholinergic, opioid or positive inotrope <20 minutes prior to occlusion, or <30 minutes after occlusion, a change in the rate of administration of an infusion between those time points and monitoring of BP by non-invasive methods and use of anticoagulants (other than heparin saline for equipment flushing). Collected data included age, sex, weight, breed, PDA occlusion technique, perioperative anesthetic drugs, type of ventilation (spontaneous or mechanical), artery cannulated, and end tidal carbon dioxide (ETCO2), anesthetic agents and body temperature at T0. HR (beats per minute, bpm) and BP (systolic and diastolic BP, mmHg) were collected at 5-minute intervals after T0 to 30 minutes post occlusion (T30). The percentage (%) of variation in HR and BP from T0, rate of change compared to previous time point (mmHg/minute) and rate of % change (%/minute) compared to previous time point were all calculated (Table 1). All interventional and surgical procedures were undertaken by a European College of Veterinary Internal Medicine (ECVIM) or European College of Veterinary Surgery/ Royal College of Veterinary Surgeons (ECVS/RCVS) Diplomate or supervised enrolled resident, and anesthesia was performed by a supervised European College of Veterinary Anaesthesia and Analgesia (ECVAA)-enrolled resident, or ECVAA Diplomate. HR was monitored with a three-lead electrocardiogram. BP was monitored with an arterial cannula placed in a peripheral artery (metatarsal or auricular), through a multiparametric monitor (Datex Ohmeda S/5 Monitor ®, General Electric Healthcare, Chalfont St Giles, United Kingdom). The transducer used in monitoring invasive BP was zeroed to atmospheric pressure prior to its use and positioned at the level of the right atrium. Statistical analysisContinuous variables and residuals from multiple comparison tests were tested for normality (Shapiro-Wilk test). Single comparisons (age and weight) were made between ACDO and SL groups with paired t-test for parametric data or Mann–Whitney U tests for nonparametric data. Gender differences were determined with Fisher’s exact test. Hemodynamic variables and calculations were compared between and within groups using a repeated measures general linear model with procedure type (ACDO or SL) and time-point post occlusion (T0–T30) as fixed factors, patient case number as a random factor nested within procedure type, and age and weight as co-variates. Interaction between procedure type and time-points was also assessed. Where significance was identified, Dunnett’s (compared to T0) or Tukey’s (compared to previous time point) post hoc tests were applied as appropriate. Due to the retrospective nature of this study and the limitations inherent to a retrospective power calculation (Zhang et al., 2019), a prospective two-sample t-test power calculation was favored. We predicted that the maximum reduction in HR in SL dogs would be 10 beats/minute more than in ACDO dogs, suggesting that sample sizes of 6 dogs per group would achieve 80% power. For all tests, significance was set at p < 0.05. Results are expressed as mean ± standard deviation for parametric data or median ± range for nonparametric data. Ethical approvalTable 1. Hemodynamic parameters calculated for HR and BP variation, including units. Tx-5 represents the value of HR or BP of a specific time point. T0 represents the value of HR or BP at occlusion time. represents the value of HR or BP of the previous time point. Rate of change Tx represents the calculated rate of change of HR or BP for a specific time point.
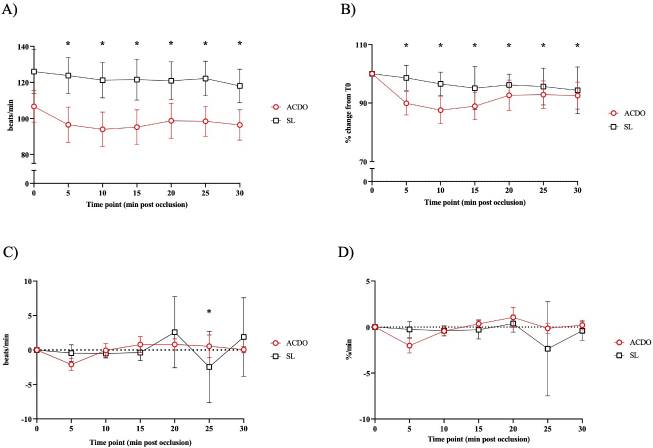
Fig. 1. Changes in HR of dogs undergoing PDA closure with SL or ACDO: (A): variation from T0; (B): percentage of change from T0; (C): rate of change; (D): rate of percentage of change. ACDO–Amplatz canine duct occluder group; min–minutes; SL–Surgical ligation group. Asterisks (*) indicate significance (p < 0.05) to T0 (graphs A and B), or to previous time point (graphs C and D) in both groups combined. This project was approved by a local veterinary ethical review committee (VERC reference 25.21). ResultsOf the 139 dogs undergoing PDA occlusion, only 41 dogs (ACDO n=32, SL n=9; Table 2) were included. The median age of all the dogs was 6 months (range 2–84), with a median weight of 10.5 kg (range 3.2–43.5). Dogs in the ACDO group were older (median 8 months, range 2–84) than dogs in the SL group (median 2 months, range 2–11) (p=0.001), and heavier (median 11.2 kg, range 3.65–43.45) than SL dogs (median 5.3 kg, range 3.2–27) (p=0.001). Most dogs were female (21/32 ACDO, 7/9 SL) in both groups (p=0.48). Various breeds were represented, but the German Shepherd Dog (GSD) was the most common breed in both groups (9/32 ACDO, 3/9 SL; Table 2). Pethidine was the most used opioid in premedication in both groups (31/32 ACDO, 6/9 SL), whilst acepromazine was the most used sedative (12/32 ACDO, 2/9 SL). Propofol was the induction agent of choice (22/32 ACDO, 7/9 SL), and sevoflurane was favored to maintain anesthesia (20/32 ACDO, 6/9 SL). For intraoperative analgesia, the commonest opioid administered to ACDO dogs was methadone (n=11/32), whereas fentanyl (5–10 mg/kg/minute, mcg kg−1 minute−1) was preferred for SL (n=5/9). Other opioids that were used are listed in Table 2. Adjunctive analgesia was provided by continuous infusion of lidocaine (10–40 mcg kg−1 minute−1) in 6/32 ACDO dogs, whereas a continuous infusion of ketamine (5–10 mcg kg−1 minute−1) fulfilled this role in 3/9 SL dogs. Invasive BP monitoring was mainly via dorsal metatarsal artery (17/32 ACDO dogs, 5/9 SL dogs), with the auricular artery being the second most used site (5/32 ACDO dogs, none in SL dogs). Only 7/35 ACDO dogs were mechanically ventilated compared to all the SL dogs. There was no difference in mean body temperature between groups at T0 (37.4°C ± 0.8°C; p=0.619). Similarly, T0 values of mean end tidal of isoflurane (ACDO 1.56% ± 0.27%, SL 1.6% ± 0.16%; p=0.984), mean end tidal of sevoflurane (ACDO 2.44% ± 0.33%, SL 2.5% ± 0.52%; p=0.206), and median ETCO2 (ACDO 47 mmHg, 39–67, SL 54 mmHg, 35–81; p=0.133) were also not significantly different between groups. Table 2. Population description (n=41).
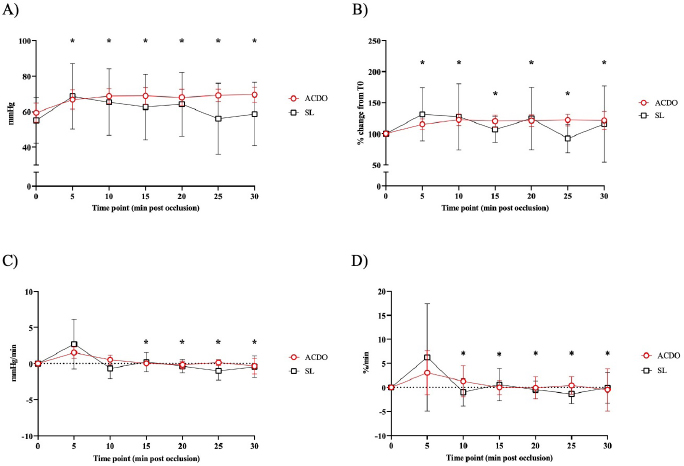
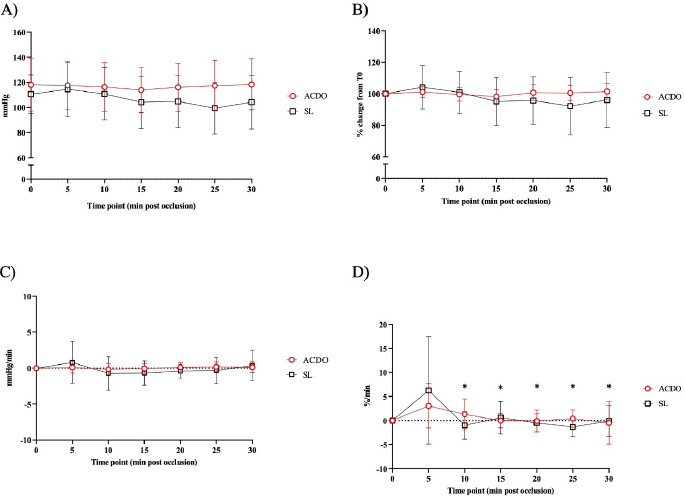
Fig. 2. Changes in diastolic BP of dogs undergoing PDA closure with SL or ACDO: (A): variation from T0; (B): percentage of change from T0; (C): rate of change; (D): rate of percentage of change. ACDO–Amplatz canine duct occluder group; min–minutes; SL–Surgical ligation group. Asterisks (*) indicate significance (p < 0.05) to T0 (graphs A and B), or to previous time point (graphs C and D) in both groups combined. Hemodynamic changesA Branham sign was observed in both groups and, between T0 and T30, no differences in HR (p=0.140), systolic (p=0.184) or diastolic (p=0.568) BP could be identified between the two groups. As the hemodynamic changes were not significantly different between group ACDO and SL, results are presented for both groups simultaneously and not separately. HR decreased by 11 bpm (5.3–16.3) by T10, a reduction of 10.4% (5.4–15.5) from T0, at a rate of 1 bpm (0–3) or 1.2%/minute (0–2.5) (p < 0.001) (Fig. 1A–D). From T10, HR increased by 3 bpm (0–5; p=0.004) (2.5%/minute, 0–4.3; p=0.002) until T20, but never reached its initial value (Fig. 1A-D). By contrast, diastolic BP increased by 9.5 mmHg (3–16) by T10, an increment of 23.5% (7.1–39.9) from baseline, at a rate of 1.5 mmHg/minute (0–3.4) or 3%/minute (0.9–7.6) (p=0.002) (Fig. 2A–D). From T15, diastolic blood pressure (DBP) started to decrease at a rate of 2 mmHg/minute (0–4; p=0.009) or 3.7%/minute (0.9–6.7; p=0.009) until T25, although a very mild increase (4%, 0–8; p=0.04), was noted at T30. Mild fluctuations in systolic BP after T0 were not significant (p=0.155) (Fig. 3A–D). Influence of age and weight on the cardiovascular changes between groupsHR at all time points in both groups combined was not independent of age (p=0.040), but all measures of HR changes were age independent (Table 3). Weight was not associated with any of the hemodynamic changes observed (Table 3). DiscussionThis study illustrates the changes in BP that initiate the Branham sign following PDA occlusion in dogs. It compares the magnitude of the response between surgical and ACDO methods. Our results show that both methods elicit a similarly mild Branham sign that is maximal 10 minutes after ductal occlusion and lasts for at least a further 20 minutes. However, we could not exclude a longer duration as the analyzed period was limited to 30 minutes. It is linked to changes in mean BP that occur as soon as the ductus is occluded and is adequate to prevent an increase in systolic BP but not diastolic BP. It is also independent of age and weight. Previous studies have described the cardiovascular changes observed following PDA ligation in dogs (Gozalo-Marcilla et al., 2012; Selmic et al., 2013), but in the two most recent, De Monte et al. (2017) identified a significant decrease in HR and increase in DBP similarly to our results, and Parisi et al. (2020) only identified a significant increase in DBP. Our study differs in three important ways. First, we included only animals with direct intra-arterial BP measurements, which is the gold-standard for continuous arterial BP measurement and allows quick detection of sudden changes in BP (MacFarlane et al., 2010; Bartels et al., 2016). Although BP readings do vary according to the artery within which BP is being measured (Acierno et al., 2015; da Cunha et al., 2017), this variation is small compared to the well-recognized measurement discrepancies with indirect methods (Seliskar et al., 2013). We believe that our intra-arterial data accurately reflect the systolic and diastolic hemodynamic consequences of PDA occlusion. Second, our data includes measurements up to 30 minutes after PDA occlusion, which is longer than those obtained by Parisi et al. (2020). This is important because regulation of BP is dependent on plasma volume as well as sympathetic tone, and profound changes in effective plasma volume occur after ductal occlusion (Epstein et al., 1953). Extending the study window by a period relevant to both methods of ductal occlusion has demonstrated that the Branham sign is maintained for this duration. Furthermore, our data suggest that the reduction in plasma volume required to permit restoration of HR to pre-occlusion levels without a consequent increase in systolic BP has not been achieved by 30 minutes. Characterization of the modification of compensatory renal mechanisms involving renin-angiotensin-aldosterone system and pressure natriuresis would require accurate measurement and collection of urine, which was beyond the remit of this study, though it would be unlikely to observe such changes during the proposed timeframe for this study. Third, our study makes a direct comparison between surgical and non-surgical methods and, contrary to our hypothesis, we were unable to demonstrate different cardiovascular consequences between the groups. We had predicted a greater Branham sign in the surgical dogs, as a consequence of a more rapid occlusion, but this was not apparent, despite adopting several mathematical determinants of magnitude or rate of change of HR and diastolic BP. We considered whether this might be a consequence of a particular anesthetic protocol, particularly one incorporating more potent opioids that could have at least partially attenuated the cardiovascular changes that typically follow occlusion of a PDA (Szilagyi, 1987; Shanazari et al., 2011). The range of opioids used tend to decrease HR and BP to a different degree (KuKanich and Wiese, 2015), and although no cardiovascular differences were found between groups, their contribution, although likely to be minimal, cannot be completely ruled out. Similarly, ketamine increases HR and BP (Haskins et al., 1985) but usually only when used at higher doses than those used in our study (Franco et al., 2018), and lidocaine exerts minimal cardiovascular effects (Ortega and Cruz, 2011; Moran-Munoz et al., 2017). Still, the contribution of these adjunctive analgesics to the hemodynamic changes observed cannot be completely excluded.
Fig. 3. Changes in systolic BP of dogs undergoing PDA closure with SL or ACDO: (A): variation from T0; (B): percentage of change from T0; (C): rate of change; (D): rate of percentage of change. ACDO–Amplatz canine duct occluder group; min–minutes; SL–Surgical ligation group. Asterisks (*) indicate significance (p < 0.05) to T0 (graphs A and B), or to previous time point (graphs C and D) in both groups combined. Table 3. p values for age and weight for both measured and calculated cardiovascular parameters, with significance set at p < 0.05.
We also examined ETCO2, temperature and the end tidal of inhalational agents at the time of closure, but these were not different between groups. We conclude that the use of different anesthetic protocols, particularly, the variation in analgesia employed, does not fully explain the mild nature of the Branham sign observed in the SL dogs. There are several alternative explanations for the similarity in Branham sign between the two groups. One possibility is that soft tissue surgeons, mindful of a potential Branham sign, have adapted their technique to ligate PDAs gradually over several minutes (Broaddus and Tillson, 2010). This approach has been adopted at our institution and could mimic the gradual closure achieved with an ACDO. Greater nociception from thoracotomy could increase the sympathetic drive in SL dogs and mitigate the Branham sign. Furthermore, we normally select larger ACDOs, with a waist to minimal ductal diameter of at or near to 2:1, rather than 1.5:1 (data not included), and a width of device at least 2 mm wider than the ampulla. This might tend to occlude ductal flow more rapidly, thus mimicking the cardiovascular effects of SL. Age and weight are commonly cited as potential cofounding variables in observational studies (Norgaard et al., 2017). These two factors are relevant to our study because weight influences circulating blood volume and therefore hemodynamic changes, whilst age is associated with autonomic maturation, particularly of the baroreceptor reflex (Hageman et al., 1986). In our institution, dogs <5 kg have been more likely to undergo SL because their femoral arteries are often not large enough to accommodate the delivery system required for ACDO deployment. However, we were unable to demonstrate an influence of age or weight on the magnitude of the Branham sign. This is of clinical relevance because new generation devices such as the Amplatzer vascular plug 2 (Wagner, 2019) and ductal occluder (Hulsman et al., 2021) can be deployed either through smaller delivery systems or venous routes, paving the way to interventional closure in an age and weight of dogs previously more likely to undergo SL. Where interventional closure is possible, it is usually adopted because SL carries a higher rate of major complications such as ductal rupture and hemorrhage (Goodrich et al., 2007; Meijer and Beijerink, 2012). Since a Branham sign is a useful real-time guide to the interventionalist that occlusion of the ductus has occurred, and age and weight do not appear to modify it, we conclude that the Branham sign also is likely to be a useful monitoring tool in smaller and younger dogs undergoing interventional ductal occlusion. The retrospective nature of this study is its major limitation. Consequently, anesthetic protocols were non-standardized although an influence on the magnitude of the Branham sign could not be identified. Additionally, despite statistically similar doses of inhalational anesthetic agents at the time of occlusion, the depth of anesthesia could have been different between patients. The smaller number of dogs in the SL group could mean that our study was underpowered to demonstrate differences between ACDO and SL dogs. For simplicity, power analysis was based on a two-sample t-test of maximum reduction in HR. The use of multiple comparison tests requires larger numbers of animals to maintain power. However, on observing the trends in the data sets, if a type II error exists, it would appear to be more likely that it masks a greater Branham sign in the ACDO dogs than the SL dogs. This is still consistent with our conclusion that the ACDO method of ductal occlusion does not elicit a lesser Branham sign than the current SL technique. ConclusionIn conclusion, our study demonstrates that a mild Branham sign is common in dogs undergoing surgical and ACDO ductal ligation and the hemodynamic changes that elicit it can be tracked over at least 30 minutes by intra-arterial BP monitoring. The magnitude of the Branham sign is not dependent on the method of ductal closure or the age or weight of the dog. Conflict of interestThe authors declare that there is no conflict of interests. Authors’ contributionsFilipe Madruga–Data collection, manuscript writing, statistical analysis. Yolanda Martinez Pereira–Original manuscript idea, manuscript revision. Ambra Panti–Critical manuscript revision. Ian Handel–Statistical analysis, manuscript revision. Geoff Culshaw–Critical manuscript revision, statistical analysis. ReferencesAcierno, M.J., Domingues, M.E., Ramos, S.J., Shelby, A.M. and da Cunha, A.F. 2015. Comparison of directly measured arterial blood pressure at various anatomic locations in anesthetized dogs. Am. J. Vet. Res. 76, 266–271. Bartels, K., Esper, S.A. and Thiele, R.H. 2016. Blood pressure monitoring for the anesthesiologist: a practical review. Anesth. Analg. 122, 1866–1879. Blossom, J.E., Bright, J.M. and Griffiths, L.G. 2010. Transvenous occlusion of patent ductus arteriosus in 56 consecutive dogs. J. Vet. Cardiol. 12, 75–84. Broaddus, K. and Tillson, M. 2010. Patent ductus arteriosus in dogs. Compend. Contin. Educ. Vet. 32, E3. Buchanan, J.W. 2001. Patent ductus arteriousus morphology, pathogenesis, types and treatment. J. Vet. Cardiol. 3, 7–16. da Cunha, A.F., Ramos, S.J., Domingues, M., Shelby, A., Beaufrere, H., Stout, R. and Acierno, M.J. 2017. Validation of noninvasive blood pressure equipment: which peripheral artery is best for comparison studies in dogs? Vet. Anaesth. Analg. 44, 1068–1075. De Monte, V., Staffieri, F., Caivano, D., Nannarone, S., Birettoni, F., Porciello, F., Di Meo, A. and Bufalari, A. 2017. Heart rate and blood pressure variations after transvascular patent ductus arteriosus occlusion in dogs. Res. Vet. Sci. 113, 73–78. Epstein, F.H., Shadle, O.W., Ferguson, T.B. and McDowell, M.E. 1953. Cardiac output and intracardiac pressures in patients with arteriovenous fistulas. J. Clin. Invest. 32, 543–547. Franco, L.G., Wilges, C.H.M., Junior, D.P., Cerejo, S.A., Nishimura, L.T. and Bittar, I.P. 2018. Effects of ketamine constant rate infusions on cardiac biomarkers and cardiac function in dogs. Vet. Anaesth. Analg. 45, 250–259. Goodrich, K.R., Kyles, A.E., Kass, P.H. and Campbell, F. 2007. Retrospective comparison of surgical ligation and transarterial catheter occlusion for treatment of patent ductus arteriosus in two hundred and four dogs (1993-2003). Vet. Surg. 36, 43–49. Gozalo-Marcilla, M., Seymour, C.J., Schauvliege, S., Bosmans, T. and Gasthuys, F. 2012. Anesthetic management for the correction of a patent ductus arteriosus by means of either surgical ligation or transarterial occlusion in dogs. Vlaams. Diergeneeskundig. Tijdschrift. 81, 17–21. Hageman, G.R., Neely, B.H. and Urthaler, F. 1986. Cardiac autonomic efferent activity during baroreflex in puppies and adult dogs. Am. J. Physiol. 251, H443–H447. Haskins, S.C., Farver, T.B. and Patz, J.D. 1985. Ketamine in dogs. Am. J. Vet. Res. 46, 1855–1860. Hulsman, A.H., Breur, J. and Szatmari, V. 2021. Low profile vascular plug for transarterial occlusion of patent ductus arteriosus in small dogs. J. Vet. Intern. Med. 35, 98–106. KuKanich, B. and Wiese, A.J. 2015. Opioids. In Veterinary anesthesia and analgesia, Eds., Grimm, K.A., Lamont, L.A., Tranquilli, W.J., Greene, S.A. and Robertson, S.A. 5th ed. Ames, Iowa: Wiley Blackwell, pp: 207–226. MacFarlane, P.D., Grint, N. and Dugdale, A. 2010. Comparison of invasive and non-invasive blood pressure monitoring during clinical anaesthesia in dogs. Vet. Res. Commun. 34, 217–227. Meijer, M. and Beijerink, N.J. 2012. Patent ductus arteriosus in the dog: a retrospective study of clinical presentation, diagnostics and comparison of interventional techniques in 102 dogs (2003-2011). Tijdschr. Diergeneeskd. 137, 376–383. Moran-Munoz, R., Valverde, A., Ibancovichi, J.A., Acevedo-Arcique, C.M., Recillas-Morales, S., Sanchez-Aparicio, P., Osorio-Avalos, J. and Chavez-Monteagudo, J.R. 2017. Cardiovascular effects of constant rate infusions of lidocaine, lidocaine and dexmedetomidine, and dexmedetomidine in dogs anesthetized at equipotent doses of sevoflurane. Can. Vet. J. 58, 729–734. Norgaard, M., Ehrenstein, V. and Vandenbroucke, J.P. 2017. Confounding in observational studies based on large health care databases: problems and potential solutions - a primer for the clinician. Clin. Epidemiol. 9, 185–193. Ortega, M. and Cruz, I. 2011. Evaluation of a constant rate infusion of lidocaine for balanced anesthesia in dogs undergoing surgery. Can. Vet. J. 52, 856–860. Parisi, C., Phillips, V., Ferreira, J., Linney, C. and Mair, A. 2020. Anaesthetic management and complications of transvascular patent ductus arteriosus occlusion in dogs. Vet. Anaesth. Analg. 47, 581–587. Pascoe, P.J. 2016. Intrathoracic surgery and interventions. In BSAVA Manual of canine and feline anaesthesia and analgesia. Eds., Duke-Novakovski, T., Vries, M.d. and Seymour, C. 3rd ed. Quedgeley, Gloucester: British Small Animal Veterinary Association. Porciello, F., Caivano, D., Giorgi, M.E., Knafelz, P., Rishniw, M., Moise, N.S., Bufalari, A., Fruganti, A. and Birettoni, F. 2014. Transesophageal echocardiography as the sole guidance for occlusion of patent ductus arteriosus using a canine ductal occluder in dogs. J. Vet. Intern. Med. 28, 1504–1512. Ranganathan, B., LeBlanc, N.L., Scollan, K.F., Townsend, K.L., Agarwal, D. and Milovancev, M. 2018. Comparison of major complication and survival rates between surgical ligation and use of a canine ductal occluder device for treatment of dogs with left-to-right shunting patent ductus arteriosus. J. Am. Vet. Med. Assoc. 253, 1046–1052. Robinson, R. and Borgeat, K. 2016. Cardiovascular disease. In BSAVA Manual of canine and feline anaesthesia and analgesia. Eds., Duke-Novakovski, T., Vries, M.d. and Seymour, C. 3rd ed. Quedgeley, Gloucester: British Small Animal Veterinary Association, pp: 283–313. Scansen, B.A. 2018. Cardiac interventions in small animals: areas of uncertainty. Vet. Clin. North Am. Small Anim. Pract. 48, 797–817. Seliskar, A., Zrimsek, P., Sredensek, J. and Petric, A.D. 2013. Comparison of high definition oscillometric and doppler ultrasound devices with invasive blood pressure in anaesthetized dogs. Vet. Anaesth. Analg. 40, 21–27. Selmic, L.E., Nelson, D.A., Saunders, A.B., Hobson, H.P. and Saunders, W.B. 2013. An intrapericardial technique for PDA ligation: surgical description and clinical outcome in 35 dogs. J. Am. Anim. Hosp. Assoc. 49, 31–40. Shanazari, A.A., Aslani, Z., Ramshini, E. and Alaei, H. 2011. Acute and chronic effects of morphine on cardiovascular system and the baroreflexes sensitivity during severe increase in blood pressure in rats. ARYA. Atheroscler. 7, 111–117. Stanley, B.J., Luis-Fuentes, V. and Darke, P.G. 2003. Comparison of the incidence of residual shunting between two surgical techniques used for ligation of patent ductus arteriosus in the dog. Vet. Surg. 32, 231–237. Szilagyi, J.E. 1987. Opioid modulation of baroreceptor reflex sensitivity in dogs. Am. J. Physiol. 252, H733–H737. Velez-Roa, S., Neubauer, J., Wissing, M., Porta, A., Somers, V.K., Unger, P. and van de Borne, P. 2004. Acute arterio-venous fistula occlusion decreases sympathetic activity and improves baroreflex control in kidney transplanted patients. Nephrol. Dial. Transplant. 19, 1606–1612. Wagner, T. 2019. Plugging the gap between ACDOs and coils: experience with the amplatz vascular plug-2. In Proceedings of the autumn meeting of the veterinary cardiovascular society. Wattanasirichaigoon, S. and Pomposelli, F.B., Jr. 1997. Branham’s sign is an exaggerated Bezold-Jarisch reflex of arteriovenous fistula. J. Vasc. Surg. 26, 171–172. Zhang, Y., Hedo, R., Rivera, A., Rull, R., Richardson, S. and Tu, X.M. 2019. Post hoc power analysis: is it an informative and meaningful analysis? Gen. Psychiatr. 32, e100069. | ||
| How to Cite this Article |
| Pubmed Style Madruga FL, Pereira YM, Panti A, Handel I, Culshaw G. Branham sign in dogs undergoing interventional patent ductus arteriosus occlusion or surgical ligation: a retrospective study.. Open Vet J. 2021; 11(4): 603-612. doi:10.5455/OVJ.2021.v11.i4.10 Web Style Madruga FL, Pereira YM, Panti A, Handel I, Culshaw G. Branham sign in dogs undergoing interventional patent ductus arteriosus occlusion or surgical ligation: a retrospective study.. https://www.openveterinaryjournal.com/?mno=86180 [Access: November 08, 2024]. doi:10.5455/OVJ.2021.v11.i4.10 AMA (American Medical Association) Style Madruga FL, Pereira YM, Panti A, Handel I, Culshaw G. Branham sign in dogs undergoing interventional patent ductus arteriosus occlusion or surgical ligation: a retrospective study.. Open Vet J. 2021; 11(4): 603-612. doi:10.5455/OVJ.2021.v11.i4.10 Vancouver/ICMJE Style Madruga FL, Pereira YM, Panti A, Handel I, Culshaw G. Branham sign in dogs undergoing interventional patent ductus arteriosus occlusion or surgical ligation: a retrospective study.. Open Vet J. (2021), [cited November 08, 2024]; 11(4): 603-612. doi:10.5455/OVJ.2021.v11.i4.10 Harvard Style Madruga, F. L., Pereira, . Y. M., Panti, . A., Handel, . I. & Culshaw, . G. (2021) Branham sign in dogs undergoing interventional patent ductus arteriosus occlusion or surgical ligation: a retrospective study.. Open Vet J, 11 (4), 603-612. doi:10.5455/OVJ.2021.v11.i4.10 Turabian Style Madruga, Filipe Lalanda, Yolanda Martinez Pereira, Ambra Panti, Ian Handel, and Geoff Culshaw. 2021. Branham sign in dogs undergoing interventional patent ductus arteriosus occlusion or surgical ligation: a retrospective study.. Open Veterinary Journal, 11 (4), 603-612. doi:10.5455/OVJ.2021.v11.i4.10 Chicago Style Madruga, Filipe Lalanda, Yolanda Martinez Pereira, Ambra Panti, Ian Handel, and Geoff Culshaw. "Branham sign in dogs undergoing interventional patent ductus arteriosus occlusion or surgical ligation: a retrospective study.." Open Veterinary Journal 11 (2021), 603-612. doi:10.5455/OVJ.2021.v11.i4.10 MLA (The Modern Language Association) Style Madruga, Filipe Lalanda, Yolanda Martinez Pereira, Ambra Panti, Ian Handel, and Geoff Culshaw. "Branham sign in dogs undergoing interventional patent ductus arteriosus occlusion or surgical ligation: a retrospective study.." Open Veterinary Journal 11.4 (2021), 603-612. Print. doi:10.5455/OVJ.2021.v11.i4.10 APA (American Psychological Association) Style Madruga, F. L., Pereira, . Y. M., Panti, . A., Handel, . I. & Culshaw, . G. (2021) Branham sign in dogs undergoing interventional patent ductus arteriosus occlusion or surgical ligation: a retrospective study.. Open Veterinary Journal, 11 (4), 603-612. doi:10.5455/OVJ.2021.v11.i4.10 |