| Case Report | ||
Open Vet J. 2020; 10(3): 308-316 doi: 10.4314/ovj.v10i3.9 Open Veterinary Journal, (2020), Vol. 10(3): 308–316 Case Report http://dx.doi.org/10.4314/ovj.v10i3.9 Use of saline contrast ultrasonography in the diagnosis of complete jugular vein occlusion in a horseAndrea Corda1,2, Nicolò Columbano1,2*, Valentina Secchi1,2, Antonio Scanu1,2, Maria Luisa Pinna Parpaglia1,2, Giovanni Mario Careddu1,2 and Eraldo Sanna Passino1,21Department of Veterinary Medicine, University of Sassari, Sassari, Italy 2Veterinary Teaching Hospital, University of Sassari, Sassari, Italy *Corresponding Author: Nicolò Columbano. Department of Veterinary Medicine, University of Sassari, Sassari, Italy. Email: ncolumbano [at] uniss.it Submitted: 25/03/2020 Accepted: 20/08/2020 Published: 15/09/2020 © 2020 Open Veterinary Journal
AbstractBackground: Thrombophlebitis and thrombosis are the most common causes of jugular vein occlusion in horses. Medical and surgical treatments aim to recanalize the occluded vessel and reduce proximal venous congestion and edema. Case Description: The present report describes a clinical case of equine jugular vein thrombosis (JVT) with complete vein occlusion diagnosed by saline contrast ultrasonography (SCU) and confirmed by contrast venography. Conclusion: Our results demonstrated that the SCU test can be easily performed and objectively interpreted using standard ultrasound equipment; it is not expensive and it does not require x-ray exposure. The SCU test is a valid tool to assess vessel patency and presence of collateral circulation in JVT. The test could therefore be used to monitor the progression of the disease and the effectiveness of therapy against JVT in horses. Keywords: Contrast, Equine, Jugular, Thrombus, Ultrasound. IntroductionJugular vein occlusion, secondary to thrombophlebitis and thrombosis, is a condition commonly encountered in equine practice. Thrombophlebitis is defined as an inflammation of the venous wall, followed by the formation of a blood clot in the lumen of the vessel (Schoster, 2017). In most cases, jugular vein thrombophlebitis (JVTP) is associated with prolonged or improper placement of indwelling catheters, poor venipuncture technique, or injections of irritating drugs that lead to mechanical or chemical injury of the vessel walls (Dickson et al., 1990; Spurlock et al., 1990; Meister et al., 1993; Schoster, 2017). However, the condition could also arise from the extension of local penetrating lesions (Matsuda et al., 2010). Patient factors may also play a role in the development of JVTP (Dolente et al., 2005; Schoster, 2017); indeed, hypercoagulable states related with gastrointestinal diseases, endotoxemia, systemic inflammatory response syndrome (SIRS), sepsis, genetic coagulation defects, neoplasia, protein-losing enteropathy and protein-losing nephropathy are associated with JVTP (Dolente et al., 2005; Dallap and Epstein, 2009; Pentelado and Correa, 2013; Epstein, 2014; Schoster, 2017). Jugular vein thrombosis (JVT) refers to the presence of a thrombus within the vein without concurrent mural inflammation; however, in horses, it often represents the long-term outcome of JVTP (Schoster, 2017). The diagnosis of JVTP or JVT is primarily based on history and physical examination. Clinical signs of JVTP include fever, depression, pain and neck stiffness, swollen, hot and painful jugular vein, dilation of facial veins and edema of the affected side. On the contrary, JVT usually manifests with a firm and cord-like jugular vein not affected by pain, with facial venous distention and edema proximal to the thrombus site. Diagnosis of JVTP and JVT is usually achieved by ultrasonography and/or venography (Gardner et al., 1991; des Lions et al., 2008; Hussni et al., 2012; Pentelado and Correa, 2013; Schoster, 2017; Borghesan et al., 2018). The choice of treatment depends on the severity of the disease and aims to reduce inflammation, prevent or treat any bacterial infection, prevent further thrombus formation, and/or dissolve an existing thrombus. To prevent thrombus formation or attempt to resolve an existing thrombus, low molecular weight heparin (Schwarzwald et al., 2002; Feige et al., 2003), acetylsalicylic acid (Schoster, 2017) and recombinant tissue plasminogen activator (Bäumer et al., 2013) have been proposed as systemic treatments. In the attempt to avoid the possible hemorrhagic consequences of thrombolytic therapy, some authors have proposed local infusion of streptokinase (des Lions et al., 2008; Pentelado and Correa, 2013). However, in severe cases, medical treatments are often ineffective, and restoration of blood flow is only achieved with complex and expensive surgical treatments (Rijkenhuizen and Van Swieten, 1998; Wiemer et al., 2005; Russell et al., 2010). Whatever the treatment, the aim of the therapy is to recanalize the occluded vessel and reduce proximal venous congestion and edema. Doppler ultrasonography and venography have been used in both experimental and natural JVTP and JVT to assess vessel patency and monitor therapy (Gardner et al., 1991; Rijkenhuizen and Van Swieten, 1998; Hussni et al., 2006, 2012; des Lions et al., 2008; Borghesan et al., 2018). Nevertheless, ultrasonography alone is often unable to correctly assess vessel patency and detect the presence of compensatory collateral circulation (Hussni et al., 2012; Borghesan et al., 2018). The present report describes a case of JVT with complete jugular vein occlusion in a horse diagnosed by saline contrast ultrasonography (SCU). Case DetailsA 5-year–old brown unbroken, Italian Standardbred, jumping horse, weighing 493 kg, was presented at the Veterinary Teaching Hospital of the University of Sassari for surgical castration under general anesthesia. During the clinical examination, before surgery, a dilatation of the right-side facial veins became evident. The right jugular vein could be palpated as a not painful, thick, and solid structure in the jugular groove extending caudally from a scare tissue localized at the level of the right mandibular angle. No manual congestion of the vessel could be achieved. A suspected clinical diagnosis of the right JVT was made. The horse did not have a history of long-term catheter placement or intravenous injection in the right jugular vein. Anamnesis reported a taming attempt with ropes made by non-professional staff. The clinical examination did not show other abnormalities. Food, but not water, was withheld for 12 hours prior to anesthesia. The horse was premedicated with detomidine (5 mg), butorphanol (5 mg), and acepromazine (5 mg) each administered intravenously (IV). After 15 minutes, anesthesia was induced with 50 mg of diazepam IV, followed immediately by 1 g of ketamine IV. When orotracheal intubation was obtained, the maintenance of anesthesia was achieved by using sevoflurane. The inspired O2 concentration (FiO2) was held above 0.4. The end-tidal concentration of sevoflurane was set from 2.3 to 2.6 to ensure an adequate plane of anesthesia. Before the surgery, the horse was treated with benzylpenicillin/dihydrostreptomycin (10,000,000 UI and 10 g, respectively) intramuscularly and 1 g of ketoprofen IV. After orchiectomy, the skin, overlying both jugular veins, was clipped and cleaned, and coupling gel was then applied to the clipped area. The ultrasonographic examination was performed with a portable unit (Logiq-e, General Electric, Fairfield, CT, USA) equipped with a wide ranging (4–12 MHz) linear transducer (8-LRS, General Electric, Fairfield, CT, USA). Both jugular veins were scanned from the thoracic inlet toward the head in both long-axis and short-axis planes. The SCU test was performed by injecting agitated saline solution, as a ultrasonographic contrast (Gómez-Ochoa et al., 2010), into a right congestive facial vein according to the following protocol: 40 ml of 0.9% NaCl solution was agitated back and forth between two 60-ml syringes connected at right angles by a three-way stopcock (Fig. 1) in order to produce echo-dense contrast microbubbles. A 20-G intravenous catheter was placed into a right congestive facial vein (Fig. 2) and then attached to the three–way stopcock via a flexible extension tube connected with the syringes. The agitated saline solution was injected at two different times, in each of which a bolus of 20 ml was injected in <5 seconds. During the first bolus injection and throughout the subsequent minute, the transducer was placed on the right jugular vein, just caudal to the thrombus. The SCU test was defined positive if microbubbles appeared caudally to the thrombus, following the contrast injection, proving the presence of thrombus recanalization. During the second bolus injection and throughout the subsequent minute, the transducer was positioned on the left jugular vein, immediately proximal to the thoracic inlet. The SCU test was defined positive if microbubbles appeared in the left jugular vein, following the contrast injection, proving the presence of collateral circulation between the right facial vein and left jugular vein. Subsequently, venographies were performed in latero-lateral and ventro-dorsal views by using 30 ml of iohexol (Omnipaque; GE Healthcare) as a contrast medium.
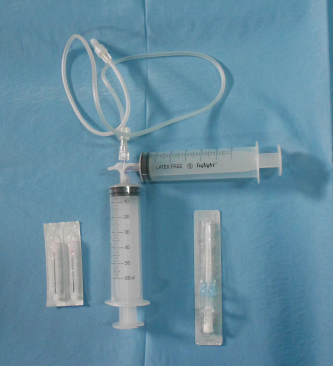
Fig. 1. The device for producing the agitated saline contrast ultrasonography. It was made by two 60-ml syringes connected at right angles by a three-way stopcock attached to a flexible extension tube.
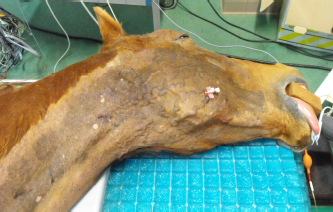
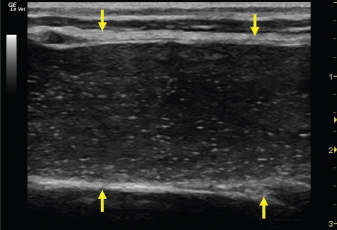
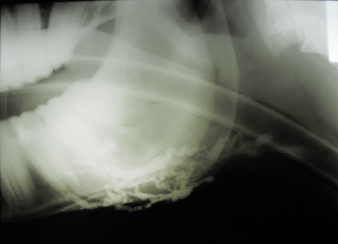
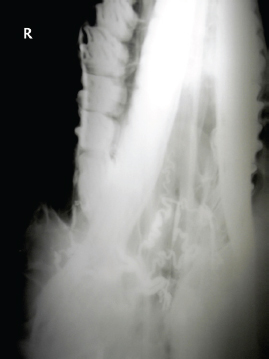
Fig. 2. A 20-G intravenous catheter placed into the right congestive facial vein in the anesthetized horse. The left jugular vein appeared as an anechoic tubular structure which collapsed under the probe pressure, the color Doppler examination revealed normal blood flow within the vessel (Fig. 3). Bi-dimensional ultrasonography of the right jugular vein showed a heterogeneous, non-cavitating, hypoechoic structure occluding a narrowed right jugular vein for about 30 cm of length localized in the proximal and mid-portion of the neck compatible with a thrombus (Fig. 4). The color Doppler examination showed blood flow through the lateral portions of the thrombus in the cranial and mid-portions of the right jugular vein (Fig. 5). Cranially to the thrombus, a marked distension of the maxillary and linguofacial veins was evident, with a turbulent and echogenic flow; the latter was compatible with blood stasis near the thrombus (Fig. 6). Caudally to the thrombus, the right jugular vein presented reduced diameter, thickened walls, and normal blood flow (Fig. 7). The cranial part of the thrombus originated from a heterogeneous scare tissue, localized at the level of the right mandibular angle (Fig. 8). After injection of the first saline contrast bolus in a right facial vein, no microbubbles appeared in the right jugular vein, caudally to the thrombus, proving the presence of a complete right jugular vein occlusion. On the contrary, after injection of the second bolus, microbubbles were visualized in the left jugular vein as small, intense, echo signals within the vein lumen, demonstrating the presence of collateral circulation that drained the blood from the right facial veins to the left jugular vein (Fig. 9). Venography results confirmed the complete occlusion of the right jugular vein and the presence of submandibular venous collaterals that connected the right facial veins with the left jugular vein (Figs. 10 and 11). Based on the previous results, the diagnosis of JVT with complete occlusion of the vessel lumen was confirmed. The horse’s owner refused any proposed medical or surgical therapy.
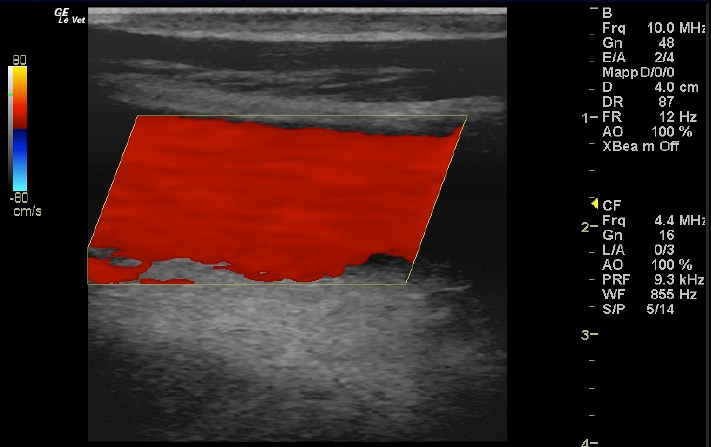
Fig. 3. Color Doppler image of the normal blood flow in the left jugular vein. Ethical approvalThe publication of this case report was approved by the Institutional Animal Care and Use Committee at the University of Sassari (Comitato Indipendente di Bioetica di Ateneo per la Sperimentazione Animale (CIBASA); protocol number 22865), according to the Italian legislation.
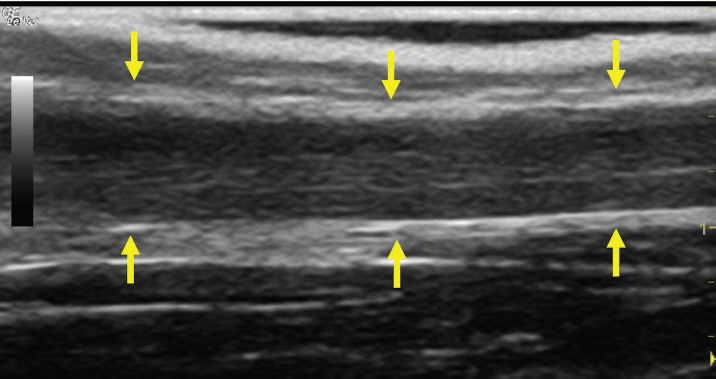
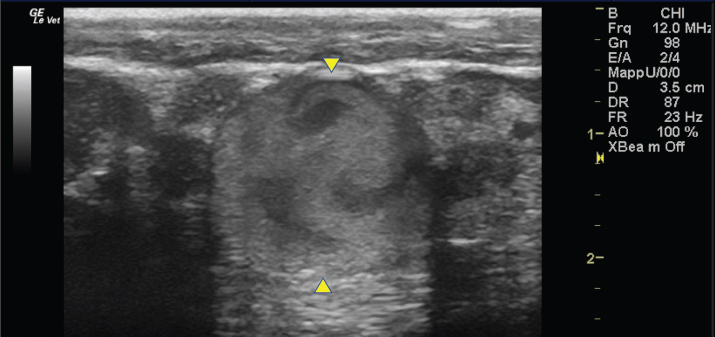
Fig. 4. Bi-dimensional ultrasonographic image of the right jugular vein in longitudinal section. The lumen of the vessel was occluded by a heterogeneous, non-cavitating, hypoechoic structure compatible with a thrombus (arrows).ç
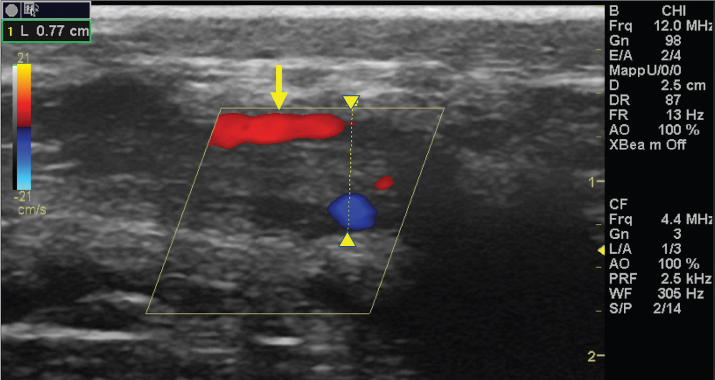
Fig. 5. Color Doppler examination of the occluded right jugular vein in longitudinal section (between arrowheads). The image shows the presence of blood flow in the periphery of the thrombus (arrow).
Fig. 6. Bi-dimensional ultrasonographic image of the dilated right maxillary vein (between arrowheads). Note the turbulent and echogenic flow compatible with blood stasis near the thrombus.
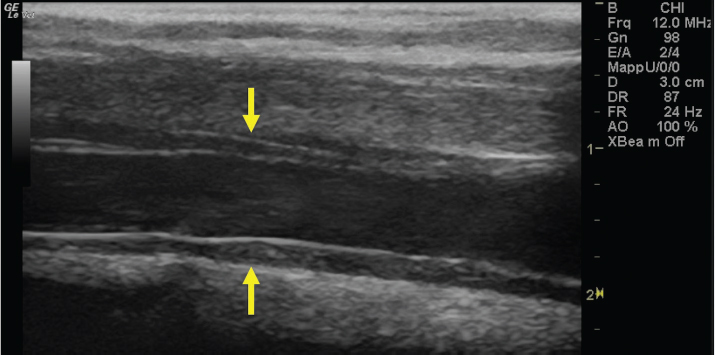
Fig. 7. Bi-dimensional ultrasonographic image of the right jugular vein, caudally to the thrombus, in longitudinal section (arrows). No microbubbles appeared caudally to the thrombus, following injection of the first saline contrast bolus. DiscussionThe description of the present clinical case shows that SCU is a valid tool to assess vessel patency and presence of collateral circulation in JVT. Two-dimensional ultrasonography is useful in diagnosing JVTP and JVT and in monitoring the progression of the disease (Gardner et al., 1991; Borghesan et al., 2018). Vessel patency is usually assessed by venography and Doppler ultrasonography (Hussni et al., 2009; Borghesan et al., 2018). However, venography requires repeated x-ray exposure and Doppler ultrasonography is highly operator and equipment-dependent. Furthermore, the Doppler color examination allows evaluating blood flow presence and direction only in small portions of the vessel at a time; therefore, when the thrombus is 15–44 cm long, as happens in the JVT of horses (Borghesan et al., 2018), it does not give a global view of the obstructed vessel. On the contrary, the SCU test allows a real distinction between total and partial vessel occlusion, it can be easily performed and objectively interpreted using standard ultrasound equipment, it is not expensive and, finally, it does not require x-ray exposure.
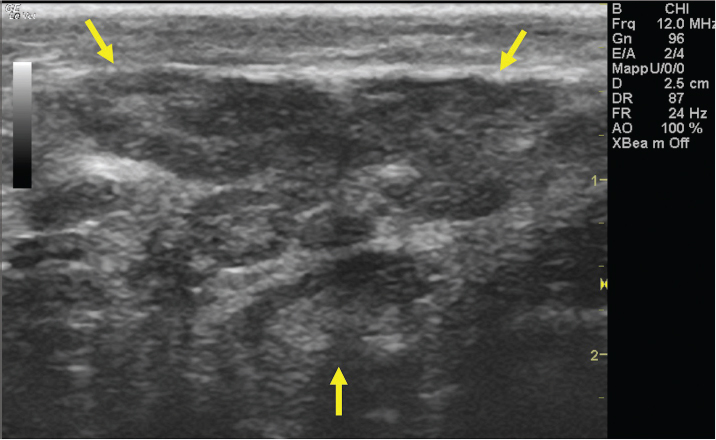
Fig. 8. Bi-dimensional ultrasonographic image of the heterogeneous, subcutaneous scare tissue localized at the level of the right mandibular angle.
Fig. 9. Bi-dimensional ultrasonographic image of the left jugular vein in longitudinal section (arrows). Microbubbles appeared as small, intense, echo signals that passed in the vein lumen following agitated saline injection.
Fig. 10. Venographic examination, latero-lateral view. Note the large number of collateral vessels draining the contrast.
Fig. 11. Venographic examination, ventro-dorsal view. Note the large number of collateral vessels draining the contrast. Saline agitated microbubbles are small air-filled microspheres that have specific acoustic properties that make them useful as a contrast agent in ultrasound imaging. The microbubbles can be easily identified by a two-dimensional ultrasonography as they appear as small hyperechoic particles passing in the lumen of the vessel. Agitated saline solution is demonstrated to be safe in both large and small animals (Bonagura and Pipers, 1983). Air microbubbles disappear in a few seconds after intravenous injection; firstly, because the solubility of air in blood is high and, secondly, because the lungs filter the large microbubbles present in the agitated saline solution (Dijkmans et al., 2004). Jugular vein occlusion, secondary to thrombophlebitis and thrombosis, is one of the most common vascular diseases in horses. The condition is usually secondary to prolonged or improper placement of intravenous catheters or injections of irritating drugs that lead to mechanical or chemical injury of the vessel walls (Dickson et al., 1990; Spurlock et al., 1990; Meister et al., 1993; Schoster, 2017). In the clinical case described in this report, a repeated trauma due to a rope tied too tight around the neck may have caused abrasions at first, with a consequent deep subcutaneous fibrosis around the venous jugular wall, and consequently thrombosis. Other authors have previously reported a case of JVTP secondary to the extension of a buccal lesion in a thoroughbred horse (Matsuda et al., 2010). Although a previous study has shown that racing performances were only slightly negatively affected by JVTP in horses (Moreau and Lavoie, 2009), jugular vein occlusion may potentially decrease the athletic performances because of impaired venous drainage of the head that can lead to brain congestion and edema as well as nasal, pharyngeal, or laryngeal mucosal congestion that could potentially cause airway obstruction during intense exercise (Moreau and Lavoie, 2009). Because of that, several medical and/or surgical therapies have been proposed to recanalize the occluded vessel and reduce proximal venous congestion. In this context, SCU could therefore be used as a tool to monitor the effectiveness of therapy and the progression of the disease. The test can be performed in sedated animals, although, in our case, we put the horse under general anesthesia because he was going to be operated. One of the main limitations of the SCU test, in jugular vein occlusion, is that it is not able to distinguish between recanalization and homolateral compensatory circulation which drains the blood from the distal to the proximal area of the thrombus. This compensatory vascular pattern has been described in experimentally induced JVTP (Borghesan et al., 2018). In the clinical case described here, we did not observe the presence of collateral vessels that joined the cranial with the caudal extremities of the thrombus. We hypothesize that this type of collateral circulation develops only in experimentally induced JVTP due to the rapidity of the occlusion process. In conclusion, the SCU test appears as a valid method to assess the vessel patency in JVT in horses. The test could be used as a method to monitor the progression of the disease and the effectiveness of the therapy. Further studies are needed to evaluate the validity of SCU on horses with naturally occurring JVTP. AcknowledgmentsThe authors would like to thank Dr. Elizabeth Vargiu and Dr. Riccardo James Vargiu for English language editing of the manuscript and Miss Rita Fenu, the Radiology Technician of the Veterinary Teaching Hospital of the University of Sassari, for her valuable technical support. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionsAndrea Corda conceived and performed the procedure, drafted and revised the manuscript, and approved the final version. Nicolò Columbano conceived and performed the procedure, drafted and revised the manuscript, and approved the final version. Valentina Secchi performed the procedure, and drafted and revised the manuscript. Antonio Scanu performed the procedure, and drafted and revised the manuscript. Maria Luisa Pinna Parpaglia drafted and revised the manuscript. Giovanni Mario Careddu performed the procedure, drafted and revised the manuscript. Eraldo Sanna Passino performed the procedure, drafted and revised the manuscript, and approved the final version. ReferencesBäumer, W., Herrling, G.M. and Feige, K. 2013. Pharmacokinetics and thrombolytic effects of the recombinant tissue-type plasminogen activator in horses. BMC Vet. Res. 9, 158. Bonagura, J.D. and Pipers, F.S. 1983. Diagnosis of cardiac lesions by contrast echocardiography. J. Am. Vet. Med. Assoc. 182, 396–402. Borghesan, A.C., Barbosa, R.G., Cerqueira, N.F., Takahira, R.K., Vulcano, L.C., Alves, A.L.G, Watanabe, M.J., Alonso, J.M., Rollo, H.A. and Hussni, C.A. 2018. Evaluation of experimental jugular thrombophlebitis in horses treated with heparin. J. Equine Vet. Sci. 69, 59–65. Dallap, S.B.L. and Epstein, K. 2009. Coagulopathy of the critically ill equine patient. J. Vet. Emerg. Crit. Care (San Antonio) 19, 53–65. des Lions, J.A., Carette, O., de Broucker, C.-A., Tainturier, B. and Ribot, X. 2008. Utilisation de la streptokinase dans le traitement des thrombophlébites de la jugulaire chez le cheval: à propos de 2 cas cliniques. Bull la Société Vétérinaire Prat Fr. 92, 17–22. Dickson, L.R., Badcoe, L.M., Burbidge, H. and Kannegieter, N.J. 1990. Jugular thrombophlebitis resulting from an anaesthetic induction technique in the horse. Equine Vet. J. 22, 177–179. Dijkmans, P.A., Juffermans, L.J.M., Musters, R.J.P., van Wamel, A., Ten Cate, F.J., van Gilst, W., Visser, C.A., de Jong, N. and Kamp, O. 2004. Microbubbles and ultrasound: from diagnosis to therapy. Eur. J. Echocardiogr. 5, 245–256. Dolente, B.A., Beech, J., Lindborg, S. and Smith, G. 2005. Evaluation of risk factors for development of catheter-associated jugular thrombophlebitis in horses: 50 cases (1993-1998). J. Am. Vet. Med. Assoc. 227, 1134–1141. Epstein, K.L. 2014. Coagulopathies in horses. Vet. Clin. North Am. Equine Pract. 30(2), 437–452. Feige, K., Schwarzwald, C.C. and Bombeli, T. 2003. Comparison of unfractioned and low molecular weight heparin for prophylaxis of coagulopathies in 52 horses with colic: a randomised double-blind clinical trial. Equine Vet. J. 35, 506–513. Gardner, S.Y., Reef, V.B. and Spencer, P.A. 1991. Ultrasonographic evaluation of horses with thrombophlebitis of the jugular vein: 46 cases (1985-1988). J. Am. Vet. Med. Assoc. 199, 370–373. Gómez-Ochoa, P., Llabrés-Díaz, F., Ruiz, S., Corda, A., Prieto, S., Sosa, I., Gregori, T. and Gascon, M. 2010. Ultrasonographic appearance of the intravascular transit of agitated saline in normal dogs following ultrasound guided percutaneous splenic injection. Vet. Radiol. Ultrasound. 51, 523–526. Hussni, C.A., Barbosa, R.G., Borghesan, A.C., Rollo, H.A., Alves, A.L.G., Watanabe, M.J., de Vasconcelos Machando, A.M. and Cerqueira, N.F. 2012. Aspectos clínicos, ultrasonográfiicos e venográfiicos da trombo flebite jugular experimental em equinos. Pesqui. Vet. Bras. 32, 595–600. Hussni, C.A., Dornbusch, P.T., Garcia Alves, A.L., Thomassian, A., De Mello Nicoletti, J.L., Pantano De Cillo, G. and Vulcano, L.C. 2006. Avaliacao clinica e angiografica da vascularizacao consecutiva a resseccao experimental da jugular de equinos. Vet. e Zootec. 13, 163–168. Hussni, C.A., Dornbusch, P.T., Yoshida, W.B., Alves, A.L.G., Nicoletti, J.L.M., Mamprim, M.J. and Vulcano, L.C. 2009. Trombectomia com cateter de Fogarty no tratamento da tromboflebite jugular experimental em eqüinos. Pesqui. Vet. Bras. 29, 45–51. Matsuda, K., Suzuki, H., Tsunoda, N. and Taniyama, H. 2010. Jugular thrombophlebitis developed from buccal ulcer in a thoroughbred horse. J. Vet. Med. Sci. 72, 913-915. Meister, D., Fürst, A., Kaegi, B., Struchen, C., Kaser-Hotz, B. and Flückiger, M. 1993. Experiences with long-term intravenous therapy using teflon catheters in 80 horses. Tierarztl. Prax. 21, 437–443. Moreau, P. and Lavoie, J.P. 2009. Evaluation of athletic performance in horses with jugular vein thrombophlebitis: 91 cases (1988-2005). J. Am. Vet. Med. Assoc. 235, 1073–1078. Pentelado, M.D.D. and Correa, L.J. 2013. Jugular thrombophlebitis in horses: a reviw of fibrinolysis, thrombus formation, and clinical managment. Can. Vet. J. 54, 65–71. Rijkenhuizen, A.B.M. and Van Swieten, H.A. 1998. Reconstruction of the jugular vein in horses with post thrombophlebitis stenosis using saphenous vein graft. Equine Vet. J. 30, 236–239. Russell, T.M., Kearney, C. and Pollock, P.J. 2010. Surgical treatment of septic jugular thrombophlebitis in nine horses. Vet. Surg. 39, 627–630. Schoster, A. 2017. Complications of intravenous catheterization in horses. Schweiz. Arch. Tierheilkd. 159, 477–485. Schwarzwald, C.C., Feige, K., Wunderli-Allenspach, H. and Braun, U. 2002. Comparison of pharmacokinetic variables for two low-molecular-weight heparins after subcutaneous administration of a single dose to horses. Am. J. Vet. Res. 63, 868–873. Spurlock, S.L., Spurlock, G.H., Parker, G. and Ward, M.V. 1990. Long-term jugular vein catheterization in horses. J. Am. Vet. Med. Assoc. 196, 425–430. Wiemer, P., Gruys, E. and van Hoeck, B. 2005. A study of seven different types of grafts for jugular vein transplantation in the horse. Res. Vet. Sci. 79, 211–217. | ||
| How to Cite this Article |
| Pubmed Style Corda A, Columbano N, Secchi V, Scanu A, Parpaglia MLP, GMC, Passino ES. Use of saline contrast ultrasonography in the diagnosis of complete jugular vein occlusion in a horse. Open Vet J. 2020; 10(3): 308-316. doi:10.4314/ovj.v10i3.9 Web Style Corda A, Columbano N, Secchi V, Scanu A, Parpaglia MLP, GMC, Passino ES. Use of saline contrast ultrasonography in the diagnosis of complete jugular vein occlusion in a horse. https://www.openveterinaryjournal.com/?mno=94496 [Access: July 27, 2024]. doi:10.4314/ovj.v10i3.9 AMA (American Medical Association) Style Corda A, Columbano N, Secchi V, Scanu A, Parpaglia MLP, GMC, Passino ES. Use of saline contrast ultrasonography in the diagnosis of complete jugular vein occlusion in a horse. Open Vet J. 2020; 10(3): 308-316. doi:10.4314/ovj.v10i3.9 Vancouver/ICMJE Style Corda A, Columbano N, Secchi V, Scanu A, Parpaglia MLP, GMC, Passino ES. Use of saline contrast ultrasonography in the diagnosis of complete jugular vein occlusion in a horse. Open Vet J. (2020), [cited July 27, 2024]; 10(3): 308-316. doi:10.4314/ovj.v10i3.9 Harvard Style Corda, A., Columbano, . N., Secchi, . V., Scanu, . A., Parpaglia, . M. L. P., , . G. M. C. & Passino, . E. S. (2020) Use of saline contrast ultrasonography in the diagnosis of complete jugular vein occlusion in a horse. Open Vet J, 10 (3), 308-316. doi:10.4314/ovj.v10i3.9 Turabian Style Corda, Andrea, Nicolo Columbano, Valentina Secchi, Antonio Scanu, Maria Luisa Pinna Parpaglia, Giovanni Mario Careddu, and Eraldo Sanna Passino. 2020. Use of saline contrast ultrasonography in the diagnosis of complete jugular vein occlusion in a horse. Open Veterinary Journal, 10 (3), 308-316. doi:10.4314/ovj.v10i3.9 Chicago Style Corda, Andrea, Nicolo Columbano, Valentina Secchi, Antonio Scanu, Maria Luisa Pinna Parpaglia, Giovanni Mario Careddu, and Eraldo Sanna Passino. "Use of saline contrast ultrasonography in the diagnosis of complete jugular vein occlusion in a horse." Open Veterinary Journal 10 (2020), 308-316. doi:10.4314/ovj.v10i3.9 MLA (The Modern Language Association) Style Corda, Andrea, Nicolo Columbano, Valentina Secchi, Antonio Scanu, Maria Luisa Pinna Parpaglia, Giovanni Mario Careddu, and Eraldo Sanna Passino. "Use of saline contrast ultrasonography in the diagnosis of complete jugular vein occlusion in a horse." Open Veterinary Journal 10.3 (2020), 308-316. Print. doi:10.4314/ovj.v10i3.9 APA (American Psychological Association) Style Corda, A., Columbano, . N., Secchi, . V., Scanu, . A., Parpaglia, . M. L. P., , . G. M. C. & Passino, . E. S. (2020) Use of saline contrast ultrasonography in the diagnosis of complete jugular vein occlusion in a horse. Open Veterinary Journal, 10 (3), 308-316. doi:10.4314/ovj.v10i3.9 |