| Research Article | ||
Open Vet J. 2023; 13(9): 1091-1098 Open Veterinary Journal, (2023), Vol. 13(9): 1091–1098 Original Research Magnetic resonance imaging characteristics of atlanto-axial subluxation in 42 dogs: Analysis of joint cavity size, subluxation distance, and craniocervical junction anomaliesKathryn Y. Bray1, Simon R. Platt2*, Marc Kent3, Natasha J. Olby4, Peter J. Early4, Christopher L. Mariani4, Karen R. Muñana4 and Shannon P. Holmes51Carolina Veterinary Specialists, Winston Salem, NC 2Vetoracle Teleneurology, Norfolk, UK 3Department of Small Animal Medicine and Surgery, University of Georgia College of Veterinary Medicine, Athens, GA 4Department of Clinical Sciences, College of Veterinary Medicine, North Carolina State University, Raleigh, NC 5AXIS—Animal Cross-Sectional Imaging Specialists, Athens, GA *Corresponding Author: Simon R. Platt. Vetoracle Teleneurology, Norfolk, UK. Email: srplatt1 [at] gmail.com Submitted: 17/03/2023 Accepted: 06/08/2023 Published: 30/09/2023 © 2023 Open Veterinary Journal
AbstractBackground: Atlanto-axial (AA) subluxation can be a complex syndrome in dogs. Accurate identification and assessment of this condition are key to providing treatment and resolution. Aim: The purpose of this retrospective study was to describe the magnetic resonance imaging (MRI) characteristics of AA subluxation and associated neurologic deficits. Methods: A multicenter review of dogs with a diagnosis of AA subluxation was conducted, evaluating signalment, neurologic grade, duration of signs, and MRI characteristics. MRI characteristics included degree of spinal cord compression and joint subluxation, integrity of odontoid ligaments, presence of a dens, spinal cord signal intensity, and presence of syringohydromyelia, hydrocephalus, and Chiari-like malformation. A control population with normal AA joints was also evaluated. MR images of 42 dogs with AA subluxation were compared to 26 age and breed-matched control dogs. Results: Affected dogs had a median age of 27 months and a median weight of 2.7 kg, and the most commonly affected breed was the Yorkshire terrier (47.5%). Spinal cord signal hyperintensity, increased AA joint size, and cross-sectional cord compression at the level of the dens and mid-body C2 were associated with AA subluxation. No associations were found between cord compression, the appearance of the dens, or cord signal intensity and neurologic grade. Affected dogs did not have a higher incidence of Chiari-like malformation, syringohydromyelia, or hydrocephalus than control dogs, and their neurologic grade was not associated with MRI findings. Lack of dens and/or odontoid ligaments was associated with larger subluxations. Conclusion: Dogs with clinical signs of AA subluxation were significantly more likely to have intramedullary hyperintensity at the level of compression ( p=0.0004), an increased AA joint cavity size ( p=0.0005), and increased spinal cord compression at the level of dens and mid-body C2 (p ≤ 0.05). The authors suggest an AA joint cavity size >1.4 mm and a subluxation distance >2.5 mm as cutoffs for MRI diagnosis of AA subluxation in dogs. No differences were noted between dogs with AA subluxation and control dogs regarding syringohydromyelia, hydrocephalus, and Chiari-like malformation. Keywords: Canine, Cervical, Congenital, Malformation, Myelopathy. IntroductionAtlanto-axial (AA) subluxation is an important cause of morbidity and mortality among small and toy-breed dogs, which are predisposed to congenital malformations of the craniocervical junction. Abnormalities associated with AA subluxation include aplasia or hypoplasia of the odontoid process, absence of the transverse ligament of the atlas or other supporting ligaments, fracture of the odontoid process, dorsal angulation, and/or separation of the dens from the axis (Oliver and Lewis, 1973; McCarthy et al., 1995; Slanina, 2015). Additional structural abnormalities reported in conjunction with AA subluxation which may impact morbidity and mortality include syringohydromyelia, incomplete ossification of the atlas, occipital dysplasia, and atlanto-occipital overlap (Cerda-Gonzalez et al., 2009). While cervical radiographs may allow preliminary diagnosis of AA subluxation, they do not demonstrate the degree of spinal cord compression or extent of congenital malformation. In humans with AA subluxation, high-resolution magnetic resonance imaging (MRI) provides reliable evaluation of the transverse and alar ligaments, as well as the spinal cord (Vetti et al., 2010). MRI in humans improves the accuracy of AA subluxation diagnosis; specifically, the combined identification of peridental effusion, lateral facet arthropathy, abnormal intramedullary intensity, and an abnormal spinolaminar line has a sensitivity of 100% and a specificity of 90% in the diagnosis of AA subluxation (Hung et al., 2010). In addition to structural abnormalities, MRI in dogs is reported to be sensitive for detecting secondary trauma to the spinal cord, as well as assessment of syrinx formation (Levistki et al., 1999). The ligamentous structures of the occipitoatlanto-axial region in the normal dog can be visualized on MRI, including the apical, alar, and transverse ligaments (Da Costa and Samii, 2010; Middleton et al., 2012). Therefore, MRI is an ideal imaging modality to evaluate the entire craniocervical junction in dogs with suspicion for, or diagnosis of, AA subluxation. The information provided by MRI of the vertebral column may be important for both diagnosis and prognosis (Sanders et al., 2004; Kent et al., 2010; Middleton et al., 2012). In a study investigating MRI characteristics of vertebral fracture or subluxation in dogs, MRI provided helpful information on the location and extent of damage to soft tissue structures and enabled assessment of spinal cord injury including parenchymal signal intensity changes, compression, spinal cord swelling, or intramedullary hemorrhage (Johnson et al., 2012). Few reports focus solely on MRI findings in dogs with AA subluxation in the veterinary literature (Warren-Smith et al., 2009; Kent et al., 2010). Aikawa et al. (2013) described the low-resolution MRI of 22 dogs with AA subluxation in a recent report on surgical technique and noted additional findings such as abnormal intramedullary signal, enlarged ventricles, caudal occipital malformation syndrome, and syringomyelia. Several dogs also had dorsal spinal cord compression identified on MRI that was not previously seen on myelography. However, to the best of the authors’ knowledge, no work has focused on the description of MRI characteristics of the AA joint in dogs with subluxation and comparison to those seen in similar clinically normal dogs. The purpose of the study reported here was to describe the MRI characteristics of dogs with AA subluxation and compare them to a control population of dogs with normal AA joints. The authors hypothesized that dogs with AA subluxation would have malformed dens, measurable increased joint space, and absent supporting ligaments. The secondary aims were to investigate and identify associations of craniocervical joint and spinal cord abnormalities in both populations of dogs. Materials and MethodsCase selectionA multicenter retrospective review of the medical records and MR images of 42 dogs with a diagnosis of AA subluxation was conducted, with case collection from 2005 to 2013. There were no age, sex, or breed restrictions. Inclusion criteria for the study required a complete clinical history, documented neurologic examination, and standard high-field (1 T or above) MR images of the AA joint to be available for independent review. A control population was identified from dogs with available MR images of a normal AA joint but diagnosed with other compressive cervical spinal conditions (primarily degenerative disc disease) and consisted of 26 dogs that were similar in age and breed to the dogs with AA subluxation. The animals included in this study were client-owned patients of specialty referral hospitals whose images were obtained during a diagnostic investigation of their neurological disorders. Dogs with a diagnosis of AA subluxation were assigned a neurologic grade from 1 to 5 similar to a modified Frankel score previously documented (Levine et al., 2009), but no distinction was made between superficial and deep nociception. The neurologic grade was therefore determined as 5=spinal hyperesthesia without neurological deficits, 4=ambulatory tetraparesis, 3=nonambulatory tetraparesis, 2=tetraplegia, or 1=tetraplegia with loss of nociception. The duration of neurologic signs was also recorded, and categorized as less than one week, between one week and one month, or longer than one month. Data collectionAll dogs had multiplanar MRI with 1.5 or 3.0 T magnetic field strength (Table 1). The MRI reviewers consisted of a board-certified neurologist, a board-certified radiologist, and a second-year neurology resident, selected from the hospital that designed the study. The images from all cases, AA subluxation, and normal AA junction control were combined and randomized. The reviewers were not provided with clinical information regarding the cases. Since the MRI studies came from multiple institutions, the individual sequences performed were variable. For all cases, T2-weighted images in sagittal, transverse, and dorsal were reviewed and included. Specific imaging characteristics were documented on a prefabricated MRI scoring spreadsheet. The images and required measurements were viewed and performed with a standard software program (Osirix Imaging Software, v 3.9.2, Pixmeo, Geneva, Switzerland). MRI characteristics were evaluated categorically and numerically. To evaluate spinal cord compression, a cross-sectional percentage of the spinal cord was calculated by outlining the spinal cord diameter at the level of the dens compared to its size at the level of the mid-body of C2 on the imaging software program (Figs. 1B and C and 2B and C). The degree of AA joint subluxation was identified as the size of the AA joint cavity, using a sagittal measurement of the distance from the ventral border of the dens or the most rostral portion of C2 to the caudal articular fovea of the atlas (Figs. 1A and 2A), and a transverse measurement from the ventral border of the dens or rostral C2 to the dorsal margin of the atlas (Figs. 1B and 2B). The integrity of apical, alar, and transverse ligaments was assessed as present (Fig. 2B), absent, or inconclusive based on the previous protocol established by Middleton et al. (2012). Table 1. Range of technical parameters used for MRI studies.
Further characterization of the dens was performed and the presence of abnormal intramedullary hyperintensity, hydrocephalus, Chiari-like malformation, and syringohydromyelia were documented (Figs. 3 and 4). The presence or absence of dens was noted, including whether the dens appeared hypoplastic. Hydrocephalus was characterized by assessment of lateral cerebral ventricular distension, when the field of view included the brain and was subjectively graded as mild, moderate, or severe. Syringohydromyelia was considered present if the central canal diameter or fluid-filled cavities in the spinal cord exceeded 2 mm, and evaluated as focal or extensive, with distinct or indistinct margins (Cappello and Rusbridge, 2007). Chiari-like malformation was classed as absent, indentation of the caudal aspect of the cerebellum, or herniated (impaction of cerebellar vermis through the foramen magnum), based on a previously reported grading scheme (Rusbridge et al., 2000). Statistical analysisAll analyses were performed using SAS V 9.2 (Cary, NC). The three MRI reviewers completed independent MRI scoring spreadsheets. Since there were three reviewers, if the scores differed significantly a consensus was reached for categorical variables, and a mean was calculated for numerical measurements. Associations of MRI characteristics with the disease process of a dog (AA subluxation or control) and neurologic grade were assessed by logistic regression. If quasi-separation of variables occurred, Firth’s penalized maximum likelihood estimation method was used to reduce bias in logistic model parameter estimation. Associations of MRI characteristics with each other were performed using Spearman and Pearson correlations, student t-tests, analysis of variance, and Kruskal–Wallis tests as appropriate based on categorical versus numerical status of data. p-values <0.05 were considered significant.
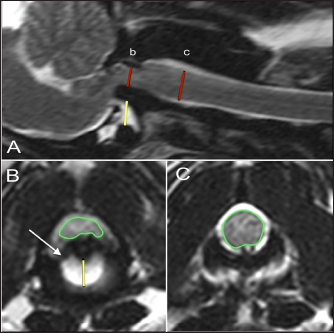
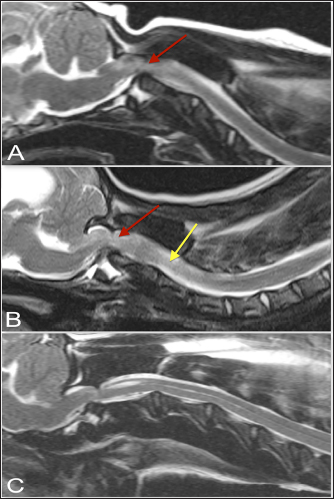
Fig. 1. T2-weighted sagittal (A) and transverse (B, C) images of a 3-year-old spayed female Maltese with acute tetraplegia (TE, 120 ms; TR, 3800 ms; slice thickness, 2.5 mm). On the sagittal image (A), the dorsoventral diameter of the spinal cord at the level of the dens (b) and mid-body of C2 (c) has been measured (red lines), as has the distance from the ventral border of the dens to the caudal articular fovea of the atlas (yellow line). On the transverse images, the cross-sectional area of the spinal cord at the level of the dens (B) and mid-body of C2 (C) has been outlined, as has the subluxation distance from the dens to the atlas (yellow line in B). This dog had dorsally and cranially displaced dens present, as well as an overlapping C1. The transverse ligament was present (white arrow in B). Ethical approvalThe animals included in this study were client-owned patients of tertiary specialty referral hospitals, not experimental subjects. As such, an international animal care and use committee (IACUC) protocol was not necessary, and the use of data was approved by the hospitals.
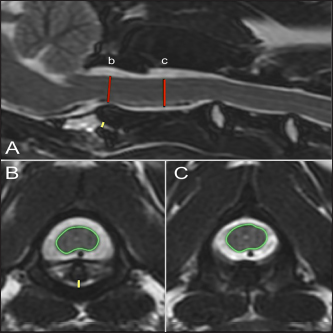
Fig. 2. T2-weighted sagittal (A) and transverse (B, C) images of a 4-year-old spayed female Maltese used as a control dog (TE, 120 ms; TR, 3800 ms; slice thickness, 2.5 mm). On the sagittal image (A), the dorsoventral diameter of the spinal cord at the level of the dens (b) and mid-body of C2 (c) has been measured (red lines), as has the distance from the ventral border of the dens to the caudal articular fovea of the atlas (yellow line). On the transverse images, the cross-sectional area of the spinal cord at the level of the dens (B) and mid-body of C2 (C) has been outlined, as has the subluxation distance from the dens to the atlas (yellow line in B). ResultsClinical findingsForty-two dogs met the selection criteria and were included in the study. All but six patients belonged to the toy group, and Yorkshire terriers made up 47.5% (19/42) of the cases. The breeds represented included: Yorkshire terrier (19/42, 45%), Chihuahua (7/42, 17%), Pomeranian (3/42, 8%), mixed breed (3/42, 8%), and two (5%) each of the following: Maltese, Toy Poodle, Dachshund, Rat terrier, and one (2%) Cavalier King Charles spaniel. The median age was 27 months (range, 3–120 months). The median weight was 2.7 kg (range, 1.1–9.3 kg). There were 23 females (17 spayed and 6 sexually intact) and 19 males (13 neutered and 6 sexually intact). The control population consisted of 26 dogs. The most common breeds were Maltese and Pomeranian, each 18.5% (5/26), with the remaining breeds: Chihuahua (4/26, 15%), Miniature Poodle (3/26, 12%), Yorkshire terrier (3/26, 12%), Miniature Pinscher (2/26, 8%), and 1 each (4%) of Dachshund, Pekingese, Miniature Schnauzer, and Shih Tzu. The median age was 96 months (range, 48–156 months). The median weight was 4.7 kg (range, 2.74–11 kg). There were 13 females, all spayed, and 13 males (1 sexually intact).
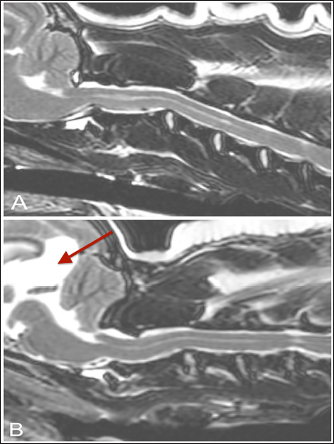
Fig. 3. T-2 weighted sagittal images of two control dogs included in this study (TE, 120 ms; TR, 3800 ms; slice thickness, 2.5 mm). (A) There is a Chiari-like malformation causing indentation of the cerebellum and syringomyelia/central canal dilatation less than 2 mm in diameter. (B) There is Chiari malformation causing impaction of the cerebellum, as well as the presence of a quadrigeminal cyst (arrow). The duration of clinical signs before evaluation in dogs with AA subluxation was variable; 28% (12/42) were affected for less than 1 week, 30% (13/42) were affected from 1 week to 1 month, and 42% (17/42) were affected for greater than 1 month. Two dogs had signs of cervical pain as the only neurologic examination abnormality (neurologic grade 5) whereas 21 dogs remained ambulatory (neurologic grade 4) and 15 dogs were nonambulatory (neurologic grade 3). Four dogs were tetraplegic, with 3 retaining nociception (neurologic grade 2), and 1 dog reported to have lost nociception (neurologic grade 1). MRI findingsNo significant differences were noted between the dogs with AA subluxation and control dogs with respect to the presence of syringohydromyelia, hydrocephalus, and Chiari-like malformation. Within the control population, 6 dogs had focal syringohydromyelia, 5 dogs had hydrocephalus (mild in 3 dogs and moderate in 2 dogs), and 14 dogs had Chiari-like malformation (11 with indentation, 3 with both herniation and indentation, and none with herniation alone). In the dogs with AA subluxation, 10 dogs had focal syringohydromyelia and 5 had diffuse syringohydromyelia. Eighteen dogs had hydrocephalus (12 mild, 3 moderate, and 3 severe), and 20 had Chiari-like malformation (17 with indentation, 3 with both herniation and indentation, and none with herniation alone).
Fig. 4. T-2 weighted sagittal images of three dogs affected by an atlanto-axial subluxation in this study (TE, 120 ms; TR, 3800 ms; slice thickness, 2.5 mm). (A) This dog with atlantoaxial subluxation has intramedullary T2 hyperintensity at the level of compression that likely indicates edema or ischemia (red arrow). Chiari malformation is absent. (B) There is Chiari malformation causing impaction of the cerebellum, with similar intramedullary hyperintensity at the level of compression as in A (red arrow) but additional pre-syrinx formation caudal to the level of spinal cord compression (yellow arrow). This dog was also graded as having moderate hydrocephalus. (C) There is Chiari malformation causing indentation of the cerebellum and focal syringomyelia greater than 2 mm in diameter. The dens were present and appeared normal in all control dogs. Just over half of the dogs with AA subluxation also had dens (22/42), with 16 dogs having hypoplastic or absent dens, and 4 dogs with inconclusive results. The apical ligament was present in the majority of control dogs (24) and inconclusive in the remaining 2 dogs. The transverse ligament was present in all control dogs. In the dogs with AA subluxation, the apical ligament was present in 14 dogs, absent in 18 dogs, and inconclusive in 10 dogs. The transverse ligament was present in 6 dogs, absent in 24 dogs, and inconclusive in 12 dogs. The alar ligaments were identified as present in one control dog; the rest were inconclusive. This was similar in the dogs with AA subluxation, of which 5 had the alar ligament present, 4 had an absence of the ligament, and the remainder (33) was inconclusive. Thus, control dogs were significantly more likely to have normally formed dens ( p=0.0221), as well as intact and normal-appearing apical ( p=0.0029), and transverse ligaments ( p < 0.0001). In the dogs with clinical signs of AA subluxation, 64% (27/42) had an abnormal intramedullary hyperintensity at the level of the AA junction. There was an association between the presence of abnormal intramedullary hyperintensity and AA joint cavity size ( p=0.0016). In addition, reviewers found that dogs with clinical signs of AA subluxation were also significantly more likely to have an increased AA joint cavity size ( p=0.0005). The AA joint cavity size in dogs with AA subluxation was a median of 2.2 mm (range, 0.613–6.9 mm) compared to control dogs with a median joint cavity size of 0.86 mm (range, 0–1.4 mm). Thirty-two dogs with AA subluxation had a joint cavity size above 1.4 mm, the upper range of the control dogs’ joint cavity size. The mean value of joint cavity size was lower for dogs with alar ligaments present compared to those dogs with alar ligaments absent ( p=0.0314). The median dens-atlas distance was 2.2 mm in control dogs (range, 1.7–2.3 mm) and 4.7 mm in dogs with AA subluxation (range, 2.7–9.7 mm). Dogs with AA subluxation that had normal-appearing dens present had a median subluxation distance of 4.3 mm (range, 2.6–8.2 mm) compared to dogs with reduced or absent dens, that had a median subluxation distance of 6.3 mm (range, 2.7–9.7 mm). This corresponded to a significantly lower subluxation distance for dogs with normal-appearing dens compared to dogs with dens appearing absent or hypoplastic ( p=0.0223). Dogs with AA subluxation were significantly more likely to have increased cross-sectional spinal cord compression at both the level of the dens or rostral C2 ( p=0.0111) and mid-body C2 ( p=0.0051). The median cross-sectional spinal cord area at the level of the dens or rostral C2 in dogs with AA subluxation was 14.4 mm (range, 5.6–31.7 mm) and at the level of mid-body C2 was 21.4 mm (range, 11.8–31.2 mm), which correlated to a median cross-sectional spinal cord compression of 33.5% (range, 13.6% to 64.4%). In the sagittal plane, dogs with AA subluxation had a median dorsoventral spinal cord diameter of 2.9 mm at the level of the dens (range, 1.1–4.8 mm) and 4.5 mm at the level of mid-body C2 (range, 2.8–7.1 mm). This correlated to a median sagittal spinal cord compression of 36.8% (range, 7.7% to 64.4%). All control dogs had sagittal plane spinal cord size measurements, with a median of 5.0 mm diameter at the dens (range, 4.0–6.5 mm), 5.2 mm diameter at mid-body of C2 (range, 4.2–6.9 mm), and median sagittal spinal cord area reduction of 3% (range, 0% to 18%). In the control dogs with transverse images of the AA articulation available, the median cross-sectional area of the spinal cord at the level of the dens was 30 mm (range, 25.5–37.4 mm) and 25.4 mm at the level of mid-body of C2 (range, 19.5–31.9 mm) with a median cross-sectional spinal cord area reduction of 0% (range, 0% to 6%). No associations were found between the neurologic grade of dogs with AA subluxation and degree of cord compression, appearance of the dens, or presence of abnormal intramedullary hyperintensity. Dens abnormalities were associated with greater subluxation distance (p=0.02) and alar ligament absence was associated with increased joint cavity size (p=0.03). DiscussionDogs with AA subluxation were significantly more likely to have intramedullary cord hyperintensity, which was present in 64% of cases. This was also associated with increased joint cavity size, which was 2.2 mm in dogs with AA subluxation compared to 0.86 mm in control dogs. There was also increased cross-sectional spinal cord compression at the level of the dens and mid-body C2 in dogs with AA subluxation, with a median cross-sectional spinal cord compression of 33.5%, ranging from 13.6% to 64.4%. Despite dogs with AA subluxation having up to 64.4% spinal cord compression, the presenting neurologic grade was not significantly associated with the severity of subluxation, which is similar to disc disease studies. The present study described the MRI characteristics of the craniocervical junction in dogs with AA subluxation compared to dogs with normal AA articulation. MRI was able to identify the degree of spinal cord compression, size of the ventral AA joint cavity, and presence of intramedullary hyperintensity, and allowed evaluation of the dens and the integrity of the ligamentous attachments. In addition, MRI evaluation allowed simultaneous assessment of coexisting conditions, such as Chiari-like malformation, hydrocephalus, and syringohydromyelia, and this study determined a lack of association of these conditions with AA subluxation. Initial diagnosis of AA subluxation in dogs can often be made with survey radiographs, with findings such as dorsal displacement of the axis, an increased distance between the arch of the atlas and spinous process of the axis, and/or dens irregularities (Geary et al., 1967; McCarthy et al., 1995; Havig et al., 2005). The AA joint angle has been previously evaluated radiographically in normal dogs and dogs with AA subluxation (McLear and Saunders, 2000); however, the extreme flexion and extension performed to measure these angles could be clinically dangerous. With the wider availability of advanced imaging modalities, improvements have been made in the assessment of AA subluxation. Parry et al. have described the use of computed tomography (CT) to evaluate atlas morphology in dogs with AA subluxation. CT imaging also allows a more detailed assessment of the dens and spinal canal; surgical planning may be improved with the development of 3-D reconstructions (Reves et al., 2013). The veterinary practitioner needs to understand that radiographs may not confirm AA subluxation in all cases and cannot demonstrate the degree of spinal cord compression. There is also a need to evaluate for additional disorders of the craniocervical junction as well as intraparenchymal lesions, including edema, hemorrhage, or syringomyelia, that cannot be seen on radiographs or CT and that may impact patient management or outcome. This reinforces the use of MRI as a more ideal imaging modality for dogs with AA subluxation (Cerda-Gonzalez and Dewey, 2010; Da Costa and Samii, 2010; Kent et al., 2010). In recent years, more attention has been paid to the anatomy of the craniocervical junction, especially ligaments and fibrocartilage (Kupczynska et al., 2012) and their appearance on MRI (Middleton et al., 2012). Since the alar ligaments provide the greatest stabilizing force of the AA ligaments in a dorsoventral direction (Reber et al., 2013), the ability to directly assess their integrity may aid in the evaluation of dogs with AA subluxation. In human studies, there are conflicting reports on the feasibility of MRI to evaluate the ligaments of the AA junction (Roy et al., 2004; Vetti et al., 2010). Hung et al. (2010) evaluated MR images of humans with anterior AA subluxation and found peridental effusion, lateral facet arthropathy, abnormal intramedullary signals, and abnormal spinolaminar line in these patients correlating with the subluxation. By contrast, no studies have been performed to correlate MRI abnormalities with AA subluxation in dogs. The population of dogs evaluated for AA subluxation in our study was similar to that reported in the literature (Geary et al., 1967; Oliver and Lewis, 1973), with 85.7% of dogs belonging to the toy group, and Yorkshire terriers making up 45.2% of dogs. The distribution of reported clinical signs has recently been summarized (Slanina, 2015), showing that approximately 24.9% of dogs presented with neck pain only, 34.1% were ambulatory with paresis, 34.5% were nonambulatory, and 6.5% were tetraplegic. Our study had a much smaller group of dogs with neck pain only (4.7%), and 47.6% were ambulatory, while 35.7% were nonambulatory and 9.5% were tetraplegic. The duration of clinical signs was quite variable in this study and almost evenly distributed between the three chosen durations for this study (less than one week, one week to one month, and greater than one month). This is similarly reflected in veterinary reports, with clinical duration reported to be from as little as 1.5 hours up to 40 months (Aikawa et al., 2013). Children who develop AA instability secondary to ligamentous laxity or dens hypoplasia are genetically predisposed via associated skeletal dysplasias or connective tissue disorders, such as Down’s syndrome or Morquio syndrome (Kim, 2013). In dogs, the existence of craniocervical junction disorders or other congenital anomalies, such as Chiari-like malformation, hydrocephalus, and syringohydromyelia, is most common in toy and small-breed dogs but are not correlated with a single morphologic abnormality (Cerda-Gonzalez and Dewey, 2010). Similarly, our findings supported the existence of these anomalous disorders in both the dogs affected with AA subluxation as well as the control population of toy breed dogs. A primary focus in humans when evaluating the atlantoaxial vertebrae involves measuring the atlanto-dens interval to confirm instability; this is the distance between the posterior edge of the anterior arch of C1 and the anterior edge of the odontoid process (Lee et al., 2002). Normal parameters for this interval have been set at less than 5 mm in children, and less than 3 mm in adults (Kim, 2013). In our population, dogs with AA subluxation had a significantly increased subluxation distance, from the atlas to the dens of 4.7 mm, compared with 2.2 mm in control dogs. The appearance or lack of the dens is a strong predictor of possible AA subluxation in dogs, especially as part of the initial radiographic evaluation. In our study, 52% (22) of the dogs with AA subluxation had dens present, with reduced or absent dens detected in 38% (16). Visibility of AA ligaments on MRI was more often accomplished with control dogs, while the alar ligament was difficult to visualize and inconclusive in 78.5% (33) of the affected dogs. This is consistent with Middleton et al. (2012), who had to reconstruct MR images by angling the dorsal scan plane to a 20° angle relative to the ventral floor of the vertebral canal to visualize the alar ligaments. Our study has several limitations. Given its retrospective nature, a standardized positioning for performing MRI on a dog with suspected AA subluxation was not utilized, which may impact the subluxation measurement. In addition, cases were collected from multiple institutions with varying MRI techniques and quality. Although we attempted to control for breed and weight, the control population was not perfectly matched with a smaller proportion of Yorkshire terriers and higher median ages and weights than the affected population. However, MRIs on clinically normal dogs were not available, so the control population was chosen from dogs with other cervical spinal cord disorders whose AA junction was included in the field of view. This does confound additional interpretation of the control group’s MRI findings, especially intramedullary hyperintensity and severity of signs. Whether dogs with AA subluxation ultimately had surgical or conservative management and their outcomes was also outside the scope of this study. This provides baseline data for future studies to correlate MRI abnormalities with successful clinical outcomes, as well as continue to further explore the benefits of MRI in assessing dogs with AA subluxation. In conclusion, dogs affected by AA subluxation do not have a higher incidence of Chiari-like malformation, syringohydromyelia, or hydrocephalus than control dogs, and their neurologic grade is not associated with MRI findings. However, lack of dens and/or odontoid ligaments is associated with larger subluxations. Additional angled dorsal MRI scan planes should be considered to identify and evaluate the ligaments of the AA junction. Based on this study’s results, the authors suggest using an AA joint cavity size >1.4 mm and a subluxation distance >2.5 mm as cutoffs for MRI diagnosis of atlantoaxial subluxation in dogs. AcknowledgmentsThis work was completed at the College of Veterinary Medicine, University of Georgia. The authors would like to thank the Colleges of Veterinary Medicine at the University of Georgia, Dr. Amy Hodshon and the University of Tennessee, Dr. Jill Narak and Auburn University, and North Carolina State University for their contribution of cases, Carolyn Harden for compiling and blinding the MR images, Dr. Deborah Keys for statistical expertise, and Drs. Allison Haley and Melissa Lewis for manuscript content review. Author contributionsThis project was conceived and designed by KB and SP, with data acquisition performed by KB, SP, MK, NO, KM, CM, and PE. Analysis and interpretation of the data were performed by KB, SH, and SP. The article was drafted by KB and SP, with revision for intellectual content performed by SP, MK, NO, SH, KM, CM, and PE. All authors gave final approval for article submission. Conflict of interestThe authors declare that there are no known conflicts of interest associated with this publication and there was no financial support necessary for this work that could have influenced the outcome. FundingThis research did not receive any funding. Data availabilityThe data used to support the primary findings of this study are included in the article. Complete datasets generated and analyzed during the study are available from the corresponding author upon reasonable request. ReferencesAikawa, T., Shibata, M. And Fujita, H. 2013. Modified ventral stabilization using positively threaded profile pins and polymethylmethacrylate for atlantoaxial instability in 49 dogs. Vet. Surg. 42, 683–692. Cappello, R. and Rusbridge, C. 2007. Report from the Chiari-like malformation and Syringomyelia Working Group Round Table. Vet. Surg. 36, 509–512. Cerda-Gonzalez, S. and Dewey, C.W. 2010. Congenital diseases of the craniocervical junction in the dog. Vet. Clin. Small Anim. 40, 121–141. Cerda-Gonzalez, S., Dewey, C.W., Scrivani, P.V. and Kline, K.L. 2009. Imaging features of atlanto-occipital overlapping in dogs. J. Vet. Rad. Ultr. 50, 264–268. Da Costa, R.C. and Samii, V.F. 2010. Advanced imaging of the spine in small animals. Vet. Clin. Small Anim. 40, 765–790. Geary, J., Oliver, J. and Hoerlein, B.F. 1967. Atlantoaxial subluxation in the canine. J. Small Anim. Pract. 8, 577–582. Havig, M.E., Cornell, K.K., Hawthorne, J.C., McDonnell, J.J. and Selcer, B.A. 2005. Evaluation of nonsurgical treatment of atlantoaxial subluxation in dogs: 19 cases (1992–2001). J. Am. Vet. Med. Assoc. 227, 257–262. Hung, S.C., Wu, H.M. and Guo, W.Y. 2010. Revisiting anterior atlantoaxial subluxation with overlooked information on MR images. Am. J. Neuroradiol. 31, 838–843. Johnson, P., Bertran, E., Dennis, R. and Taeymans, O. 2012. Magnetic resonance imaging characteristics of suspected vertebral instability associated with fracture or subluxation in eleven dogs. Vet. Radiol. Ultra. 53, 552–559. Kent, M., Eagleson, J.S., Neravanda, D., Schatzberg, S.J., Gruenenfelder, F.I. and Platt, S.R. 2010. Intraaxial spinal cord hemorrhage secondary to atlantoaxial subluxation in a dog. J. Am. Anim. Hosp. Assoc. 46, 132–137. Kim, H.J. 2013. Cervical spinal anomalies in children and adolescents. Curr. Opin. Pediatr. 25, 72–77. Kupczynska, M., Wieladek, A. and Janczyk, P. 2012. Craniocervical junction in dogs revisited—new ligaments and confirmed presence of enthesis fibrocartilage. Res. Vet. Sci. 92, 356–361. Lee, S.C., Lui, T.N. and Lee, S.T. 2002. Atlantoaxial rotary subluxation in skeletally immature patients. Brit. J. Neurosurg. 16, 154–157. Levine, J.M., Fosgate, G.T., Chen, A.V., Rushing, R., Ngheim, P.P., Platt, S.R., Bagley, R.S., Kent, M., Hicks, D.G., Young, B.D. and Schatzberg, S. 2009. Magnetic resonance imaging in dogs with neurologic impairment due to acute thoracic and lumbar intervertebral disk herniation. J. Vet. Intern. Med. 23, 1220–1226. Levistki, R.E., Lipsitz, D. and Chauvet, A.E. 1999. Magnetic resonance imaging of the cervical spine in 27 dogs. J. Vet. Rad. Ultr. 40, 332–341. McCarthy, R.J., Lewis, D.D. and Hosgood, G. 1995. Atlantoaxial subluxation in dogs. Compend. Contin. Educ. Pract. Vet. 17, 215–227. McLear, R.C. and Saunders, H.M. 2000. Atlantoaxial mobility in the dog. Vet. Radiol. Ultra. 41, 558. Middleton, G., Hillman, D.J., Trichel, J., Bragulla, H.H. and Gaschen, L. 2012. Magnetic resonance imaging of the ligamentous structures of the occipitoatlantoaxial region in the dog. J. Vet. Rad. Ultra. 53, 545–551. Oliver, J.E. and Lewis, R.E. 1973. Lesions of the atlas and axis in dogs. J. Am. Anim. Hosp. Assoc. 9, 304–313. Reber, K., Burki, A., Reves, N.V., Stoffel, M., Gendron, K., Ferguson, S.J. and Forterre, F. 2013. Biomechanical evaluation of the stabilizing function of the atlantoaxial ligaments under shear loading: a canine cadaveric study. Vet. Surg. 42, 918–923. Reves, N.V., Stahl, C., Stoffel, M., Bali, M. and Forterre, F. 2013. CT scan-based determination of optimal bone corridor for atlantoaxial ventral screw fixation in miniature breed dogs. Vet. Surg. 42, 819–824. Roy, S., Hol, P.K., Laerum, L.T. and Tillung, T. 2004. Pitfalls of magnetic resonance imaging of alar ligament. Neuroradiology 46, 392–398. Rusbridge, C., MacSweeny, J.F., Davies, J.V., Chandler, K., Fitzmaurice, S.N., Dennis, R., Cappello, R. and Wheeler, S.J. 2000. Syringohydromyelia in Cavalier King Charles Spaniels. J. Am. Anim. Hosp. Assoc. 3, 34–41. Sanders, S.G., Bagley, R.S., Silver, G.M., Moore, M. and Tucker, R.L. 2004. Outcomes and complications associated with ventral screws, pins, and polymethylmethacrylate for atlantoaxial instability in 12 dogs. J. Am. Anim. Hosp. Assoc. 40, 204–210. Slanina, M.C. 2015. Atlantoaxial instability. Vet. Clin. North Am. Sm. Anim. Pract. 46, 265–275. Warren-Smith, C.M., Kneissl, S., Benigni, L., Kenny, P.J. and Lamb, C.R. 2009. Incomplete ossification of the atlas in dogs with cervical signs. Vet. Radiol. Ultra. 50, 635–638. Vetti, N., Alsing, R., Krakenes, J., Rorvik, J., Gilhus, N.E., Brun, J.G. and Espeland, A. 2010. MRI of the transverse and alar ligaments in rheumatoid arthritis: feasibility and relations to atlantoaxial subluxation and disease activity. Neuroradiology 52, 215–223. | ||
| How to Cite this Article |
| Pubmed Style Bray KY, Platt SR, Kent M, Olby NJ, Early PJ, Mariani CL, Muñana KR, Holmes SP. Magnetic resonance imaging characteristics of atlanto-axial subluxation in 42 dogs: Analysis of joint cavity size, subluxation distance, and craniocervical junction anomalies. Open Vet J. 2023; 13(9): 1091-1098. doi:10.5455/OVJ.2023.v13.i9.4 Web Style Bray KY, Platt SR, Kent M, Olby NJ, Early PJ, Mariani CL, Muñana KR, Holmes SP. Magnetic resonance imaging characteristics of atlanto-axial subluxation in 42 dogs: Analysis of joint cavity size, subluxation distance, and craniocervical junction anomalies. https://www.openveterinaryjournal.com/?mno=142045 [Access: May 19, 2024]. doi:10.5455/OVJ.2023.v13.i9.4 AMA (American Medical Association) Style Bray KY, Platt SR, Kent M, Olby NJ, Early PJ, Mariani CL, Muñana KR, Holmes SP. Magnetic resonance imaging characteristics of atlanto-axial subluxation in 42 dogs: Analysis of joint cavity size, subluxation distance, and craniocervical junction anomalies. Open Vet J. 2023; 13(9): 1091-1098. doi:10.5455/OVJ.2023.v13.i9.4 Vancouver/ICMJE Style Bray KY, Platt SR, Kent M, Olby NJ, Early PJ, Mariani CL, Muñana KR, Holmes SP. Magnetic resonance imaging characteristics of atlanto-axial subluxation in 42 dogs: Analysis of joint cavity size, subluxation distance, and craniocervical junction anomalies. Open Vet J. (2023), [cited May 19, 2024]; 13(9): 1091-1098. doi:10.5455/OVJ.2023.v13.i9.4 Harvard Style Bray, K. Y., Platt, . S. R., Kent, . M., Olby, . N. J., Early, . P. J., Mariani, . C. L., Muñana, . K. R. & Holmes, . S. P. (2023) Magnetic resonance imaging characteristics of atlanto-axial subluxation in 42 dogs: Analysis of joint cavity size, subluxation distance, and craniocervical junction anomalies. Open Vet J, 13 (9), 1091-1098. doi:10.5455/OVJ.2023.v13.i9.4 Turabian Style Bray, Kathryn Y., Simon R. Platt, Marc Kent, Natasha J. Olby, Peter J. Early, Christopher L. Mariani, Karen R. Muñana, and Shannon P. Holmes. 2023. Magnetic resonance imaging characteristics of atlanto-axial subluxation in 42 dogs: Analysis of joint cavity size, subluxation distance, and craniocervical junction anomalies. Open Veterinary Journal, 13 (9), 1091-1098. doi:10.5455/OVJ.2023.v13.i9.4 Chicago Style Bray, Kathryn Y., Simon R. Platt, Marc Kent, Natasha J. Olby, Peter J. Early, Christopher L. Mariani, Karen R. Muñana, and Shannon P. Holmes. "Magnetic resonance imaging characteristics of atlanto-axial subluxation in 42 dogs: Analysis of joint cavity size, subluxation distance, and craniocervical junction anomalies." Open Veterinary Journal 13 (2023), 1091-1098. doi:10.5455/OVJ.2023.v13.i9.4 MLA (The Modern Language Association) Style Bray, Kathryn Y., Simon R. Platt, Marc Kent, Natasha J. Olby, Peter J. Early, Christopher L. Mariani, Karen R. Muñana, and Shannon P. Holmes. "Magnetic resonance imaging characteristics of atlanto-axial subluxation in 42 dogs: Analysis of joint cavity size, subluxation distance, and craniocervical junction anomalies." Open Veterinary Journal 13.9 (2023), 1091-1098. Print. doi:10.5455/OVJ.2023.v13.i9.4 APA (American Psychological Association) Style Bray, K. Y., Platt, . S. R., Kent, . M., Olby, . N. J., Early, . P. J., Mariani, . C. L., Muñana, . K. R. & Holmes, . S. P. (2023) Magnetic resonance imaging characteristics of atlanto-axial subluxation in 42 dogs: Analysis of joint cavity size, subluxation distance, and craniocervical junction anomalies. Open Veterinary Journal, 13 (9), 1091-1098. doi:10.5455/OVJ.2023.v13.i9.4 |