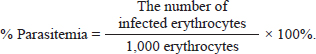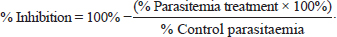| Research Article | ||
Open Vet J. 2023; 13(9): 1116-1123 Open Veterinary Journal, (2023), Vol. 13(9): 1116–1123 Original Research In vitro antimalarial activity of Syzygium cumini fruit fractionLilik Maslachah1* and Neny Purwitasari21Division of Basic Veterinary Medicine, Veterinary Pharmacy, Faculty of Veterinary Medicine, University Airlangga, Surabaya, Indonesia 2Department of Pharmaceutical Sciences, Faculty of Pharmacy, University Airlangga, Surabaya, Indonesia *Corresponding Author: Lilik Maslachah. Division of Basic Veterinary Medicine, Veterinary Pharmacy, Faculty of Veterinary Medicine, University Airlangga, Surabaya, Indonesia. Email: lilik.maslachah [at] fkh.unair.ac.id Submitted: 17/05/2023 Accepted: 10/08/2023 Published: 30/09/2023 © 2023 Open Veterinary Journal
AbstractBackground: Malaria is still one of the most severe public health problems worldwide. The development of treatment, prevention, and control of malaria is one of the substantial problems in the world. Aims: To investigate the in vitro antimalarial activity of Syzygium cumini methanol fruit fraction. Methods: Syzygium cumini L fruit powder was macerated with methanol (PA) and the extract obtained was fractionated using the liquid–liquid partition method with n-hexane, ethyl acetate, butanol, chloroform, methanol, and water solvents. In vitro antimalarial assay was conducted using the culture of Plasmodium falciparum 3D7 strain culture that had reached >5% growth and was examined for IC50 values using a 24-well microplate in duplicate. Each treatment and control well contained 1,080 μl of complete media. Well, number 1 was added with 120 μl fraction, and then the solution was diluted until it reached 0.01, 0.1, 1, 10, and 100 μg/ml the final concentration in the microtiter well. The control only contained complete media and infected erythrocytes without the addition of anti-malarial drugs. The microplate was incubated for 48 hours. After 48 hours, a thin blood smear was made fixed with methanol and stained with 20% Giemsa for 20 minutes to determine the IC50 value by plotting sample concentrations and percentage of parasitemia in Excel. Results: The IC50 values of ethyl acetate fraction, n.hexane fraction, butanol fraction, and water fraction were 1.189, 76.996, 1,769, and 15.058 μg/ml, respectively. Whereases the IC50 values of C1 fraction (mix fraction from chloroform: methanol 100:0 and 90:10) and C4 fraction (mix fraction from chloroform: methanol 20:80, 10:90, and 0:100) were 100.126 and 1.015 μg/ml, respectively. The results showed that the IC50 value of ethyl acetate, butanol, and C4 fraction were lower than 10μg/ml and were considered as good activity (strong antimalarial activity). Conclusion: The ethyl acetate, butanol and C4 subfraction from S. cumini fruit have the potential to be developed as an antimalarial agent. Keywords: Infectious disease, Malaria, IC50, Syzygium cumini L fruit. IntroductionMalaria is still one of the most severe public health problems worldwide. The development of treatment, prevention, and control of malaria is one of the substantial problems in the world. The use of common antimalarial drugs has been challenged variously due to the development of parasite resistance to some antimalarial drugs. There is also no effective vaccine to control malaria infection due to the complex life cycle of the Plasmodium parasite (Batista et al., 2009). The problem of antimalarial drug resistance increases and there are difficulties in accessing effective antimalarial drugs, due to the spread of malaria-resistant multidrug parasites, so it is urgently needed to develop new antimalarial drugs from natural materials. One of the many medicinal plants found in Indonesia is juwet (Syzygium cumini). The results of a study showed that S. cumini has strong radical scavenging and antioxidant activity (Zhang and Lin, 2009). Phenolic compounds and flavonoids contained in S. cumini have antioxidant and anti-inflammatory activities (Borges et al., 2017). The anti-malarial activities of flavonoids inhibit the fatty acid biosynthesis (FASII) of the parasite as well as inhibiting the influx of L-glutamine and myoinositol into infected erythrocytes (Rudrapal and Chetia, 2016). The use of some parts of the S. cumini plant as an antimalarial showed that S. cumini fruit as a therapeutic adjuvant has a better inhibitory effect on Plasmodium as compared to the leaves and bark (Maslachah and Sugihartuti, 2018; Maslachah et al., 2020). Determining the antimalarial activity of compounds in S. cumini L fruit can be made by in vitro. In vitro antimalarial testing is a test that does not involve the intervention of the host physiological factor, so the results obtained are the result of a parasite that is directly exposed to the test material and can be examined for inhibition of growth and maturation into schizont (Maji, 2018). The result of in vitro testing on malarial material can be determined based on the value of 50 concentration inhibitors (IC50), so this research was conducted with the fraction of S. cumini L fruit as in vitro antimalarial assay against Plasmodium falciparum 3D7 strain to determine the IC50 value. Materials and MethodsMaterialsThe solvents used to make S. cumini fractionation were n-hexane (Merck), chloroform (Merck), Methanol (Merck), Butanol (Merck), ethyl acetate (Merck), and aquadest. Materials used to make P. falciparum culture media and to make in vitro test solutions were aquadest, HEPES buffer (Sigma), RPMI 1640 (Gibco BRL, USA), sodium bicarbonate, gentamicin, plasma, and human erythrocytes, DMSO (Sigma-Aldrich), Giemsa dyes, methanol, and emersion oil. Plasmodium isolateIsolate of P. falciparum 3D7 (Chloroquine-sensitive) strain used in this study from the University Faculty of Pharmacy Airlangga Surabaya Indonesia. Plant materialSyzygium cumini L fruit was obtained from Lumajang city of East Java, Indonesia, and was identified in the Herbal laboratory of Materia Medica, Batu with letter number 067/1873/102.20/2023. Preparation of fruit S. cumini L extractThe cleaned fruit is dried by aerating it in the open air. After drying, it is ground to a fine powder. Syzygium cumini L fruit powder was macerated with 96% methanol (PA) for 3 × 24 hours then the filtrate was evaporated with rotavapor at 50°C and then dried using a freeze dryer to produce a thick extract. Preparation of Syzygium fruit cumini L fractionExtract fractionation uses the solid-liquid method with n-hexane, ethyl acetate, butanol, and water solvents. The filtrate results are evaporated. After drying, all fractions were weighed to find out each weight. Extract fractionation with chloroform and methanol was carried out by vacuum liquid chromatography, methanol extract of 5 g, was put in a porcelain cup. Silica gel 60 GF254 weighs as much as 20 g and takes a third part to be mixed into the extract and crushed until silica and extract form a homogeneous powder. The elution process uses eluents with a series of motion phases as follows: CHCl3: MeOH (100:0), CHCl3: MeOH (90:10), CHCl3: MeOH (80:20), CHCl3: MeOH (70:30) and CHCl3: MeOH (60:40), CHCl3: MeOH (50: 50), CHCl3: MeOH (40:60), CHCl3: MeOH (30:70), CHCl3: MeOH (20:80), CHCl3: MeOH (10:90), and CHCl3: MeOH (0:100). The fractions obtained were evaporated and conducted for thin layer chromatography (TLC) investigation using mobile phase chloroform: methanol 7:3. Fractions that have the same Rf value and spot color are combined into one mixture. In vitro testing procedureIn vitro activity antimalarial test was conducted using the culture of P. falciparum 3D7 strain. Plasmodium falciparum 3D7 strain which had been stored in liquid nitrogen was thawed using the Rowe method. In vitro culture using the Trager and Jensen method. O-type human red blood cells, hematocrit 5% in RPMI 1640 medium (GIBCO BRL, USA), supplemented with 22.3 mM HEPES (sigma), Hypoxanthine sodium bicarbonate with 10% plasma from human O-blood type. In vitro tests were carried out in good culture 24 with 1% parasitemia (1 ml suspension/well). For the analysis of sample concentrations starting from 0.01, 0.1, 1, 10, and 100 μg/ml. The culture was incubated at 37°C for 48 hours, a thin blood smear was made fixed with methanol and stained with Giemsa staining 20% for 20 minutes. Then washed with water and dried. After that, the percentage of parasitemia P. falciparum 3D7 strain was calculated by counting the number of infected erythrocyte cells per 1,000 erythrocyte cells under a light microscope at 1,000× magnification. Plasmodium falciparum 3D7 strain culture that had reached >5% growth was examined for IC50 values using a 24-well microplate in duplicate. The serial concentration of fractions that we tested for antimalarial activity were 0.01, 0.1, 1, 10, and 100 μg/ml. The final concentration in the microtiter well with two repetitions. The control only contained complete media and infected erythrocytes without the addition of anti-malarial drugs. The microplate was incubated for 48 hours. After 48 hours a thin blood smear was fixed with methanol, stained with 20% Giemsa for 20 minutes, washed with water, and dried. Calculation percentage of parasitemia and percentage of growth inhibitionThe percentage of parasitemia is calculated by the following (Ljungstrom et al., 2004; Garcia et al., 2008):
The calculation of parasitemia percentage was made in 1,000 erythrocytes using a light microscope with 1,000× magnification, the growth and the percentage of inhibition were done with the following equation (Inbaneson et al., 2013):
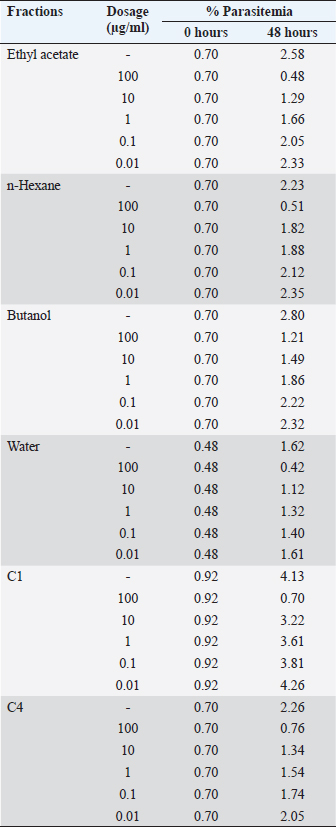
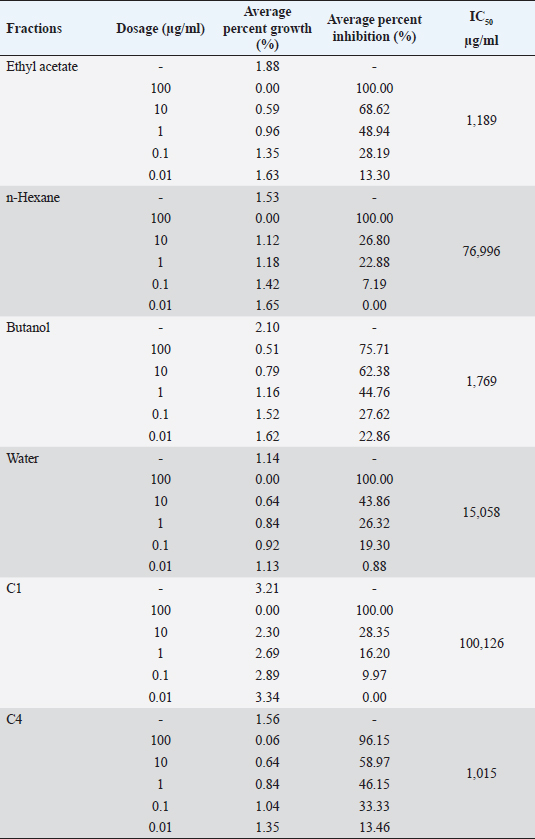
Then, the SPSS 15 probit analysis program is used to determine the IC50 value. ResultsResults of activity test of antimalarial in vitro with the culture of P. falciparum strain 3D7 using the Trager and Jensen (1976) methods. The results of the percentage of parasitemia, percentage of growth, percentage of inhibition, and IC50 P. falciparum 3D7 are shown in Tables 1 and 2. Results extract fractionated with technique partition liquid–liquid. The results of the fractionation process graded from the fraction test are shown in Table 3. Table 1. The average percent of parasitemia of P. falciparum 3D7 gives a fraction of S. cumini L extract in vitro.
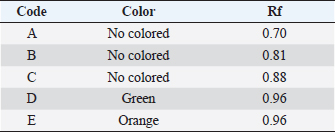
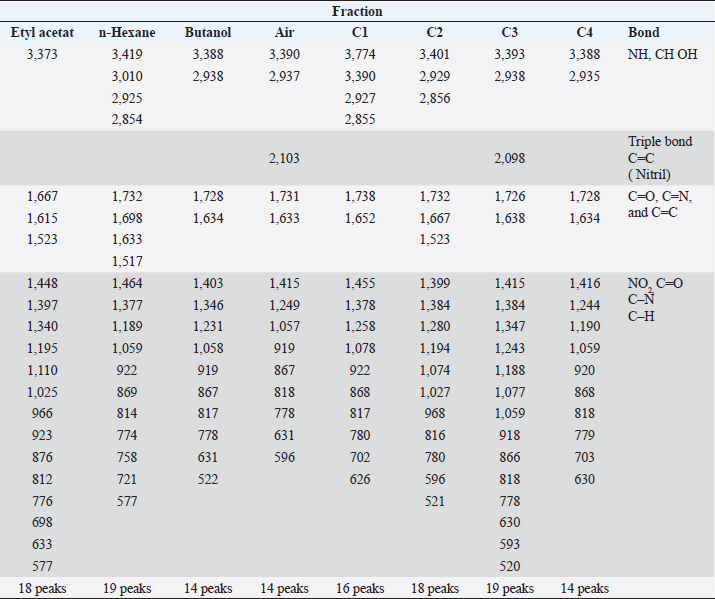
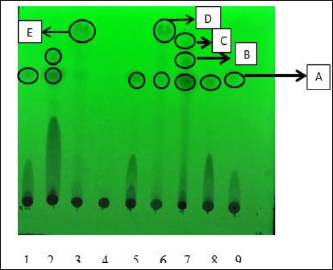
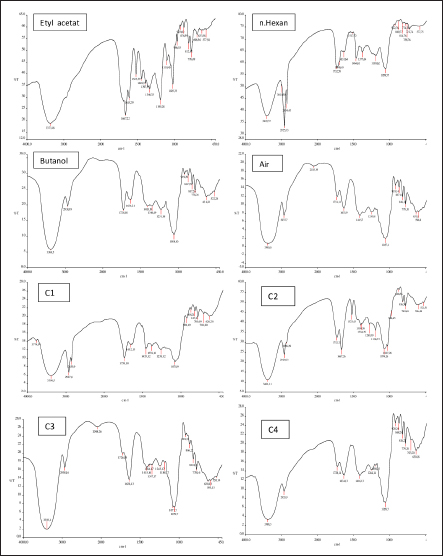
Results TLC observations and Wavenumber (cm−1) of several extract fractions of S. cumini L are shown in Tables 4 and 5. Results profile TLC chromatogram with eluent ethyl acetate: chloroform (7:3). Results identification to spots that appear were done with sighting stain citric borate, and then checked with UV lamp on Lambda 254 and 366 nm. The results show (A). Rf 0.70, colorless, (B) Rf 0.81, colorless, (C) Rf 0.88, colorless, (D) Rf 0.96, green, and E) Rf 0.96 orange color (Fig. 1). The results of Fourier transform infra red (FTIR) ethyl acetate were 18 peaks, N-hexane 19 peaks, butanol 14 peaks, water 14 peaks, C1 16 peaks, C4 14 peaks. FTIR spectra of several extract fractions of S. cumini L (Fig. 2). DiscussionThe results of the antimalarial activity test showed that the C4 fraction (chloroform: methanol with a ratio of 20:80, 10:90, and 0:1,000 had the highest antimalarial activity with IC50 value of 1.015 μg/ml, then ethyl acetate with IC50 value 1.189 μg/ml, Butanol with IC50 value 1.769 μg/ml, water with IC50 value 15.058 μg/ml, n-hexane with IC50 value of 76.996, fraction C1 (chloroform: methanol with a ratio of 100:0 and 90:10) with IC50 value 100.126 μg/ml and C2 (chloroform: methanol; 80:20,70:30) and C3 (chloroform: methanol; 60:40, 50:5, 40:60, and 30:70) fraction not detected. A testing sample is considered to be a strong antimalarial inhibition if it has an IC50 value less than 10 μg/ml, while moderate if the IC50 value is 10–50 μg /ml and low activity if the IC50 ranges from 50 to 100 μg/ml and if the IC50 is above 100 μg/ml, is declared less active (inactive). From these data, it is shown that the antimalarial activity of the C4, ethyl acetate, and butanol fractions has strong antimalarial activity while the water fraction was moderate and the n-hexane fraction was low activity and the C1 fraction was less active (inactive) (Dolabela et al., 2008). Fractionation was carried out using the different polarity of solvents such as n-hexane, ethyl acetate, butanol, and water as well as chloroform and methanol to extract the active ingredients and to separate the active compounds from S. cumini fruit extract. Syzygium cumini contains a variety of compounds that have different polarities, such as triterpenoids, polyphenols, flavonoids, and anthocyanins. A previous study reported that in vitro antimalarial activity test on clove extract showed an association of antioxidant effects of phenol compounds and their derivatives such as flavonoids and tannins produced from clove plants with the ability to inhibit P. falciparum growth) (Brahmam et al., 2018; Dharmawati, 2019). Methanol extract of S. cumini contains alkaloids, amino acids, flavonoids, glycosides, phytosterols, saponins, steroids, tannins, and triterpenoids. The results of chemical studies reported that there is a relationship between the form of chemical conformation the antimalarial activity against Plasmodium vivax and P. falciparum (Kumar et al., 2009; Oliveira et al., 2009; Onguéné et al., 2013). The alkaloid component of Strychnos malacoclados bark also has antimalarial activity (Tchinda et al. 2011). Elfita et al. (2011) stated that 7-hydroxypiranopiridin-4-one alkaloid compound (C8H7NO2) as antimalarial potential, isolated from the microbial endophytic microbial (Andographis paniculata Nees) IC50 value of 0.201 μM. Several nonalkaloid compounds that act as antimalarials have been reported. Batista et al. (2009) suggested that antimalarial compounds are found in terpene, limonoid, flavonoid, chromon, xanton, antraquinone, and miscellaneous groups. Sutthivaiyakit et al. (2009) suggested that the compound components of diterpene, sesquiterpenes, and sesquiterpenicumarin from Jatropha integerrima plants have antimalarial activity in vitro. Uchôa et al. (2010) group of triterpenoid compounds from the Cecropia pachystachya plant are antimalarial. Antimalarial activity of lactone sesquiterpenes in vitro against P. falciparum was reported by Sulsen et al. (2011) on Ambrosia tenuifolia plant and Toyang et al. (2013) on Vernonia guineensis (Asteraceae). Artemicia annua plant has an antimalarial active compound namely artemisinin which belongs to the group of sesquiterpenes compounds (Guo, 2016). Some other compounds are proven to be antimalarial β-karyofilen (Kamaraj et al., 2017). Gallic acid derivatives (octyl gallate) with hydroxyl groups are found in the aromatic ring (Arsianti et al., 2017). Methyl galat (Arsianti et al., 2018), coumarin (Pingaew et al., 2014; Tanjung et al., 2016; Beaufay et al., 2017). Table 2. Percent growth, percent inhibition, and IC50 P. falciparum 3D7 administration of S. cumini L extract fraction in vitro.
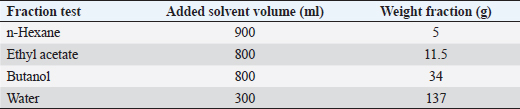
Table 3. Fractionation results graded extract methanol fruit S. cumini L.
Table 4. Results TLC observations.
Table 5. Wavenumber (cm−1) of several extract fractions of S. cumini L.
Fig. 1. Chromatogram of TLC results with UV lamp on Lambda 254 and 366 nm. (1) Methanol extract; (2) ethyl fraction acetate; (3) fraction n-hexane; (4) water fraction; (5) fraction butanol; (6) fraction C1; (7) C2 fraction; (8) C3 faction; and (9) C4 fraction.
Fig. 2. FTIR spectra of several extract fractions of S. cumini L. The antimalarial activity of these antioxidant potential compounds which have low molecular weight, and can interfere with the redox metabolism of malaria parasites, acts as an inhibitor of the formation of antioxidant enzymes and can interfere with the formation of hemozoin. Also, the nature of a compound determines its activity. The active compound contained in the test fraction (supposed as flavonoid) has semipolar properties so that it is easier to penetrate the semipolar layer than the nonpolar lipid layer which makes it more difficult for the compound to enter the cell membrane (Becker et al., 2004). The activity of flavonoids as an antimalarial with the mechanism of action by inhibiting the biosynthesis of fatty acids (FAS II) pathway, which is in the parasite’s apicoplast such as luteolin-7-O-β-d- glucopyranoside, reported as the first natural product to target the plasmodial FAB I enzyme which regulates the FAS II pathway (Waller et al., 2003). Some flavonoids also show the ability to inhibit l-glutamine and myoinositol influx in infected erythrocytes or work with interference with hemin degradation (Elford, 1986). ConclusionThe ethyl acetate, butanol and C4 fraction from S. cumini fruit has the potential to be developed as an antimalarial agent. Further identification of the active compounds of these fractions is to be investigated. AcknowledgmentsThe authors would like to thank the Ministry of Higher Education on Research and Technology (Kemenristek Dikti ) for the funding support of the DRPM research grant the PTUPT research 2019 with contract number 661/UN3.14/LT/2019. Conflict of interestThe authors declare that there is no conflict of interest. Authors contributionsResearch project leader and coordinating research, designed study, analyzed data, drafted the paper and corresponding author by Lilik Maslachah. Processing, examination, and analysis of in vitro data and reading the final manuscript by Neny Purwitasari. All authors contributed to the manuscript’s reading, reviewing, revising, and approving the final version. FundingThe funding support of the DRPM research grant the PTUPT research 2019 from the Ministry of Higher Education on Research and Technology. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesArsianti, A., Astuty, H., Fadilah, Bahtiar, A., Tanimoto, H. and Kakiuchi K. 2017. Design and screening of gallic acid derivatives as inhibitors of malarial dihydrofolatereductase by in silico docking. Asian J. Pharm. Clin. Res. 10(2), 330–334. Arsianti, A., Astuty, H., Fadilah, Simadibrata, D.M., Adyasa, Z.M., Daniel, A., Bahtiar, A., Tanimoto, H. and Kakiuchi, K. 2018. Synthesis and in vitro antimalarial activity of alkyl esters of gallate as a growth inhibitor of Plasmodium falciparum. Orient. J. Chem. 34(2), 655–662. Batista, R., Júnior, A.D.J.S. and Oliveira, A.B.D. 2009. Plant derived antimalarial agents: new leads and efficient phytomedicines. II. Non-alkaloidal natural products. Molecules 14(8), 3037–3072. Becker, K., Tilley, L., Vennerstrom, J.L., Roberts, D., Rogerson, S. and Ginsburg, H. 2004. Oxidative stress in malaria parasite-infected erythrocytes: host–parasite interactions. Intern. J. Parasitol. 34, 163–189. Beaufay, C., Hérent, M.F., Quetin-Leclercq, J. and Bero, J. 2017. In vivo anti-malarial activity and toxicity studies of triterpenic esters isolated form Keetia leucantha and crude extracts. Malar. J. 16(406), 1–8. Borges, R.M., Bitencourt, P.E.R., Stein, C.S., Bochi, G.V., Boligon, A., Moresco, R.N. and Moretto, M.B. 2017. Leaves and seeds of Syzygium cumini extracts produce significant attenuation of 2,2 azobis-2-amidinopropane dihydrochloride-induced toxicity via modulation of ectoenzymes and antioxidant activities. J. Appl. Pharm. Sci. 7(06), 37–48. Brahmam, P., Sunita, K. and Babu, B.H. 2018. Phytochemical investigation of plants Limonia acidissima (L.) and Pergularia daemia (L.) from Prakasam district, Andhra Pradesh, India. Eur. J. Biomed. Pharm. Sci. 5(5), 977–983. Dharmawati, M.T. 2019. Antimalarial testing using clove plants (Syzygium aromaticu) Merr & Perry (in vitro studies, in vivo and screening of active compounds). Dissertations, Institute of Agriculture, Bogor Graduate School, Bogor, Indonesia. Dolabela, M.F., Oliveira, S.G., Nascimento, J.M., Peres, J.M., Wagner, H., Povo, MM. and de Oliveira, A.B. 2008. In vitro antiplasmodial activity of extract and constituents from Esenbeckia febrifuga a plant traditionally used to treat malaria in the Brazilian Amazon. Phytomedicine 1, 367–372. Elfita, M., Munawar, S. and Oktasari, A. 2011. Antimalarial compounds from the bitter endophytic fungi (Andographis paniculata Nees). Nat. Ind. 13(2), 123–129. Elford, B.C. 1986. L-Glutamine influx in malaria-infected erythrocytes: a target for antimalarials. Parasitol. Today 2(11), 309–312. Garcia, C.R., de Azevedo, M.F., Wunderlich, G., Budu, A., Young J.A. and Bannister, L. 2008. Plasmodium in the post genomic era: new insight into molecular cell biology of malaria parasites. Int. Rev. Cell Mol. Biol. 266, 85–156. Guo, Z. 2016. Artemisinin anti-malarial drugs in China. Acta Pharm. Sin. B. 6(2), 115–124. Inbaneson, S.J., Sundaram, A. and Ravikumar, S. 2013. In vitro antiplasmodial activity of PDDS-coated metal oxide nanoparticles against Plasmodium falciparum. Appl. Nanosci. 3, 197–201. Kamaraj, C., Balasubramani, G., Siva, C., Raja, M., Balasubramanian, V., Raja, R.K., Tamilselvan, S., Giovanni, B. and Perumal, P. 2017. Ag nanoparticles synthesized using b-caryophyllene isolated from Murraya koenigii antimalarial (Plasmodium falciparum 3D7) and anticancer activity (A549 and HeLa cell Lines). J. Clust. Sci. 28, 1667–1684. Kumar, A., Ilavarasan, R., Jayachandran, T., Decaraman, M., Aravindhan, P., Padmanabhan, N. and Krishnan, M.R.V. 2009. Phytochemical investigation in a tropical plant Syzygium cumini from Kattuppalayam, Erode District, Tamil Nadu, South India. Pak. J. Nutr. 8(1), 83–85. Ljungstrom, I., Perlmann, H., Schlictherle, M., Scherf, A. and Wahlgren, M. 2004. Methods in malaria research, 4th ed. University Boulevard, Manassas, Virginia. ISBN.0-930009-64-9. pp: 1–240. Maji, A.K. 2018. Drug susceptibility testing methods of antimalarial agents. Trop. Parasitol. 8(2), 70-76. Maslachah, L. and Sugihartuti, R. 2018. Potency Syzygium cumini L as adjuvant therapy on mice model malaria. Iraqi J. Vet. Sci. 31(1), 73–80. Maslachah, L., Sugihartuti, R., Wahjuni, R.S. and Yustinasari, L.T. 2020. Adjuvant therapy of Syzygium cumini leaf and fruit extract nanoparticles in mice (Mus musculus) infected by Plasmodium berghei. Indian Vet. J. 97(2), 33–36. Oliveira, A.B., Dolabela, M.F., Braga, F.C., Jácome, R.L.R.P., Varotti, F.P. and Póvoa, M.M. 2009. Plant-derived antimalarial agents: new leads and efficient phytomedicines. Part I. Alkaloids. An. Acad. Bras. Cienc. 81(4), 715–740. Onguéné, P.A., Ntie-Kang, F., Lifongo, L.L., Ndom, J.C., Sippl, W. and Mbaze, L.M. 2013. The potential of anti-malarial compounds derived from African medicinal plants, part I: a pharmacological evaluation of alkaloids and terpenoids. Malar. J. 12(449), 1–25. Pingaew, R., Saekee, A., Mandi, P., Nantasenamat, C., Prachayasittikul, S., Ruchirawat, S. and Prachayasittikul, V. 2014. Synthesis, biological evaluation and molecular docking of novel chalconee-coumarin hybrids as anticancer and antimalarial agents. Eur. J. Med. Chem. 85, 65–76. Rudrapal, M. and Chetia, D. 2016. Plant flavonoids as a potential source of future antimalarial leads. Syst. Rev. Pharm. 8, 13–18. Sulsen, V., Yappu, D.G., Laurella, L., Anesini, C., Turba, A.G., Martino, V. and Muschietti, L. 2011. In vitro antiplasmodial activity of sesquiterpene lactones from Ambrosia tenuifolia. Evid. Based Complement. Altern. Med. 2011, 1–4. Sutthivaiyakit, S., Mongkolvisut, W., Prabpai, S. and Kongsaeree, P. 2009. Diterpenes, sesquiterpenes, and a sesquiterpene-coumarin conjugate from Jatropha integerrima. J. Nat. Prod. 72(11), 2024–2027. Tanjung, M., Saputri, R.D., Fitriati, F.F. and Tjahjandarie, T.S. 2016. Antimalarial and antioxidant activities of isoprenylated coumarins from the stem bark of Mesua borneensis L. J. Biol. Act. Prod. Nat. 6(2), 95–100. Tchinda, A.T., Ngono, A.R.N., Tamze, V., Jonville, M.C., Cao, M., Angenot, L. and Frédérich, M. 2011. Antiplasmodial alkaloids from the stem bark of Strychnos malacoclados. Planta Med. 78, 377–382. Toyang, N.J., Krause, M.A., Fairhurst, R.M., Tane, P., Bryant, J. and Verpoorte, R. 2013. Antiplasmodial activity of sesquiterpene lactones and a sucrose ester from Vernonia guineensis Benth. (Asteraceae). J. Ethnopharm. 147, 618–621. Trager, W. and Jensen, J.B. 1976. Human malaria parasites in continuous culture. Science 193, 673–675. Uchôa, V.T., Paula, R.C.D., Krettli, L.G., Santana, A.E.G. and Krettli, A.U. 2010. Antimalarial activity of compounds and mixed fractions of Cecropia pachystachya. Drug Dev. Res. 71, 82–91. Waller, R.F., Ralph, S.A., Reed M.B, Su, V., Douglas, J.D. and Minnikin, D.E.A. 2003. Type II pathway for fatty acid biosynthesis presents drug targets in Plasmodium falciparum. Antimicrob. Agent Chemother. 47(1), 297–301. Zhang, L.L. and Lin, Y.M. 2009. Antioxidant tannins from Syzygium cumini fruit. Afr. J. Biotechnol. 8(10), 2301–2309. | ||
| How to Cite this Article |
| Pubmed Style Maslachah L, Purwitasari N. In Vitro antimalarial activity of Syzygium cumini fruit fraction. Open Vet J. 2023; 13(9): 1116-1123. doi:10.5455/OVJ.2023.v13.i9.7 Web Style Maslachah L, Purwitasari N. In Vitro antimalarial activity of Syzygium cumini fruit fraction. https://www.openveterinaryjournal.com/?mno=151625 [Access: May 28, 2024]. doi:10.5455/OVJ.2023.v13.i9.7 AMA (American Medical Association) Style Maslachah L, Purwitasari N. In Vitro antimalarial activity of Syzygium cumini fruit fraction. Open Vet J. 2023; 13(9): 1116-1123. doi:10.5455/OVJ.2023.v13.i9.7 Vancouver/ICMJE Style Maslachah L, Purwitasari N. In Vitro antimalarial activity of Syzygium cumini fruit fraction. Open Vet J. (2023), [cited May 28, 2024]; 13(9): 1116-1123. doi:10.5455/OVJ.2023.v13.i9.7 Harvard Style Maslachah, L. & Purwitasari, . N. (2023) In Vitro antimalarial activity of Syzygium cumini fruit fraction. Open Vet J, 13 (9), 1116-1123. doi:10.5455/OVJ.2023.v13.i9.7 Turabian Style Maslachah, Lilik, and Neny Purwitasari. 2023. In Vitro antimalarial activity of Syzygium cumini fruit fraction. Open Veterinary Journal, 13 (9), 1116-1123. doi:10.5455/OVJ.2023.v13.i9.7 Chicago Style Maslachah, Lilik, and Neny Purwitasari. "In Vitro antimalarial activity of Syzygium cumini fruit fraction." Open Veterinary Journal 13 (2023), 1116-1123. doi:10.5455/OVJ.2023.v13.i9.7 MLA (The Modern Language Association) Style Maslachah, Lilik, and Neny Purwitasari. "In Vitro antimalarial activity of Syzygium cumini fruit fraction." Open Veterinary Journal 13.9 (2023), 1116-1123. Print. doi:10.5455/OVJ.2023.v13.i9.7 APA (American Psychological Association) Style Maslachah, L. & Purwitasari, . N. (2023) In Vitro antimalarial activity of Syzygium cumini fruit fraction. Open Veterinary Journal, 13 (9), 1116-1123. doi:10.5455/OVJ.2023.v13.i9.7 |