| Research Article | ||
Open Vet J. 2023; 13(9): 1141-1149 Open Veterinary Journal, (2023), Vol. 13(9): 1141-1149 Original Research Percutaneous ultrasonic debridement of equine tendinopathy and desmopathy: A report of 10 casesTed P. Vlahos*Yellowstone Equine Hospital, PC, 356 W. Yellowstone Avenue, Cody, Wyoming 82414, USA *Corresponding Author: Ted P. Vlahos. Yellowstone Equine Hospital, PC, 356 W. Yellowstone Avenue, Cody, Wyoming 82414, USA. Email: drv [at] yellowstoneequine.com Submitted: 27/05/2023 Accepted: 14/08/2023 Published: 30/09/2023 © 2023 Open Veterinary Journal
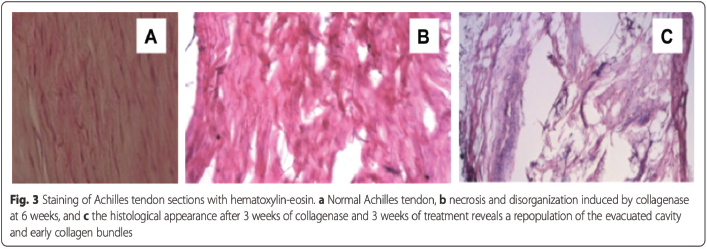
AbstractBackground: Tendinopathy and desmopathy are significant causes of morbidity in horses. Aim: To evaluate the use of percutaneous ultrasonic debridement (PUD) as a treatment for chronic tendinopathy and desmopathy in the horse. Methods: Eight adult horses with 10 affected limbs presented for lameness, ranging from 60–700 days postinjury. Diagnostic ultrasound identified the following: suspensory branch desmitis (n=1), suspensory body desmitis (n=2), Achilles tendinopathy (n=1), desmitis of the accessory ligament of the deep digital flexor (DDF) tendon (n =1), DDF tendinopathy (n=2), and superficial digital flexor tendinopathy (n=3). All horses had demonstrated lameness ranging from grade 1 to 4 [American Association of Equine Practitioners (AAEP) scale], with a mean pretreatment grade of 2.7. All horses underwent PUD using the Tenex Health TX® System. Results: Follow-up results were available from 6 to 41 months (mean, 23.2 months). Follow-up ultrasound imaging demonstrated improvement in fiber alignment and architectural change in all cases. All horses had a reduction in lameness from the treated tendon or ligament (AAEP grade 0–1; mean AAEP grade, 0.2) following a single treatment; lameness completely resolved in 8 of 10 treated limbs. No adverse events occurred in any case. No horses in this study developed a recurrence of their original lesion. Conclusion: Horses in this study demonstrated improvement following the PUD procedure. The procedure was well-tolerated and safe. Removal of tendinopathic scar tissue with PUD resulted in a return to function and without recurrence of the original lesion in all horses. Keywords: Chronic, Debridement, Desmopathy, Equine, Tendinopathy. IntroductionTendinopathy and desmopathy are debilitating pathologies in the equine athlete and are among the leading causes of morbidity (Hogan and Bramlage, 1995; Dowling et al., 2000; Dyson, 2004; Kashashima et al., 2004; Tnibar, 2007; White and Hewes, 2008; Ely et al., 2009; Thorpe et al., 2010; Clegg, 2012; Dakin, 2016; O’Brien et al., 2021; Ribitsch et al., 2021). The equine superficial digital flexor tendon (SDFT) has a maximal strain of approximately 16% and is the structure most at risk for injury in racehorses with supraphysiological loads, with the incidence of injury occurrence up to 43% (Tnibar, 2007; Dyson and Murray, 2012; Ribitsch et al., 2021). This pathology is analogous to human tendinosis or chronic repetitive overuse type injuries; these are much more common than acute injuries. Tendinosis results from repetitive microtrauma that exceeds the capacity of the tendon to repair. When examined histologically, this type of tendon injury is often characterized by tendon degeneration without evidence of an appropriate cellular inflammatory response with little to no prostaglandin-mediated inflammation, and repair seems to be, in the long run, inadequate and unpredictable (Plevin and McLellan, 2014; O’Brien et al., 2021; Ribitsch et al., 2021). In horses that compete at speed, there is evidence of similar strain-induced tendinopathy that results in progressive degenerative changes in the SDFT (often bilaterally). Such cases often manifest as acute tendinitis, as sudden peak forces can easily overcome the strength of the degenerated tendon. This type of injury can occur at any time, including turnout, but is most likely when a horse is maximally loading the tendon during training or competition. In racehorses, SDFT disease accounts for up to 90% of all tendon and ligament injuries; the remainder being predominantly suspensory ligament injuries (Dowling et al., 2000; Ely et al., 2009; Ribitsch et al., 2021). These lesions are often career-ending events. In 2-year-old thoroughbred racehorses, almost 10% were affected by suspensory branch desmitis (SBD) (Kamineni et al., 2019). SBD in thoroughbreds in race training led to 3.2 times less likelihood of starting a race as a 2-year-old, and 3.6 times less likely as a 3-year-old (Plevin and McLellan, 2014). In the nonracing equine athlete, repetitive use and overstrain can lead to devastating fibrosis and recalcitrant tendinopathy or desmopathy, which can have a significant negative impact on the horse’s utility. Equine tendon and ligament healing is initiated due to the disruption of the tissue matrix, which leads to hemorrhage and pronounced inflammation. Tendon healing occurs in three distinct, but partially overlapping phases (Abate et al., 2009; O’Brien et al., 2021; Ribitsch et al., 2021). The acute inflammatory phase occurs initially after injury and lasts from 3 to 7 days postinjury. The initial process involves hematoma formation and platelet activation, followed by an influx of erythrocytes, neutrophils, macrophages, monocytes, proteolytic enzymes, and proinflammatory cytokines. Phagocytosis of necrotic tissue begins in the first 24 hours. The proliferative phase then lasts between 5 and 21 days, where fibroblasts begin the process of collagen production. The maturation and remodeling phase, also known as the reparative phase, occurs after the proliferative phase and may last up to a year or more after injury (Abate et al., 2009; O’Brien et al., 2021; Ribitsch et al., 2021). Repair of the equine adult tendon is slow and inefficient due to low cellularity and vascularity. Tendon repair results in the synthesis of scar tissue which has a composition of approximately 50% type III collagen, compared to only 10% type III collagen in the normal tendon. As the remodeling phase continues, there is an incomplete return to type I collagen as the fibrous scar matures. The new type III collagen contains extensive cross-linking, resulting in a stiffer, and functionally inferior tissue compared to that of the normal tendon. The fibrotic scarring in the tendon is likely responsible for the 23%–67% of horses that experience re-injury of a tendon (within 2 years of their initial injury) following conservative treatment methods (Dyson, 2004; O’Brien et al., 2021; Ribitsch et al., 2021). In addition, continued use of a compromised tendon results in chronic repetitive microtrauma that exceeds repair capacity, resulting in scar deposition and tendon degeneration. Prolonged recurrent injury to the inelastic tendon can result in recalcitrant tissue (Abate et al., 2009; Elattrache and Morrey, 2013; Koh et al., 2013; Dakin et al., 2014; Barnes et al., 2015; Freed et al., 2019; Ribitsch et al., 2021). Traditional treatment modalities for equine tendinopathy and desmopathy have run the gamut, ranging from conservative management rest and nonsteroidal anti-inflammatory drugs (NSAIDS)] to the invasive (pin firing and blistering, tendon splitting), none of which have stood the test of time. More recently, newer technological advances have been employed in the treatment of a variety of soft tissue injuries. These include injections with polysulfated glycosaminoglycans, hyaluronate, platelet-rich plasma, amnion, mesenchymal stem cells, urinary bladder matrix scaffolds, autologous serum products, and steroids. Other treatment modalities include extracorporeal shock wave therapy and regenerative laser therapy. Surgeries have included percutaneous tendon splitting, lateral plantar nerve neurectomy, SDFT accessory ligament desmotomy, tenoscopic debridement of damaged tendon within a sheath, and fasciotomy (Hogan and Bramlage, 1995; Dyson, 2004; Richardson et al., 2007; Tnibar, 2007; White and Hewes, 2008; Dyson and Murray, 2012; Hu and Bramlage, 2014; Ribitsch et al., 2021). Phacoemulsification surgery, using ultrasonic energy to sculpt and emulsify degenerative tissue, was developed in 1967 and revolutionized the treatment of visual loss in humans due to cataracts (Kelman, 1994). The procedure gained widespread acceptance in the 1990s and remains the gold standard as a minimally invasive outpatient procedure with extraordinary safety and efficacy. In 2010, Mayo Clinic researchers Dr. Gill and Dr. Morrey modified the technology to apply to the treatment of tendinopathy and fasciitis. The Tenex Health TX® System (Tenex Health, Lake Forest, CA) uses high-frequency ultrasonic energy to effectively tenotomize the diseased tendon by a cavitation effect. Kamineni et al. (2015) created a collagenase-injected rabbit Achilles tendinopathy model. Subjects subsequently underwent percutaneous ultrasonic debridement (PUD) with the Tenex Health TX® System under ultrasound guidance. Their results demonstrated normalized qualitative and semi-quantitative collagen profiles, minimal residual tendinopathic debris, and improved histological appearance following a single treatment, with little adverse effect on normal tissue (Fig. 1).
Fig. 1. Kamineni et al., 2015; J. Orth. Surg. Res. (with permission). Table 1. Tenex Health TX® MicroTip® specifications.
Table 2. Case summaries of PUD of tendinopathy and desmopathy using the Tenex Health TX® System.
The US Food and Drug Administration (FDA) approved the Tenex Health TX® System as a class II medical device for the treatment of refractory lateral epicondylitis (tennis elbow), Achilles’ tendinopathy, plantar fasciitis and fibroma, and tendonitis of the shoulder, knee, and gluteal tendons in human patients (Tenex Health, Lake Forest, CA). In 2018, a larger diameter microtip was introduced with FDA approval to remove bone and calcific tendinopathic tissue. As of 2023, more than 160,000 human patients have been treated with the Tenex procedure, and fewer than 30 adverse events (mostly localized swelling) requiring medical attention were reported (Morrey B, personal communication, 2023; Tenex Health, Lake Forest, CA, File Data, 2023). The risk/benefit has been exceptional, with efficacy between 80% and 95% following a single procedure performed under local anesthesia (Avella and Smith, 2012; Barnes, 2013; Koh et al., 2013; Nanos and Malanga, 2015; Patel, 2015; Morrey, 2017, 2018; Freed et al., 2019; Walrod et al., 2020). PUD of tendinopathic tissue in human patients has demonstrated the advantage of excising nonstructural debris, thereby allowing regenerative cells to populate the newly cleared space (Koh et al., 2013; Elattrache and Morrey, 2013; Barnes et al., 2015; Kamineni et al., 2015; Patel, 2015 ; Patel et al., 2015; Seng et al., 2016; Ostrom and Joseph, 2018; Freed et al., 2019). In addition, the collagen isomer profile present at the site of injury returns to normal following a single procedure, with no significant adverse effects on normal tissue (O’Daly et al., 2009; Kamineni et al., 2015). In fact, the technique has proven successful in some instances of failed surgical intervention (Nanos and Malanga, 2015). Kamineni et al. (2019) described the first case of successful PUD with stem cell therapy in a 2-year-old thoroughbred racehorse, who was unable to train due to recalcitrant desmopathy despite extensive conservative management. Following the PUD procedure, the horse demonstrated consistent race training and performance, winning two races with no re-injury for 3 years following surgery.
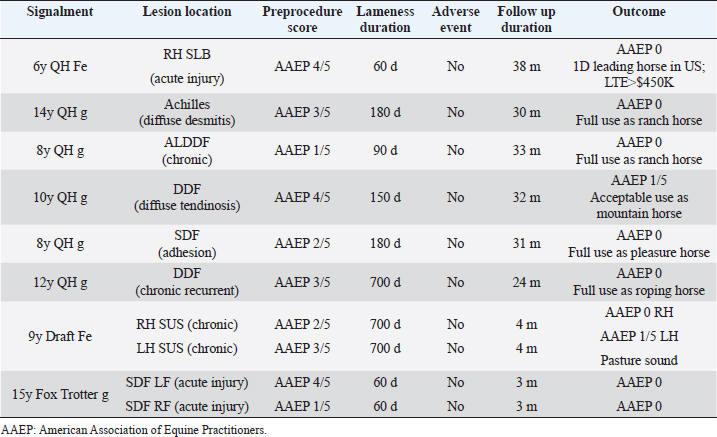
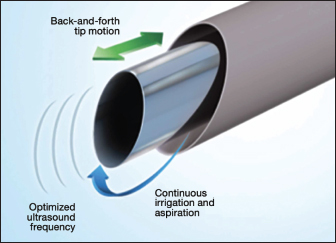
Fig. 2. Tenex Health TX® MicroTip®. Material and MethodsCase selectionEight horses with 10 affected limbs underwent the PUD procedure, ranging from 60 to 700 days postinjury (Table 2). Structures affected included suspensory ligament branch (SLB) (n=1), suspensory body (SUS) (n=2), Achilles tendon (n=1), accessory ligament of the deep digital flexor (DDF) tendon (ALDDF) (n=1), DDF (n=2), and SDF (n=3). All horses demonstrated lameness ranging from grade 1 to 4 [American Association of Equine Practitioners (AAEP)], with a mean pretreatment lameness grade of 2.7.
Fig. 3. Outer sleeve of ultrasonic probe irrigates and cools field. A hollow probe allows debris from the tenotomized tendon to be aspirated from the field. The treatment station consists of an energy module, a 500-ml bag of saline connected to a pump/aspiration cassette, and a sterile probe. The energy module has low, medium, and high settings; medium is typically used to manage tendinopathy itself, and high is used to remove calcification.
Fig. 4. Tenex Health TX® control console.
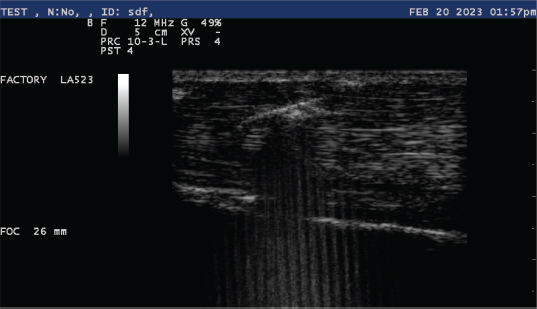
Fig. 5. Ultrasonic appearance of Tenex TX MicroTip® into a tendon.
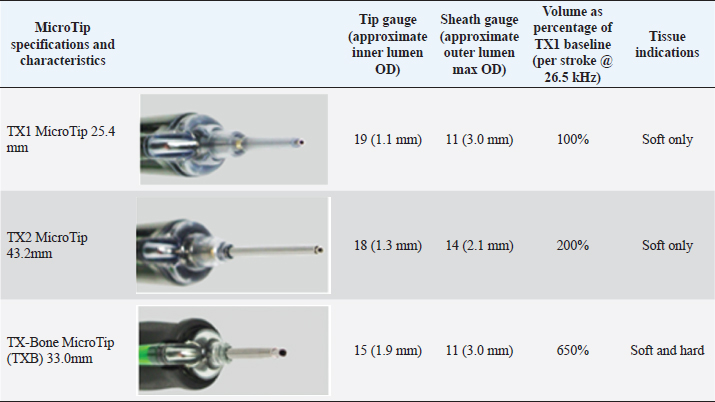
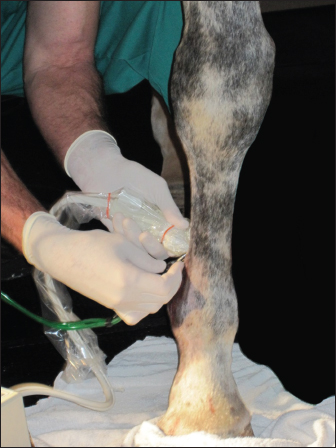
Fig. 6. Ultrasonic percutaneous tenotomy using the Tenex Health TX® System in a standing horse (photo courtesy Dr. W. R. Redding). EquipmentThe PUD procedure was performed using the Tenex Health TX® System, which employs ultrasonic energy at 26.5 kHz to debride the tendon or ligament of necrotic scar tissue and calcific tendon and bone, which is aspirated through the ensheathed micro tip (Figs. 2 and 3). Various energy settings on the Tenex control console may be used based on the type of tissue to be treated (Fig. 4). In general, a high setting was used to remove bone and calcific tendon tissue, and the medium setting was used to remove tendinopathic tissue. The low setting was not used in our study. The aspiration setting was selected to match the energy setting. The TX MicroTip® was similarly selected based on the target tissue (Table 1). The TX1 MicroTip® has a working length of 25.4 mm, the TX2 MicroTip® working length is 43.2 mm, and the TX-Bone MicroTip® (TXB) has a length of 33.0 mm. Both the TX1 and TX2 were used for tendinopathy. The TXB was selected for calcitrant tendinosis; it is also indicated for the removal of bone (enthuses and osteochondroma). The TX2 MicroTip® has an inner lumen gauge of 18 (outside diameter 1.3 mm) and a sheath gauge of 14 (2.1 mm). Sterile saline or lactated Ringer's solution is essential to provide continual cooling of the TX MicroTip® during cutting; a volume of approximately 500 ml was sufficient for most procedures. Anesthesia and patient preparationPUD with the Tenex Health TX ® system was performed in the standing sedated horse with regional anesthesia (n=2), or in the recumbent horse (n=6); determining factors included lesion location and patient temperament. The lesion to be treated was verified with high-frequency diagnostic ultrasound imaging (12 MHz). The patient and ultrasound probe were then aseptically prepared for the procedure.
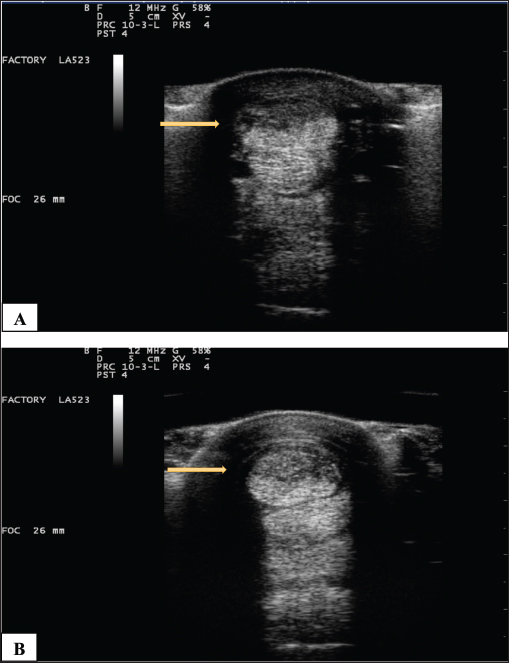
Fig. 7. (A) Pretreatment and B) 70-day post-Tenex® treatment of a severe SDF lesion (arrows). ProcedureOnce the location of the lesion was confirmed with ultrasound imaging, the footswitch was activated to prime the handpiece with sterile fluid, leaving the TX Micro Tip® cover in place. A 3–5 mm stab incision was made in the skin, and with the footswitch activated, the TX MicroTip® was introduced and directed into the pathologic tissue parallel to the normal fibers with ultrasound guidance (Fig. 5). When performed in the standing horse, it was desirable to have the treated limb de-weighted to facilitate microtip entry and aspiration of debris. The TX MicroTip® was advanced in a slow in-and-out motion while the tissue was debrided and aspirated (Fig. 6). In cases where lesion size exceeded the microtip length, separate stab incisions were made to facilitate microtip placement into the entire affected area. When the TX MicroTip® was placed directly against a structure, it would occasionally stop oscillating. When this occurred, simply backing out the microtip 1–2 mm allowed the ultrasonic debridement to resume. The microtip was activated in the tissue for 3–5 seconds, followed by a 3–5 seconds period with the footswitch off to prevent overheating of the microtip; this process was repeated until the debridement was complete. The duration of the procedure ranged from 1 to 5 minutes. Sutures were not required; a sterile dressing was applied over the stab incisions. Postoperative managementHorses received postoperative NSAIDS for the first 72 hours following the procedure and sterile bandages were maintained on the surgical sites for 2 weeks. All horses were confined to a stall for the first 2 weeks, with hand walking for 5 minutes once daily beginning 48 hours postoperative. Ultrasound examination was performed by the author at 4, 8, and 12 weeks following the procedure. Rehabilitation times varied depending on the extent and type of the initial lesion; in general, horses were confined to a small pen (approximately 30 × 30 ft) from 2 to 8 weeks following the procedure, followed by a small paddock turnout. Walking under the saddle resumed in all horses 12 weeks following the procedure. Ethical approvalThe author has adhered to the Principles of Veterinary Medical Ethics of the American Veterinary Medical Association. ResultsNo adverse events occurred in any case. Results were available from 6 to 41 months (mean, 23.2 months). Follow-up ultrasound imaging demonstrated improvement in fiber alignment and architectural change following treatment in all cases (Fig. 7). All horses had a reduction in lameness from the treated tendon or ligament (AAEP grade 0–1; mean grade 0.2); lameness completely resolved in 8 of 10 treated limbs. All horses returned to their previous level of use. No horses in this study developed a recurrence of their original lesion. DiscussionPrevious treatment modalities for tendinopathy and desmopathy have been directed at a) evacuating a hematoma in the acutely injured structure, b) functional lengthening of the muscle-tendon unit (SDFT accessory ligament desmotomy), c) decreasing pain (neurectomy and fasciotomy), d) methods to increase circulation, and e) the instillation of autologous blood products or biologicals directly into a lesion (Hogan and Bramlage, 1995; Richardson et al., 2007; Tnibar, 2007; White and Hewes, 2008; Abate et al., 2009; Avella and Smith, 2012; Clegg, 2012; Dyson and Murray, 2012; Dakin et al., 2014; Hu and Bramlage, 2014; Plevin and McLellan, 2014; Ribitsch et al., 2021). No previous modality has been targeted, nor effective, at physically removing tendinopathic scar tissue. PUD of tendinopathic tissue using the Tenex Health TX® System uses ultrasonic energy to effectively debride and aspirate the pathologic tissue, allowing populations of healthy collagen to fill in the voided tissue space, and do so at normal collagen ratios (Kamineni et al., 2015). By operating in a closed fluid system, normal tissue is not affected by the procedure (Morrey, 2014; Kamineni et al., 2015; Morrey, 2018). As demonstrated in humans, the procedure is safe and well-tolerated (O’Daly et al., 2009; Morrey, 2018; Walrod et al., 2020). Horses in this study demonstrated improvement with no adverse events, and complete resolution of lameness in 8 of 10 treated limbs following a single treatment. The procedure may be performed in almost any tendon or ligament, and the ease of operation in the standing or recumbent horse makes the process suitable for a variety of clinical or ambulatory settings. The removal of diseased tissue with PUD resulted in a return to function and without recurrent tendinopathy or desmopathy in treated horses. The limitations of this report included the following: a limited number of cases, various lesions and signalments, and no control group, statistical analysis, or blinded observers. ConclusionPUD and aspiration of diseased tendon and ligament tissue using the Tenex Health TX® System is a safe, effective, and well-tolerated procedure. In this preliminary study, PUD using the Tenex Health TX® System was safely applied to eight horses and all horses showed improvement. A controlled (clinical) study is indicated to determine the effect of this novel treatment of equine tendinopathy and desmopathy. AcknowledgmentDr. Vlahos performed the PUD procedure in all cases and wrote the article. The author thanks Dr. Bernard Morrey, MD for reviewing the manuscript. Conflict of interestThe author declares that his spouse is a distributor for Tenex Health. FundingThis research did not receive any form of funding from any entity. Data availabilityThe data generated are available from the author upon reasonable request. ReferencesAbate, M., Gravare-Silbernaglek, K., Siljeholm, C., Dilorio, A., DeAmicis, D., Salini, V., Werner, S. and Paganelli, R. 2009. Pathogenesis of teninopathies: inflammation or degeneration? Arthritis Res Ther. 11(3), 235. Avella, C. and Smith, R. 2012. Diagnosis and management of tendon and ligament disorders. In Equine surgery, 4th ed. Eds., Auer, J.A. and Stick, J.A. St. Louis, MO: Elsevier, pp: 1157–1179. Barnes, D. 2013. Ultrasonic energy in tendon treatment. Oper. Tech. Orthop. 23, 78–83. Barnes, D., Beckley, J.M. and Smith. J. 2015. Percutaneous ultrasonic tenotomy for chronic elbow tendinosis: a prospective study. J. Shoulder Elbow Surg. 24, 67–73. Clegg, P.D. 2012. Musculoskeletal disease and injury, now and in the future. Part 2: Tendon and ligament injuries. Equine Vet. J. 44, 371–375. Dakin, S.G. 2016. A review of the healing process in equine superficial digital flexor tendon tendinopathy. Equine Vet. Educ. 29, 516–520. Dakin, S.G., Dudhia, J. and Smith, R.K.W. 2014. Resolving an inflammatory concept: the importance of inflammation and resolution in tendinopathy. Vet. Immunol. Immunopathol. 15, 121–127. Dowling, B., Dart, A., Hodgson, D. and Smith R.K.W. 2000. Superficial digital flexor tendonitis in the horse. Equine Vet. J. 32, 369–378. Dyson, S.J. 2004. Medical management of superficial digital flexor tendonitis: a comparative study in 219 horses (1992-2000). Equine Vet. J. 36, 415–419. Dyson, S. and Murray, R. 2012. Management of hindlimb proximal suspensory desmopathy by neurectomy of the deep branch of the lateral plantar nerve and plantar fasciotomy: 155 horses (2003-2008). Equine Vet. J. 44, 361–367. Elattrache, N. and Morrey, B. 2013. Percutaneous ultrasonic tenotomy as a treatment for chronic patellar tendinopathy-jumper’s knee. Oper. Tech. Orthopedics 23(2), 98–103. Ely, E.R., Avella, C.S., Price, J.S., Smith, R.K., Wood, J.L. and Verheyen, K.L. 2009. Descriptive epidemiology of fracture, tendon and suspensory ligament injuries in national hunt racehorses in training. Equine Vet. J. 41, 372–378. Freed, L., Ellis, M.B., Johnson, K. and Haddon, T. 2019. Fasciotomy and surgical tenotomy for chronic achilles insertional tendinopathy: a retrospective study using ultrasound-guided percutaneous microresection. JAPMA 109(1), 1–8. Hogan, P.M. and Bramlage, L.R. 1995. Transection of the accessory ligament of the superficial digital flexor tendon for treatment of tendinitis: long term results in 61 standardbred racehorses (1985-1992). Equine Vet. J. 27(3), 221–226. Hu, A.J. and Bramlage, L.R. 2014. Racing performance of Thoroughbreds with superficial digital flexor tendonitis treated with desmotomy of the accessory ligament of the superficial digital flexor tendon: 332 cases (1989-2003). J. Am. Vet. Med. Assoc. 244(12), 1441–1448. Kamineni, S., Butterfield, T. and Sinai, A. 2015. Percutaneous ultrasonic debridement of tendinopathy—a pilot Achilles rabbit model. J. Orth. Surg. Res. 10, 70. Kamineni, S., Ruggles, A. and Hamza, A. 2019. Ultrasonic debridement with stem cell therapy of suspensory branch desmitis in an equine patient. Open Vet. J. 9(1), 54–57. Kashashima, Y., Takahashi, T., Smith, R.K.W., Goodship, A.E., Kuwano, A., Ueno, T. and Hirano, S. 2004. Prevalence of superficial digital flexor tendonitis and suspensory desmitis in Japanese Thoroughbred flat racehorses in 1999. Equine Vet. J. 36, 346–350. Kelman, C.D. 1994. The history and development of phacoemulsification. Int. Ophthalmol. Clin. 34(2), 1–12. Koh, J.S., Mohan, P.C., Howe, T.S., Lee, B.P., Chia, S.L., Yang, Z. and Morrey, B.F. 2013. Fasciotomy and surgical tenotomy for recalcitrant lateral elbow tendinopathy; early clinical experience with a novel device for minimally invasive percutaneous microresection. Am. J. Sports Med. 41, 636–644. Morrey, B.F. 2017. Ultrasonic percutaneous tenotomy for chronic refractory tendinopathy. In Morrey’s the elbow and its disorders, 5th ed. Eds., Morrey, B.F., Sanchez-Sotelo, J. and Morrey, M.E. St. Louis, MO: Elsevier, pp: 582–587. Morrey, B.F. 2018. A new safe and effective treatment for chronic refractory tendinopathy. Orthop. Rheum. Open Access J. 11(5), 1–5. Morrey, B.F. 2014. Percutaneous ultrasound tenotomy for epicondylitis. In Master’s techniques in orthopedic surgery: the elbow, 3rd ed. Ed., Morrey, B.F. Philadelphia, PA: Wolters Kluwer Health, pp: 339–346. Nanos, K.N. and Malanga, G.A. 2015. Treatment of patellar tendinopathy refractory to surgical management using percutaneous ultrasonic tenotomy and platelet rich plasma: a case presentation. PM R 7(12), 1300–1305. O’Brien, C., Marr, N. and Thorpe, C. 2021. Microdamage in the equine superficial digital flexor tendon. Equine Vet. J. 53, 417–430. O’Daly, B.J., Morris, E., Gavin, G.P., O’Byrne, J. and McGuinness, G.B. 2009. High-power low-frequency ultrasound: a review of tissue dissection and ablation in medicine and surgery. J. Mater. Process. Technol. 200(1–3), 38–58. Ostrom, E. and Joseph, A. 2018. Percutaneous ultrasound tenotomy using the Tenex System on the adductor longus tendon: a pilot case series. MOJ Sports Med. 2(1), 00035. Patel, M.M. 2015. A novel treatment for refractory plantar fasciitis. Am. J. Orthop. 44, 107–110. Patel, M., Patel, S.M., Patel, S. and Daynes, J. 2015. A pilot study of a novel treatment method for refractory painful plantar fibromas. Austin. J. Orthopaed. Rheumatol. 2(2), 1014. Plevin, S. and McLellan, J. 2014. The effect of insertional suspensory branch desmitis on racing performance in juvenile thoroughbred racehorses. Equine Vet. J. 46(4), 451–457. Ribitsch, I., Oreff, G.L. and Jenner, F. 2021. Regenerative medicine for equine musculoskeletal diseases. Animals 11(1), 234. Richardson, L.E., Dudhia, J., Clegg, P.D. and Smith, R. 2007. Stem cells in veterinary medicine-attempts at regenerating equine tendon after injury. Trends Biotechnol. 25, 409–416. Seng, C., Mohan, P.C., Koh, S.B., Howe, T.S., Lim, Y., Lee, P. and Morrey, B. 2016. Ultrasonic percutaneous tenotomy for recalcitrant lateral elbow tendinopathy: Sustainability and sonographic progression at 3 years. Am. J. Sports Med. 44(2), 504–510. Thorpe, C.T., Clegg, P.D. and Birch, H.L. 2010. A review of tendon injury: why is the equine superficial digital flexor tendon most at risk? Equine Vet. J. 42, 174–180. Tnibar, A. 2007. Ultrasound-aided tendon and ligament surgery in the horse. Equine Vet. Ed. 19(8), 435–443. Walrod, B., Turner, W. and Pauls, K. 2020. Rapid and successful rehabilitation and return to play for a D1 gymnast after treatment of lateral epicondylitis with ultrasound guided percutaneous tenotomy (Tenex). Am. J. Sports Sci. 5(1), 1–4. White, N.A. and Hewes, C.A. 2008. Treatment of suspensory ligament desmopathy. Proc. Am. Assoc. Equine Pract. 54, 502–507. | ||
| How to Cite this Article |
| Pubmed Style Ted Phillip Vlahos. Percutaneous ultrasonic debridement of equine tendinopathy and desmopathy: A report of 10 cases. Open Vet J. 2023; 13(9): 1141-1149. doi:10.5455/OVJ.2023.v13.i9.10 Web Style Ted Phillip Vlahos. Percutaneous ultrasonic debridement of equine tendinopathy and desmopathy: A report of 10 cases. https://www.openveterinaryjournal.com/?mno=154748 [Access: May 29, 2024]. doi:10.5455/OVJ.2023.v13.i9.10 AMA (American Medical Association) Style Ted Phillip Vlahos. Percutaneous ultrasonic debridement of equine tendinopathy and desmopathy: A report of 10 cases. Open Vet J. 2023; 13(9): 1141-1149. doi:10.5455/OVJ.2023.v13.i9.10 Vancouver/ICMJE Style Ted Phillip Vlahos. Percutaneous ultrasonic debridement of equine tendinopathy and desmopathy: A report of 10 cases. Open Vet J. (2023), [cited May 29, 2024]; 13(9): 1141-1149. doi:10.5455/OVJ.2023.v13.i9.10 Harvard Style Ted Phillip Vlahos (2023) Percutaneous ultrasonic debridement of equine tendinopathy and desmopathy: A report of 10 cases. Open Vet J, 13 (9), 1141-1149. doi:10.5455/OVJ.2023.v13.i9.10 Turabian Style Ted Phillip Vlahos. 2023. Percutaneous ultrasonic debridement of equine tendinopathy and desmopathy: A report of 10 cases. Open Veterinary Journal, 13 (9), 1141-1149. doi:10.5455/OVJ.2023.v13.i9.10 Chicago Style Ted Phillip Vlahos. "Percutaneous ultrasonic debridement of equine tendinopathy and desmopathy: A report of 10 cases." Open Veterinary Journal 13 (2023), 1141-1149. doi:10.5455/OVJ.2023.v13.i9.10 MLA (The Modern Language Association) Style Ted Phillip Vlahos. "Percutaneous ultrasonic debridement of equine tendinopathy and desmopathy: A report of 10 cases." Open Veterinary Journal 13.9 (2023), 1141-1149. Print. doi:10.5455/OVJ.2023.v13.i9.10 APA (American Psychological Association) Style Ted Phillip Vlahos (2023) Percutaneous ultrasonic debridement of equine tendinopathy and desmopathy: A report of 10 cases. Open Veterinary Journal, 13 (9), 1141-1149. doi:10.5455/OVJ.2023.v13.i9.10 |