| Research Article | ||
Open Vet J. 2023; 13(9): 1175-1183 Open Veterinary Journal, (2023), Vol. 13(9): 1175–1183 Original Research Comparative evaluation of some techniques used for the detection of rabies virusDalia N. Hegazy1*, Ebtesam N. Hosseny1, Amal S. M. Abo-Senna1, Zeinab T. Salem2 and Mohamed H. Khodeir21Faculty of Science, Al-Azhar University Girl’s Branch, Nasr, Egypt 2Department of Pet Animal Vaccine Research, Veterinary Serum and Vaccine Research Institute, Cairo, Egypt *Corresponding Author: Dalia N. Hegazy. Faculty of Science, Al-Azhar University Girl’s Branch, Nasr, Egypt. Email: Dalianasr94 [at] yahoo.com Submitted: 01/07/2023 Accepted: 27/08/2023 Published: 30/09/2023 © 2023 Open Veterinary Journal
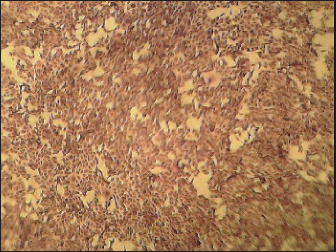
AbstractBackground: Neurotropic viruses in the family Rhabdoviridae, genus Lyssavirus, are what cause rabies, an acute, progressive, and highly lethal encephalomyelitis. Aim: Evaluation of the used diagnostic techniques to determine the most simple; rapid and accurate test for rabies virus (RABV) recognition in different specimens aiming to reach a rapid diagnosis as a step aid in the disease control and to prevent or even minimize the suspected hazard. Method: The used techniques included an infection trial of Swiss mice with the mice-adapted challenge rabies virus followed by the detection of the virus in the infected mices’ brains. Virus detection was carried out through the application BHK21 cell line infection; fluorescent antibody technique; latex agglutination test (LAT); direct enzyme-linked immunosorbent assay (ELISA); rabies antigen detection kit ELISA; conventional polymerase chain reaction (PCR). Results: It was found that virus inoculation in mice and BHK21 cell lines needs 5–7 days with positivity of 90% and 100%, respectively. Rapid antigen kit was able to detect rabies antigen in mice brains suspension and BHK21 infected fluid within 3–5 minutes with percentages of 60% and 55.5%, respectively. In 1–1.5 hours, the direct fluorescent antibody method (DFAT) detected 90% and 100% of the rabies antigen in BHK21 cell line infection and brain impressions, respectively. Latex agglutination showed clear results with 88.8% with BHK21 infected fluid within 3–5 minutes while it did not carry out on brain emulsions to prevent falsely positive results brought on by the presence of tissue fragments. Conventional one-step PCR revealed 100% positivity with either brain or cell culture preparations within 2 days. Direct ELISA showed 88.8% positivity with BHK21 infected fluid with 1 day of work. Conclusion: Mice inoculation test, cell culture infection; DFAT and PCR are the most accurate techniques for the detection of RABV with a positivity of 90%–100% followed by LAT and ELISA with a positivity of 88.8%, and lastly, rabies antigen ELISA kit (RAK) with a positivity of 55.5%–60% taking in consideration the required time for each. In addition, the positivity % of the applied tests revealed their sensitivity and specificity. Keywords: Rabies virus, Mice inoculation, Latex agglutination, Fluorescent antibody technique, PCR. IntroductionLyssavirus is a member of the Rhabdoviridae family and is responsible for the neurotropic viruses that cause rabies, an acute progressive, deadly encephalomyelitis. All lyssaviruses, which are prevalent across the world, cause rabies (Rupprecht et al., 2009). Rabies virus (RABV) is a type of RNA virus that belongs to the Rhabdoviridae family and the Lyssavirus genus of the Mononegavirales order (Calisher and Ellison, 2012). The virus particles are bullet-shaped and have a diameter of 75 nm and a total length of 200 nm (Williamson, 2010). The five structural proteins that make up the RABV genome are the nucleocapsid (N) protein (having great importance in infection and diagnosis), a phosphoprotein (P), matrix (M) protein, glycoprotein (G), and RNA-dependent RNA polymerase (L), which is composed of a nonsegmented, single negative strand molecule of RNA of roughly 12 kb (Craig Greene, 2012). The illnesses brought on by several virus species cannot be distinguished clinically (Scheffer et al., 2014). It is possible to identify rabies in animals using any part of the affected brain. However, the test must incorporate tissue from at least two different parts of the brain, specifically the cerebellum and brain stem, to rule out rabies (Swanepoel et al., 1993). Numerous methods, such as direct fluorescent antibody, inoculation of mice, infection of tissue cultures, and polymerase chain reaction (PCR), can be used to identify animal rabies. All of these techniques have the WHO’s approval (Bourhy et al., 2005). Only laboratory studies on central nervous system tissue extracted from the skull, ideally performed postmortem, can confirm a diagnosis (McElhinney et al., 2008). By agglutination on a glass plate with latex particles loaded in gamma globulin, the antigen of the RABV can be recognized. An examination of matched saliva-brain samples from 238 dogs revealed that the LA test (it is fixed) using saliva was 99% specific and 95% susceptible when compared to the FAT on smears of the brain. The LA examination has a few advantages over the traditional FAT, including simplicity and the lack of the requirement to slaughter the animal first (Kasempimolporn et al., 2000). The fluorescent antibody test (FAT) on brain tissue is considered a “gold standard” method in rabies. The mice inoculation testing (MIT), which has been employed as a means of improving quality procedures for the FAT in the past, has critical defects. The sensitivity of the FAT, when performed in a lab by trained personnel, is 99.78%. However, the FAT needs pricy chemicals and equipment, skilled workers, and necropsy supplies. Once the decomposition of brain specimens begins, the test’s sensitivity is significantly decreased. A diagnosis of rabies in circumstances when a human is suspected before death can be made using a variety of methods. It is possible to isolate the virus from patients’, Corneal fluid tears, saliva, and urine by inoculating mice, but this procedure remains unreliable and takes not less than a week. A positive FAT test demonstrating the presence of rabies viral antigen in salivary, corneal, or nuchal skin biopsy samples acts as a diagnostic (Bryceson et al., 1975). Compared to brain biopsy, these methods are less invasive. The most sensitive test, excluding brain biopsies, is still immunohistochemical analysis of a skin biopsy sample, but the results are dependent on the disease stage during the biopsy (Blenden et al., 1986). Late-stage biopsy yields better outcomes. More delicate, but demanding and costly technologies PCR, for example, has produced good outcomes for determining the presence of the antigen of viruses in cerebrospinal fluid (CSF) (Servat et al., 2007), brain, and saliva specimens (Crepin et al., 1998). To recognize RABV antigens (rVNAs) in the salivary glands and brain, enzyme immunoassays and dot hybridization have been developed (Ermine et al., 1988). As of yet, no test has been utilized to substitute MIT and FAT for routine diagnosis. It is due to both technological and logistical concerns. The methods indicated above showed no substantial except for PCR and benefit over FAT, which were unable to alleviate the problem caused by decomposing specimens. PCR was more sensitive in detecting the presence of many notifiable diseases but was too costly making it unsuitable for routine diagnosis in developing nations. Enzyme-linked immunosorbent assay (ELISA) was discovered to be easy, fast, standardized, and reliable for routine hospital diagnosis, and it may be beneficial for huge-scale epidemiological research of cryptosporidiosis (El-Settawy and Fathy, 2012). A comparison of the accuracy and specificity of several tests found that “serum ELISA” and “milk ELISA” were excellent screening tests, and “milk PCR” was a “confirmatory test” for MAP infection. A combination of milk ELISA and milk PCR may be utilized to screen for and diagnose JD in lactating/dairy herds of cattle under Indian conditions (Garg et al., 2015). In the US, the rapid fluorescence focus inhibition test (RFFIT) is frequently used to assess rVNA, or antibodies that neutralize the RABV. Results from RFFIT have consistently been repeatable and trustworthy. Although the test has been modified over time to use a smaller amount of reagents and samples, a 50 μl minimum volume of test serum is required. Small experimental animals, such as mice, are routinely tested for rVNA to conduct pathogenesis studies, even though the lowest volume for a traditional RFFIT may be difficult to acquire, especially in repetitive sample circumstances. Previously, a micro-neutralization test was performed devised to solve this issue. The micro-neutralization test was compared to the RFFIT in the current investigation, this employed 129 mice serum specimens from rabid vaccine research. The micro-neutralization test had 100% specificity, 97.5% sensitivity, and 98% concordance with a cut-off value of 0.1 IU/ml. All samples over the cut-off had a geometric mean titer of 2.0 IU/ml using RFFIT and 3.4 IU/ml using the micro-neutralization test, demonstrating that RFFIT titers are not similar to micro-neutralization titers. Based on four rVNA-positive hamster serum samples, the intra-assay coefficient of variability was 24%, and the inter-assay coefficient of variability was 30.4%. These findings support the use of the micro-neutralization test to identify RABV-neutralizing antibodies (Smith and Gilbert, 2017). In comparison to ELISA, IFAT was more sensitive (75 vs. 70%) for the identification of subclinical infection, although ELISA was more accurate (98 vs. 97%) for the diagnosis of clinical leishmaniosis (Persichetti et al., 2017). The basic goal of the present investigation was to identify the most reliable, straightforward, and quick test for detecting the RABV in various samples to achieve a speedy diagnosis as a step aid in disease control. Materials and MethodsRabies virusThe mice’s brain was infected with the challenge rabies virus strain (CVS). It is used to infect mice in experiments and has a titer of 103.5 MLD50/mice (minimum lethal dose). The Department of Pet Animal Vaccine Research (DPAVR), Veterinary Serum and Vaccine Research Institute (VSVRI), Abasia Cairo, contributed to this virus. AntiseraDPAVR and VSVRI provided the anti-rabies serum that was unconjugated and conjugated with fluorescein isothiocyanate (FITC) and implemented in serological assays to find the RABV. Cell cultureVSVRI provided the baby hamster kidney cell line (BHK21), which was employed in viral neutralization tests and RABV detection using the direct fluorescent antibody technique (FAT). MiceTwenty weaned, 3–4-week-old Albino Swiss mice were obtained by the DPAVR and VSVRI and utilized for RABV infection investigations. Used primerTable 1 lists the oligonucleotide primers available from Metabion Germany. Latex beads polystyreneIt is an aqueous suspension, solids content 10%, lot 17H0803 supplied by Sigma Chemical Company. Experimental infection of miceThere were 20 mice total, divided into 2 groups of 10. The intramuscular MIT was carried out as before described (Abdulazeez et al., 2020). Group-1 was inoculated intramuscularly in the hind limb with 0.03 ml of 10% suspension of CVS (approximately 1 × 107 infectious particles of RABV). Group-2 was kept without infection as test control. Monitoring clinical symptoms and death allowed for the evaluation of illness progression. On days 5, 7, 9, 11, and 13 after infection, 3 mice were selected at random from each group and sacrificed. Mice brain tissues were collected from all animals and phosphate buffer was kept at 20°C before being utilized for enzyme assays, RNA/DNA extractions, and FAT. Direct fluorescent antibody test (DFAT)The rabies antigen was detected using the direct fluorescent antibody test (DFAT), which has previously been published (Ehizibolo et al., 2013). Brain tissues were extracted, and preparations for impression smears were created. A fluorescent isothiocyanate-labeled anti-RABV was used to construct the test sample, as well as the positive and negative control slides (supplied by the DPAVR). A fluorescence microscope (Zeiss, Axioskop, Germany) with a magnification of 20× was used to examine the slides for the presence of rabies antigen. Rapid rabies Ag test kitIt was supplied by Bio Note, Inc., Gyeonggi-do, Korea, and used for the detection of rabies antigen in brain samples of experimentally infected mice and BHK-21 cell line. Latex testTo find the RABV or its antibodies, it was performed by the manufacturer’s instructions. Viral RNA extractionUsing a QIAamp® Viral RNA Kit, total RNA isolated from the RABV (ERA strain) as a positive control and negative control for BHK-21. The extracted RNA was stored at −80°C until usage after being eluted in 60 l of the AVE kit elution buffer. Identification of RABVnucleic acid using conventional RT-PCRThe manufacturer’s recommendations were followed to identify the RABV using conventional one-step RT-PCR. Using a particular primer, RT-PCR was performed to amplify the viral VP1-RNA (N gene), as shown in Table 1. The procedure was carried out in a reaction with a volume of 50 l, which contained 10 l of RNA template, 4 μl of gene-specific primers at a concentration of 1 M, 2 μl of dNTP (mix), 2 μl of enzyme mixture, 10 μl of 5× RT buffer, and RNase-free water. The following cycling program, consisting of 40 cycles of 94°C for 15 seconds and 60°C for 55 seconds, was used to complete up to 50 μl. Ethical approvalThe National Research Centre in Egypt’s Medical and Veterinary Research Ethics Committee gave its approval (No. 20/053) for the treatment and usage of the animals. ResultsMice infected with rabies CVS in an experiment revealed that 90% (9 out of 10) mice experimentally infected with the CVS of rabies showed rabies signs by the fourth day characterized by struggling gait, arched back; erected tail, off food followed by paralysis of the tail and rear limbs and death on the sixth to seventh day postinfection. These findings were obtained within 5 to 10 days. Application of the slid rapid rabies antigen detection test confirmed the presence of RABV in 60% (6 out of 10) brain suspensions prepared from dead infected mice showing a clear positive line up to brain suspension dilution 1:100 while the negative sample did not (Fig. 1). Testing of infected cell culture fluids showed fewer positive results (55.5%=5/9) with the same dilutions within 3–5 minutes. Direct fluorescent antibody technique (DFAT) carried out on brain impression smears from dead mice showed strong positive results represented by apple green spots confirming the presence of rabies (Fig. 2) with 90% positivity (9 out of 10 brain smears diluted up to 1:10,000 within 1–1.5 hours. A negative brain smear showed a negative reaction (Fig. 3) as a dark background. Application of DFAT on BHK-21 cell line inoculated with brain suspensions of infected mice showed intracytoplasmic apple green reactions in nine out of nine (100%) (Fig. 4), while normal cell culture did not show any fluorescent reaction (Fig. 5). This test needs 3–5 days to infect the cells and their preparation for DFAT. Table 1. Primers sequences, target genes, and amplicon sizes.
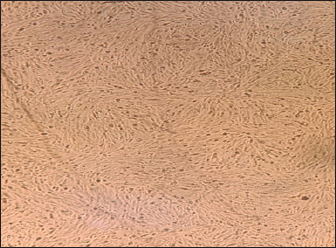
Noninfected BHK21 cell line (Fig. 6) showed normal cell shape and normal growth behavior while the RABV (obtained from infected mice brain) cytopathic effect (CPE) in mice demonstrated BHK21 cell line inoculated with the prepared brain suspension of nine infected showed specific CPE of RABV by the second day postinfection distinguished by rounding of cells followed by lysis in the cell and separation from the culture surface (Fig. 7) within 4–7 days postcell infection.
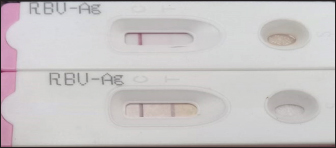
Fig. 1. A-Negative test. B-Positive test clear line.
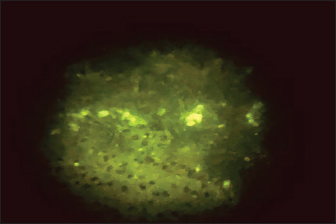
Fig. 2. Positive DFAT carried out on brain impression of experimentally infected dead infected mice showing apple green spots.
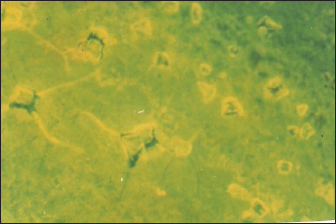
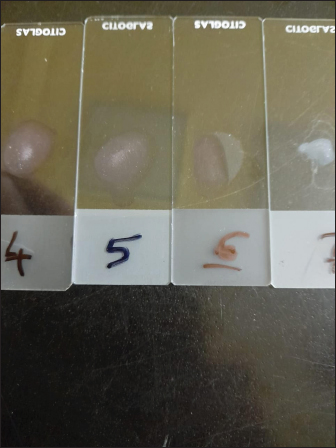
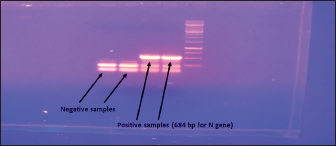
Fig. 3. Negative DFAT carried out on brain impression of healthy mice. Latex agglutination test (LAT) was able to detect RABV in BHK-21 infected virus harvest showing clear agglutinations within 3–5 minutes up to a dilution 1:1,000 (Fig. 8). Unclear agglutinations were noticed with higher antigen dilutions. Positive results were obtained in eight samples out of nine cell culture-infected fluids. Conventional RT-PCR indicated the presence of RABV in one chosen brain sample from dead experimentally infected mice and one BHK-21 cell line infected fluids showing positive bands at 684 bp for the N gene (Fig. 9). This technique is specific, sensitive and needs 2 days. RT-PCR is the most commonly used technology for detecting RABV RNA in people for intravital diagnosis of rabies.
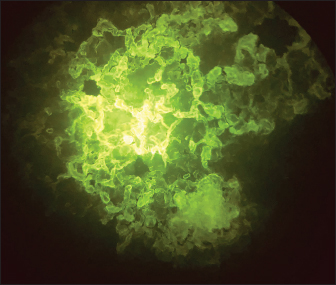
Fig. 4. Positive DFAT on RABV-infected BHK-21 cell line showing intracytoplasmic apple green reaction.
Fig. 5. Negative DFAT applied on normal BHK-21 cell line.
Fig. 6. Normal BHK-21 cell line.
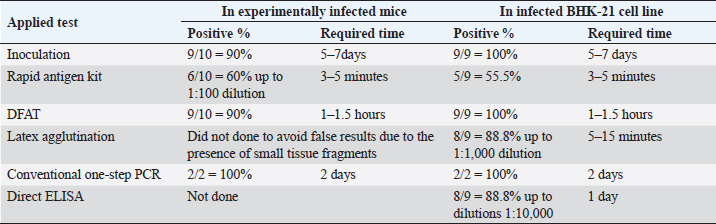
Fig. 7. RABV infected BHK-21 cell line showing cell rounding. 7-Direct ELISA applied on 9 tissue culture-infected fluids showed that 8 samples out of 9 (88.8%) were positive up to dilutions of 1:10,000. Table 2 summarizes the results of RABV detection in mice and BHK-21 cell culture, indicating that virus inoculation in mice and BHK21 cell lines need 5–7 days with positivity of 90% and 100%, respectively. Rapid antigen kit was able to detect rabies antigen in mice brains suspension and BHK21 infected fluid with 3–5 minutes with a percentage of 60 and 55.5, respectively. DFAT detected rabies antigen in brain impressions and infected BHK21 cell lines with 90% and 100%, respectively, within 1–1.5 hours. Latex agglutination showed clear results with 88.8% with BHK21 infected fluid within 3–5 minutes while it did not carry out on brain emulsions to avoid false positive results due to the presence of tissue fragments. Conventional one-step PCR revealed 100% positivity with either brain or cell culture preparations within 2 days. Direct ELISA showed 88.8% positivity with BHK21 infected fluid with 1 day of work.
Fig. 8. 4 and 5=clear agglutination; 6=unclear agglutination; 7=negative agglutination.
Fig. 9. Conventional one-step Rt-PCR. DiscussionThe zoonotic viral disease rabies, which is primarily spread by dog bites, affects humans. Lack of proper laboratory facilities and/or challenges in submitting samples from remote places to laboratories make it difficult to diagnose diseases. Simple testing may improve surveillance and control efforts. The main goal of this research is to determine the RABV incidence test that is the most precise, sensitive, specific, and quick. Table 2. Detection of RABV.
90% (9 out of 10) of the mice that were experimentally infected with the CVS of rabies in the present research displayed rabies symptoms, which is completely consistent with the findings of (Albehwar, 2009; OIE 2012), who reported that experimentally infected mice with rabies experienced hind limb paralysis by the fourth day and died by the sixth day while documenting their findings for 6 to 10 days. Slid rapid rabies antigen detection confirmed 60% positive results in brain samples and 55.5% in infected cell culture fluids. In this regard, antigen rapid rabies Ag test kit has been shown to identify rabies viral antigen in canine, bovine, and raccoon dog saliva and brain homogenates (Manufactured by BioNote, Inc. 2-9 Seogu-dong, Hwaseong-si, Gyeonggi-do, Korea 445-170 Tel.: +82 31 211 0516, Fax.: +82 31 8003 0618 http://www.bionote.co.kr). Also, it was suggested it is suitable for confirming clinical rabies cases to enable quick responses and improve rabies surveillance (Tenzin et al., 2020). The FITC-labeled antibodies to RABV are employed in the direct fluorescent antibody test, which is the gold standard for routine sensitive and specific lyssavirus postmortem identification (Mayes and Rupprecht, 2015). The FA test is also the most popular way to diagnose rabies infection in both humans and animals since it is reliable and results are frequently available within 30 minutes of receiving the specimen (WHO, 2018). In this study, the DFAT carried out on brain impressions of dead experimentally infected mice and infected BHK21 cell lines showed strong positive results with 90% and 100%, respectively, within 1–1.5 hours. Infection of the BHK21 cell line showed 100% positivity of infected mice brain suspension resulting in specific CPE of RABV by the second day postinfection as cell rounding followed by cell lysis and detachment of the cell sheet. These results match what was described by Consales et al. (1990), Knezevic (2008), and Hassanzadeh et al. (2011), who found that RABV could be harvested from BHK cell culture with similar findings at around 48 hours of infection. Regarding LAT 88.8% positivity was recorded with infected BHK-21 fluid showing clear agglutinations within 3–5 minutes. In this respect, LAT was cited as being uncomplicated for carrying out and requiring the least amount of laboratory equipment (Perrin et al., 1988; Madhusudana and Saraswati, 2003). In addition, it has been proven that the simple LAT has the benefits of being quick, straightforward to perform, simple to interpret, and useful as an on-site diagnostic technique for estimating rabies antibodies in horses (Saengseesom et al., 2010; Kasempimolporn et al., 2014). Saliva samples and brain samples acquired using various ways can also be analyzed by this technology, agreed with the results of conventional PCR, which reached 100% sensitivity (David et al., 2002; Rojas Anaya et al., 2006; Dacheux et al., 2008). Direct ELISA applied on nine tissue culture-infected fluids showed that eight samples out of nine (88.8%) were positive. According to the study, monoclonal or polyclonal antibodies can be used in samples to identify RABV antigens using ELISA (Perrin and Sureau, 1987; Saxena et al., 1989). It is sensitive and specific (Mani and Madhusudana, 2013). PCR, on the other hand, was more sensitive in detecting the presence of cryptosporidium but was prohibitively expensive, making it unsuitable for routine diagnosis in developing countries (El-Settawy and Fathy, 2012). ELISA is a simple, quick, reliable, and standardized method for routine diagnosis, particularly in hospitals, and it may be beneficial in large-scale epidemiological studies of cryptosporidiosis. Combining ELISA with PCR as a standard screening and disease diagnosis method could be employed (Garg et al., 2015). Comparing the current carried out laboratory techniques; the above obtained data could suggest to be agree with other findings revealing that PCR is more expensive and takes more time than ELISA (Servat et al., 2007). The LA test has certain advantages over the traditional FAT since it is less complicated (Kasempimolporn et al., 2000). The fluorescent-antibody test (FAT) on brain tissue is considered the “gold standard” for rabies diagnosis. Although it has significant shortcomings, the inoculation test of mice (MIT) has historically been used as a quality monitoring tool for the FAT. When performed in a lab by skilled personnel, the FAT has a sensitivity of 99.78%. However, the FAT needs high-end materials, tools, skilled technicians, and necropsy supplies. For identifying rabies viral antigen in the brain (Servat et al., 2007), CSF, and saliva specimens, more sensitive but more expensive approaches, such as PCR, have yielded satisfactory (Crepin et al., 1998). There is currently no test that has been utilized in place of FAT for standard diagnosis. This is due to technical and logistical concerns. Except for PCR, none of the technologies listed above significantly outperformed FAT and were unable to address the issue brought on by degraded specimens. ConclusionAccording to our findings, the most reliable methods for detecting the RABV are the MIT, cell culture infection, DFAT, and PCR, with positivity rates of 90%–100%, followed by LAT and ELISA with a positivity rate of 88.8%, and RAK with a positivity rate of 55.5%–60%, taking into account the time needed for each. The positivity percentage of the used tests also demonstrated their sensitivity and specificity. AcknowledgmentThe authors would like to express their gratitude to the staff of the DPAVR; VSVRI, Abasia, Cairo, for their assistance. Conflict of interestThe authors disclose that they have no competing interests. FundingThere was no external funding for the study. Data availabilityThis published article contains all of the data gathered and analyzed throughout the experiment. Authors’ contributionsAll authors contributed to administrative support, study material provision, and final manuscript approval, as well as data collection and assembly, data analysis and interpretation, and paper writing. The final manuscript was read and approved by all authors. ReferencesAbdulazeez, M., Kia, G.S.N., Abarshi, M.M., Muhammad, A., Ojedapo, C.E., Atawodi, J.C., Dantong, D. and Kwaga, J.K.P. 2020. Induction of rabies virus infection in mice brain may up and down regulate type II interferon gamma via epigenetic modifications. Metab. Brain. Dis. 35(5), 819–827. Albehwar, A.M.A. 2009. Studies on prophylactic and emergency vaccination of farm animals against rabies. Ph.D. Thesis (Infectious Diseases), Faculty of Veterinary Medicine, Cairo University, Cairo, Egypt. Blenden, D.C., Creech, W. and Torres-Angel, M.J. 1986. Use of immunofluorescence examination to detect rabies virus antigen in the skin of human with clinical encephalitis. J. Infect. Dis. 15(4), 698–701. Bourhy, H., Cowley, J.A., Larrous, F., Holmes, E.C. and Walker, P.J. 2005. Phylogenetic relationships among rhabdoviruses inferred using the L polymerase gene. J. Gen. Virol. 86(Pt 10), 2849–2858. Bryceson, A.D.M., Greenwood, B.M. and Warrell, D.A. 1975. Demonstration during life of rabies antigen in humans. J. Infect. Dis. 13(1), 71–74. Calisher, C.H. and Ellison, J. 2012. The other rabies viruses: the emergence and importance of Lyssaviruses from bats and other vertebrates. Travel. Med. Infect. Dis. 10(2), 69–79. Consales, C.A., Mendonca, R.Z., Gallina, N.M. and Pereira, C.A. 1990. Cytopathic effect induced by rabies virus in McCoy cells. Virol. Methods. 27(3), 277–285. Craig Greene. 2012. Infectious diseases of the dog and cat, 4th ed. Elsevier. Crepin, P., Audry, L., Rotivel, Y., Gacoin, A., Caroff, C. and Bourhy, H. 1998. Intravital diagnosis of human rabies by PCR using saliva and cerebrospinal fluid. J. Clin. Microbiol. 36, 1117–1121. Dacheux, L., Reynes, J.M., Buchy, P., Sivuth, O., Diop, B.M., Rousset, D., Rathat, C., Jolly, N., Dufourcq, J.B., Nareth, C., Diop, S., Iehlé, C., Rajerison, R., Sadorge, C. and Bourhy, H. 2008. A reliable diagnosis of human rabies based on analysis of skin biopsy specimens. Clin. Infect. Dis. 47(11), 1410–1417. David, D., Yakobson, B., Rotenberg, D., Dveres, N., Davidson, I. and Stram, Y. 2002. Rabies virus detection by RT-PCR in decomposed naturally infected brains. Vet. Microbiol. 87, 111–118. Ehizibolo, D., Nwosuh, C., Ehizibolo, E. and Kia, G. 2013. Comparison of the fluorescent antibody test and direct microscopic examination for rabies diagnosis at the National Veterinary Research Institute, Vom, Nigeria. Afr. J. Biomed. Res. 12(1), 73–76. El-Settawy, M.A. and Fathy, G.M. 2012. Evaluation and comparison of PCR, Coproantigen ELISA and microscopy for diagnosis of Cryptosporidium in human diarrheic specimen. J. Am. Sci. 8(12), 1378–1385. Ermine, A., Tordo, N. and Tsiang, H. 1988. Rapid diagnosis of rabies infection by means of a dot hybridization assay. Mol. Cell. Probes. 2, 75–82. Garg, R., Patil, P.K., Singh, S.V., Sharma, S., Gandham, R.K., Singh, A.V., Filia, G., Singh, P.K., Jayaraman, S., Gupta, S., Chaubey, K.K., Tiwari, R., Saminathan, M., Dhama, K. and Sohal, J.S. 2015. Comparative evaluation of different test combinations for diagnosis of Mycobacterium avium subspecies paratuberculosis infecting dairy herds in India. Biomed. Res. Int. 2015, 983978. Hassanzadeh, M.S., Zavareh, A.M., Shokrgozar, A., Ramezani, A. and Fayaz, A. 2011. High vero cell density and rabies virus proliferation on fibracel disks versus cytodex-1 in spinner flask. Pak. J. Biol. Sci. 14(7), 441–448. Jackson, A.C. 2013. Rabies, 3rd ed. Toronto, Canada: Elsevier Inc., Academic Press. Kasempimolporn, S., Chaiyabutr, N. and Sitprija, V. 2014. Demonstration of rabies antibody by a latex agglutination test. In Current laboratory techniques in rabies diagnosis, research and prevention. Eds., Rupprecht, C. and Nagarajan T. Elsevier, pp: 141–145. Kasempimolporn, S., Saengseesom, W., Lumlertdacha, B. and Siriraj, V. 2000. Detection of rabies virus antigen in dog saliva using a latex agglutination test. J. Clin. Microbiol. 38(8), 3098–3099. Knezevic, I. 2008. BHK-21 cell culture rabies vaccine: lmmunogenicity of a candidate vaccine for humans. Develop. Biol. 131, 421–429. Madhusudana, S.N. and Saraswati, S. 2003. Development and evaluation of a latex agglutination test for rabies antibodies. J. Clin. Virol. 27, 129–135. Mani, R.S. and Madhusudana, S.N. 2013. Laboratory diagnosis of human rabies: recent advances. Sci. World. J. 2013, e569712. Mayes, B. and Rupprecht, C.E. 2015. Direct fluorescent antibody test for rabies diagnosis research. In Current laboratory techniques in rabies diagnosis, research and prevention, volume 2. Eds., Rupprecht, C. and Nagarajan, T. Cambridge, MA: Academic Press, pp: 83–92. Mcelhinney, L.M., Fooks, A.R. and Radford, A.D. 2008. Diagnostic tools for the detection of rabies virus. Euro. J. Comp. Anim. Pract. 18, 224–231. OIE. 2012. Manual of diagnostic tests and vaccines for terrestrial animals. OIE. Available via http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2.01.13_RABIES.pdf Perrin, P. and Sureau, P.A. 1987. Collaborative study of an experimental kit for rapid rabies enzyme immunodiagnosis (RREID). Bull. World. Health. Organ. 65, 489–493. Perrin, P., Versmisse, P. and Sureau, P. 1988. A rabies agglutination test (RAT) for rabies antibody detection. J. Biol. Stand. 16, 281–286. Persichetti, M.F., Solano-Gallego, L., Vullo, A., Masucci, M., Marty, P., Delaunay, P., Vitale, F. and Pennisi, M.G. 2017. Diagnostic performance of ELISA, IFAT and Western blot for the detection of anti-Leishmania infantum antibodies in cats using a Bayesian analysis without a gold standard. Parasit. Vectors. 10(1), 119. Rojas Anaya, E., Loza-Rubio, E., Banda Ruiz, V.M. and Hernández Baumgarten, E. 2006. Use of reverse transcription-polymerase chain reaction to determine the stability of rabies virus genome in brains kept at room temperature. J. Vet. Diagn. Invest. 18(1), 98–101. Rupprecht, C.E., Briggs, D., Brown, C.M., Franka, R., Katz, S.L., Kerr, H.D., Lett, S., Levis, R., Meltzer, M.I., Schaffner, W. and Cieslak, P.R. 2009. Evidence for a 4-dose vaccine schedule for human rabies post-exposure prophylaxis in previously non-vaccinated individuals. Vaccine 27(51), 7141–7148. Saengseesom, W., Kasempimolporn, S., Akesowan, S., Ouisuwan, S. and Sitprija, V. 2010. Use of latex agglutination test to determine rabies antibodies in production of rabies antisera in horses. Southeast. Asian. J. Trop. Med. Public. Health. 41(6), 1387–1392. Saxena, S.N., Madhusudana, S.N., Tripathi, K.K., Gupta, P. and Ahuja, S. 1989. Evaluation of the new rapid rabies immunodiagnosis technique. Indian. J. Med. Res. 89, 445–448. Scheffer, K.C., Iamamoto, K., Asano, K.M., Mori, E., Garcia, A.I.E., Achkar, S.M. and de Oliveira Fahl, W. 2014. Murciélagos hematófagos como reservorios de la rabia. Rev. Peru. Med. Exp. Salud. Publica. 31(2), 302–309. Servat, A., Feyssaguet, M., Blanchard, I., Morize, J.L., Schereffer, J.L., Boue, F. and Cliquet, F. 2007. A quantitative indirect ELISA to monitor the effectiveness of rabies vaccination in domestic and wild carnivores. J. Immunol. Methods. 318(1–2), 1–10. Shoji, Y.S., Nakamichi, K., Kurane, I., Sakai, T. and Morimoto, K. 2004. Generation and characterization of P gene-deficient rabies virus. Virology 318(1), 295–305. Smith, T.G. and Gilbert, A.T. 2017. Comparison of a micro-neutralization test with the rapid fluorescent focus inhibition test for measuring rabies virus neutralizing antibodies. Trop. Med. Infect. Dis. 2(3), 24. Swanepoel, R., Barnard, B.J., Meredith, C.D., Bishop, G.C., Brückner, G.K., Foggin, C.M. and Hübschle, O.J. 1993. Rabies in southern Africa. Onderstepoort. J. Vet. Res. 60(4), 325–346. Tenzin, T., Lhamo, K., Rai, P.B., Tshering, D., Jamtsho, P., Namgyal, J., Wangdi, T., Letho, S., Rai, T., Jamtsho, S., Dorji, C., Rinchen, S., Lungten, L., Wangmo, K., Lungten, L., Wangchuk, P., Gempo, T., Jigme, K., Phuntshok, K., Tenzinla, T., Gurung, R.B. and Dukpa, K. 2020. Evaluation of a rapid immunochromatographic test kit to the gold standard fluorescent antibody test for diagnosis of rabies in animals in Bhutan. BMC. Vet. Res. 16(1), 183. Williamson, J.G. 2010. Rabies: symptoms, treatment and prevention. New York, NY: Nova Science Publishers, Inc. WHO. 2018. An indirect fluorescent antibody test for the detection of rabies virus immunoglobulin G and immunoglobulin M antibodies; Laboratory Techniques in rabies Chapter 21. Geneva, Switzerland: WHO. | ||
| How to Cite this Article |
| Pubmed Style Hegazy DN, Hosseny E, Abo-Senna A, Salem Z, Khodeir M. Comparative evaluation of some techniques used for detection of rabies virus. Open Vet J. 2023; 13(9): 1175-1183. doi:10.5455/OVJ.2023.v13.i9.13 Web Style Hegazy DN, Hosseny E, Abo-Senna A, Salem Z, Khodeir M. Comparative evaluation of some techniques used for detection of rabies virus. https://www.openveterinaryjournal.com/?mno=159494 [Access: May 19, 2024]. doi:10.5455/OVJ.2023.v13.i9.13 AMA (American Medical Association) Style Hegazy DN, Hosseny E, Abo-Senna A, Salem Z, Khodeir M. Comparative evaluation of some techniques used for detection of rabies virus. Open Vet J. 2023; 13(9): 1175-1183. doi:10.5455/OVJ.2023.v13.i9.13 Vancouver/ICMJE Style Hegazy DN, Hosseny E, Abo-Senna A, Salem Z, Khodeir M. Comparative evaluation of some techniques used for detection of rabies virus. Open Vet J. (2023), [cited May 19, 2024]; 13(9): 1175-1183. doi:10.5455/OVJ.2023.v13.i9.13 Harvard Style Hegazy, D. N., Hosseny, . E., Abo-Senna, . A., Salem, . Z. & Khodeir, . M. (2023) Comparative evaluation of some techniques used for detection of rabies virus. Open Vet J, 13 (9), 1175-1183. doi:10.5455/OVJ.2023.v13.i9.13 Turabian Style Hegazy, Dalia Nasr, Ebtesam Hosseny, Amal Abo-Senna, Zeinab Salem, and Mohamed Khodeir. 2023. Comparative evaluation of some techniques used for detection of rabies virus. Open Veterinary Journal, 13 (9), 1175-1183. doi:10.5455/OVJ.2023.v13.i9.13 Chicago Style Hegazy, Dalia Nasr, Ebtesam Hosseny, Amal Abo-Senna, Zeinab Salem, and Mohamed Khodeir. "Comparative evaluation of some techniques used for detection of rabies virus." Open Veterinary Journal 13 (2023), 1175-1183. doi:10.5455/OVJ.2023.v13.i9.13 MLA (The Modern Language Association) Style Hegazy, Dalia Nasr, Ebtesam Hosseny, Amal Abo-Senna, Zeinab Salem, and Mohamed Khodeir. "Comparative evaluation of some techniques used for detection of rabies virus." Open Veterinary Journal 13.9 (2023), 1175-1183. Print. doi:10.5455/OVJ.2023.v13.i9.13 APA (American Psychological Association) Style Hegazy, D. N., Hosseny, . E., Abo-Senna, . A., Salem, . Z. & Khodeir, . M. (2023) Comparative evaluation of some techniques used for detection of rabies virus. Open Veterinary Journal, 13 (9), 1175-1183. doi:10.5455/OVJ.2023.v13.i9.13 |